Expression Profiling of Flavonoid Biosynthesis Genes and Secondary Metabolites Accumulation in Populus under Drought Stress
Abstract
1. Introduction
2. Results
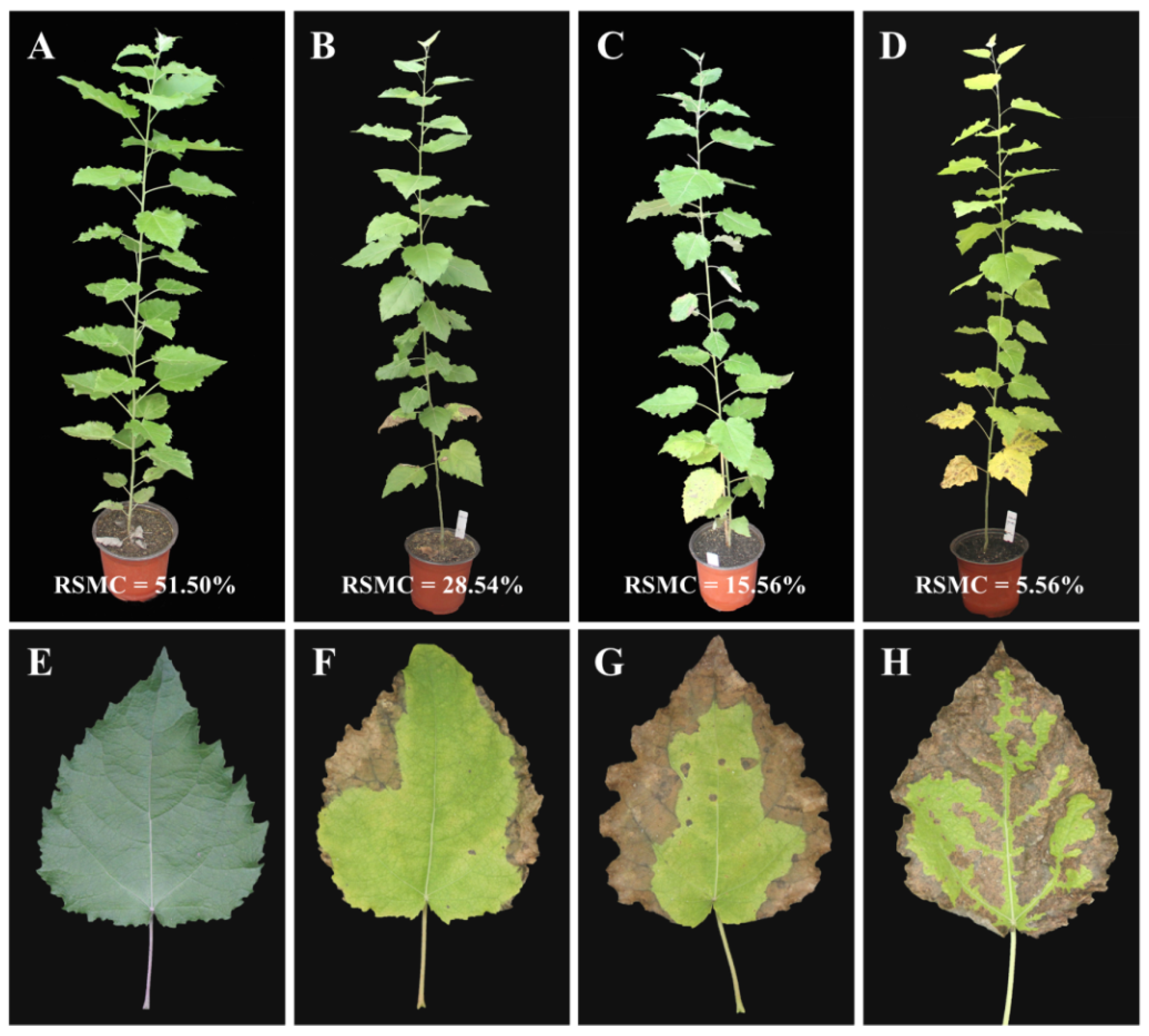
2.1. Morphological Changes in Poplar Leaves under Drought Stress
2.2. Photosynthesis under Drought Stress in Poplar
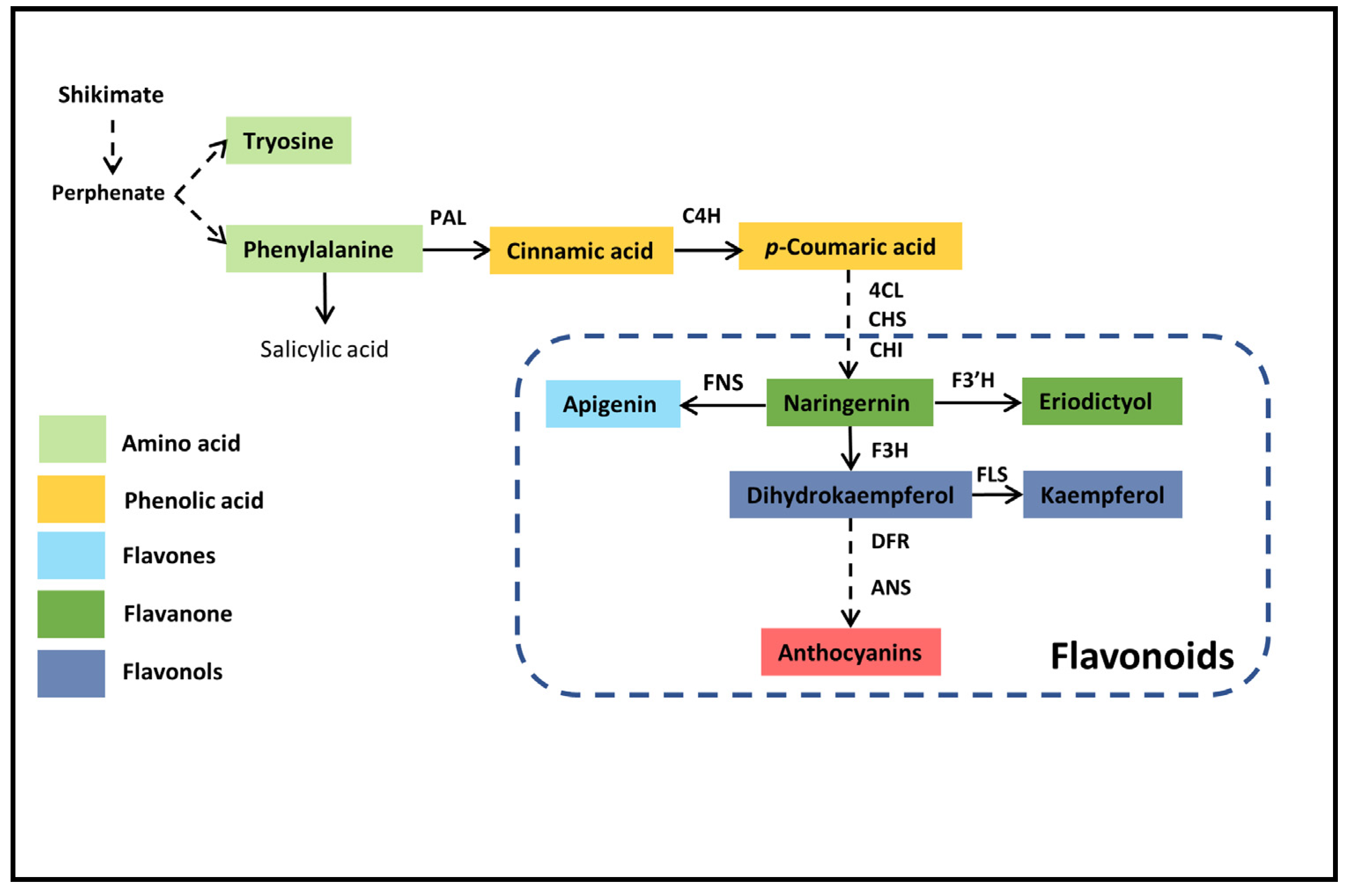
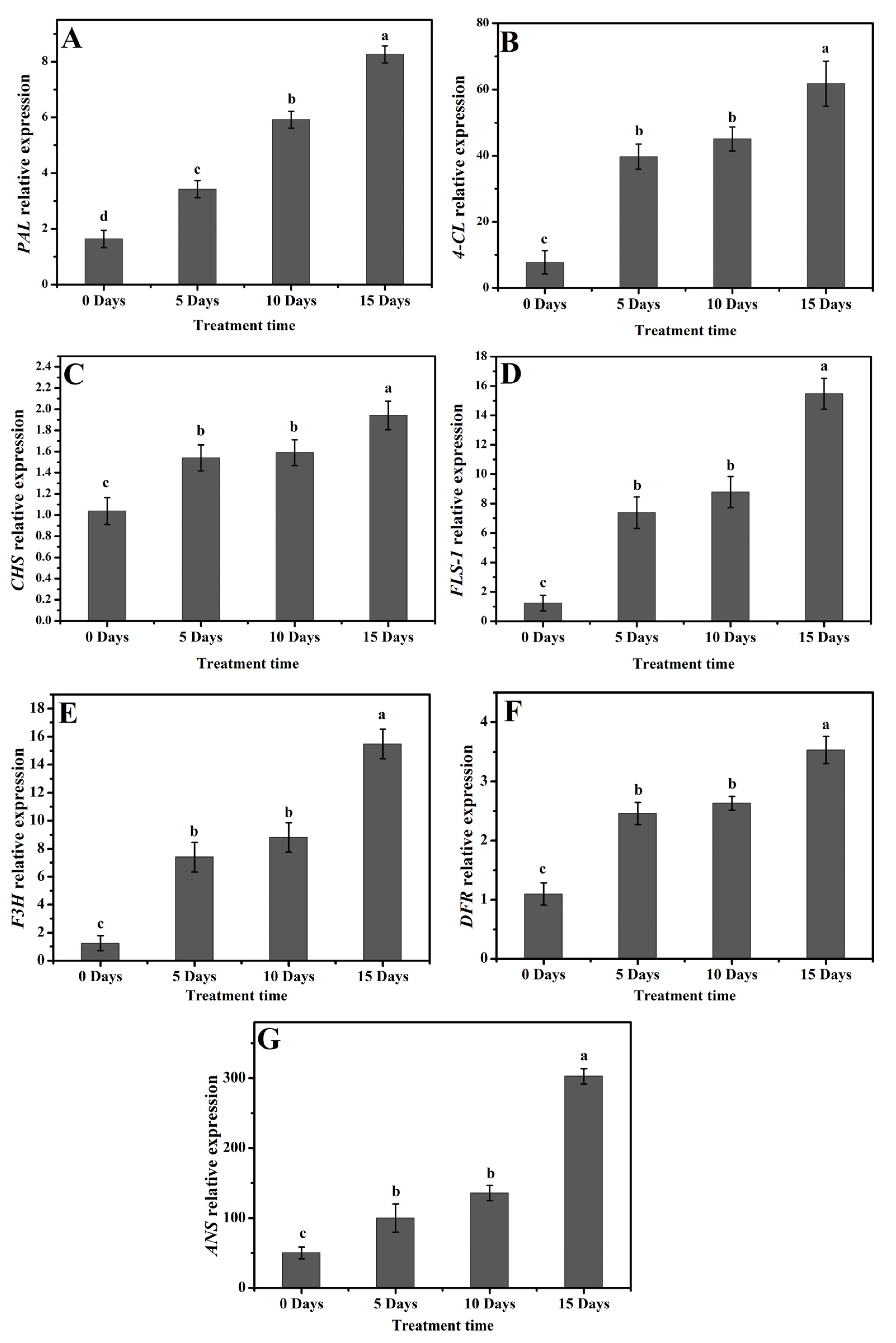
2.3. Expression Profiling of Flavonoid Biosynthesis Genes under Drought Stress in Poplar Leaves
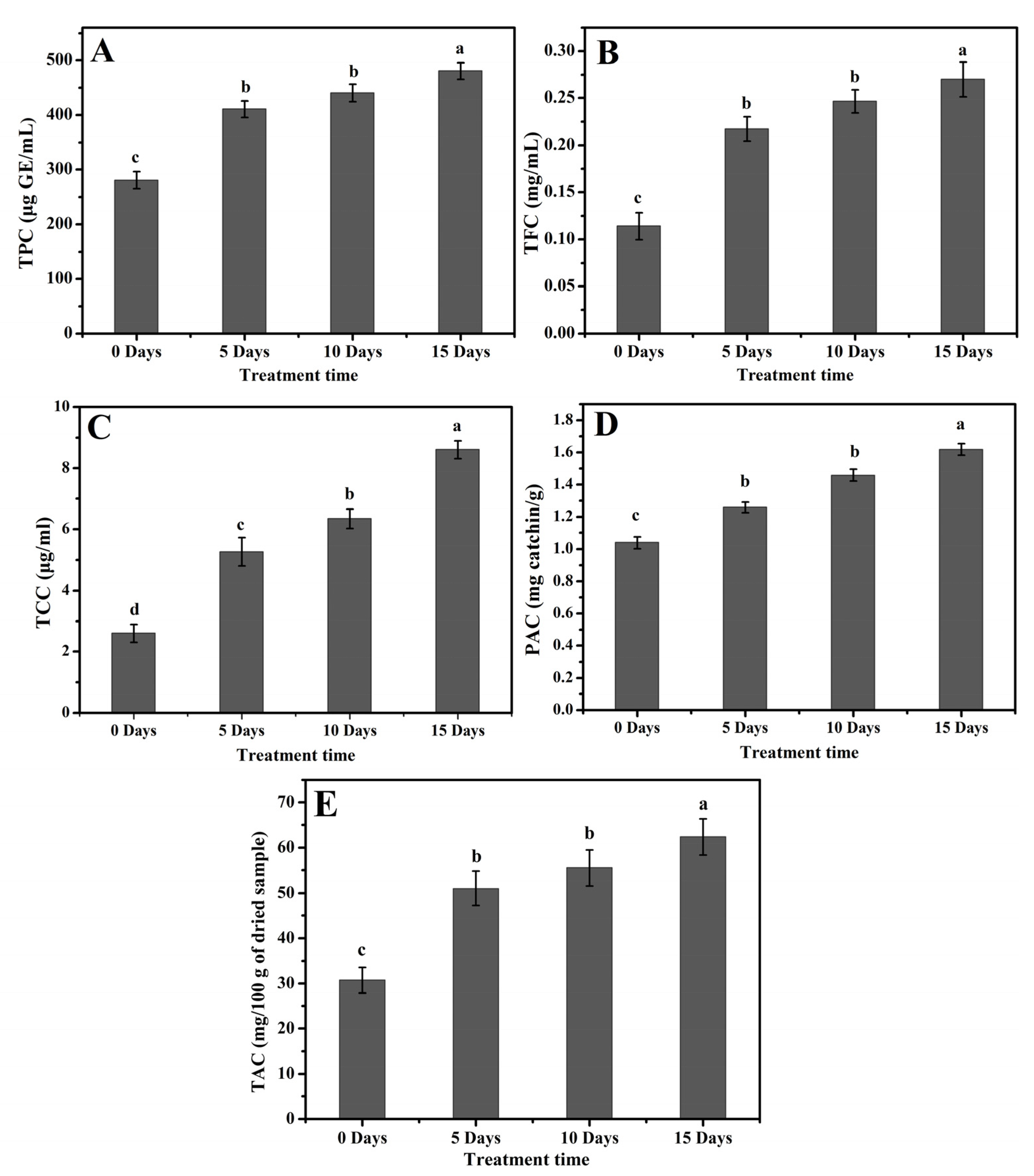
2.4. Accumulation of Total Phenolics, Flavonoids, and Carotenoids Content in Poplar Leaves under Drought Stress
2.5. Accumulation of PAC and TAC in Poplar Leaves under Drought Stress
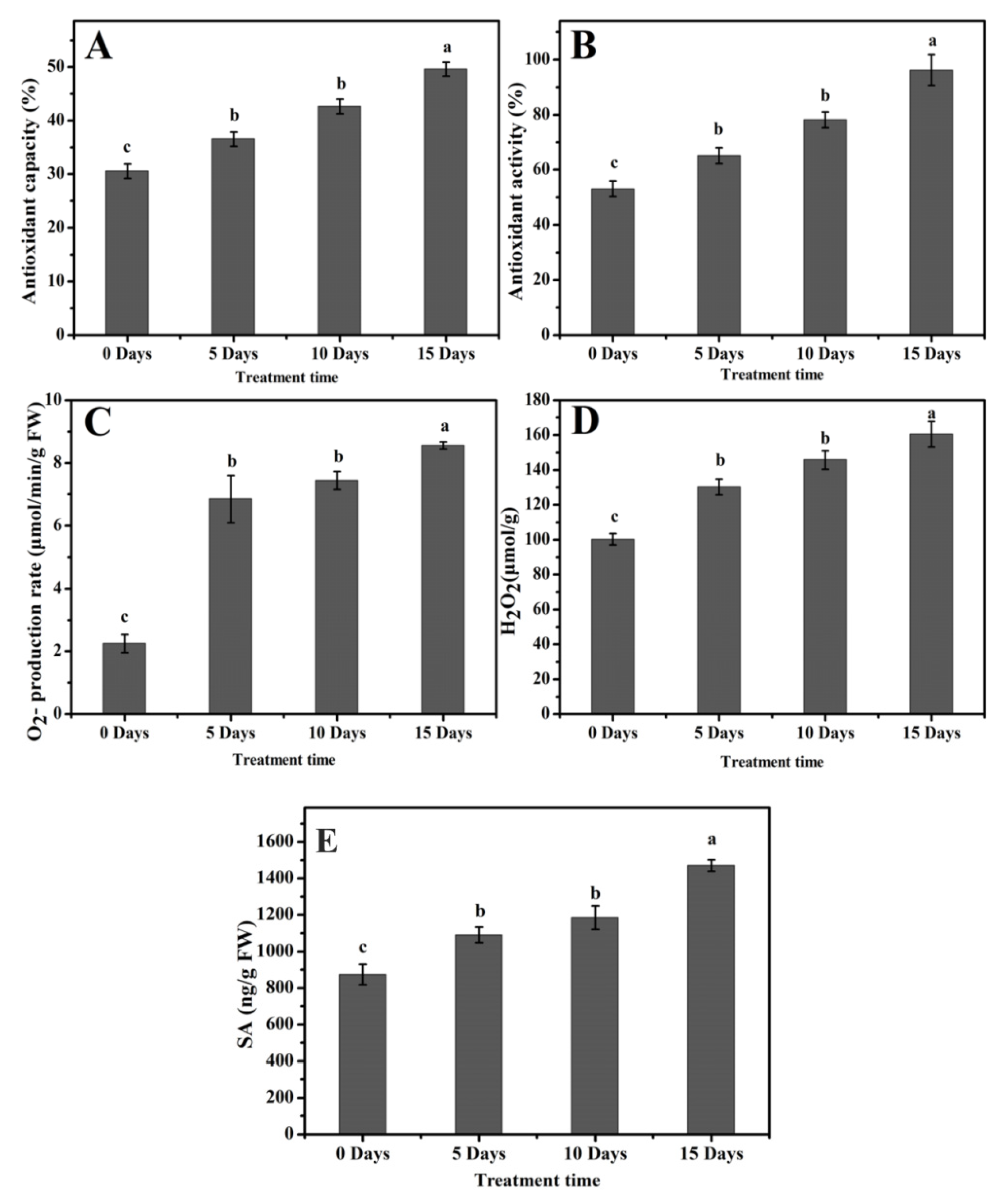
2.6. Enhanced Antioxidant Capacity and Antioxidant Activity
2.7. High Accumulation of O2−, H2O2, and Salicylic Acid under Drought Stress
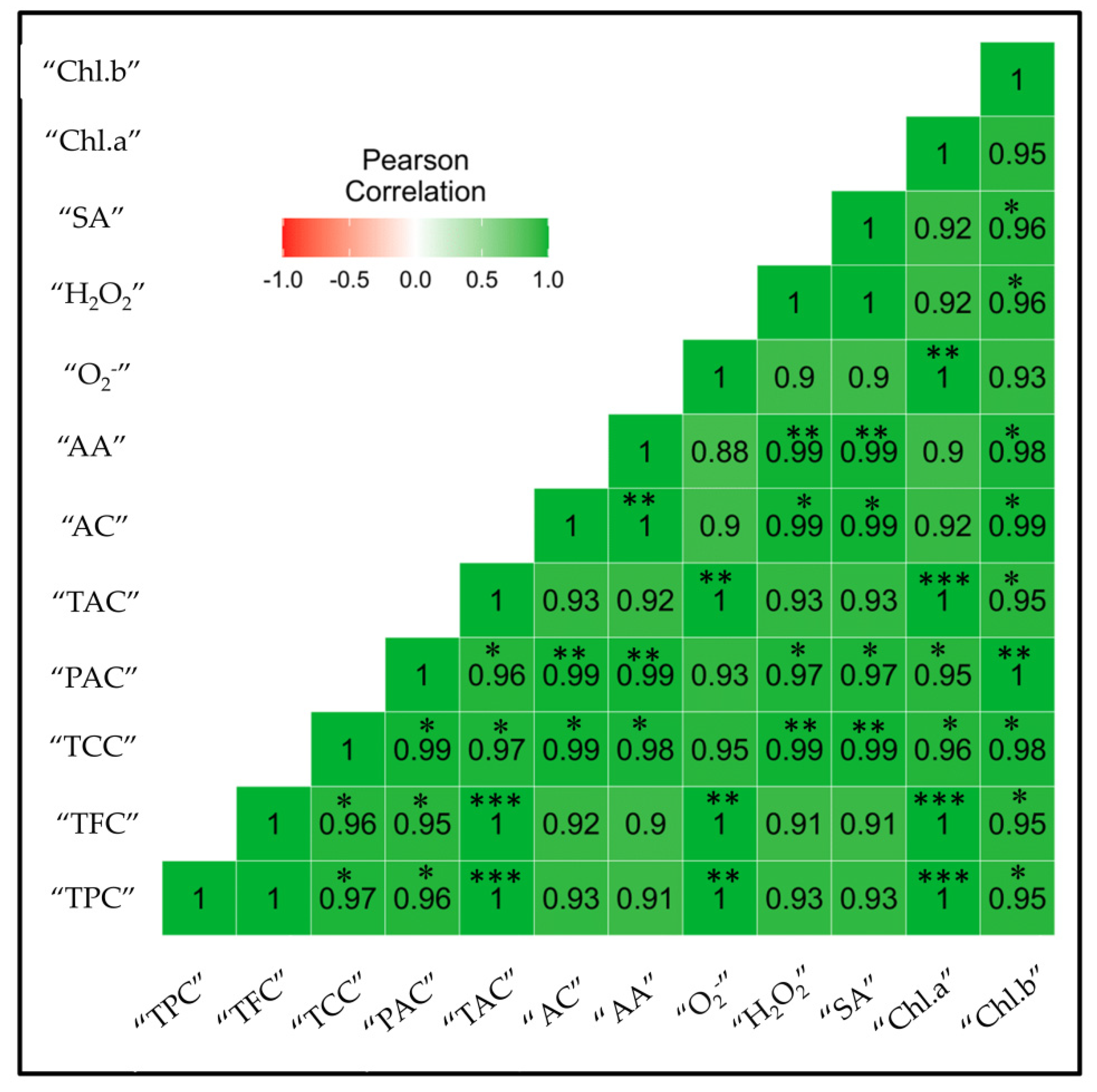
2.8. Correlation Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Drought Stress Treatment
4.3. Measurement of Photosynthetic Parameters
Chl b (mg/L) = A645 × 22.9 − A663 × 4.68
4.4. Total RNA Extraction cDNA Synthesis and qRT-PCR
4.5. Extraction and Evaluation of Secondary Metabolites from Poplar Leaves
4.5.1. Total Flavonoids Content and Total Phenolics Content
4.5.2. Total Carotenoids Content
4.5.3. Proanthocyanidins and Total Anthocyanin Content
4.6. Investigation of Antioxidants Capacity, Antioxidants Activity, and H2O2 and O2− Production under Drought Stress
4.7. Estimation and Evaluation of Salicylic Acid
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Shafroth, P.B.; Stromberg, J.C.; Patten, D.T. Woody riparian vegetation response to different alluvial water table regimes. West. N. Am. Nat. 2000, 60, 66–76. [Google Scholar]
- Ma, D.; Sun, D.; Wang, C.; Li, Y.; Guo, T. Expression of flavonoid biosynthesis genes and accumulation of flavonoid in wheat leaves in response to drought stress. Plant Physiol. Biochem. 2014, 80, 60–66. [Google Scholar] [CrossRef]
- Li, C.; Wang, K. Differences in drought responses of three contrasting Eucalyptus microtheca F. Muell. populations. For. Ecol. Manag. 2003, 179, 377–385. [Google Scholar] [CrossRef]
- Yin, C.; Wang, X.; Duana, B.; Luob, J.; Li, C. Early growth, dry matter allocation and water use efficiency of two sympatric Populus species as affected by water stress. Environ. Exp. Bot. 2005, 53, 315–322. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, N.; Li, C. Physiological and growth responses of Populus davidiana ecotypes to different soil water contents. J. Arid Environ. 2005, 60, 567–579. [Google Scholar] [CrossRef]
- Salem, N.; Msaada, K.; Dhifi, W.; Limam, F.; Marzouk, B. Effect of salinity on plant growth and biological activities of Carthamus tinctorius L. extracts at two flowering stages. Acta Physiol. Plant 2014, 36, 433–445. [Google Scholar] [CrossRef]
- Pirbalouti, A.G.; Malekpoor, F.; Salimi, A.; Golparvar, A. Exogenous application of chitosan on biochemical and physiological characteristics, phenolic content and antioxidant activity of two species of basil (Ocimum ciliatum and Ocimum basilicum) under reduced irrigation. Sci. Hortic. 2017, 217, 114–122. [Google Scholar] [CrossRef]
- Zhang, W.; Cao, Z.; Xie, Z.; Lang, D.; Zhou, L.; Chu, Y.; Zhao, Q.; Zhang, X.; Zhao, Y. Effect of water stress on roots biomass and secondary metabolites in the medicinal plant Stellaria dichotoma L. var. lanceolata Bge. Sci. Hortic. 2017, 224, 180–185. [Google Scholar] [CrossRef]
- Kozlowski, T.T.; Pallardy, S. Acclimation and adaptive responses of woody plants to environmetal stresses. Bot. Rev. 2002, 68, 279–334. [Google Scholar] [CrossRef]
- Ruiz-Sánchez, M.C.; Domingo, R.; Pérez-Pastor, A. Daily variations in water relations of apricot trees under different irrigation regimes. Biol. Plant 2007, 51, 735–740. [Google Scholar] [CrossRef]
- Shvaleva, A.L.; Costa, E.; Silva, F.; Breia, E.; Jouve, J.; Hausman, J.F.; Almeida, M.H.; Maroco, J.P.; Rodrigues, M.L.; Pereira, J.S.; et al. Metabolic responses to water deficit in two Eucalyptus globulus clones with contrasting drought sensitivity. Tree Physiol. 2006, 26, 239–248. [Google Scholar] [CrossRef]
- Horváth, E.; Pál, M.; Szalai, G.; Páldi, E.; Janda, T. Exogenous 4-hydroxybenzoic acid and salicylic acid modulate the effect of short-term drought and freezing stress on wheat plants. Biol. Plant 2007, 51, 480–487. [Google Scholar] [CrossRef]
- Ren, J.; Dai, W.; Xuan, Z.; Yao, Y.; Korpelainen, H.; Li, C. The effect of drought and enhanced UV-B radiation on the growth and physiological traits of two contrasting poplar species. For. Ecol. Manag. 2007, 239, 112–119. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, X.; Li, C. Growth, abscisic acid content, and carbon isotope composition in wheat cultivars grown under different soil moisture. Biol. Plant 2007, 51, 181–184. [Google Scholar] [CrossRef]
- Tardieu, F.; Tuberosa, R. Dissection and modeling of abiotic tolerance plants. Curr. Opin. Plant Biol. 2010, 13, 206–212. [Google Scholar] [CrossRef]
- Valliyodan, B.; Nguyen, H.T. Understanding regulatory networks and engineering for enhanced drought tolerance in plants. Curr. Opin. Plant Biol. 2006, 9, 189–195. [Google Scholar] [CrossRef]
- Rosa, D.D.; Furtado, E.L.; Boava, L.P.; Marino, C.L.; Mori, E.S.; Guerrini, I.A.; Veline, E.D.; Wilcken, C.F. Eucalyptus ESTs involved in mechanisms against plant pathogens and environmental stresses. Summa Phytopathol. 2010, 36, 282–290. [Google Scholar] [CrossRef]
- Daayf, F.; El-Hadrami, A.; El-Bebany, A.F.; Henriquez, M.A.; Yao, Z.; Derksen, H.; El-Hadrami, I.; Adam, L.R. Phenolic Compounds in Plant Defense and Pathogen Counter-Defense Mechanisms. In Recent Advances in Polyphenol Research; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2012; pp. 191–208. ISBN 9781118299753. [Google Scholar]
- Ferreyra, M.L.F.; Rius, S.P.; Casati, P. Flavonoids: Biosynthesis, biological functions, and biotechnological applications. Front. Plant Sci. 2012, 3, 222. [Google Scholar]
- Treutter, D. Significance of flavonoids in plant resistance: A review. Environ. Chem. Lett. 2006, 4, 147–157. [Google Scholar] [CrossRef]
- Shih, C.H.; Chu, H.; Tang, L.K.; Sakamoto, W.; Maekawa, M.; Chu, I.K.; Wang, M.; Lo, C. Functional characterization of key structural genes in rice flavonoid biosynthesis. Planta 2008, 228, 1043–1054. [Google Scholar] [CrossRef] [PubMed]
- Constabel, C.P.; Lindroth, R. The Impact of Genomics on Advances in Herbivore Defense and Secondary Metabolism in Populus. In Genetics and Genomics of Populus; Jansson, S., Bhalerao, R., Groover, A., Eds.; Springer: New York, NY, USA, 2010; pp. 279–305. ISBN 978-1-4419-1541-2. [Google Scholar]
- Daniels, W.; Rautenbach, F.; Marnewick, J.L.; Valentine, A.J.; Babajide, O.J.; Mabusela, W.T. Environmental stress effect on the phytochemistry and antioxidant activity of a South African bulbous geophyte, Gethyllis multifolia L. Bolus. S. Afr. J. Bot. 2015, 96, 29–36. [Google Scholar] [CrossRef]
- Bistgani, Z.E.; Siadat, S.A.; Bakhshandeh, A.; Pirbalouti, A.G.; Hashemi, M. Morpho-physiological and phytochemical traits of (Thymus daenensis Celak.) in response to deficit irrigation and chitosan application. Acta Physiol. Plant 2017, 39, 231. [Google Scholar] [CrossRef]
- Vosoughi, N.; Gomarian, M.; Pirbalouti, A.G.; Khaghani, S.; Malekpoor, F. Essential oil composition and total phenolic, flavonoid contents, and antioxidant activity of sage (Salvia officinalis L.) extract under chitosan application and irrigation frequencies. Ind. Crops Prod. 2018, 117, 366–374. [Google Scholar] [CrossRef]
- Markham, K.R.; Tanner, G.J.; Caasi-Lit, M.; Whitecross, M.I.; Nayudu, M.; Mitchell, K.A. Possible protective role for 3′, 4′-dihydroxyflavones induced by enhanced UV-B in a UV-tolerant rice cultivar. Phytochemistry 1998, 49, 1913–1919. [Google Scholar] [CrossRef]
- Khoyerdi, F.F.; Shamshiri, M.H.; Estaji, A. Changes in some physiological and osmotic parameters of several pistachio genotypes under drought stress. Sci. Hortic. 2016, 198, 44–51. [Google Scholar] [CrossRef]
- Gharibi, S.; Tabatabaei, B.E.; Saeidi, G.; Goli, S.A. Effect of Drought Stress on Total Phenolic, Lipid Peroxidation, and Antioxidant Activity of Achillea Species. Appl. Biochem. Biotechnol. 2016, 178, 796–809. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; Deavours, B.E.; de Fáltima, Â.; Naoumkina, M.; Dixon, R.A.; Sumner, L.W. Integrated metabolite and transcript profiling identify a biosynthetic mechanism for hispidol in Medicago truncatula cell cultures. Plant Physiol. 2009, 151, 1096–1113. [Google Scholar] [CrossRef] [PubMed]
- Hall, R.; Heybroek, H. Biology of Populus and its implications for management and conservation. For. Sci. 1997, 43, 457–458. [Google Scholar]
- Brunner, A.M.; Busov, V.B.; Strauss, S.H. Poplar genome sequence: Functional genomics in an ecologically dominant plant species. Trends Plant Sci. 2004, 9, 49–56. [Google Scholar] [CrossRef]
- Tuskan, G.A.; Difazio, S.; Jansson, S.; Bohlmann, J.; Grigoriev, I.; Hellsten, U.; Putnam, N.; Ralph, S.; Rombauts, S.; Salamov, A.; et al. The genome of black cottonwood, Populus trichocarpa (Torr. & Gray). Science 2006, 313, 1596–1604. [Google Scholar]
- Monclus, R.; Dreyer, E.; Villar, M.; Delmotte, F.M.; Delay, D.; Petit, J.; Barbaroux, C.; Le Thiec, D.; Bréchet, C.; Brignolas, F. Impact of drought on productivity and water use efficiency in 29 genotypes of Populus deltoides × Populus nigra. New Phytol. 2006, 169, 765–777. [Google Scholar] [CrossRef]
- Yin, C.; Duan, B.; Wang, X.; Li, C. Morphological and physiological responses of two contrasting Poplar species to drought stress and exogenous abscisic acid application. Plant Sci. 2004, 167, 1091–1097. [Google Scholar] [CrossRef]
- Taylor, G. Populus: Arabidopsis for forestry. Do we need a model tree? Ann. Bot. 2002, 90, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Mader, M.; Le Paslier, M.C.; Bounon, R.; Berard, A.; Rampant, P.F.; Fladung, M.; Leple, J.C.; Kersten, B. Whole-genome draft assembly of Populus tremula × P. alba clone INRA 717-1B4. Silvae Genet. 2016, 65, 74–79. [Google Scholar] [CrossRef]
- Bai, Q.; Duan, B.; Ma, J.; Fen, Y.; Sun, S.; Long, Q.; Lv, J.; Wan, D. Coexpression of PalbHLH1 and PalMYB90 genes from Populus alba enhances pathogen resistance in poplar by increasing the flavonoid content. Front. Plant Sci. 2020, 10, 1772. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Chen, M.; Zhou, H.; Zhou, X.; Wang, Y. Metabolite profiles of Populus in response to pathogen stress. Biochem. Biophys. Res. Commun. 2015, 465, 421–426. [Google Scholar] [CrossRef]
- Syvertsen, J.P.; Garcia-Sanchez, F. Multiple abiotic stresses occurring with salinity stress in citrus. Environ. Exp. Bot. 2014, 103, 128–137. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Augmentation of leaf color parameters, pigments, vitamins, phenolic acids, flavonoids and antioxidant activity in selected Amaranthus tricolor under salinity stress. Sci. Rep. 2018, 8, 12349. [Google Scholar] [CrossRef]
- Yu, J.J.; Jin, X.; Sun, X.M.; Gao, T.X.; Chen, X.M.; She, Y.M.; Jiang, T.B.; Chen, S.X.; Dai, S.J. Hydrogen peroxide response in leaves of Poplar (Populus simonii × Populus nigra) revealed from physiological and proteomic analyses. Int. J. Mol. Sci. 2017, 18, 2085. [Google Scholar] [CrossRef]
- Roby, G.; Harbertson, J.F.; Adams, D.A.; Matthews, M.A. Berry size and vine water deficits as factors in winegrape composition: Anthocyanins and tannins. Aust. J. Grape Wine Res. 2004, 10, 100–107. [Google Scholar] [CrossRef]
- Lenka, S.K.; Katiyar, A.; Chinnusamy, V.; Bansal, K.C. Comparative analysis of drought-responsive transcriptome in Indica rice genotypes with contrasting drought tolerance. Plant Biotechnol. J. 2011, 9, 315–327. [Google Scholar] [CrossRef]
- Vasquez-Robinet, C.; Mane, S.P.; Ulanov, A.V.; Watkinson, J.I.; Stromberg, V.K.; De Koeyer, D.; Schafleitner, R.; Willmot, D.B.; Bonierbale, M.; Bohnert, H.J.; et al. Physiological and molecular adaptations to drought in Andean potato genotypes. J. Exp. Bot. 2008, 59, 2109–2123. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Gu, M.; Lai, Z.; Fan, B.; Shi, K.; Zhou, Y.; Yu, J.; Chen, Z. Functional analysis of the Arabidopsis PAL gene family in plant growth, development, and response to environmental stress. Plant Physiol. 2010, 153, 1526–1538. [Google Scholar] [CrossRef]
- Dempsey, D.M.A.; Vlot, A.C.; Wildermuth, M.C.; Klessig, D.F. Salicylic acid biosynthesis and metabolism. Arab. Book 2011, 9, e0156. [Google Scholar] [CrossRef]
- Liu, M.L.; Li, X.R.; Liu, Y.B.; Cao, B. Regulation of flavanone 3-hydroxylase gene involved in the flavonoid biosynthesis pathway in response to UV-B radiation and drought stress in the desert plant, Reaumuria soongorica. Plant Physiol. Biochem. 2013, 73, 161–167. [Google Scholar] [CrossRef]
- Castellarin, S.D.; Matthews, M.A.; Di Gaspero, G.; Gambetta, G.A. Water deficits accelerate ripening and induce changes in gene expression regulating flavonoid biosynthesis in grape berries. Planta 2007, 227, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.G.; Lee, E.R.; Ahn, J.H. Analysis of flavonoid contents and expression of flavonoid biosynthetic genes in Populus euramericana Guinier in response to abiotic stress. J. Korean Soc. Appl. Biol. Chem. 2012, 80, 60–66. [Google Scholar] [CrossRef]
- Arbona, V.; Manzi, M.; Ollas, C.; Gomez-Cadenas, A. Metabolomics as a tool to investigate abiotic stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 4885–4911. [Google Scholar] [CrossRef]
- Fraser, C.M.; Chapple, C. The phenylpropanoid pathway in Arabidopsis. Arab. Book 2011, 9, e0152. [Google Scholar] [CrossRef]
- Hussain, S.; Rao, M.J.; Anjum, M.A.; Ejaz, S.; Zakir, I.; Ali, M.A.; Ahmad, N.; Ahmad, S. Oxidative Stress and Antioxidant defense in plants under drought conditions. In Plant Abiotic Stress Tolerance: Agronomic, Molecular and Biotechnological Approaches; Hasanuzzaman, M., Hakeem, K.R., Nahar, K., Alharby, H.F., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 207–219. [Google Scholar]
- Laxa, M.; Liebthal, M.; Telman, W.; Chibani, K.; Dietz, K.-J. The role of the plant antioxidant system in drought tolerance. Antioxidants 2019, 8, 94. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.J.; Xu, Y.; Huang, Y.; Tang, X.; Deng, X.; Xu, Q. Ectopic expression of citrus UDP-GLUCOSYL TRANSFERASE gene enhances anthocyanin and proanthocyanidins contents and confers high light tolerance in Arabidopsis. BMC Plant Biol. 2019, 19, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Khalid, M.F.; Saqib, M.; Ahmad, S.; Zafar, W.; Rao, M.J.; Morillon, R.; Anjum, M.A. Drought tolerance in citrus rootstocks is associated with better antioxidant defense mechanism. Acta Physiol. Plant 2018, 40, 135. [Google Scholar] [CrossRef]
- Okello, O.P.; Nawiri, M.P.; Musila, W.; Gweyi-Onyango, J.P. Water stress effect on total antioxiant activity and total phenolic content of Solanum scabrum and Solanum scabrum in Kiambu, Kenya. Int. J. Biochem. Res. Rev. 2017, 17, 1–9. [Google Scholar] [CrossRef][Green Version]
- Alothman, M.; Bhat, R.; Karim, A. Antioxidant capacity and phenolic content of selected tropical fruits from Malaysia, extracted with different solvents. Food Chem. 2009, 115, 785–788. [Google Scholar] [CrossRef]
- Miao, J.; Li, X.; Zhao, C.; Gao, X.; Wang, Y.; Gao, W. Active compounds, antioxidant activity and α -glucosidase inhibitory activity of different varieties of Chaenomeles fruits. Food Chem. 2018, 248, 330–339. [Google Scholar] [CrossRef]
- Abdallah, S.B.; Rabhi, M.; Harbaoui, F.; Zar-kalai, F.; Lachâal, M.; Karray-Bouraoui, N. Distribution of phenolic compounds and antioxidant activity between young and old leaves of Carthamus tinctorius L. and their induction by salt stress. Acta Physiol. Plant 2013, 35, 1161–1169. [Google Scholar] [CrossRef]
- Hudz, N.; Ivanova, R.; Brindza, J.; Grygorieva, O.; Schubertová, Z.; Ivanišová, E. Approaches to the determination of antioxidant activity of extracts from bee bread and safflower leaves and flowers. Potravin. Slovak J. Food Sci. 2017, 11, 480–488. [Google Scholar]
- Salem, N.; Msaada, K.; Hamdaoui, G.; Limam, F.; Marzouk, B. Variation in phenolic composition and antioxidant activity during flower development of safflower (Carthamus tinctorius L.). J. Agric. Food Chem. 2011, 59, 4455–4463. [Google Scholar] [CrossRef]
- Ramel, F.; Birtic, S.; Ginies, C.; Soubigou-Taconnat, L.; Triantaphylides, C.; Havaux, M. Carotenoid oxidation products are stress signals that mediate gene responses to singlet oxygen in plants. Proc. Natl. Acad. Sci. USA 2012, 109, 5535–5540. [Google Scholar] [CrossRef]
- Hou, X.; Rivers, J.; León, P.; McQuinn, R.P.; Pogson, B.J. Synthesis and function of apocarotenoid signals in plants. Trends Plant Sci. 2016, 21, 792–803. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Chen, S.; Zhao, Q.; Wang, T.; Yang, C.; Diaz, C.; Sun, G.; Dai, S. Physiological and proteomic analysis of salinity tolerance in Puccinellia tenuiflora. J. Proteome Res. 2011, 10, 3852–3870. [Google Scholar] [CrossRef]
- Sumanta, N.; Haque, C.I.; Nishika, J.; Suprakash, R. Spectrophotometric analysis of chlorophylls and carotenoids from commonly grown fern species by using various extracting solvents spectrophotometric analysis of chlorophylls and carotenoids from commonly grown fern species by using various extracting solvents. Res. J. Chem. Sci. 2014, 4, 63–69. [Google Scholar]
- Pfaffl, M.W.; Horgan, G.W.; Dempfle, L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002, 30, e36. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Liu, Y.; Tian, L.; Wan, M.; Zheng, B.; Shi, X. Involvement of Populus CLEL peptides in root development. Tree Physiol. 2019, 39, 1907–1921. [Google Scholar] [CrossRef]
- Velioglu, Y.S.; Mazza, G.; Gao, L.; Oomah, B.D. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J. Agric. Food Chem. 1998, 46, 4113–4117. [Google Scholar] [CrossRef]
- Dewanto, V.; Wu, X.; Adom, K.K.; Liu, R.H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef]
- Broadhurst, R.B.; Jones, W.T. Analysis of condensed tannins using acidified vanillin. J. Sci. Food Agric. 1978, 29, 788–794. [Google Scholar] [CrossRef]
- Nakata, M.; Ohme-Takagi, M. Quantification of anthocyanin content. Bio-Protocol 2014, 4, e1098. [Google Scholar] [CrossRef]
- Özgen, M.; Scheerens, J.C.; Neil Reese, R.; Miller, R.A. Total phenolic, anthocyanin contents and antioxidant capacity of selected elderberry (Sambucus canadensis L.) accessions. Pharm. Mag. 2010, 6, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, M.; Guo, Q.; Wang, G.; Gong, J.; Xu, Y.; Wang, W. Manipulation of monoubiquitin improves chilling tolerance in transgenic tobacco (Nicotiana tabacum). Plant Physiol. Biochem. 2014, 75, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Warrier, R.; Paul, M.; Vineetha, M. Estimation of salicylic acid in Eucalyptus leaves using spectrophotometric methods. Genet. Plant Physiol. 2013, 3, 90–97. [Google Scholar]

| Photosythetic Parameters | F Ratio | p-Value |
|---|---|---|
| Pn | 83.07 | 0.001 *** |
| Ci | 83.07 | 0.001 *** |
| gs | 133.37 | 0.001 *** |
| Tr | 227.71 | 0.001 *** |
| Chl a | 107.39 | 0.001 *** |
| Chl b | 9.14 | 0.0058 |
| Secondary Metabolites | F Ratio | p-Value |
|---|---|---|
| TPC | 146.45 | 0.001 *** |
| TFC | 106.74 | 0.001 *** |
| TCC | 153.74 | 0.001 *** |
| PAC | 202.61 | 0.001 *** |
| TAC | 308.46 | 0.001 *** |
| AC | 240.60 | 0.001 *** |
| AA | 85.82 | 0.001 *** |
| O2- | 122.85 | 0.001 *** |
| H2O2 | 150.40 | 0.001 *** |
| SA | 257.54 | 0.001 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, U.; Rao, M.J.; Qi, C.; Xie, Q.; Noushahi, H.A.; Yaseen, M.; Shi, X.; Zheng, B. Expression Profiling of Flavonoid Biosynthesis Genes and Secondary Metabolites Accumulation in Populus under Drought Stress. Molecules 2021, 26, 5546. https://doi.org/10.3390/molecules26185546
Ahmed U, Rao MJ, Qi C, Xie Q, Noushahi HA, Yaseen M, Shi X, Zheng B. Expression Profiling of Flavonoid Biosynthesis Genes and Secondary Metabolites Accumulation in Populus under Drought Stress. Molecules. 2021; 26(18):5546. https://doi.org/10.3390/molecules26185546
Chicago/Turabian StyleAhmed, Umair, Muhammad Junaid Rao, Cheng Qi, Qi Xie, Hamza Armghan Noushahi, Muhammad Yaseen, Xueping Shi, and Bo Zheng. 2021. "Expression Profiling of Flavonoid Biosynthesis Genes and Secondary Metabolites Accumulation in Populus under Drought Stress" Molecules 26, no. 18: 5546. https://doi.org/10.3390/molecules26185546
APA StyleAhmed, U., Rao, M. J., Qi, C., Xie, Q., Noushahi, H. A., Yaseen, M., Shi, X., & Zheng, B. (2021). Expression Profiling of Flavonoid Biosynthesis Genes and Secondary Metabolites Accumulation in Populus under Drought Stress. Molecules, 26(18), 5546. https://doi.org/10.3390/molecules26185546









