Optimization of Taxol Extraction Process Using Response Surface Methodology and Investigation of Temporal and Spatial Distribution of Taxol in Taxus mairei
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Taxol Extraction
2.3. Taxol Content Determination
2.4. Taxol Content Calculation
2.5. Single-Factor Experiment Design
2.6. Response Surface Experiment Design
2.7. Statistical Analysis
3. Results
3.1. The Influence of Four Factors on Yield of Taxol
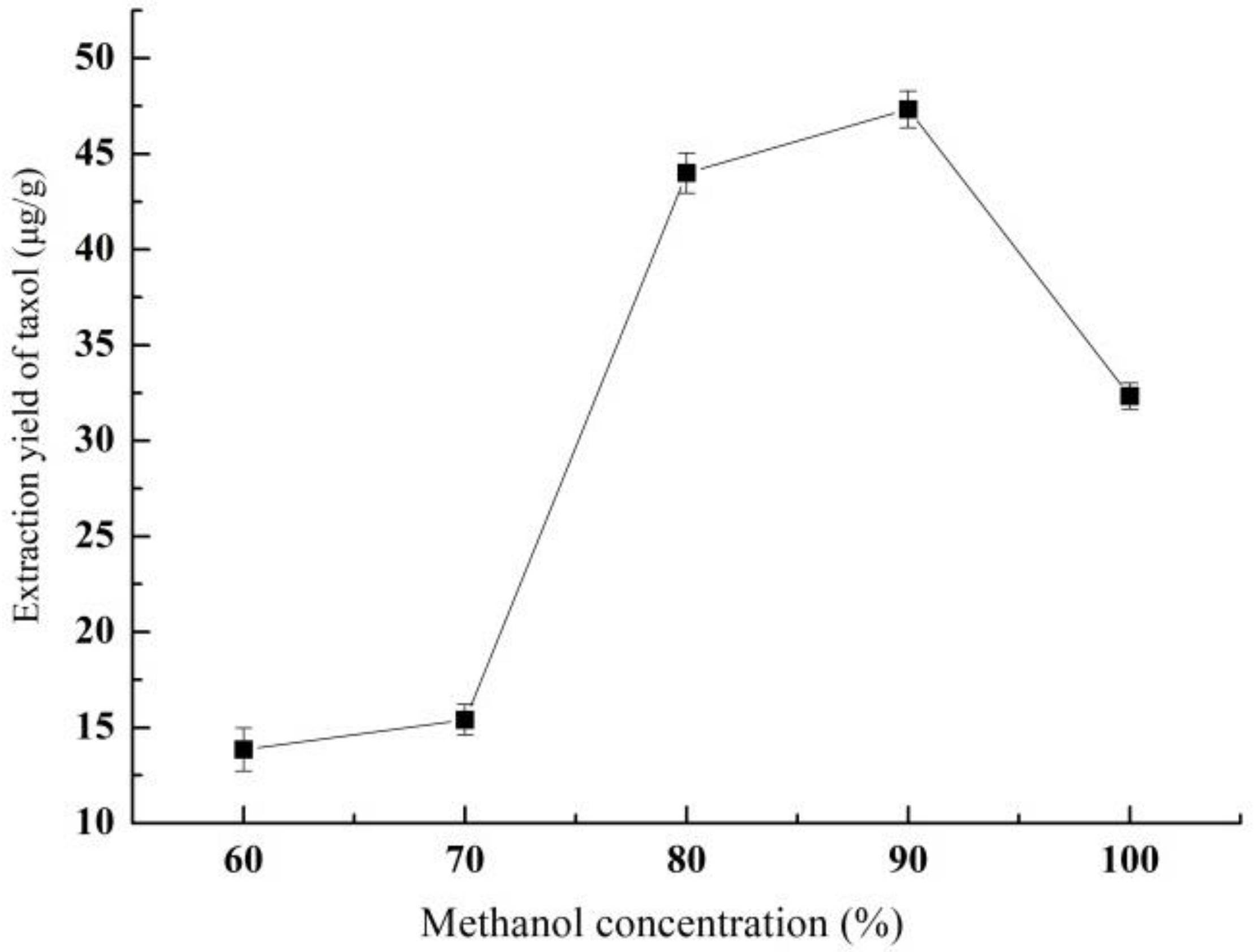
3.1.1. Effect of Methanol Concentration on Yield of Taxol
3.1.2. Effect of Solid-Liquid Ratio on Yield of Taxol
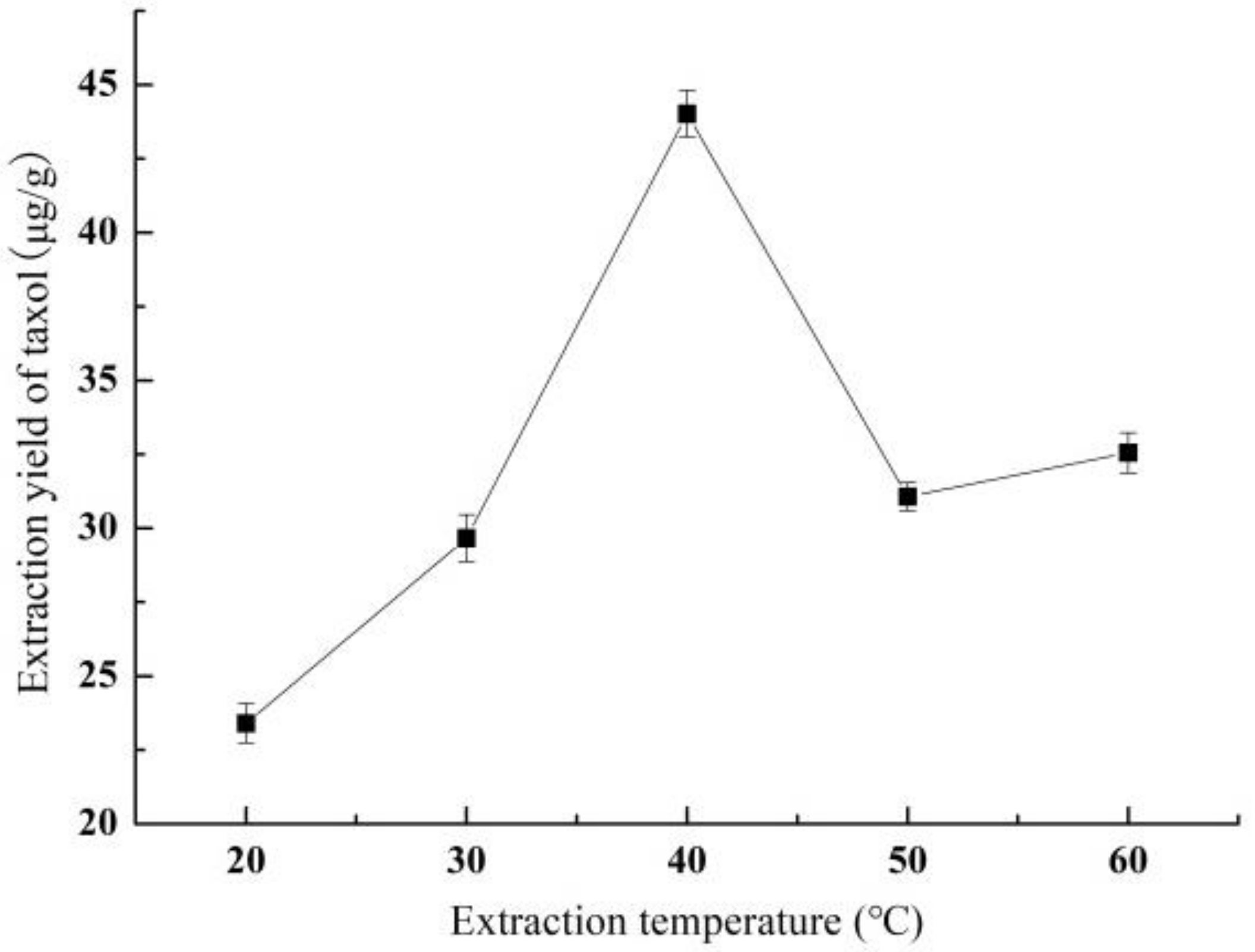
3.1.3. Effect of Extraction Temperature on Yield of Taxol
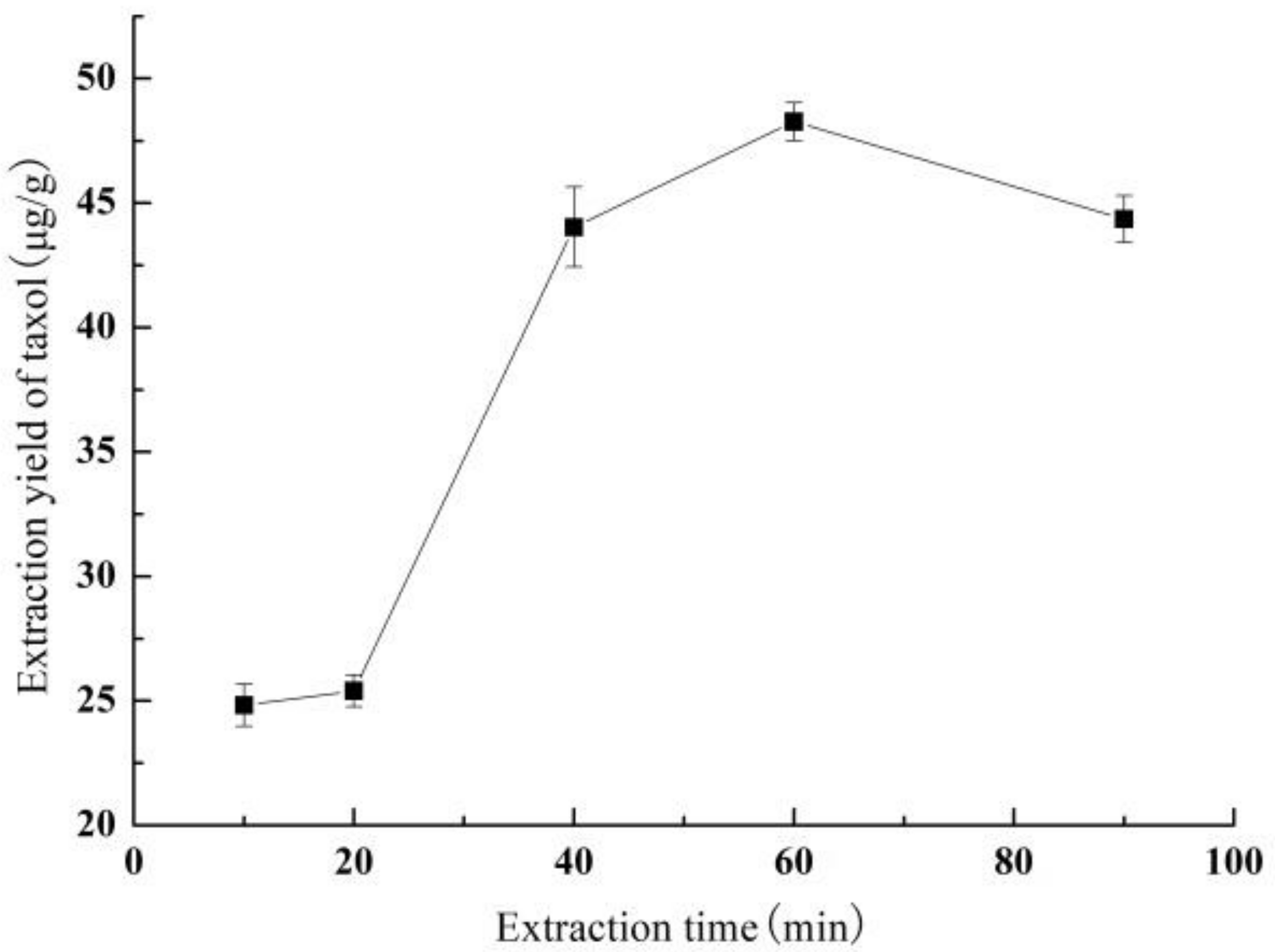
3.1.4. Effect of Extraction Time on Yield of Taxol
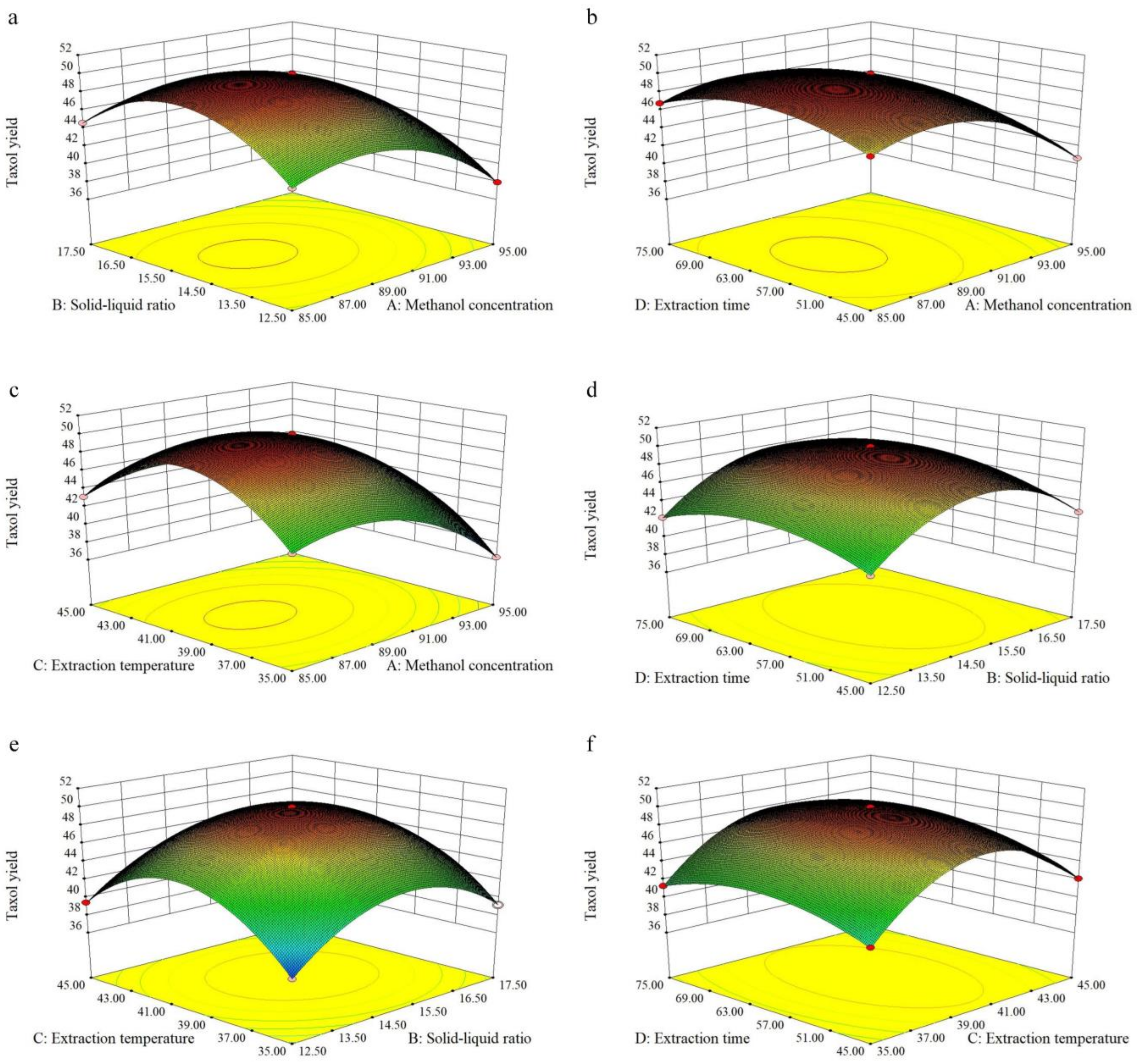
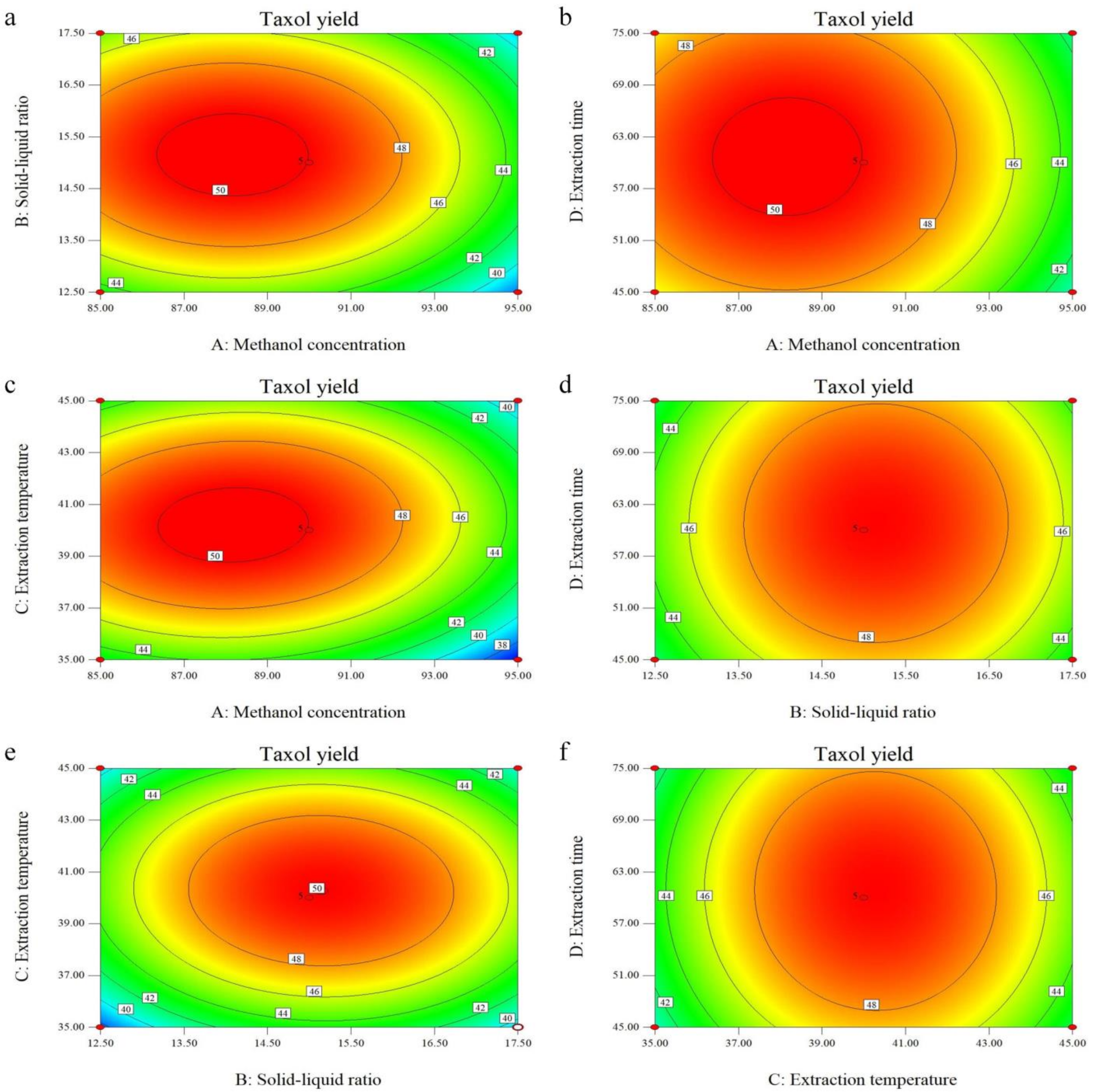
3.2. Optimization of the Extraction by RSM
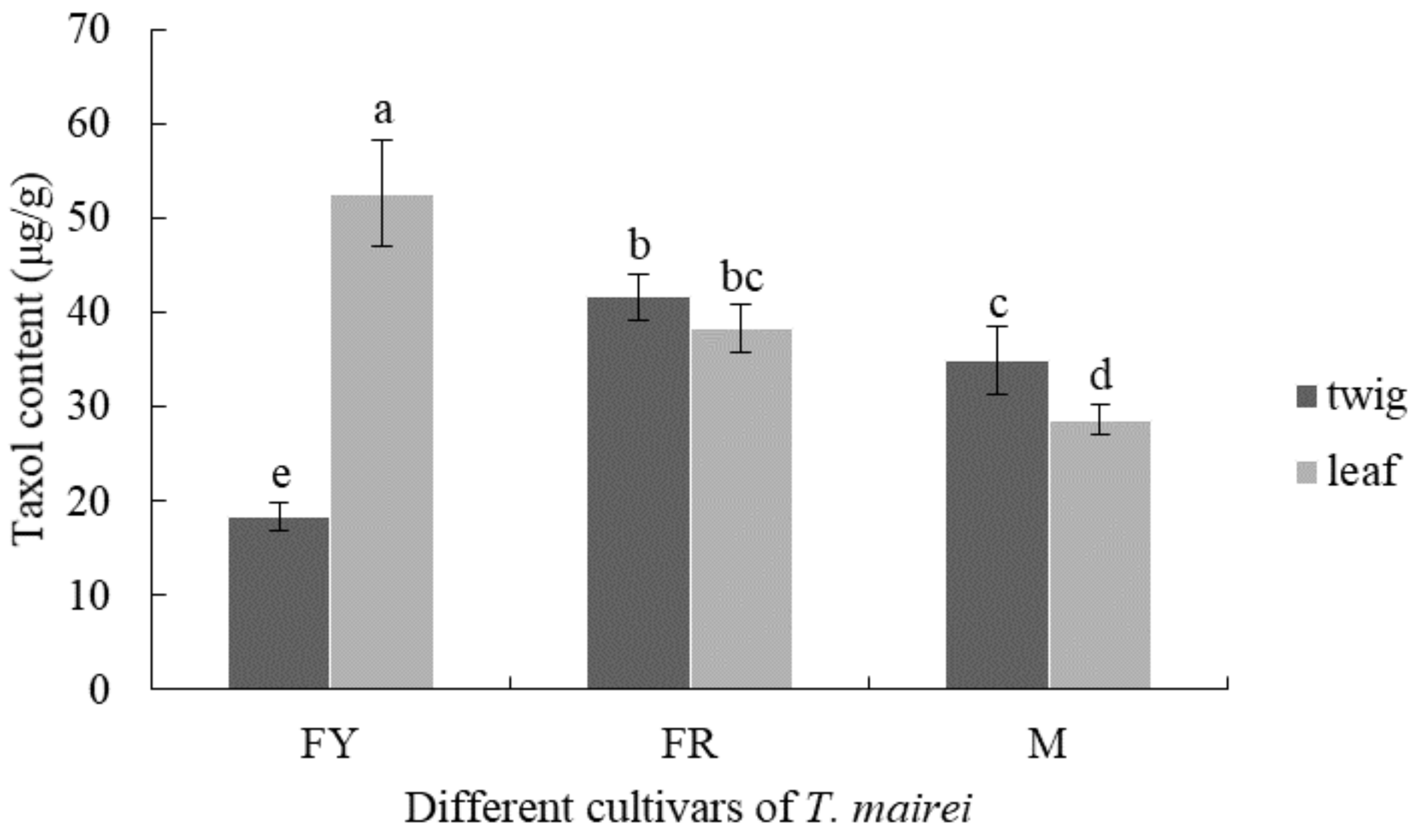
3.3. Comparison of Taxol Content from Different T. mairei Cultivars
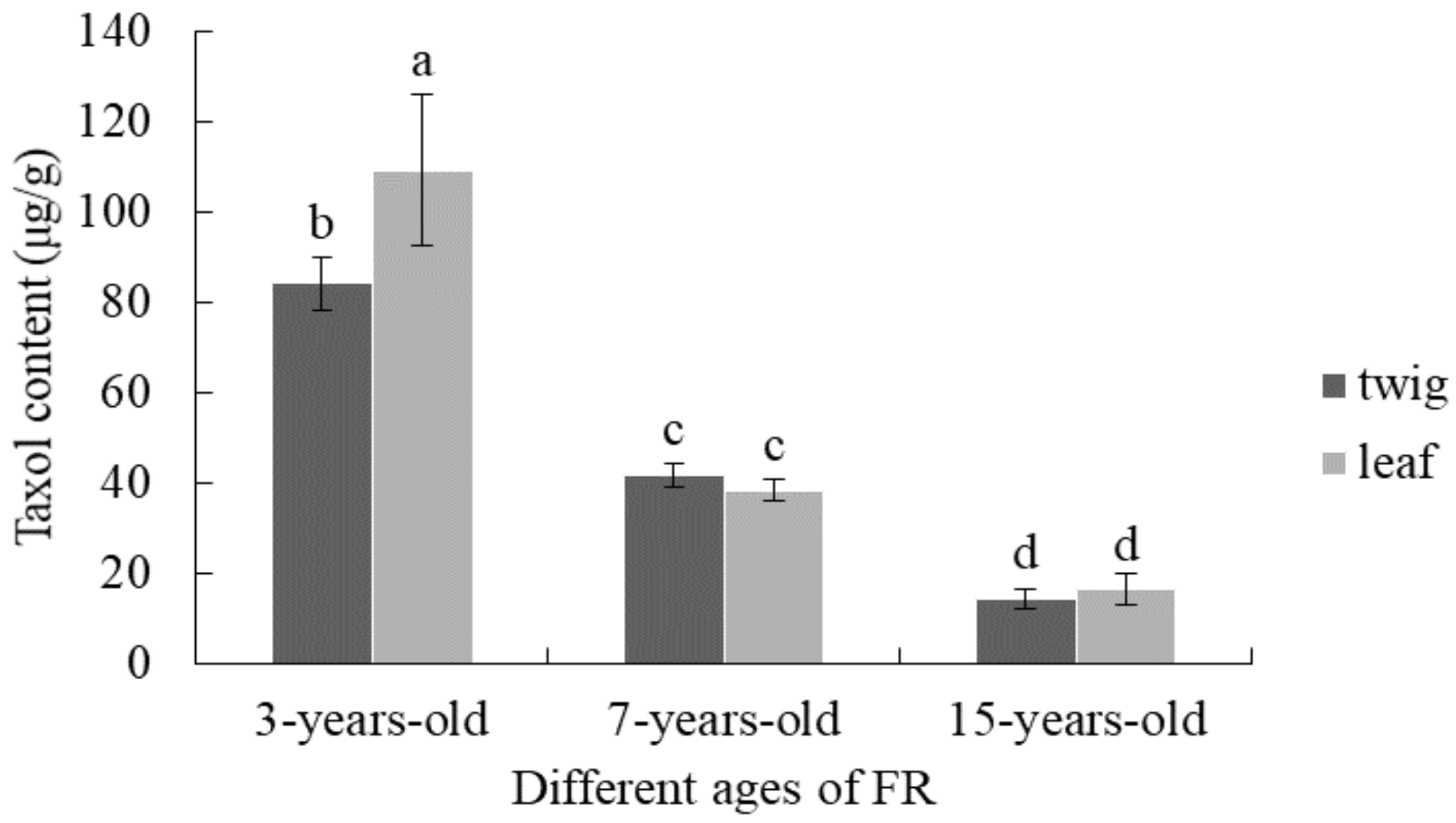
3.4. Comparison of Taxol Content from T. mairei with Different Tree Ages
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Wani, M.C.; Taylor, H.L.; Wall, M.E.; Coggon, P.; McPhail, A.T. Plant antitumor agents. VI. Isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J. Am. Chem. Soc. 1971, 93, 2325–2327. [Google Scholar] [CrossRef] [PubMed]
- Croteau, R.; Ketchum, R.E.B.; Long, R.M.; Kaspera, R.; Wildung, M.R. Taxol biosynthesis and molecular genetics. Phytochem. Rev. 2006, 5, 75–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wani, M.C.; Horwitz, S.B. Nature as a Remarkable Chemist: A personal story of the discovery and development of Taxol. Anti-Cancer Drug 2014, 25, 482. [Google Scholar] [CrossRef] [Green Version]
- Kuang, X.J.; Sun, S.J.; Wei, J.H.; Li, Y.; Sun, C. Iso-Seq analysis of the Taxus cuspidata transcriptome reveals the complexity of Taxol biosynthesis. BMC Plant Biol. 2019, 19, 210. [Google Scholar] [CrossRef] [Green Version]
- You, L.F.; Wei, T.; Zheng, Q.W.; Lin, J.F.; Huang, J.J. Activity Essential Residue Analysis of Taxoid 10β-O-Acetyl Transferase for Enzymatic Synthesis of Baccatin. Appl. Biochem. Biotech. 2018, 186, 949–959. [Google Scholar] [CrossRef] [PubMed]
- Nicolaou, K.C.; Yang, Z.; Liu, J.J.; Ueno, H.; Nantermet, P.G.; Guy, R.K.; Claiborne, C.F.; Renaud, J.; Couladouros, E.A.; Paulvannan, K.; et al. Total synthesis of taxol. Nature 1994, 367, 630–634. [Google Scholar] [CrossRef]
- Khosroushahi, A.Y.; Valizadeh, M.; Ghasempour, A.; Khosrowshahli, M.; Naghdibadi, H.; Dadpour, M.R.; Omidi, Y. Improved Taxol production by combination of inducing factors in suspension cell culture of Taxus baccata. Cell Biol. Int. 2006, 30, 262–269. [Google Scholar] [CrossRef]
- Qiao, W.C.; Tang, T.H.; Ling, F. Comparative transcriptome analysis of a taxol-producing endophytic fungus, Aspergillus aculeatinus Tax-6, and its mutant strain. Sci. Rep. 2020, 10, 10558. [Google Scholar] [CrossRef] [PubMed]
- Nazhand, A.; Durazzo, A.; Lucarini, M.; Mobilia, M.A.; Omri, B.; Santini, A. Rewiring cellular metabolism for heterologous biosynthesis of Taxol. Nat. Prod. Res. 2020, 34, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.D.; Cao, J.S.; Bao, S.S.; Shao, G.S.; Wang, Z.H.; Qin, B.Y.; Wang, T.; Fu, Y.J. Preparation and application of modified three-dimensional cellulose microspheres for paclitaxel targeted separation. J. Chromatogr. A 2021, 1655, 462487. [Google Scholar] [CrossRef] [PubMed]
- Lopes, M.P.; Galinha, C.F.; Crespo, J.G.; Velizarov, S. Optimisation of arsenate removal from water by an integrated ion-exchange membrane process coupled with Fe co-precipitation. Sep. Purif. Technol. 2020, 246, 116894. [Google Scholar]
- Liu, P.; Li, W.R.; Hu, Z.Z.; Qin, X.G.; Liu, G. Isolation, purification, identification, and stability of anthocyanins from Lycium ruthenicum Murr. LWT FOOD Sci. Technol. 2020, 126, 109334. [Google Scholar] [CrossRef]
- Papoutsis, K.; Pristijono, P.; Golding, J.B.; Stathopoulos, C.E.; Bowyer, M.C.; Scarlett, C.J.; Vuong, Q.V. Optimizing a sustainable ultrasound-assisted extraction method for the recovery of polyphenols from lemon by-products: Comparison with hot water and organic solvent extractions. Eur. Food Res. Technol. 2018, 244, 1353–1365. [Google Scholar] [CrossRef] [Green Version]
- Zou, T.B.; Wang, M.; Gan, R.Y.; Ling, W.H. Optimization of ultrasound-assisted extraction of anthocyanins from mulberry, using response surface methodology. Int. J. Mol. Sci. 2011, 12, 3006–3017. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Lee, A.Y.; Lee, A.R.; Choi, G.; Kim, H.K. Optimization of ultrasound-assisted extraction of glycyrrhizic acid from licorice using response surface methodology. Intger. Med. Res. 2017, 6, 388–394. [Google Scholar] [CrossRef]
- Pu, G.Z.; Lv, Y.N.; Xu, G.P.; Zeng, D.J.; Huang, Y.Q. Research progress on karst tiankeng ecosystems. Bot. Rev. 2017, 83, 5–37. [Google Scholar] [CrossRef]
- Wang, T.; Chen, Y.M.; Zhuang, W.B.; Zhang, F.J.; Shu, X.C.; Wang, Z.; Yang, Q. Transcriptome sequencing reveals regulatory mechanisms of taxol synthesis in Taxus wallichiana var. Mairei. Int. J. Genom. 2019, 2019, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, D.C.; Ge, G.B.; Xiao, P.G.; Zhang, Y.Y.; Yang, L. The first insight into the tissue specific taxus transcriptome via Illumina second generation sequencing. PLoS ONE 2011, 6, e21220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, C.N.; Guo, H.; Zhang, Y.Y.; Song, Y.B.; Pi, E.; Yu, C.L.; Zhang, L.; Dong, M.; Zheng, B.S.; Wang, H.Z.; et al. Identification of potential genes that contributed to the variation in the taxoid contents between two Taxus species (Taxus media and Taxus mairei). Tree Physiol. 2017, 37, 1659–1671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, J.P.; Cao, J.; Zhang, X.H.; Huang, J.Z.; Kong, T.; Tong, S.; Tian, Z.Y.; Xie, Y.L.; Xu, R.; Zhu, J.H. Optimization of ionic liquid based ultrasonic assisted extraction of puerarin from radix puerariaelobatae by response surface methodology. Food Chem. 2012, 135, 2299–2306. [Google Scholar] [CrossRef] [PubMed]
- Gallego-Jara, J.; Lozano-Terol, G.; Sola-Martínez, R.A.; Cánovas-Díaz, M.; de Diego Puente, T. A compressive review about taxol: History and future challenges. Molecules 2020, 25, 5986. [Google Scholar] [CrossRef] [PubMed]
- Kayan, B.; Gizir, A.M.; Kalderis, D. Ultrasonic-assisted extraction of 10-deacetylbaccatin III from Taxus baccata L.: Optimization using response surface methodology. J. Iran. Chem. Soc. 2021, 18, 37–45. [Google Scholar]
- Sadeghi-Aliabadi, H.; Asghari, G.; Mostafavi, S.A.; Esmaeili, A. Solvent optimization on Taxol extraction from Taxus baccata L., using HPLC and LC-MS. DARU J. Pharm. Sci. 2015, 17, 192–198. [Google Scholar]
- Kang, H.J.; Kim, J.H. Removal of residual chloroform from amorphous paclitaxel pretreated by alcohol. Korean J. Chem. Eng. 2019, 36, 1965–1970. [Google Scholar] [CrossRef]
- Lin, H.; Zhang, Y.; Han, M.; Yang, L. Aqueous ionic liquid based ultrasonic assisted extraction of eight ginsenosides from ginseng root. Ultrason. Sonochem. 2013, 20, 680–684. [Google Scholar] [CrossRef]
- Wang, L.T.; Lu, W.H.; Li, J.L.; Hu, J.X.; Ding, R.F.; Lv, M.; Wang, Q.B. Optimization of ultrasonic-assisted extraction and purification of zeaxanthin and lutein in corn gluten meal. Molecules 2019, 24, 2994. [Google Scholar] [CrossRef] [Green Version]
- Wen, C.; Zhang, J.X.; Zhang, H.H.; Dzah, C.S.; Zandile, M.; Duan, Y.Q.; Ma, H.; Luo, X.P. Advances in ultrasound assisted extraction of bioactive compounds from cash crops—A review. Ultrason. Sonochem. 2018, 48, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Ha, G.S.; Kim, J.H. Kinetic and thermodynamic characteristics of ultrasound-assisted extraction for recovery of paclitaxel from biomass. Process. Biochem. 2016, 51, 1664–1673. [Google Scholar] [CrossRef]
- Xu, X.Q.; Gu, Y.W.; Huang, W.; Chen, D.K.; Zhang, C.; Yang, X. Structural Optimization of Steel—Epoxy Asphalt Pavement Based on Orthogonal Design and GA—BP Algorithm. Crystals 2021, 11, 417. [Google Scholar] [CrossRef]
- Kim, B.; Choi, Y.; Choi, J.; Shin, Y.; Lee, S. Effect of surfactant on wetting due to fouling in membrane distillation membrane: Application of response surface methodology (RSM) and artificial neural networks (ANN). Korean J. Chem. Eng. 2020, 37, 1–10. [Google Scholar] [CrossRef]
- Boyadzhieva, S.S.; Georgieva, S.S.; Angelov, G. Optimization of the extraction of natural antioxidants from avocado seeds. Bulg. Chem. Commun. 2018, 50, 80–84. [Google Scholar]
- Boyadzhieva, S.S.; Georgieva, S.S.; Angelov, G. Recovery of antioxidant phenolic compounds from avocado peels by solvent extraction. Bulg. Chem. Commun. 2018, 50, 83–89. [Google Scholar]
- Yu, C.N.; Luo, X.J.; Zhan, X.R.; Hao, J.; Zhang, L.; Song, Y.B.; Shen, C.J.; Dong, M. Comparative metabolomics reveals the metabolic variations between two endangered Taxus species (T. fuana and T. yunnanensis) in the Himalayas. BMC Plant Biol. 2018, 18, 197. [Google Scholar]
- Fu, L.; Wang, Q.; Zhang, M.J.; Zheng, Y.H.; Wu, M.Y.; Lan, Z.Y.; Pu, J.S.; Zhang, H.W.; Chen, F.; Su, W.T. Electrochemical sex determination of dioecious plants using polydopamine-functionalized graphene sheets. Front. Chem. 2020, 8, 92. [Google Scholar] [CrossRef]
- Nadeem, M.; Rikhari, H.C.; Kumar, A.; Palni, L.M.S.; Nandi, S.K. Taxol content in the bark of Himalayan yew in relation to tree age and sex. Phytochemistry 2002, 60, 627–631. [Google Scholar] [CrossRef]
- Neto, A.G.F.; DiCosmo, F. Distribution and amounts of taxol in different shoot parts of Taxus cuspidata. Planta Med. 1992, 58, 464–466. [Google Scholar] [CrossRef]
- Fang, Y.X.; Hua, Y.L.; Tian, X.J. Spatial Variation of the Taxol Content in Taxus chinensis var. mairei. Chin. Agric. Sci. Bull. 2015, 31, 19–22. [Google Scholar]

| Factor | Level | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| Methanol concentration (%) | 85 | 90 | 95 |
| Solid-liquid ratio (g/L) | 12.5 | 15 | 17.5 |
| Extraction temperature (°C) | 35 | 40 | 60 |
| Extraction time (min) | 45 | 60 | 75 |
| No. | Methanol Concentration (%) | Solid-Liquid Ratio (g/mL) | Extraction Temperature (°C) | Extraction Time (min) | Taxol Yield (μg/g) |
|---|---|---|---|---|---|
| 1 | 90 | 15 | 35 | 45 | 40.72 |
| 2 | 85 | 15 | 40 | 75 | 46.81 |
| 3 | 90 | 15 | 40 | 60 | 49.99 |
| 4 | 90 | 12.5 | 45 | 60 | 39.46 |
| 5 | 85 | 17.5 | 40 | 60 | 44.65 |
| 6 | 95 | 15 | 35 | 60 | 36.27 |
| 7 | 90 | 15 | 40 | 60 | 49.94 |
| 8 | 90 | 12.5 | 35 | 60 | 37.52 |
| 9 | 95 | 15 | 40 | 75 | 41.38 |
| 10 | 85 | 12.5 | 40 | 60 | 43.32 |
| 11 | 90 | 15 | 40 | 60 | 50.01 |
| 12 | 90 | 12.5 | 40 | 75 | 42.21 |
| 13 | 90 | 15 | 40 | 60 | 49.93 |
| 14 | 95 | 15 | 40 | 45 | 40.71 |
| 15 | 90 | 15 | 45 | 75 | 42.51 |
| 16 | 90 | 15 | 35 | 75 | 41.34 |
| 17 | 85 | 15 | 45 | 60 | 43.16 |
| 18 | 95 | 17.5 | 40 | 60 | 38.96 |
| 19 | 85 | 15 | 35 | 60 | 42.82 |
| 20 | 90 | 17.5 | 35 | 60 | 39.18 |
| 21 | 90 | 17.5 | 45 | 60 | 40.08 |
| 22 | 90 | 17.5 | 40 | 45 | 42.85 |
| 23 | 90 | 17.5 | 40 | 75 | 43.58 |
| 24 | 90 | 12.5 | 40 | 45 | 41.86 |
| 25 | 90 | 15 | 45 | 45 | 42.18 |
| 26 | 85 | 15 | 40 | 45 | 46.47 |
| 27 | 90 | 15 | 40 | 60 | 49.98 |
| 28 | 95 | 15 | 45 | 60 | 38.56 |
| 29 | 95 | 12.5 | 40 | 60 | 37.96 |
| Source | Degree of Freedom | Sum of Squares | Mean Square | F-Value | p-Value | Significance |
|---|---|---|---|---|---|---|
| model | 14 | 463.52 | 33.11 | 17,960.09 | <0.0001 | *** |
| A | 1 | 92.91 | 92.91 | 50,398.74 | <0.0001 | *** |
| B | 1 | 4.05 | 4.05 | 2196.10 | <0.0001 | *** |
| C | 1 | 5.47 | 5.47 | 2965.90 | <0.0001 | *** |
| D | 1 | 0.77 | 0.77 | 417.77 | <0.0001 | *** |
| AB | 1 | 0.03 | 0.03 | 14.77 | 0.0018 | ** |
| AC | 1 | 0.95 | 0.95 | 515.68 | <0.0001 | *** |
| AD | 1 | 0.03 | 0.03 | 14.77 | 0.0018 | ** |
| BC | 1 | 0.27 | 0.27 | 146.68 | <0.0001 | *** |
| BD | 1 | 0.04 | 0.04 | 19.58 | 0.0006 | *** |
| CD | 1 | 0.02 | 0.02 | 11.41 | 0.0045 | ** |
| A2 | 1 | 93.23 | 93.23 | 50,575.76 | <0.0001 | *** |
| B2 | 1 | 160.30 | 160.30 | 86,957.85 | <0.0001 | *** |
| C2 | 1 | 229.64 | 229.64 | 124,569.44 | <0.0001 | *** |
| D2 | 1 | 35.75 | 35.75 | 19,390.49 | <0.0001 | *** |
| Residual | 14 | 0.03 | 0.00 | - | - | - |
| Lack of fit | 10 | 0.02 | 0.00 | 1.84 | 0.2913 | - |
| Pure error | 4 | 0.00 | 0.00 | - | - | - |
| Total | 28 | 463.55 | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Chen, Y.; Ma, Y.; Wang, Z.; Wang, T.; Xie, Y. Optimization of Taxol Extraction Process Using Response Surface Methodology and Investigation of Temporal and Spatial Distribution of Taxol in Taxus mairei. Molecules 2021, 26, 5485. https://doi.org/10.3390/molecules26185485
Li L, Chen Y, Ma Y, Wang Z, Wang T, Xie Y. Optimization of Taxol Extraction Process Using Response Surface Methodology and Investigation of Temporal and Spatial Distribution of Taxol in Taxus mairei. Molecules. 2021; 26(18):5485. https://doi.org/10.3390/molecules26185485
Chicago/Turabian StyleLi, Lingyu, Yiming Chen, Yingli Ma, Zhong Wang, Tao Wang, and Yinfeng Xie. 2021. "Optimization of Taxol Extraction Process Using Response Surface Methodology and Investigation of Temporal and Spatial Distribution of Taxol in Taxus mairei" Molecules 26, no. 18: 5485. https://doi.org/10.3390/molecules26185485
APA StyleLi, L., Chen, Y., Ma, Y., Wang, Z., Wang, T., & Xie, Y. (2021). Optimization of Taxol Extraction Process Using Response Surface Methodology and Investigation of Temporal and Spatial Distribution of Taxol in Taxus mairei. Molecules, 26(18), 5485. https://doi.org/10.3390/molecules26185485






