Bimodal Ultrasound and X-ray Bioimaging Properties of Particulate Calcium Fluoride Biomaterial
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physicochemical Characterization
2.1.1. Scanning Electron Microscopy Analysis
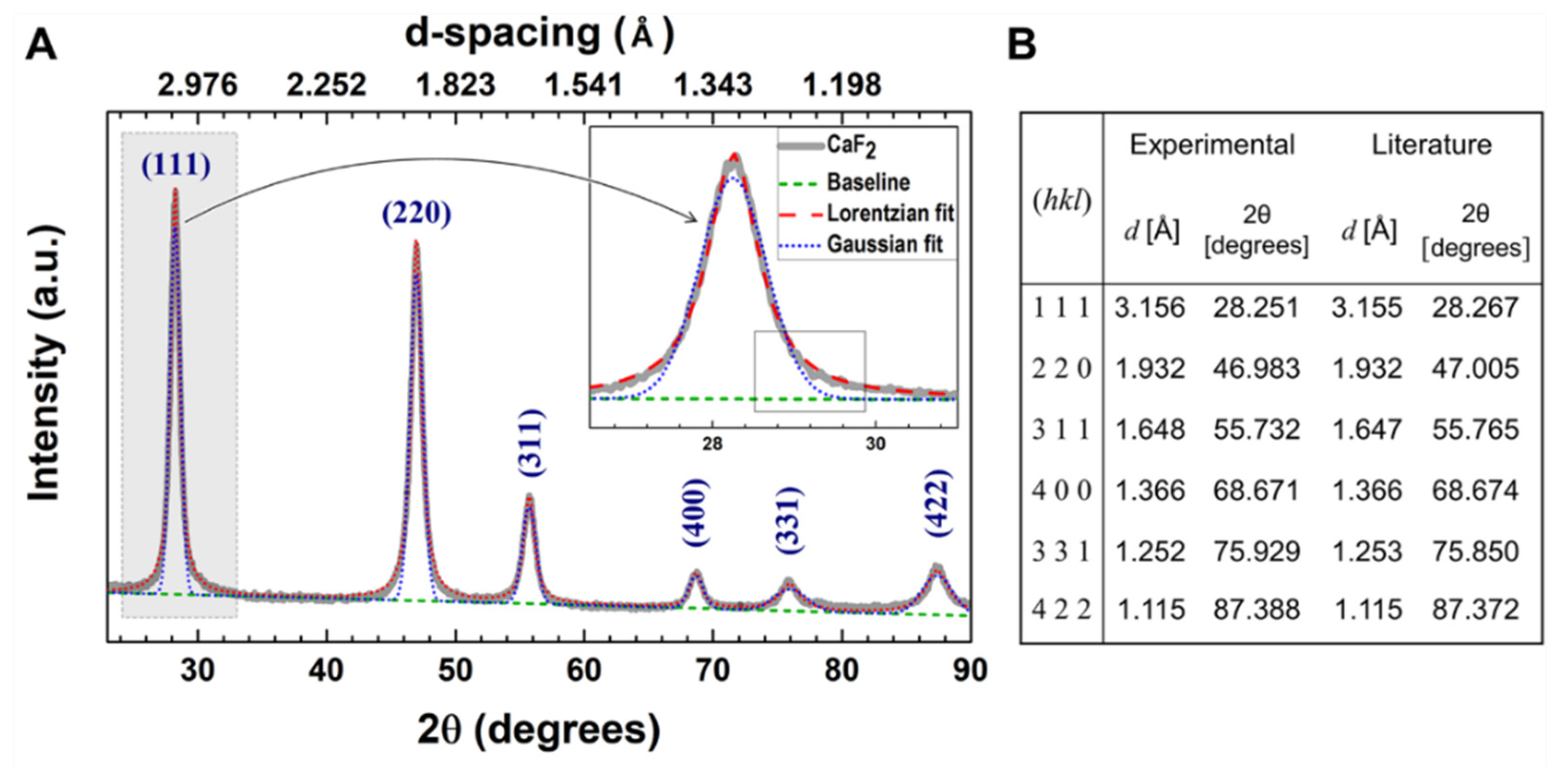
2.1.2. X-ray Diffraction
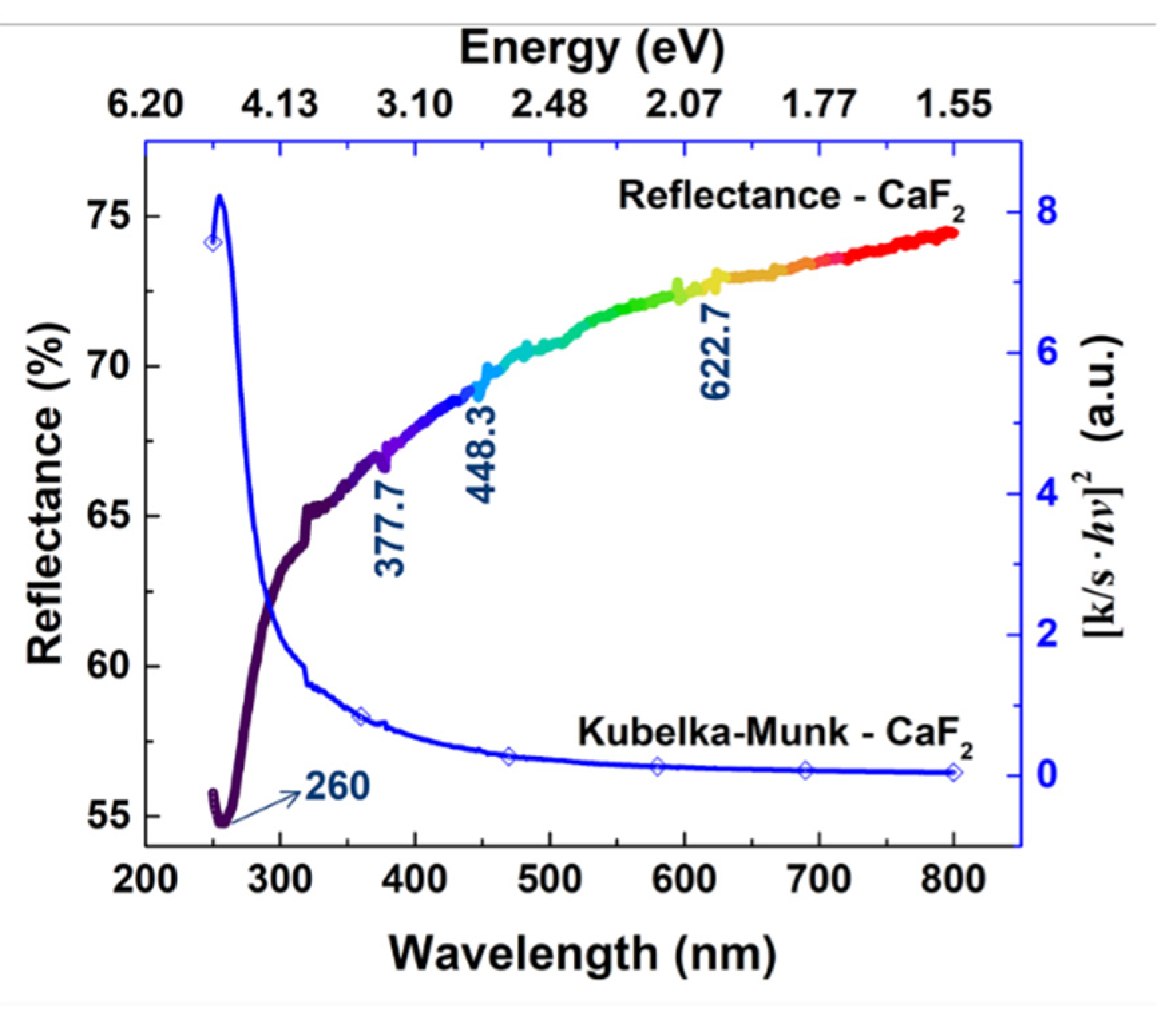
2.1.3. Diffuse Reflectance UV-Vis Spectroscopy
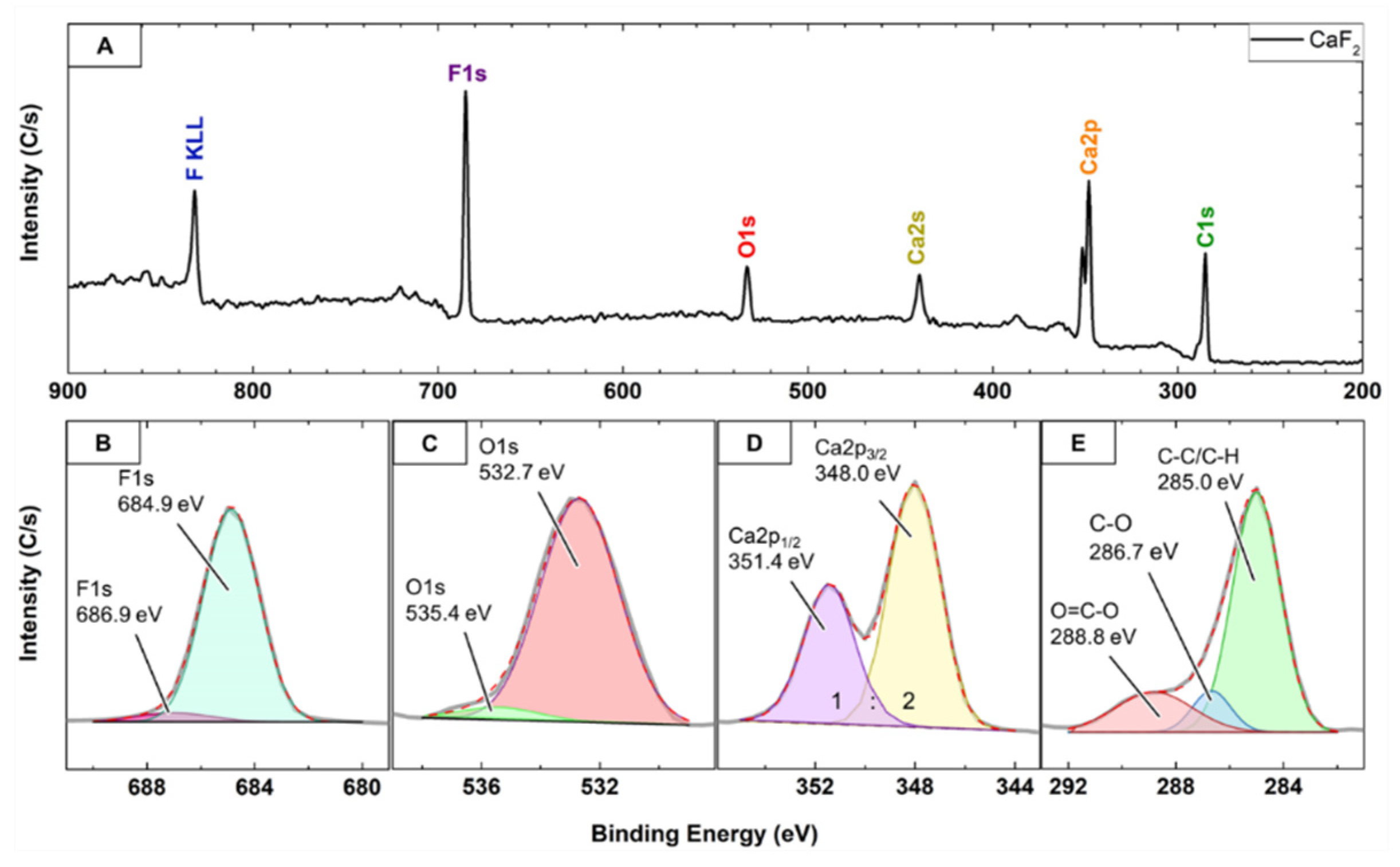
2.1.4. X-ray Photoelectron Spectroscopy
2.2. Biological Properties of Part-CaF2
2.2.1. In Vitro Cytotoxicity
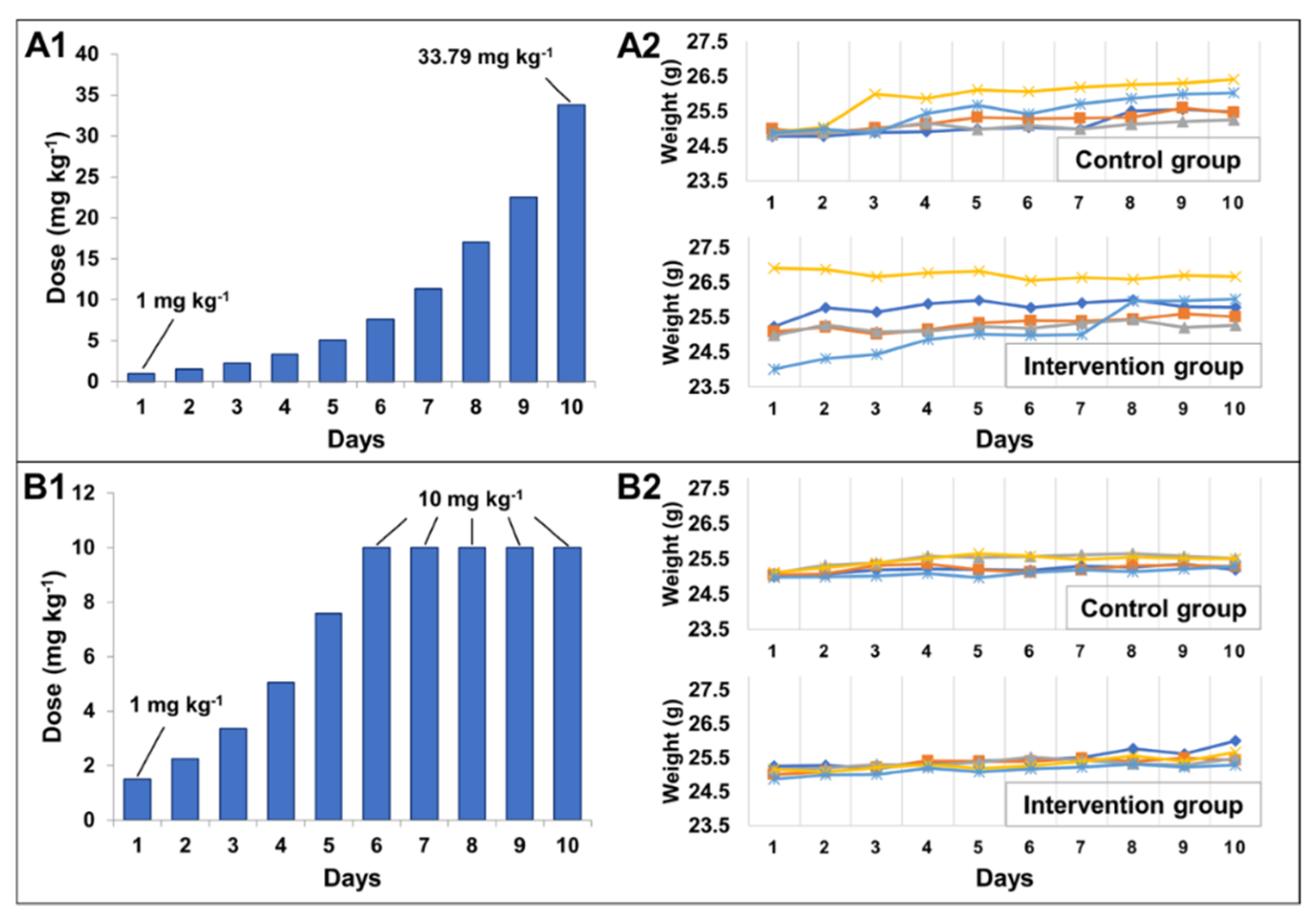
2.2.2. Determination of Maximum Tolerated Dose
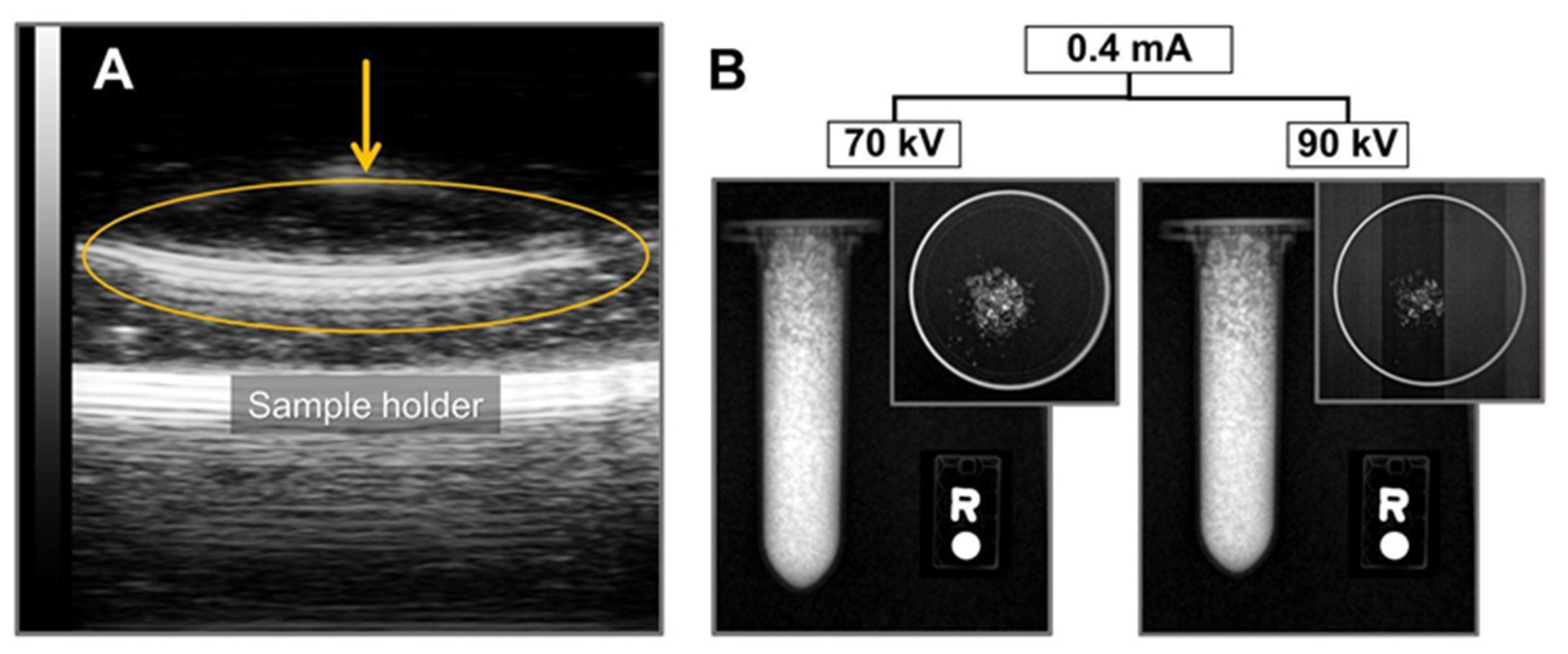
2.3. Ultrasound and X-ray Imaging
3. Materials and Methods
3.1. Preparation of Particulate CaF2
3.2. Physico-chemical Characterization
3.2.1. Scanning Electron Microscopy—Energy Dispersive X-ray Spectroscopy (SEM-EDX)
3.2.2. X-ray Diffraction
3.2.3. Diffuse Reflectance UV-Vis Spectroscopy
3.2.4. X-ray Photoelectron Spectroscopy (XPS)
3.3. Biological Properties of Part-CaF2
3.3.1. In Vitro Cytotoxicity
3.3.2. Maximum Tolerated Dose (MTD)
3.4. Ultrasound and X-ray Imaging Using Part-CaF2
4. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Nelson, T.R.; Pretorius, D.H. Three-Dimensional Ultrasound Imaging. Ultrasound Med. Biol. 1998, 24, 1243–1270. [Google Scholar] [CrossRef]
- Bercovich, E.; Javitt, M.C. Medical Imaging: From Roentgen to the Digital Revolution, and Beyond. Rambam Maimonides Med. J. 2018, 9, e0034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallyn, J.; Anton, N.; Akram, S.; Vandamme, T.F. Biomedical Imaging: Principles, Technologies, Clinical Aspects, Contrast Agents, Limitations and Future Trends in Nanomedicines. Pharm. Res. 2019, 36, 78. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.A.; Brahme, A. Development of phase-contrast X-ray imaging techniques and potential medical applications. Phys. Med. 2008; 24, 129–148. [Google Scholar]
- Lusic, H.; Grinstaff, M.W. X-ray-computed tomography contrast agents. Chem. Rev. 2013, 113, 1641–1666. [Google Scholar] [CrossRef] [Green Version]
- Shibaguchi, H.; Tsuru, H.; Kuroki, M.; Kuroki, M. Sonodynamic Cancer Therapy: A Non-invasive and Repeatable Approach Using Low-intensity Ultrasound with a Sonosensitizer. Anticancer Res. 2011, 31, 2425–2430. [Google Scholar]
- Chen, H.; Zhou, X.; Gao, Y.; Zheng, B.; Tang, F.; Huang, J. Recent progress in development of new sonosensitizers for sonodynamic cancer therapy. Drug Discov. Today 2014, 19, 502–509. [Google Scholar] [CrossRef]
- Ignee, A.; Atkinson, N.S.; Schuessler, G.; Dietrich, C.F. Ultrasound contrast agents. Endosc. Ultrasound 2016, 5, 355–362. [Google Scholar] [CrossRef] [Green Version]
- Alexis, F.; Pridgen, E.; Molnar, L.K.; Farokhzad, O.C. Factors Affecting the Clearance and Biodistribution of Polymeric Nanoparticles. Mol. Pharm. 2008, 5, 505–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolishetti, N.; Alexis, F.; Pridgen, E.M.; Farokhzad, O.C. Biodistribution and Pharmacokinetics of Nanoprobes. In Nanoplatform-Based Molecular Imaging; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 75–104. [Google Scholar]
- Chingo Aimacaña, C.M.; Quinchiguango Perez, D.A.; Rocha Pinto, S.; Debut, A.; Attia, M.F.; Santos-Oliveira, R.; Whitehead, D.C.; Terencio, T.; Alexis, F.; Dahoumane, S.A. Polytetrafluoroethylene-like Nanoparticles as a Promising Contrast Agent for Dual Modal Ultrasound and X-ray Bioimaging. ACS Biomater. Sci. Eng. 2021, 7, 1181–1191. [Google Scholar] [CrossRef]
- Sasidharan, S.; Jayasree, A.; Fazal, S.; Koyakutty, M.; Nair, S.V.; Menon, D. Ambient temperature synthesis of citrate stabilized and biofunctionalized, fluorescent calcium fluoride nanocrystals for targeted labeling of cancer cells. Biomater. Sci. 2013, 1, 294–305. [Google Scholar] [CrossRef]
- Prasad, S.; Ganisetti, S.; Jana, A.; Kant, S.; Sinha, P.K.; Tripathy, S.; Illath, K.; Ajithkumar, T.G.; Annapurna, K.; Allu, A.R.; et al. Elucidating the effect of CaF2 on structure, biocompatibility and antibacterial properties of S53P4 glass. J. Alloys Compd. 2020, 831, 154704. [Google Scholar] [CrossRef]
- Afseth, J.; Ekstrand, J.; Hagelid, P. Dissolution of calcium fluoride tablets in vitro and bioavailability in man. Scand. J. Dent. Res. 1987, 95, 191–192. [Google Scholar] [CrossRef] [PubMed]
- Larsen, M.J.; Ravnholt, G. Dissolution of Various Calcium Fluoride Preparations in Inorganic Solutions and in Stimulated Human Saliva. Caries Res. 1994, 28, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Chow, L.C. Preparation and properties of nano-sized calcium fluoride for dental applications. Dent. Mater. 2008, 24, 111–116. [Google Scholar] [CrossRef] [Green Version]
- Ghafar, H.; Khan, M.I.; Sarwar, H.S.; Yaqoob, S.; Hussain, S.Z.; Tariq, I.; Madni, A.U.; Shahnaz, G.; Sohail, M.F. Development and Characterization of Bioadhesive Film Embedded with Lignocaine and Calcium Fluoride Nanoparticles. AAPS PharmSciTech 2020, 21, 60. [Google Scholar] [CrossRef]
- Bala, W.A.; Benitha, V.S.; Jeyasubramanian, K.; Hikku, G.S.; Sankar, P.; Kumar, S.V. Investigation of anti-bacterial activity and cytotoxicity of calcium fluoride nanoparticles. J. Fluor. Chem. 2017, 193, 38–44. [Google Scholar] [CrossRef]
- Kulshrestha, S.; Khan, S.; Hasan, S.; Khan, M.E.; Misba, L.; Khan, A.U. Calcium fluoride nanoparticles induced suppression of Streptococcus mutans biofilm: An in vitro and in vivo approach. Appl. Microbiol. Biotechnol. 2016, 100, 1901–1914. [Google Scholar] [CrossRef]
- Jeong, S.H.; Shin, D.Y.; Kang, I.K.; Song, E.H.; Seong, Y.J.; Park, J.U.; Kim, H.E. Effective Wound Healing by Antibacterial and Bioactive Calcium-Fluoride-Containing Composite Hydrogel Dressings Prepared Using in Situ Precipitation. ACS Biomater. Sci. Eng. 2018, 4, 2380–2389. [Google Scholar] [CrossRef]
- Ashur, I.; Allouche-Arnon, H.; Bar-Shir, A. Calcium Fluoride Nanocrystals: Tracers for In Vivo 19F Magnetic Resonance Imaging. Angew. Chem. Int. Ed. Engl. 2018, 57, 7478–7482. [Google Scholar] [CrossRef]
- Wei, J.; Zheng, W.; Shang, X.; Li, R.; Huang, P.; Liu, Y.; Gong, Z.; Zhou, S.; Chen, Z.; Chen, X. Mn2+-activated calcium fluoride nanoprobes for time-resolved photoluminescence biosensing. Sci. China Mater. 2019, 62, 130–137. [Google Scholar] [CrossRef] [Green Version]
- Straßer, M.; Schrauth, J.H.X.; Dembski, S.; Haddad, D.; Ahrens, B.; Schweizer, S.; Christ, B.; Cubukova, A.; Metzger, M.; Walles, H.; et al. Calcium fluoride based multifunctional nanoparticles for multimodal imaging. Beilstein J. Nanotechnol. 2017, 8, 1484–1493. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.H.; Moreau, J.L.; Sun, L.; Chow, L.C. Novel CaF2 nanocomposite with high strength and fluoride ion release. J. Dent. Res. 2010, 89, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Azami, M.; Jalilifiroozinezhad, S.; Mozafari, M.; Rabiee, M. Synthesis and solubility of calcium fluoride/hydroxy-fluorapatite nanocrystals for dental applications. Ceram. Int. 2011, 37, 2007–2014. [Google Scholar] [CrossRef]
- Mitwalli, H.; Balhaddad, A.A.; AlSahafi, R.; Oates, T.W.; Melo, M.A.S.; Xu, H.H.K.; Weir, M.D. Novel CaF2 Nanocomposites with Antibacterial Function and Fluoride and Calcium Ion Release to Inhibit Oral Biofilm and Protect Teeth. J. Funct. Biomater. 2020, 11, 56. [Google Scholar] [CrossRef]
- Klibanov, A.L. Targeted delivery of gas-filled microspheres, contrast agents for ultrasound imaging. Adv. Drug Deliv. Rev. 1999, 37, 139–157. [Google Scholar] [CrossRef]
- Chandra Sekhar Reddy, K.; Chingakham, C.; Gupta, B.; Shiva Prasad, M.; Atchuta, S.R.; Sakthivel, S. Single compound in-situ synthesis of core-shell CaF2 nanoparticles based broad band antireflective coatings for solar energy conversion. Sol. Energy 2019, 190, 119–125. [Google Scholar] [CrossRef]
- Pandurangappa, C.; Lakshminarasappa, B.N. Optical absorption and Photoluminescence studies in Gamma-irradiated nanocrystalline CaF2. J. Nanomed. Nanotechnol. 2011, 2, 2. [Google Scholar] [CrossRef]
- Bezerra, C.d.S.; Valerio, M.E.G. Structural and optical study of CaF2 nanoparticles produced by a microwave-assisted hydrothermal method. Phys. B Condens. Matter 2016, 501, 106–112. [Google Scholar] [CrossRef]
- Il’ves, V.G.; Sokovnin, S.Y.; Zuev, M.G.; Uimin, M.A.; Rähn, M.; Kozlova, J.; Sammelselg, V. Effect of Annealing on Structural, Textural, Thermal, Magnetic, and Luminescence Properties of Calcium Fluoride Nanoparticles. Phys. Solid State 2019, 61, 2200–2217. [Google Scholar] [CrossRef]
- Motevalizadeh, L.; Heidary, Z.; Ebrahimizadeh Abrishami, M. Facile template-free hydrothermal synthesis and microstrain measurement of ZnO nanorods. Bull. Mater. Sci. 2014, 37, 397–405. [Google Scholar] [CrossRef]
- Pandurangappa, C.; Lakshminarasappa, B.N. Optical studies of samarium-doped fluoride nanoparticles. Philos. Mag. 2011, 91, 4486–4494. [Google Scholar] [CrossRef]
- Taurozzi, J.S.; Hackley, V.A.; Wiesner, M.R. Ultrasonic dispersion of nanoparticles for environmental, health and safety assessment—Issues and recommendations. Nanotoxicology 2011, 5, 711–729. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J.D.; Rizzello, L.; Avila-Olias, M.; Gaitzsch, J.; Contini, C.; Magon, M.S.; Renshaw, S.A.; Battaglia, G. Purification of Nanoparticles by Size and Shape. Sci. Rep. 2016, 6, 27494. [Google Scholar] [CrossRef] [PubMed]
- Molaiyan, P.; Witter, R. Surface defect-enhanced conductivity of calcium fluoride for electrochemical applications. Mater. Des. Process. Comm 2019, 1, e44. [Google Scholar] [CrossRef] [Green Version]
- Kumar, G.A.; Chen, C.W.; Ballato, J.; Riman, R.E. Optical Characterization of Infrared Emitting Rare-Earth-Doped Fluoride Nanocrystals and Their Transparent Nanocomposites. Chem. Mater. 2007, 19, 1523–1528. [Google Scholar] [CrossRef]
- Heaton, R.A.; Lin, C.C. Electronic energy-band structure of the calcium fluoride crystal. Phys. Rev. B 1980, 22, 3629–3638. [Google Scholar] [CrossRef]
- Suturin, S.M.; Banshchikov, A.G.; Sokolov, N.S.; Tyaginov, S.E.; Vexler, M.I. Static current-voltage characteristics of Au/CaF2/n-Si(111) MIS tunneling structures. Semiconductors 2009, 42, 1304–1308. [Google Scholar] [CrossRef]
- Budyanto, S.; Kuo, Y.-L.; Liu, J.C. Adsorption and precipitation of fluoride on calcite nanoparticles: A spectroscopic study. Sep. Purif. Technol. 2015, 150, 325–331. [Google Scholar] [CrossRef]
- Fujii, E.; Kawabata, K.; Yoshimatsu, H.; Hayakawa, S.; Tsuru, K.; Osaka, A. Structure and Biomineralization of Calcium Silicate Glasses Containing Fluoride Ions. J. Ceram. Soc. Jpn. 2003, 111, 762–766. [Google Scholar] [CrossRef] [Green Version]
- Gerth, H.U.; Dammaschke, T.; Schafer, E.; Zuchner, H. A three layer structure model of fluoridated enamel containing CaF2, Ca(OH)2 and FAp. Dent. Mater. 2007, 23, 1521–1528. [Google Scholar] [CrossRef] [PubMed]
- Borisyuk, P.V.; Vasilyev, O.S.; Krasavin, A.V.; Lebedinskii, Y.Y.; Troyan, V.I.; Tkalya, E.V. Band structure and decay channels of thorium-229 low-lying isomeric state for ensemble of thorium atoms adsorbed on calcium fluoride. Phys. Status Solidi C 2015, 12, 1333–1337. [Google Scholar] [CrossRef] [Green Version]
- Ono, K. A Comprehensive Report on Ultrasonic Attenuation of Engineering Materials, Including Metals, Ceramics, Polymers, Fiber-Reinforced Composites, Wood, and Rocks. Appl. Sci. 2020, 10, 2230. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Wang, G.; Guo, Y.; Kang, F.; Huang, Y.; Bo, D. Microwave-assisted synthesis and characterization of hierarchically structured calcium fluoride. Mater. Res. Bull. 2012, 47, 3965–3970. [Google Scholar] [CrossRef]
- Łukomska-Szymańska, M.; Zarzycka, B.; Grzegorczyk, J.; Sokołowski, K.; Półtorak, K.; Sokołowski, J.; Łapińska, B. Antibacterial Properties of Calcium Fluoride-Based Composite Materials: In Vitro Study. BioMed Res. Int. 2016, 2016, 1048320. [Google Scholar]
- Malviya, D.; Pawade, V.B.; Bhanvase, B.A. Ultrasound assisted synthesis of CaF2:Eu3+ phosphor nanoparticles. Luminescence 2019, 34, 426–431. [Google Scholar] [CrossRef]
- Wang, J.; Miao, W.; Li, Y.; Yao, H.; Li, Z. Water-soluble Ln3+-doped calcium fluoride nanocrystals: Controlled synthesis and luminescence properties. Mater. Lett. 2009, 63, 1794–1796. [Google Scholar] [CrossRef]
- Xu, H.H.; Moreau, J.L.; Sun, L.; Chow, L.C. Strength and fluoride release characteristics of a calcium fluoride based dental nanocomposite. Biomaterials 2008, 29, 4261–4267. [Google Scholar] [CrossRef] [Green Version]
- Nath, D.; Singh, F.; Das, R. X-ray diffraction analysis by Williamson-Hall, Halder-Wagner and size-strain plot methods of CdSe nanoparticles-a comparative study. Mater. Chem. Phys. 2020, 239, 122021. [Google Scholar] [CrossRef]
- Aston, W.J.; Hope, D.E.; Nowak, A.K.; Robinson, B.W.; Lake, R.A.; Lesterhuis, W.J. A systematic investigation of the maximum tolerated dose of cytotoxic chemotherapy with and without supportive care in mice. BMC Cancer 2017, 17, 684. [Google Scholar] [CrossRef] [Green Version]


| Score | 0 | 1 | 2 | |
|---|---|---|---|---|
| Appearance | Weight loss | Steady weight | >10% weight loss | 15–20% weight loss |
| Coat | Normal | Mild ruffled coat | Moderate ruffled coat | |
| Body condition | Normal | Thin | Excessive loss fat (>15%) | |
| Body posture | Normal | Hunched | Hunched and still | |
| Movement | Normal | Reduced/slow | Reluctant to move when touched | |
| Activity | Proximity to others | Close contact | Separate | Completely separate |
| Other | Injection site | Normal | Redness | Redness and swelling |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chingo Aimacaña, C.M.; Pila, K.O.; Quinchiguango Perez, D.A.; Debut, A.; Attia, M.F.; Santos-Oliveira, R.; Whitehead, D.C.; Reinoso, C.; Alexis, F.; Dahoumane, S.A. Bimodal Ultrasound and X-ray Bioimaging Properties of Particulate Calcium Fluoride Biomaterial. Molecules 2021, 26, 5447. https://doi.org/10.3390/molecules26185447
Chingo Aimacaña CM, Pila KO, Quinchiguango Perez DA, Debut A, Attia MF, Santos-Oliveira R, Whitehead DC, Reinoso C, Alexis F, Dahoumane SA. Bimodal Ultrasound and X-ray Bioimaging Properties of Particulate Calcium Fluoride Biomaterial. Molecules. 2021; 26(18):5447. https://doi.org/10.3390/molecules26185447
Chicago/Turabian StyleChingo Aimacaña, Cristhian Marcelo, Kevin O. Pila, Dilan A. Quinchiguango Perez, Alexis Debut, Mohamed F. Attia, Ralph Santos-Oliveira, Daniel C. Whitehead, Carlos Reinoso, Frank Alexis, and Si Amar Dahoumane. 2021. "Bimodal Ultrasound and X-ray Bioimaging Properties of Particulate Calcium Fluoride Biomaterial" Molecules 26, no. 18: 5447. https://doi.org/10.3390/molecules26185447
APA StyleChingo Aimacaña, C. M., Pila, K. O., Quinchiguango Perez, D. A., Debut, A., Attia, M. F., Santos-Oliveira, R., Whitehead, D. C., Reinoso, C., Alexis, F., & Dahoumane, S. A. (2021). Bimodal Ultrasound and X-ray Bioimaging Properties of Particulate Calcium Fluoride Biomaterial. Molecules, 26(18), 5447. https://doi.org/10.3390/molecules26185447











