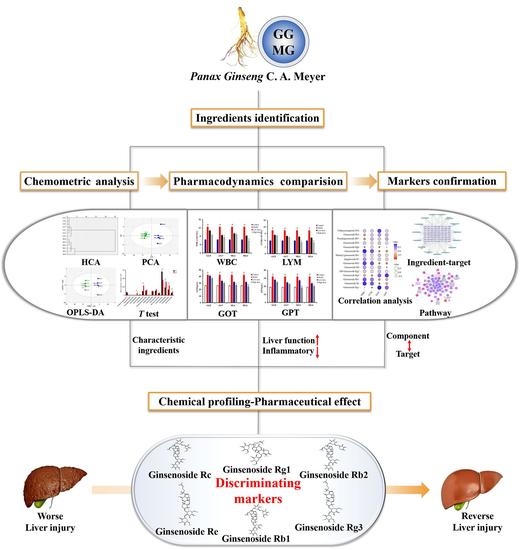
An Integrated Mutually Oriented “Chemical Profiling–Pharmaceutical Effect” Strategy for Screening Discriminating Markers of Underlying Hepatoprotective Effects to Distinguish Garden-Cultivated from Mountain-Cultivated Ginseng
Abstract
:1. Introduction
2. Results and Discussion
2.1. Qualitative and Semi-Quantitative Analysis of Ingredients in Ginseng with HPLC Q-TOF/MS
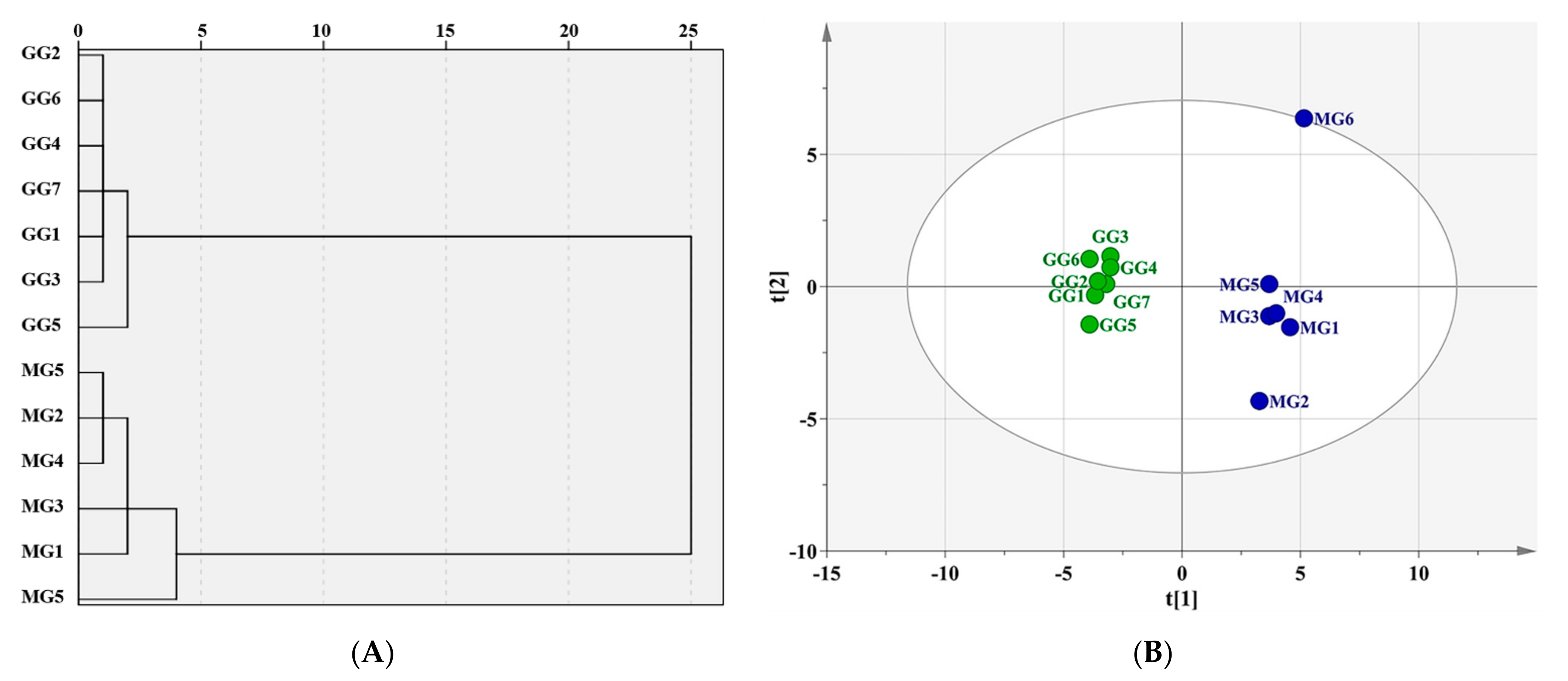
2.2. Exploration of Characteristic Components for Differentiating GG from MG by Chemometric Analysis
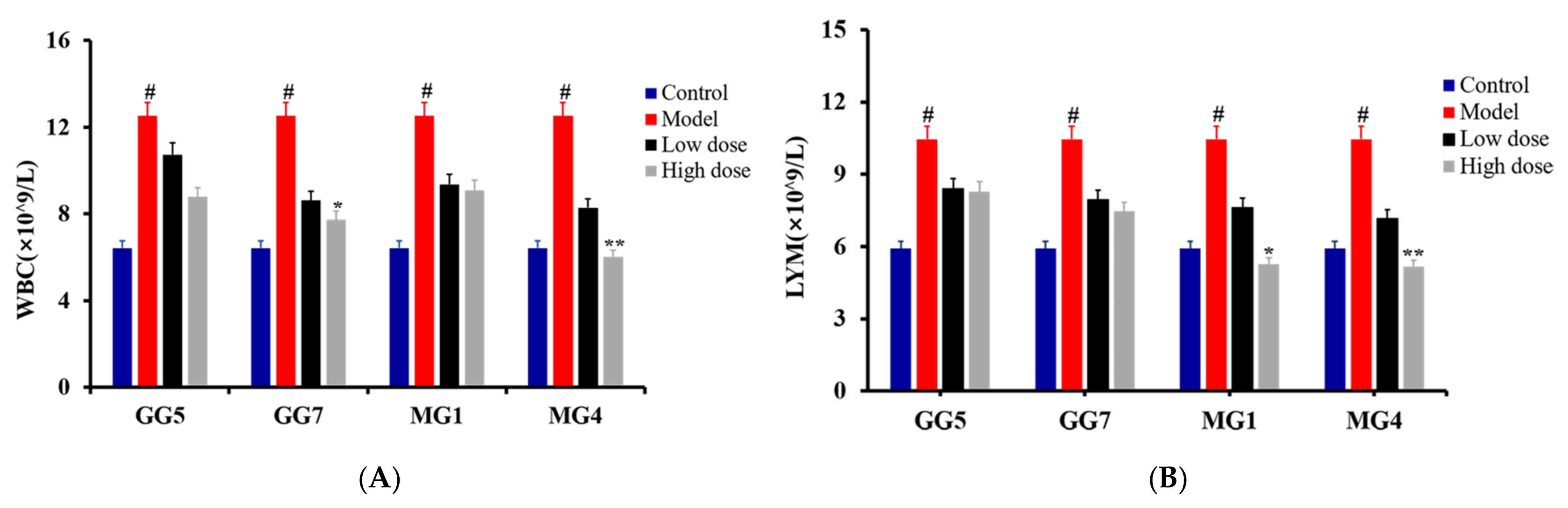
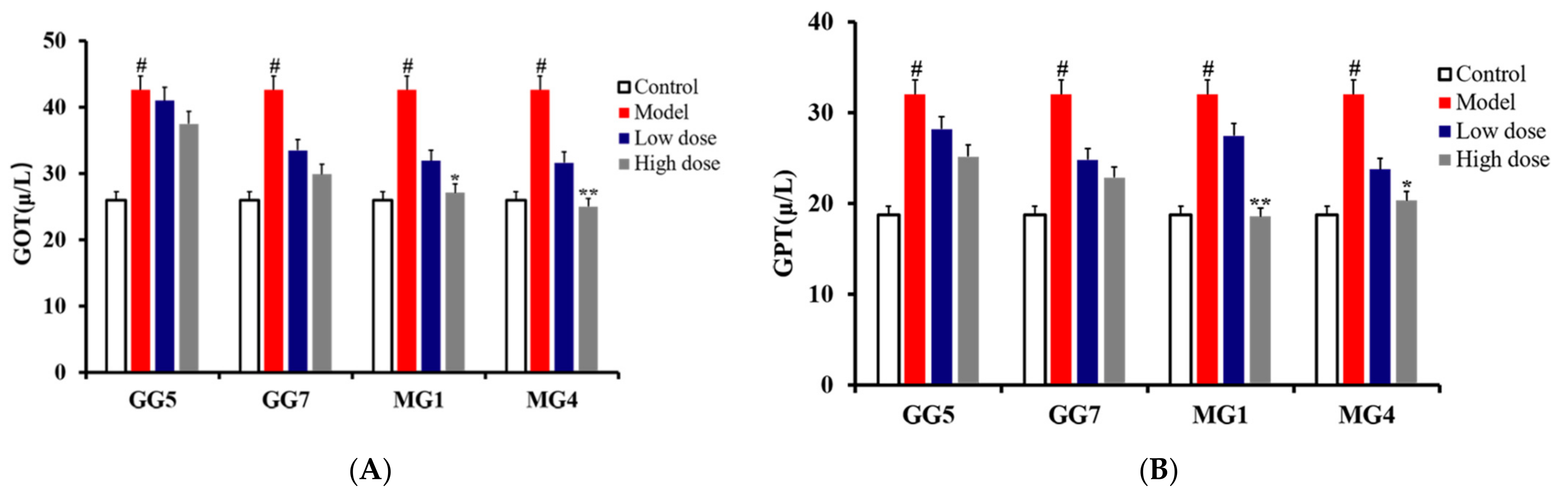
2.3. Comparative Study of the Pharmacodynamics of GG and MG in Paclitaxel-Induced Liver Injury
2.4. Discovery of Pharmacodynamic-Based Markers to Distinguish GG from MG
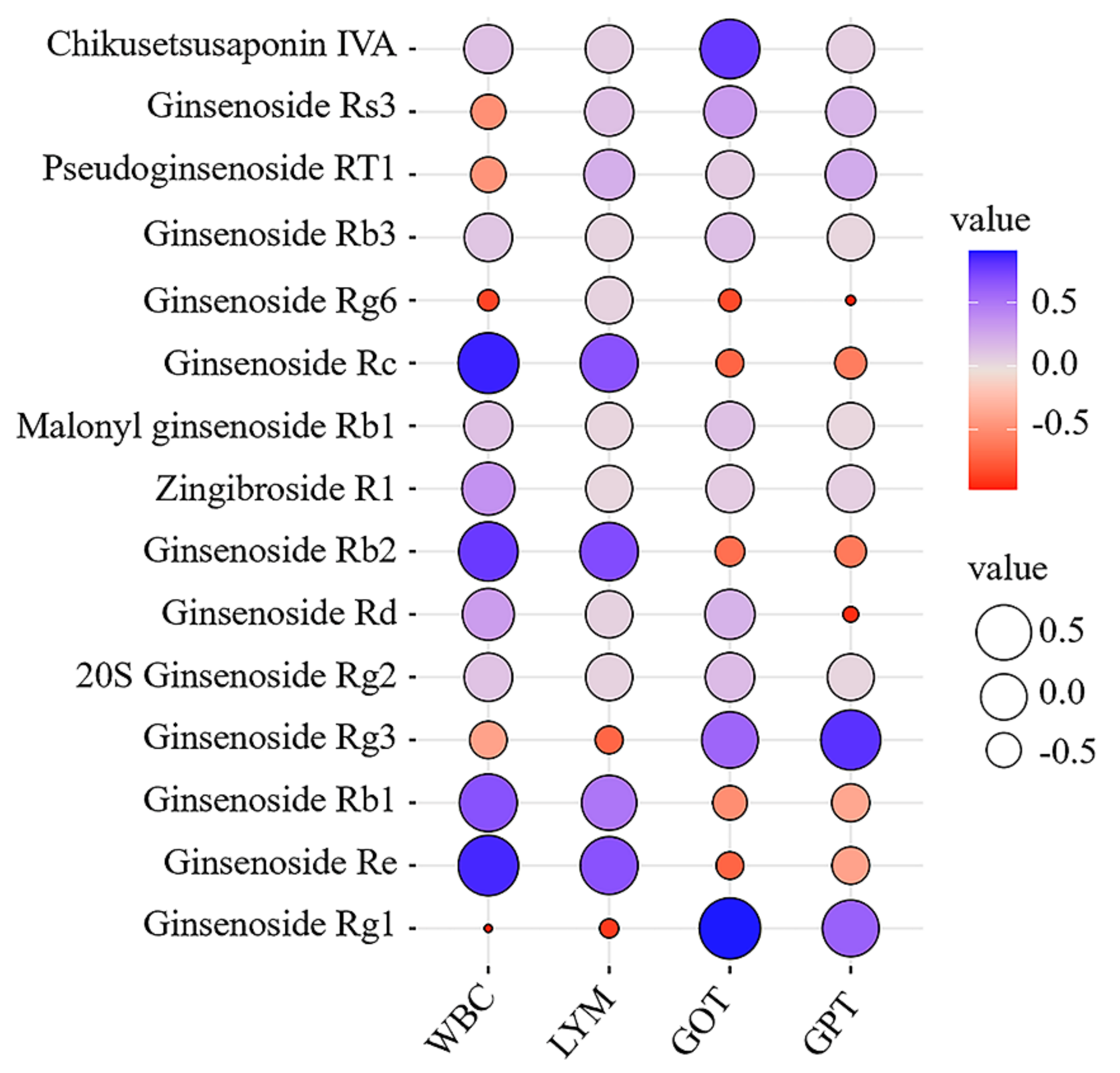
2.4.1. Correlation Analysis between Characteristic Components and Pharmacodynamic Indicators
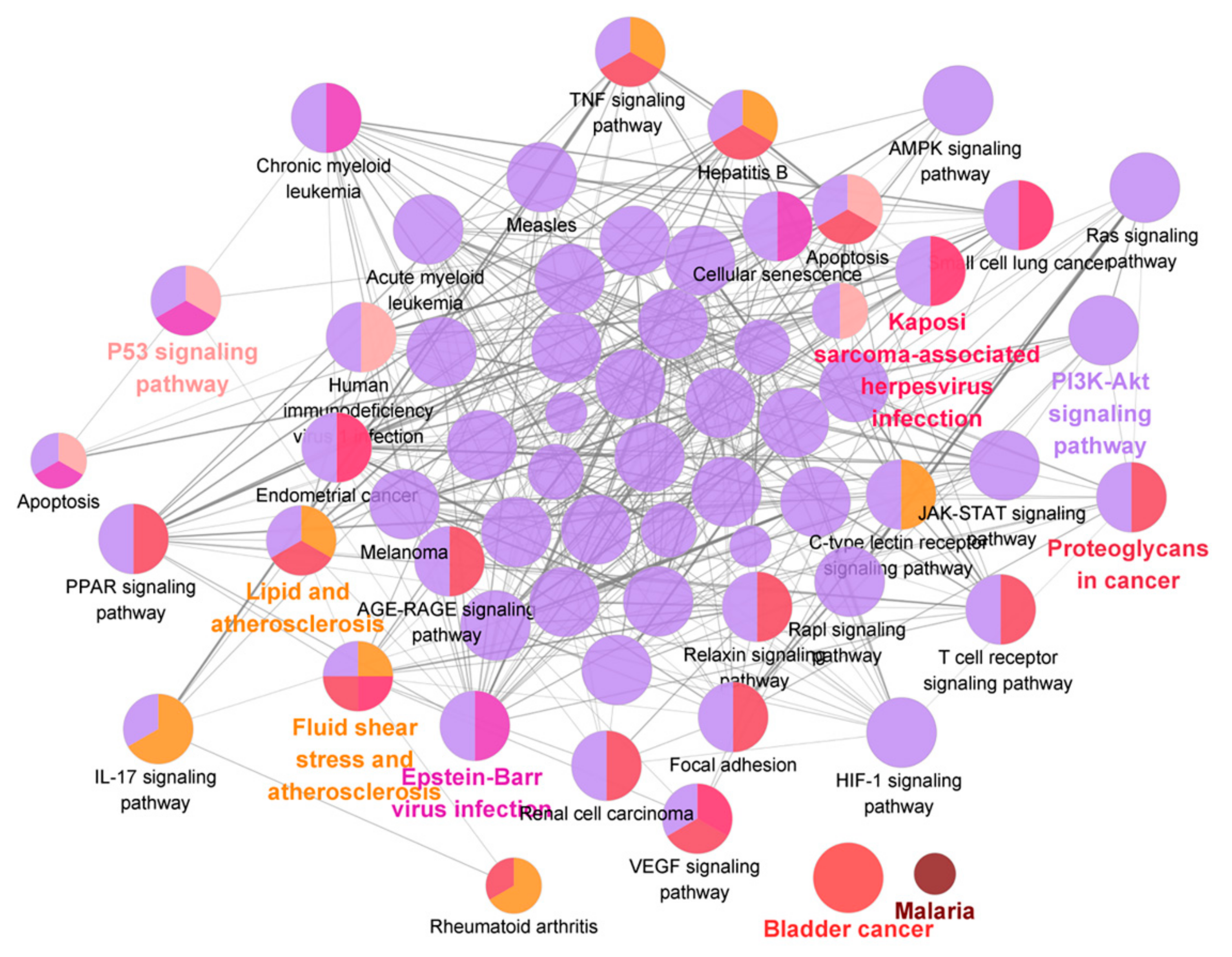
2.4.2. Identification of Effective Representative Substances of GG and MG Using Bioinformatic Analysis
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. HPLC Q-TOF/MS Analysis
3.2.1. Sample Preparation
3.2.2. HPLC Q-TOF/MS Conditions
3.3. Animals
3.4. Detection of Pharmacodynamic Indicators
3.5. Bioinformatics Analysis Process
3.6. Statistical and Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Guo, M.; Shao, S.; Wang, D.; Zhao, D.; Wang, M. Recent progress in polysaccharides from Panax ginseng C. A. Meyer. Food Funct. 2021, 12, 494–518. [Google Scholar] [CrossRef]
- Ki, S.H.; Yang, J.H.; Ku, S.K.; Kim, S.C.; Kim, Y.W.; Cho, I.J. Red ginseng extract protects against carbon tetrachloride-induced liver fibrosis. J. Ginseng Res. 2013, 37, 45–53. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Liu, H.; Zhang, M.; Li, C.; Li, G. Spectrum-effect relationship between UPLC fingerprints and anti-lung cancer effect of Panax ginseng. Phytochem. Anal. 2020, 32, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Park, S.B.; Park, G.H.; Um, Y.; Kim, H.N.; Song, H.M.; Kim, N.; Kim, H.-S.; Jeong, J.B. Wood-cultivated ginseng exerts anti-inflammatory effect in LPS-stimulated RAW264.7 cells. Int. J. Biol. Macromol. 2018, 116, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Song, Y.; Hyun, G.H.; Long, N.P.; Park, J.H.; Hsieh, Y.S.Y.; Kwon, S.W. Characterization and Antioxidant Activity Determination of Neutral and Acidic Polysaccharides from Panax Ginseng C. A. Meyer. Molecules 2020, 25, 791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, Y.C.; Tae, S.W.; Seo, Y.Y.; Ike, C.d.P.; Yoon, J.C.; Hyung, S.A.; Yong, S.L.; Gu, Y.Y.; Jae, H.C. Red Ginseng Supplementation More Effectively Alleviates Psychological than Physical Fatigue. J. Ginseng Res. 2011, 35, 331–338. [Google Scholar]
- Zhu, H.; Long, M.-H.; Wu, J.; Wang, M.-M.; Li, X.-Y.; Shen, H.; Xu, J.-D.; Zhou, L.; Fang, Z.-J.; Luo, Y.; et al. Ginseng alleviates cyclophosphamide-induced hepatotoxicity via reversing disordered homeostasis of glutathione and bile acid. Sci. Rep. 2015, 5, 17536. [Google Scholar] [CrossRef] [PubMed]
- Lam, P.; Cheung, F.; Tan, H.Y.; Wang, N.; Yuen, M.F.; Feng, Y. Hepatoprotective Effects of Chinese Medicinal Herbs: A Focus on Anti-Inflammatory and Anti-Oxidative Activities. Int. J. Mol. Sci. 2016, 17, 465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, A.-H.; Shimaa, I.S.; Safaa, I.K.; Eman, S.E.-S.; Hosny, A.E.F.; Shafika, A.E. Panax ginseng is superior to vitamin E as a hepatoprotector against cyclophosphamide-induced liver damage. Complement. Ther. Med. 2019, 46, 95–102. [Google Scholar]
- Wang, W.; Wang, S.; Liu, J.; Cai, E.; Zhu, H.; He, Z.; Gao, Y.; Li, P.; Zhao, Y. Sesquiterpenoids from the root of Panax Ginseng protect CCl 4 –induced acute liver injury by anti-inflammatory and anti-oxidative capabilities in mice. Biomed. Pharmacother. 2018, 102, 412–419. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Y.; Li, H.; Zhao, Y.; Cai, E.; Zhu, H.; Li, P.; Liu, J. Protective Effects of Sesquiterpenoids from the Root of Panax ginseng on Fulminant Liver Injury Induced by Lipopolysaccharide/D-galactosamine. J. Agric. Food Chem. 2018, 66, 7758–7763. [Google Scholar] [CrossRef]
- Pan, H.-Y.; Qu, Y.; Zhang, J.-K.; Kang, T.-G.; Dou, D.-Q. Antioxidant activity of Ginseng cultivated under mountainous forest with different growing years. J. Ginseng Res. 2013, 37, 355–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Yoo, J.-M.; Kim, J.S.; Kim, S.-G.; Park, J.E.; Seok, Y.M.; Son, J.-H.; Kim, H.J.; Faurot, K.R. Anticancer Effect of Mountain Ginseng on Human Breast Cancer: Comparison with Farm-Cultivated Ginseng. Alternat. Med. 2020, 2020, 2584783. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.-F.; Cheng, X.-L.; Lin, Q.-H.; Li, S.-S.; Jia, Z.; Han, T.; Lin, R.-C.; Wang, D.; Wei, F.; Li, X.-R. Identification of mountain-cultivated ginseng and cultivated ginseng using UPLC/oa-TOF MSE with a multivariate statistical sample-profiling strategy. J. Ginseng Res. 2016, 40, 344–350. [Google Scholar] [CrossRef]
- Zhu, L.; Luan, X.; Yuan, Y.; Dou, D.; Huang, L. The characteristics of ginsenosides and oligosaccharides in mountain- and garden-cultivated Ginseng. J. Sci. Food Agric. 2021, 101, 1491–1498. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-Y.; Kim, J.; Shrestha, S.; Seo, K.-H.; Lee, Y.-H.; Noh, H.-J.; Kim, G.-S.; Kim, Y.-B.; Kim, S.-Y.; Baek, N.-I. Quality Evaluation of Panax ginseng Roots Using a Rapid Resolution LC-QTOF/MS-Based Metabolomics Approach. Molecules 2013, 18, 14849–14861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Li, S.; Liang, Y.; Liu, R.; Lv, X.; Zhang, Q.; Xu, H.; Bi, K.; Li, Z.; Li, Q. A systematic strategy for uncovering quality marker of Asari Radix et Rhizoma on alleviating inflammation based chemometrics analysis of components. J. Chromatogr. A 2021, 1642, 461960. [Google Scholar] [CrossRef]
- Liang, G.; Yang, J.; Liu, T.; Wang, S.; Wen, Y.; Han, C.; Huang, Y.; Wang, R.; Wang, Y.; Hu, L.; et al. A multi-strategy platform for quality control and Q-markers screen of Chaiqin chengqi decoction. Phytomedicine 2021, 85, 153525. [Google Scholar] [CrossRef]
- Wu, P.; Dong, X.-M.; Song, G.-Q.; Wei, M.-M.; Fang, C.; Zheng, F.-B.; Zhao, Y.-J.; Lu, H.-Q.; Cheng, L.-H.; Zhou, J.-L.; et al. Bioactivity-guided discovery of quality control markers in rhizomes of Curcuma wenyujin based on spectrum-effect relationship against human lung cancer cells. Phytomedicine 2021, 86, 153559. [Google Scholar] [CrossRef]
- Chen, R.; He, J.; Tong, X.; Tang, L.; Liu, M.; Lam, C.W.K. The Hedyotis diffusa Willd. (Rubiaceae): A Review on Phytochemistry, Pharmacology, Quality Control and Pharmacokinetics. Molecules 2016, 21, 710. [Google Scholar] [CrossRef] [PubMed]
- Dilek, K.; Banu, E.; Osman, N.K. The protective effect of beta-1,3-D-glucan on taxol-induced hepatotoxicity: A histopathological and stereological study. Drug Chem. Toxicol. 2010, 33, 8–16. [Google Scholar]
- Wang, X.; Liu, J.; Yang, X.; Zhang, Q.; Zhang, Y.; Li, Q.; Bi, K. Development of a systematic strategy for the global identification and classification of the chemical constituents and metabolites of Kai-Xin-San based on liquid chromatography with quadrupole time-of-flight mass spectrometry combined with multiple data-processing approaches. J. Sep. Sci. 2018, 41, 2672–2680. [Google Scholar] [PubMed]
- Zuo, T.; Zhang, C.; Li, W.; Wang, H.; Hu, Y.; Yang, W.; Jia, L.; Wang, X.; Gao, X.; Guo, D. Offline two-dimensional liquid chromatography coupled with ion mobility-quadrupole time-of-flight mass spectrometry enabling four-dimensional separation and characterization of the multicomponents from white ginseng and red ginseng. J. Pharm. Anal. 2020, 10, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Ji, S.-H.; Lee, Y.-S.; Jin, C.D.; Choi, B.-R.; Kim, G.-S.; Baek, N.-I.; Lee, D.Y. Mass Spectrometry Based Profiling and Imaging of Various Ginsenosides from Panax ginseng Roots at Different Ages. Int. J. Mol. Sci. 2017, 18, 1114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yen, F.-L.; Wu, T.-H.; Lin, L.-T.; Lin, C.-C. Hepatoprotective and antioxidant effects of Cuscuta chinensis against acetaminophen-induced hepatotoxicity in rats. J. Ethnopharmacol. 2007, 111, 123–128. [Google Scholar] [CrossRef]
- Chen, T.-M.; Subeq, Y.-M.; Lee, R.-P.; Chiou, T.-W.; Hsu, B.-G. Single dose intravenous thioacetamide administration as a model of acute liver damage in rats. Int. J. Exp. Pathol. 2008, 89, 223–231. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Liu, L.; Pan, Y.; Hu, Y.; Yang, P.; Liao, M. Protective and therapeutic effects of nanoliposomal quercetin on acute liver injury in rats. BMC Pharmacol. Toxicol. 2020, 21, 11. [Google Scholar] [CrossRef] [Green Version]
- Badr, G.; Sayed, E.A.; Waly, H.; Hassan, K.A.-H.; Mahmoud, M.H.; Selamoglu, Z. The Therapeutic Mechanisms of Propolis Against CCl4 -Mediated Liver Injury by Mediating Apoptosis of Activated Hepatic Stellate Cells and Improving the Hepatic Architecture through PI3K/AKT/mTOR, TGF-β/Smad2, Bcl2/BAX/P53 and iNOS Signaling Pathways. Cell. Physiol. Biochem. 2019, 53, 301–322. [Google Scholar]
- Nesma, M.E.A.E.-N.; Dalia, O.S.; Sawsan, S.M.; Salwa, M.N.; Rania, M.A.; Marwa, M.S.; Hanan, S.E.-A. Olmesartan attenuates type 2 diabetes-associated liver injury: Cross-talk of AGE/RAGE/JNK, STAT3/SCOS3 and RAS signaling pathways. Eur. J. Pharmacol. 2020, 874, 173010. [Google Scholar]
- Zhu, Q.; Wang, H.; Jiang, B.; Ni, X.; Jiang, L.; Li, C.; Wang, X.; Zhang, F.; Ke, B.; Lu, L. Loss of ATF3 exacerbates liver damage through the activation of mTOR/p70S6K/ HIF-1α signaling pathway in liver inflammatory injury. Cell Death Dis. 2018, 9, 910. [Google Scholar] [CrossRef] [Green Version]
- Jing, Z.-T.; Liu, W.; Xue, C.-R.; Wu, S.-X.; Chen, W.-N.; Lin, X.-J.; Lin, X. AKT activator SC79 protects hepatocytes from TNF-α-mediated apoptosis and alleviates d-Gal/LPS-induced liver injury. Am. J. Physiol.-Gastr. L 2019, 316, 387–396. [Google Scholar] [CrossRef]
- Orfila, C.; Lepert, J.-C.; Alric, L.; Carrera, G.; Béraud, M.; Pipy, B. Immunohistochemical distribution of activated nuclear factor kappaB and peroxisome proliferator-activated receptors in carbon tetrachloride-induced chronic liver injury in rats. Histochem. Cell Biol. 2005, 123, 585–593. [Google Scholar] [CrossRef]
- Yao, Y.-L.; Han, X.; Song, J.; Zhang, J.; Li, Y.-M.; Lian, L.-H.; Wu, Y.-L.; Nan, J.-X. Acanthoic acid protectsagainst ethanol-induced liver injury: Possible role of AMPK activation and IRAK4 inhibition. Toxicol. Lett. 2017, 281, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Ning, C.; Gao, X.; Wang, C.; Huo, X.; Liu, Z.; Sun, H.; Yang, X.; Sun, P.; Ma, X.; Meng, Q.; et al. Protective effects of ginsenoside Rg1 against lipopolysaccharide/d-galactosamine-induced acute liver injury in mice through inhibiting toll-like receptor 4 signaling pathway. Int. Immunopharmacol. 2018, 61, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Zhou, P.; Qu, M.; Dai, Z.; Zhang, X.; Zhang, C.; Dong, X.; Sun, G.; Sun, X. Ginsenoside Re Attenuates High Glucose-Induced RF/6A Injury via Regulating PI3K/AKT Inhibited HIF-1a/VEGF Signaling Pathway. Front. Pharmacol. 2020, 11, 695. [Google Scholar] [CrossRef]
- Yang, J.; Li, S.; Wang, L.; Du, F.; Zhou, X.; Song, Q.; Zhao, J.; Fang, R. Ginsenoside Rg3 Attenuates Lipopolysaccharide-Induced Acute Lung Injury via MerTK-Dependent Activation of the PI3K/AKT/mTOR Pathway. Front. Pharmacol. 2018, 9, 850–863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, S.; Leng, J.; Xu, X.-Y.; Jiang, S.; Wang, Y.-P.; Yan, X.-T.; Liu, Z.; Chen, C.; Wang, Z.; Li, W. Ginsenoside Rb1, A Major Saponin from Panax ginseng, Exerts Protective Effects Against Acetaminophen-Induced Hepatotoxicity in Mice. Am. J. Chin. Med. 2019, 47, 1815–1831. [Google Scholar] [CrossRef]
- Dai, S.; Hong, Y.; Xu, J.; Lin, Y.; Si, Q.; Gu, X. Ginsenoside Rb2 promotes glucose metabolism and attenuates fat accumulation via AKT-dependent mechanisms. Biomed. Pharmacother 2018, 100, 93–100. [Google Scholar] [CrossRef]
- Tao, Y.; Man, H.R.; Lee, J.; Kim, S.H.; Yang, Y.; Kim, H.G.; Kim, Y.; Kim, C.; Kwak, Y.-S.; Kim, J.-H.; et al. Ginsenoside Rc from Korean Red Ginseng (Panax ginseng C.A. Meyer) Attenuates Inflammatory Symptoms of Gastritis, Hepatitis and Arthritis. Am. J. Chin. Med. 2016, 44, 595–615. [Google Scholar]



| NO. | Origins | Years |
|---|---|---|
| MG1 | Liaoning, Benxi | 8 |
| MG2 | Liaoning, Benxi | 8–9 |
| MG3 | Liaoning, Huanren | 14 |
| MG4 | Liaoning, Huanren | 17 |
| MG5 | Liaoning, Shenyang | 10 |
| MG6 | Liaoning, Shenyang | 15 |
| GG1 | Liaoning, Jinzhou | 4 |
| GG2 | Liaoning, Fushun | 4 |
| GG3 | Liaoning, Jinzhou | 5 |
| GG4 | Liaoning, Fushun | 5 |
| GG5 | Liaoning, Jinzhou | 6 |
| GG6 | Liaoning, Jinzhou | 4 |
| GG7 | Liaoning, Fushun | 6 |
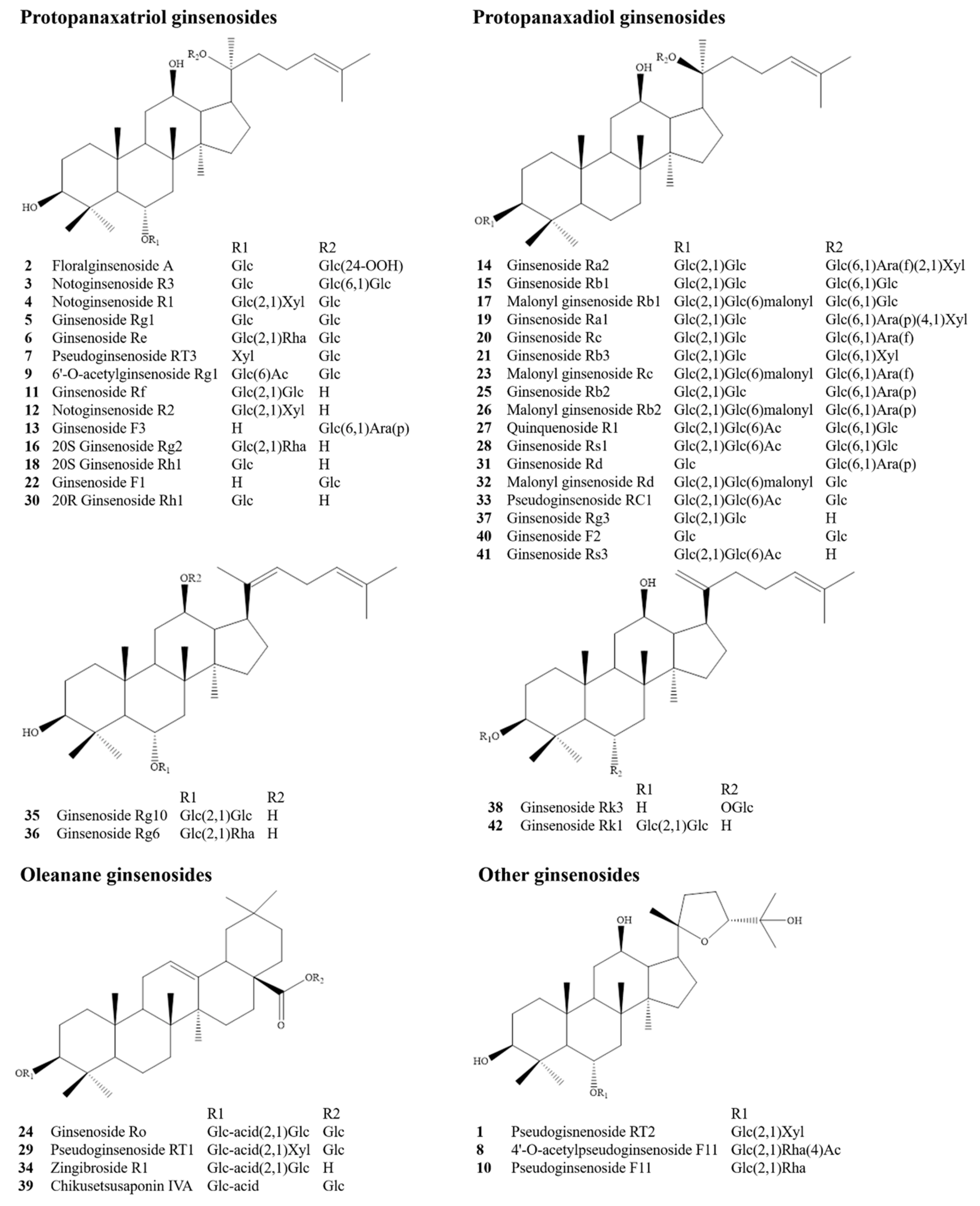
| No. | Identification | Formula | Adduct Ion | m/z | Error (ppm) | Retention Time (min) | Fragment Ions (m/z) |
|---|---|---|---|---|---|---|---|
| 1 | Pseudogisnenoside RT2 | C41H70O14 | [COOH]+ | 831.4726 | −1.3 | 14.45 | 161.0451, 491.3708, 653.4280, 785.4686 |
| 2 | Floralginsenoside A | C42H72O16 | [H]− | 831.4726 | −2.7 | 14.51 | 161.0451, 491.3708, 653.4280, 785.4686 |
| 3 | Notoginsenoside R3 | C48H82O19 | [COOH]+ | 1007.542 | −0.5 | 15.03 | 637.4349, 781.4764, 799.4853, 961.5411 |
| 4 | Notoginsenoside R1 | C47H80O18 | [COOH]+ | 977.5311 | −0.5 | 15.34 | 131.0352, 475.3777, 637.4310, 799.4866 |
| 5 | Ginsenoside Rg1 | C42H72O14 | [COOH]+ | 845.4898 | 0.5 | 15.87 | 161.0455, 475.3782, 637.4317, 799.4862 |
| 6 | Ginsenoside Re | C48H82O18 | [COOH]+ | 991.5478 | 0.5 | 16.07 | 161.0462, 179.0564, 475.3810, 619.4249 |
| 7 | Pseudoginsenoside RT3 | C41H70O13 | [COOH]+ | 815.4792 | 0.6 | 17.82 | 391.2720, 475.3806, 553.3349, 637.4354 |
| 8 | 4’-O-acetylpseudoginsenoside F11 | C44H74O15 | [COOH]+ | 887.5006 | 0.8 | 17.92 | 161.0434, 391.2863, 475.3884, 619.4274 |
| 9 | 6’-O-acetylginsenoside Rg1 | C44H74O15 | [COOH]+ | 887.5014 | 1.8 | 19.22 | 475.3800, 619.4013, 637.4266, 781.4766 |
| 10 | Pseudoginsenoside F11 * | C42H72O14 | [H]− | 799.485 | 0.2 | 20.21 | 161.0455, 415.3218, 491.3768, 653.4331 |
| 11 | Ginsenoside Rf | C42H72O14 | [COOH]+ | 845.4899 | 0.6 | 20.57 | 161.0468,415.3225, 653.4201, 799.4903 |
| 12 | Notoginsenoside R2 | C41H70O13 | [COOH]+ | 815.4793 | 0.7 | 21.49 | 475.3778, 619.4207, 637.4388, 769.4760 |
| 13 | Ginsenoside F3 | C41H70O13 | [COOH]+ | 815.4793 | 0.7 | 21.58 | 161.0463, 457.3778, 475.3778, 619.4207 |
| 14 | Ginsenoside Ra2 ** | C58H98O26 | [H]− | 1209.6292 | 1.5 | 21.83 | 149.0419, 323.1010, 621.4420, 783.4917 |
| 15 | Ginsenoside Rb1 | C54H92O23 | [COOH]+ | 1153.599 | −0.9 | 22.50 | 161.0469, 221.0674, 459.3756, 621.4323 |
| 16 | 20S Ginsenoside Rg2 | C42H72O13 | [COOH]+ | 829.4951 | 0.8 | 22.67 | 391.2899, 475.3792, 619.4224, 637.4360 |
| 17 | Malonyl ginsenoside Rb1 | C57H94O26 | [H]− | 1193.595 | −1.1 | 22.93 | 179.0562, 783.4874, 927.5361, 945.5423 |
| 18 | 20S Ginsenoside Rh1 | C36H62O9 | [COOH]+ | 683.4369 | 0.6 | 22.94 | 391.2749, 457.3619, 475.3830, 637.4316 |
| 19 | Ginsenoside Ra1 ** | C58H98O26 | [H]− | 1209.6282 | 0.7 | 23.08 | 621.4382, 783.4733, 945.5652, 1077.5902 |
| 20 | Ginsenoside Rc | C53H90O22 | [COOH]+ | 1123.59 | 0 | 23.44 | 149.0461, 621.4406, 765.4835, 783.4940 |
| 21 | Ginsenoside Rb3 | C53H90O22 | [COOH]+ | 1123.589 | −0.9 | 23.85 | 621.4402, 783.4904, 945.5373, 1077.5851 |
| 22 | Ginsenoside F1 | C36H62O9 | [COOH]+ | 683.4365 | −0.1 | 23.91 | 161.0456, 391.2859, 475.3801, 637.4345 |
| 23 | Malonyl ginsenoside Rc | C56H92O25 | [H]− | 1163.584 | −0.9 | 23.94 | 783.4904, 945.5373, 1059.5737, 1077.5851 |
| 24 | Ginsenoside Ro | C48H76O19 | [COOH]+ | 1001.496 | 0.9 | 24.4 | 455.3498, 523.3764, 569.3854, 613.3544 |
| 25 | Ginsenoside Rb2 | C53H90O22 | [COOH]+ | 1123.59 | 0.2 | 24.8 | 621.4420, 783.4967, 945.5501, 1077.5889 |
| 26 | Malonyl ginsenoside Rb2 | C56H92O25 | [H]− | 1163.584 | −0.9 | 24.83 | 459.3823, 621.4387, 783.4917, 945.5454 |
| 27 | Quinquenoside R1 | C56H94O24 | [COOH]+ | 1195.609 | −0.9 | 25.05 | 179.0564, 323.0999, 621.4222, 783.4717 |
| 28 | Ginsenoside Rs1 | C55H92O23 | [H]− | 1119.593 | −2.1 | 25.4 | 1077.5807, 945.5515, 783.4877, 621.4542 |
| 29 | Pseudoginsenoside RT1 | C47H74O18 | [H]− | 925.4797 | −0.5 | 25.48 | 523.4065, 569.3840, 613.3785, 701.4410 |
| 30 | 20R Ginsenoside Rh1 | C36H62O9 | [COOH]+ | 683.437 | 0.7 | 25.89 | 391.2854, 457.3704, 475.3805, 637.4319 |
| 31 | Ginsenoside Rd | C48H82O18 | [COOH]+ | 991.5474 | 0.2 | 26.65 | 161.0465, 621.4410, 783.4907,945.5418 |
| 32 | Malonyl ginsenoside Rd | C51H84O21 | [H]− | 1031.543 | −0.6 | 26.97 | 621.4394, 765.4816, 783.4937, 927.5336 |
| 33 | Pseudoginsenoside RC1 | C50H84O19 | [COOH]+ | 1033.552 | −5.4 | 27.44 | 161.0453, 459.3802, 621.4449, 765.4792 |
| 34 | Zingibroside R1 | C42H66O14 | [H]− | 793.4373 | −0.8 | 27.45 | 455.3508, 569.3859, 613.3767, 631.3851 |
| 35 | Ginsenoside Rg10 | C42H70O13 | [COOH]+ | 827.4781 | −0.8 | 29.98 | 161.0415, 221.0695, 619.4169, 781.4760 |
| 36 | Ginsenoside Rg6 | C42H70O12 | [COOH]+ | 811.4842 | 0.5 | 31.86 | 161.0447, 457.3617, 601.4126, 619.4218 |
| 37 | Ginsenoside Rg3 | C42H72O13 | [COOH]+ | 829.4949 | 0.6 | 32.10 | 161.0468, 459.3864, 621.4404,783.4958 |
| 38 | Ginsenoside Rk3 | C36H60O8 | [COOH]+ | 665.4262 | 0.4 | 32.37 | 161.0457, 457.3588, 619.4184, 665.4284 |
| 39 | Chikusetsusaponin IVA | C42H66O14 | [H]− | 793.438 | 0 | 32.59 | 455.3530, 569.3874, 613.3763, 793.4408 |
| 40 | Ginsenoside F2 | C42H72O13 | [COOH]+ | 829.4949 | 0.6 | 32.73 | 161.0460, 459.3853, 621.4396,783.4938 |
| 41 | Ginsenoside Rs3 | C44H74O14 | [COOH]+ | 871.5011 | −4.4 | 32.92 | 161.0461, 375.2889, 459.3834, 621.4326 |
| 42 | Ginsenoside Rk1 | C42H70O12 | [COOH]+ | 811.4845 | 0.8 | 34.20 | 161.0460, 537.3988, 603.4275, 765.4791 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Zhang, Y.; Yang, P.; Tong, M.; Xing, L.; Zhang, Q.; Bi, K.; Li, Q. An Integrated Mutually Oriented “Chemical Profiling–Pharmaceutical Effect” Strategy for Screening Discriminating Markers of Underlying Hepatoprotective Effects to Distinguish Garden-Cultivated from Mountain-Cultivated Ginseng. Molecules 2021, 26, 5456. https://doi.org/10.3390/molecules26185456
Li S, Zhang Y, Yang P, Tong M, Xing L, Zhang Q, Bi K, Li Q. An Integrated Mutually Oriented “Chemical Profiling–Pharmaceutical Effect” Strategy for Screening Discriminating Markers of Underlying Hepatoprotective Effects to Distinguish Garden-Cultivated from Mountain-Cultivated Ginseng. Molecules. 2021; 26(18):5456. https://doi.org/10.3390/molecules26185456
Chicago/Turabian StyleLi, Saiyu, Yiwen Zhang, Panpan Yang, Minghui Tong, Luwen Xing, Qian Zhang, Kaishun Bi, and Qing Li. 2021. "An Integrated Mutually Oriented “Chemical Profiling–Pharmaceutical Effect” Strategy for Screening Discriminating Markers of Underlying Hepatoprotective Effects to Distinguish Garden-Cultivated from Mountain-Cultivated Ginseng" Molecules 26, no. 18: 5456. https://doi.org/10.3390/molecules26185456
APA StyleLi, S., Zhang, Y., Yang, P., Tong, M., Xing, L., Zhang, Q., Bi, K., & Li, Q. (2021). An Integrated Mutually Oriented “Chemical Profiling–Pharmaceutical Effect” Strategy for Screening Discriminating Markers of Underlying Hepatoprotective Effects to Distinguish Garden-Cultivated from Mountain-Cultivated Ginseng. Molecules, 26(18), 5456. https://doi.org/10.3390/molecules26185456