The Influence of Propolis on Dental Plaque Reduction and the Correlation between Dental Plaque and Severity of COVID-19 Complications—A Literature Review
Abstract
1. Introduction
2. Materials and Methods
- Is there any association between COVID-19 and oral health?
- Could there be a connection between dental plaque-related periodontal disease and the severity of SARS-Co-V-2 infection?
- Is there any junction among oral health, cytokine storm and COVID-19 complications?
- What is the effect of propolis on dental plaque reduction?
- What is the impact of propolis on the prevention of SARS-Co-V-2 infection?
3. Mechanism and Risk Factors of Biofilm Formation
4. The role of Dental Plaque in The Etiology of Dental Caries and Periodontitis
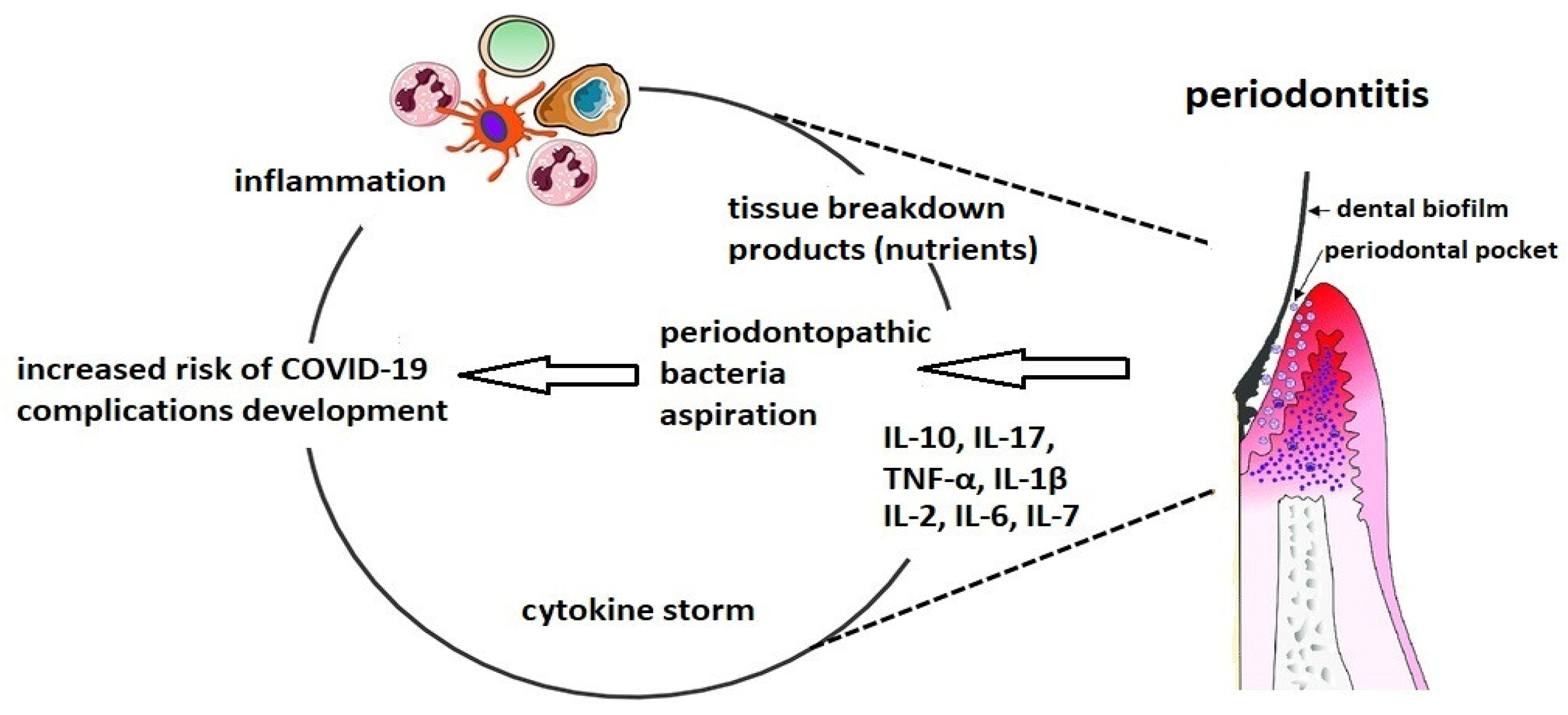
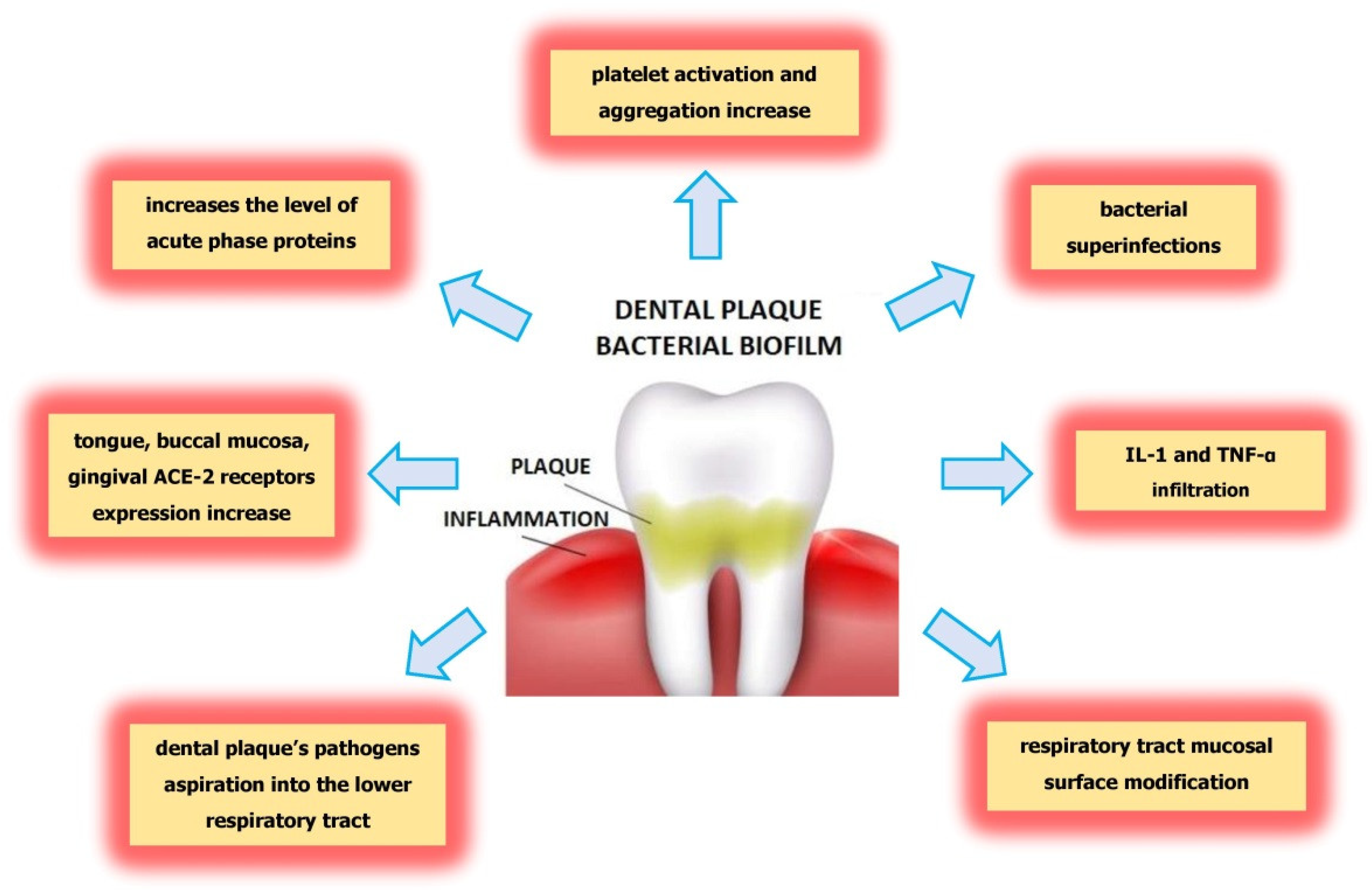
5. Possible Influence of Dental Plaque on Severe COVID-19 Complications
6. The Impact of Propolis on Dental Plaque Reduction
| Active substances. | Action | Literature |
|---|---|---|
| apigenin | inhibition the activity of glucosyltransferase | [41,49] |
| t-farnesol, apigenin, pinocembrin, artepillin C | antibacterial action | [31,32,41] |
| quercetin, apigenin, pinocembrin, caffeic acid phenethyl ester (CAPE) | reduction of biofilm formation | [33] |
7. The Impact of Propolis in Prevention against SARS-CoV-2
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Graves:, D.; Corrêa, J.; Silva, T. The Oral Microbiota Is Modified by Systemic Diseases. J. Dent. Res. 2018, 98, 148–156. [Google Scholar] [CrossRef]
- Yumoto, H.; Hirota, K.; Hirao, K.; Ninomiya, M.; Murakami, K.; Fujii, H.; Miyake, Y. The Pathogenic Factors from Oral Streptococci for Systemic Diseases. Int. J. Mol. Sci. 2019, 20, 4571. [Google Scholar] [CrossRef]
- Kleinstein, S.; Nelson, K.; Freire, M. Inflammatory Networks Linking Oral Microbiome with Systemic Health and Disease. J. Dent. Res. 2020, 99, 1131–1139. [Google Scholar] [CrossRef]
- Senpuku, H.; Tuna, E.B.; Nagasawa, R.; Nakao, R.; Ohnishi, M. The inhibitory effects of polypyrrole on the biofilm formation of Streptococcus mutans. PLOS ONE 2019, 14, e0225584. [Google Scholar] [CrossRef]
- Baeza, M.; Morales, A.; Cisterna, C.; Cavalla, F.; Jara, G.; Isamitt, Y.; Pino, P.; Gamonal, J. Effect of periodontal treatment in patients with periodontitis and diabetes: Systematic review and meta-analysis. J. Appl. Oral Sci. 2020, 28, e20190248. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Tao, Y.; Cao, Y.; Zhou, Y.; Lin, H. Streptococcus mutans Membrane Vesicles Harboring Glucosyltransferases Augment Candida albicans Biofilm Development. Front. Microbiol. 2020, 11, 581184. [Google Scholar] [CrossRef]
- Bowen, W.; Koo, H. Biology of Streptococcus mutans-Derived Glucosyltransferases: Role in Extracellular Matrix Formation of Cariogenic Biofilms. Caries Res. 2011, 45, 69–86. [Google Scholar] [CrossRef]
- Dunne, W.M. Bacterial Adhesion: Seen Any Good Biofilms Lately? Clin. Microbiol. Rev. 2002, 15, 155–166. [Google Scholar] [CrossRef]
- Pan, W.; Wang, Q.; Chen, Q. The cytokine network involved in the host immune response to periodontitis. Int. J. Oral Sci. 2019, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wojtkowska, A.A.; Wysokiński, A. Periodontitis and prevalence of cardiovascular diseases. Chor. Serca I Naczyń 2015, 12, 289–294. [Google Scholar]
- Kaur, N.; Kaur, N.; Sarangal, V. A study to evaluate the correlation of serum albumin levels with chronic periodontitis. Ind. J. Dent. Res. 2015, 26, 11–14. [Google Scholar] [CrossRef]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; Northwell COVID-19 Research Consortium. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized with COVID-19 in the New York City Area. JAMA 2020, 323, 2052–2059. [Google Scholar] [CrossRef]
- Knypl, K. Pharmacotherapy of arterial hypertension: Antagonists of angiotensin receptors AT1. Med. Rodz. 2001, 2, 58–60. [Google Scholar]
- Xu, H.; Zhong, L.; Deng, J.; Peng, J.; Dan, H.; Zeng, X.; Li, T.; Chen, Q. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int. J. Oral Sci. 2020, 12, 8. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Watanabe, N.; Kamio, N.; Kobayashi, R.; Iinuma, T.; Imai, K. Aspiration of periodontopathic bacteria due to poor oral hygiene potentially contributes to the aggravation of COVID-19. J. Oral Sci. 2021, 63, 1–3. [Google Scholar] [CrossRef]
- Takahashi, Y.; Watanabe, N.; Kamio, N.; Yokoe, S.; Suzuki, R.; Sato, S.; Iinuma, T.; Imai, K. Expression of the SARS-CoV-2 Receptor ACE2 and Proinflammatory Cytokines Induced by the Periodontopathic Bacterium Fusobacterium nucleatum in Human Respiratory Epithelial Cells. Int. J. Mol. Sci. 2021, 22, 1352. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Mohindra, R.; Chauhan, P.; Singla, V.; Goyal, K.; Sahni, V.; Gaur, R.; Verma, D.; Ghosh, A.; Soni, R.; et al. SARS-CoV-2 Detection in Gingival Crevicular Fluid. J. Dent. Res. 2021, 100, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Liccardo, D.; Cannavo, A.; Spagnuolo, G.; Ferrara, N.; Cittadini, A.; Rengo, C.; Rengo, G. Periodontal Disease: A Risk Factor for Diabetes and Cardiovascular Disease. Int. J. Mol. Sci. 2019, 20, 1414. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Hojyo, S.; Uchida, M.; Tanaka, K.; Hasebe, R.; Tanaka, Y.; Murakami, M.; Hirano, T. How COVID-19 induces cytokine storm with high mortality. Inflamm. Regen. 2020, 40, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sukumar, K.; Tadepalli, A. Nexus between COVID-19 and periodontal disease. J. Int. Med Res. 2021, 49, 3000605211002695. [Google Scholar] [CrossRef]
- Botros, N.; Iyer, P.; Ojcius, D.M. Is there an association between oral health and severity of COVID-19 complications? Biomed. J. 2020, 43, 325–327. [Google Scholar] [CrossRef]
- Praczyk, Ł.; Hoffmann, K.; Bryl, W. Galectin 3 as a Biomarker in Cardiovascular Diseases. HYGIEA Public Health 2019, 54, 75–79. Available online: http://www.h-ph.pl/pdf/hyg-2019/hyg-2019-2-075.pdf (accessed on 4 March 2021).
- Kazancioglu, S.; Yilmaz, F.M.; Bastug, A.; Ozbay, B.O.; Aydos, O.; Yücel, Ç.; Bodur, H.; Yilmaz, G. Assessment of Galectin-1, Galectin-3, and PGE2 Levels in Patients with COVID-19. Jpn. J. Infect. Dis. 2021, JJID.2021.020. [Google Scholar] [CrossRef]
- Kara, C.; Çelen, K.; Dede, F.O.; Gökmenoğlu, C.; Kara, N.B. Is periodontal disease a risk factor for developing severe Covid-19 infection? The potential role of Galectin-3. Exp. Biol. Med. 2020, 245, 1425–1427. [Google Scholar] [CrossRef]
- Liu, F.-T. Galectins: A New Family of Regulators of Inflammation. Clin. Immunol. 2000, 97, 79–88. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, F.L.; Gatto, M.; Bassi, N.; Luisetto, R.; Ghirardello, A.; Punzi, L.; Doria, A. Galectin-3 in autoimmunity and auto-immune diseases. Exp. Biol. Med. 2015, 240, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- Argüeso, P.; Panjwani, N. Focus on Molecules: Galectin-3. Exp. Eye Res. 2011, 92, 2–3. [Google Scholar] [CrossRef]
- Cao, W.; Li, T. COVID-19: Towards understanding of pathogenesis. Cell Res. 2020, 30, 367–369. [Google Scholar] [CrossRef] [PubMed]
- Herzberg, M.C.; Meyer, M.W. Effects of Oral Flora on Platelets: Possible Consequences in Cardiovascular Disease. J. Periodontol. 1996, 67, 1138–1142. [Google Scholar] [CrossRef]
- Dikshit, S. Fibrinogen Degradation Products and Periodontitis: Deciphering the Connection. J. Clin. Diagn. Res. 2015, 9, ZC10–ZC12. [Google Scholar] [CrossRef]
- Almeida-da-Silva, C.L.C.; Alpagot, T.; Zhu, Y.; Lee, S.S.; Roberts, B.P.; Hung, S.-C.; Tang, N.; Ojcius, D.M. Chlamydia pneu-moniae is present in the dental plaque of periodontitis patients and stimulates an inflammatory response in gingival epithelial cells. Microb. Cell 2019, 6, 197–208. [Google Scholar] [CrossRef]
- Gomes-Filho, I.; Passos-Soares, J.; Da Cruz, S.S. Respiratory disease and the role of oral bacteria. J. Oral Microbiol. 2010, 2, 5811. [Google Scholar] [CrossRef]
- Varanat, M.; Haase, E.; Kay, J.; Scannapieco, F. Activation of the TREM-1 pathway in human monocytes by periodontal pathogens and oral commensal bacteria. Mol. Oral Microbiol. 2017, 32, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Sampson, V.; Kamona, N.; Sampson, A. Could there be a link between oral hygiene and the severity of SARS-CoV-2 infections? Br. Dent. J. 2020, 228, 971–975. [Google Scholar] [CrossRef]
- Paju, S.; A Scannapieco, F. Oral biofilms, periodontitis, and pulmonary infections. Oral Dis. 2007, 13, 508–512. [Google Scholar] [CrossRef]
- Yang, L.-C.; Suen, Y.-J.; Wang, Y.-H.; Lin, T.-C.; Yu, H.-C.; Chang, Y.-C. The Association of Periodontal Treatment and Decreased Pneumonia: A Nationwide Population-Based Cohort Study. Int. J. Environ. Res. Public Health 2020, 17, 356. [Google Scholar] [CrossRef] [PubMed]
- Anjum, S.I.; Ullah, A.; Khan, K.A.; Attaullah, M.; Khan, H.; Ali, H.; Bashir, M.A.; Tahir, M.; Ansari, M.J.; Ghramh, H.A.; et al. Composition and functional properties of propolis (bee glue): A review. Saudi J. Biol. Sci. 2019, 26, 1695–1703. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, C.-P.; Wang, K.; Li, G.Q.; Hu, F.-L. Recent Advances in the Chemical Composition of Propolis. Molecules 2014, 19, 19610–19632. [Google Scholar] [CrossRef] [PubMed]
- Eroglu, N.; Akkus, S.; Yaman, M.; Asci, B.; Silici, S. Amino Acid and Vitamin Content of Propolis Collected by Native Caucasican Honeybees. J. Apic. Sci. 2016, 60, 101–110. [Google Scholar] [CrossRef]
- Shahinozzaman, M.; Basak, B.; Emran, R.; Rozario, P.; Obanda, D.N. Artepillin C: A comprehensive review of its chemistry, bioavailability, and pharmacological properties. Phytotherapy 2020, 147, 104775. [Google Scholar] [CrossRef]
- Paulino, N.; Abreu, S.R.L.; Uto, Y.; Koyama, D.; Nagasawa, H.; Hori, H.; Dirsch, V.; Vollmar, A.M.; Scremin, A.; Bretz, W.A. Anti-inflammatory effects of a bioavailable compound, Artepillin C, in Brazilian propolis. Eur. J. Pharmacol. 2008, 587, 296–301. [Google Scholar] [CrossRef]
- Veloz, J.J.; Alvear, M.; Salazar, L.A. Antimicrobial and Antibiofilm Activity against Streptococcus mutans of Individual and Mixtures of the Main Polyphenolic Compounds Found in Chilean Propolis. BioMed Res. Int. 2019, 2019, 1–7. [Google Scholar] [CrossRef]
- Dziedzic, A.; Kubina, R.; Wojtyczka, R.D.; Kabała-Dzik, A.; Tanasiewicz, M.; Morawiec, T. The Antibacterial Effect of Ethanol Extract of Polish Propolis on Mutans Streptococci and Lactobacilli Isolated from Saliva. Evid.-Based Complement. Altern. Med. 2013, 2013, 1–12. [Google Scholar] [CrossRef]
- Elbaz, G.; Elsayad, I. Comparison if the antimicrobial effect of Egyptian propolis vs New Zealand propolis on Streptococcus mutans and Lactobacilli in saliva. Oral Health Prev. Dent. 2012, 10, 155–160. Available online: http://www.quintpub.com/userhome/ohpd/ohpd_2012_02_s0155.pdf (accessed on 14 February 2021). [PubMed]
- Ikeno, K.; Ikeno, T.; Miyazawa, C. Effects of Propolis on Dental Caries in Rats. Caries Res. 1991, 25, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.; Cury, J.A.; Rosalen, P.L.; Ambrosano, G.M.; Ikegaki, M.; Park, Y.K. Effect of a Mouthrinse Containing Selected Propolis on 3-Day Dental Plaque Accumulation and Polysaccharide Formation. Caries Res. 2002, 36, 445–448. [Google Scholar] [CrossRef] [PubMed]
- Listyasari, N.A.; Santoso, O. Inhibition of dental plaque formation by toothpaste containing propolis. Dent. J. (Maj. Kedokt. Gigi) 2012, 45, 208–211. [Google Scholar] [CrossRef][Green Version]
- Kaushik, N.; Subramani, C.; Anang, S.; Muthumohan, R.; Shalimar; Nayak, B.; Ranjith-Kumar, C.T.; Surjit, M. Zinc Salts Block Hepatitis E Virus Replication by Inhibiting the Activity of Viral RNA-Dependent RNA Polymerase. J. Virol. 2017, 91, e00754-17. [Google Scholar] [CrossRef]
- Guler, H.I.; Tatar, G.; Yildiz, O.; Belduz, A.O.; Kolayli, S. Investigation of potential inhibitor properties of ethanolic propolis extracts against ACE-II receptors for COVID-19 treatment by molecular docking study. Arch. Microbiol. 2021, 203, 3557–3564. [Google Scholar] [CrossRef]
- Beserra, F.P.; Gushiken, L.F.S.; Hussni, M.F.; Ribeiro, V.P.; Bonamin, F.; Jackson, C.J.; Pellizzon, C.H.; Bastos, J.K. Artepillin C as an outstanding phenolic compound of Brazilian green propolis for disease treatment: A review on pharmacological aspects. Phytother. Res. 2021, 35, 2274–2286. [Google Scholar] [CrossRef] [PubMed]
- Maruta, H.; He, H. PAK1-blockers: Potential Therapeutics against COVID-19. Med. Drug Discov. 2020, 6, 100039. [Google Scholar] [CrossRef]
- Zulhendri, F.; Felitti, R.; Fearnley, J.; Ravalia, M. The use of propolis in dentistry, oral health, and medicine: A review. J. Oral Biosci. 2021, 63, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Refaat, H.; Mady, F.M.; Sarhan, H.A.; Rateb, H.S.; Alaaeldin, E. Optimization and evaluation of propolis liposomes as a promising therapeutic approach for COVID-19. Int. J. Pharm. 2021, 592, 120028. [Google Scholar] [CrossRef] [PubMed]
- Te Velthuis, A.J.W.; van den Worm, S.H.E.; Sims, A.C.; Baric, R.S.; Snijder, E.J.; Van Hemert, M.J. Zn(2+) inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010, 6, e1001176. [Google Scholar] [CrossRef]
- Dabbagh-Bazarbachi, H.; Clergeaud, G.; Quesada, I.M.; Ortiz, M.; Sullivan, C.O.; Fernández-Larrea, J.B. Zinc Ionophore Activity of Quercetin and Epigallocatechin-gallate: From Hepa 1-6 Cells to a Liposome Model. J. Agric. Food Chem. 2014, 62, 8085–8093. [Google Scholar] [CrossRef]
| Active Substances | Action | Literature |
|---|---|---|
| artepillin C, caffeic acid phenethyl ester (CAPE) | inhibition of PAK-1 activation | [40,41] |
| rutin, myricetin, caffeic acid phenethyl ester (CAPE), | inhibition of ACE2 receptors | [42] |
| caffeic acid, rutin, chrysin, kaemferol, galangin, fisetin, lupeol, 10-hydroxyl-2-decenoic acid | inhibition of viral replication | [38,41] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurek-Górecka, A.; Walczyńska-Dragon, K.; Felitti, R.; Nitecka-Buchta, A.; Baron, S.; Olczyk, P. The Influence of Propolis on Dental Plaque Reduction and the Correlation between Dental Plaque and Severity of COVID-19 Complications—A Literature Review. Molecules 2021, 26, 5516. https://doi.org/10.3390/molecules26185516
Kurek-Górecka A, Walczyńska-Dragon K, Felitti R, Nitecka-Buchta A, Baron S, Olczyk P. The Influence of Propolis on Dental Plaque Reduction and the Correlation between Dental Plaque and Severity of COVID-19 Complications—A Literature Review. Molecules. 2021; 26(18):5516. https://doi.org/10.3390/molecules26185516
Chicago/Turabian StyleKurek-Górecka, Anna, Karolina Walczyńska-Dragon, Rafael Felitti, Aleksandra Nitecka-Buchta, Stefan Baron, and Paweł Olczyk. 2021. "The Influence of Propolis on Dental Plaque Reduction and the Correlation between Dental Plaque and Severity of COVID-19 Complications—A Literature Review" Molecules 26, no. 18: 5516. https://doi.org/10.3390/molecules26185516
APA StyleKurek-Górecka, A., Walczyńska-Dragon, K., Felitti, R., Nitecka-Buchta, A., Baron, S., & Olczyk, P. (2021). The Influence of Propolis on Dental Plaque Reduction and the Correlation between Dental Plaque and Severity of COVID-19 Complications—A Literature Review. Molecules, 26(18), 5516. https://doi.org/10.3390/molecules26185516








