2.1. Synthesis of Protected Adenosine Complexes
The synthesis employs oxidative addition of brominated adenosine to platinum(0). Accordingly, the reaction of proligand 2′,3′,5′-tri-
O-acetyl-8-bromoadenosine (
BrAdeAc) [
8,
9] with Pt(PPh
3)
4 in toluene, at 100 °C, affords the adenosine complex
1 (
Scheme 1) in 76% isolated yield. Protection of the ribose is required, since the reaction with unprotected nucleoside leads to a mixture of products. Complex
1 was characterized by NMR spectroscopy. In the
1H NMR of
1, the C’1-H of the ribose ring appears as a doublet at 6.93 ppm, shifted by 0.85 ppm with respect to the ligand precursor
BrAdeAc. The most significant effect of the metalation is on the chemical shift of the NH
2 protons, which undergo an upfield shift of 1.25 ppm with respect to
BrAdeAc.
In the
13C{
1H} NMR, the C8 carbon resonates at 152.1 ppm, a value similar to other platinum analogues [
6], while the ribose ring of
1 gives rise to five signals between δ 62.7 and 89.0 ppm. The
31P{
1H} NMR spectrum confirms the expected trans connectivity already described for similar platinum compounds [
10,
11]. The two phosphorous atoms are located in two non-interconverting diastereotopic sites, due to the presence of the chiral ribose unit combined with the hindered rotation around the Pt–C8 bond. For this reason, it is possible to observe the coupling between the two phosphorus nuclei, with an AB spin system at 19.29 and 18.13 ppm featuring a very substantial rooftop effect and a
2JP,P = 435 Hz for both. Additionally, we observe two slightly different coupling constants with
195Pt, with a value of
1JPA,Pt = 2754 Hz and
1JPB,Pt = 2735 Hz. For
195Pt NMR, it is not possible to distinguish the different couplings of P
A and P
B and a triplet is observed at −4485 ppm, with a coupling constant of
1JPt,P = 2735 Hz, consistent with values determined by
31P{
1H} NMR.
Compound
1 reacts readily at N7 in the presence of acid or methyl iodide, leading to the formation of compounds
2 and
3, respectively (
Scheme 2). This reactivity parallels the one found for palladium and platinum analogues [
6,
7].
Both complexes 2 and 3 were characterized by NMR spectroscopy. For complex 2, the 1H NMR shows the new N7-H at 10.66 ppm, and significant variations for the NH2 protons, which undergo a downfield shift of 0.93 ppm, relatively to 1. For compound 3, the new N7-CH3 resonates at 3.67 ppm and, as with 2, the NH2 protons undergo a downfield shift of 1.44 ppm to 6.39 ppm. In the 13C{1H}, for the protic NHC 2, the carbenic carbon (C8) appears as a triplet at 163.6 ppm (2JC,P = 9.5 Hz), with a downfield shift of 11 ppm, reflecting the formation of the NHC. This is also the case for 3, with C8 at 163.8 ppm (2JC,P = 9.0 Hz). Similarly to 1, the 31P{1H} spectra of complexes 2 and 3 show the presence of AB spin systems due to the coupling of the two phosphorus. The AB quartet resonates at 16.15, 15.26 ppm for 2, and at 16.12, 15.04 ppm for 3, with 2JP,P = 414 Hz and 2JP,P = 413 Hz, respectively. From the 195Pt satellites, it is evident that in both 2 and 3, the two phosphorus nuclei also have different coupling constants with the platinum centre, with 1JPA,Pt = 2433 Hz; 1JPB,Pt = 2423 Hz for 2, and 1JPA,Pt = 2415 Hz; 1JPB,Pt = 2408 Hz for 3. In the 195Pt NMR spectra, only one coupling constant is observed in both cases, and compounds 2 and 3 show triplets at −4498 (1JPt,P = 2421 Hz) and −4500 (1JPt,P = 2408 Hz) ppm, with 1JPt,P consistent with those determined by 31P NMR. This is a decrease of 1JPt,P of approximately 300 Hz relatively to 1 for both, which comes as result of carbene formation, which will be further discussed in a following section.
Crystals of compound
2 were obtained from a saturated chloroform solution and were analysed by single-crystal X-ray diffraction. Unfortunately, the X-ray analysis revealed that the crystals presented racemic twinning and thermal disorder, which prevented the refinement in the chiral space group P1, only allowing the structure refinement with full data convergence in the centrosymmetric space group P−1. Still, its molecular structure, which fully agrees with the remaining chemical analysis, is depicted in
Figure S1. The platinum centre in
2 displays a square planar geometry, where the sum of the four cis-L–Pt–L’ angles is 360.3(3)°. The plane of the adenine ligand is nearly perpendicular to the coordination plane of the metal centre (dihedral angle of 92.8(3)°). All distances and angles are within the expected values for similar compounds [
5].
The formation of complexes
2 and
3 is indicative of the increased nucleophilic character of N7, which becomes the most nucleophilic position in complex
1. This increased nucleophilic character is in line with our previous findings regarding the stability of C8-platinated guanosine derivatives. For these compounds, we have demonstrated that metalation induces a higher stability of the nucleoside towards hydrolysis [
5,
6,
7]. This higher stability is a consequence of a stronger N9-C1′ bond, itself a result of the increased nucleophilic character of the N9 upon metalation. This is due to the electron donation from the metal centre to the adjacent nitrogen of the C-bonded heterocycle [
5,
6,
12,
13,
14,
15,
16]. This is also the case for N7, where electron donation renders the N7 more prone to methylation and protonation. While in the case of guanosine, N7 is the most nucleophilic site, irrespectively of metalation at C8; for the adenosine fragment in
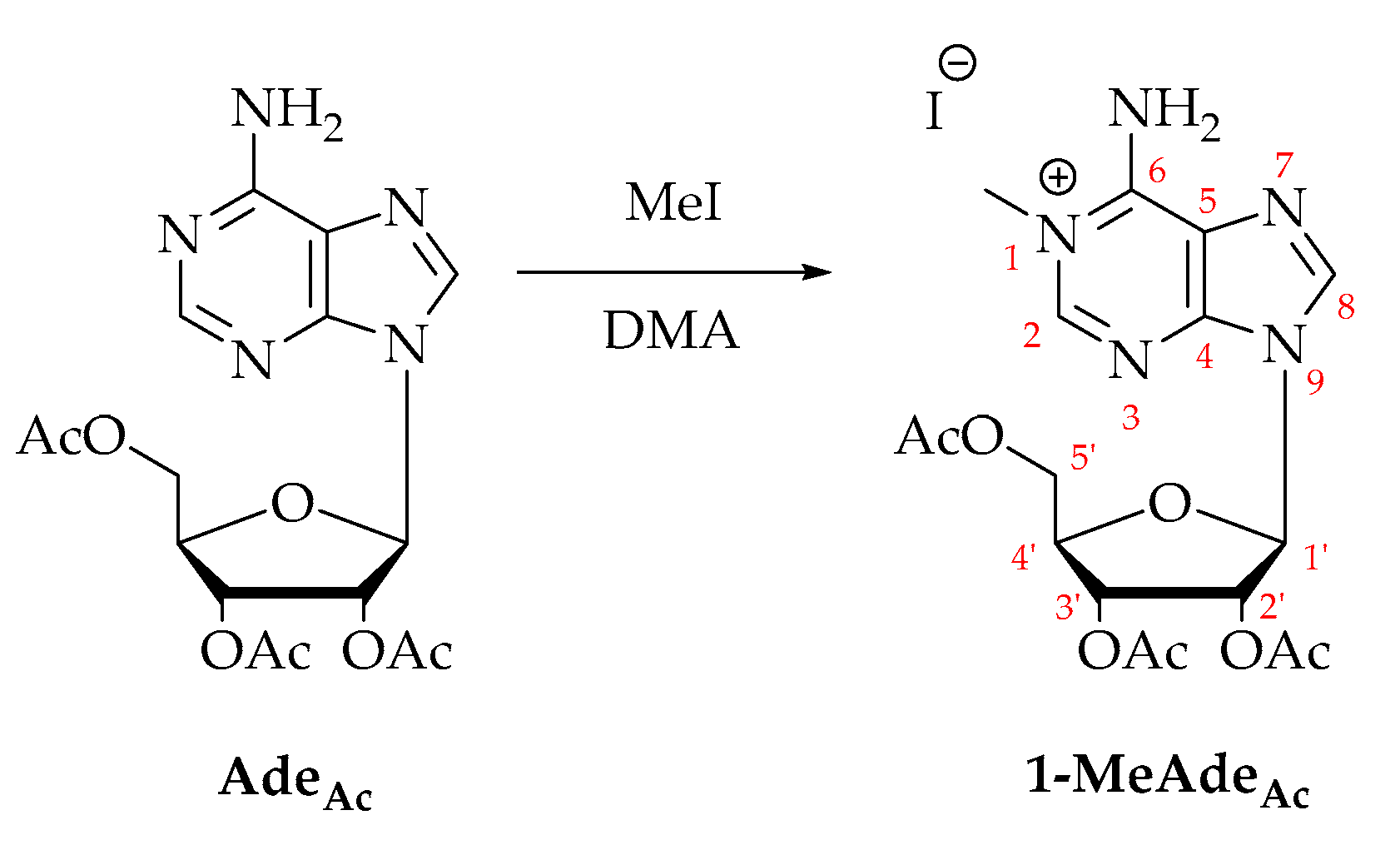
1, the higher nucleophilic character of N7 is in striking contrast with what is observed for the free nucleoside. Indeed, the most nucleophilic position in adenosine is N1 and when adenosine is reacted with methyl iodide, 1-methyladenosine is formed exclusively (
Scheme 3). We can conclude that platination at C8 induces a change in charge distribution within the adenine ring which can be further used to expand the reactivity of the nucleoside.
Importantly, for complex
1, we were unable to determine if metalation induced a higher stability to the adenosine fragment. When adenosine and complex
1 are heated at 100 °C in DMF-
d7 for several hours, both compounds remain stable and no decomposition is noted, contrasting with what we observed for guanosine complexes [
5], for which the metalated nucleoside is far more stable.
2.2. Synthesis of Deprotected Adenosine Complexes
One of the main issues when synthesizing organometallic nucleosides is the need to protect the sugar moiety. While for protected sugars it is possible to direct the synthesis and improve efficiency, without protection, the synthesis can lead to a myriad of compounds. However, subsequent deprotection can compromise the integrity of the formed complex and lead to decomposition. Indeed, Messere et al. showed that a palladium complex C-bound thymidine decomposes upon deprotection [
17]. There are nonetheless reports for the functionalization of nucleosides via cross-coupling with late transition metals that do not require protection [
18], although the corresponding metalated intermediates have not yet, to the best of our knowledge, been isolated.
We have recently reported a successful methodology for the deprotection of palladium and platinum compounds based on guanosine that does not compromise the stability of the M–C bond and does not promote the hydrolysis of the sugar [
5,
6]. This methodology makes use of acidic ethanolic solutions and can also lead to the direct formation of protic NHCs by concomitant protonation of N7. Unfortunately, when the same methodology was employed with adenosine complex
1, deprotection was not efficient and led to a mixture of compounds. We were able to circumvent this issue by using ammonium hydroxide solutions, upon which the synthesis of compound
4 was achieved efficiently, with 66% isolated yield (
Scheme 4). Again, this deprotection methodology does not compromise the Pt–C bond or the glycosidic bond, as confirmed by NMR.
Compound 4 was characterized by NMR spectroscopy in DMSO-d6, due to the higher resolution achieved in this solvent as compared to CDCl3. Compounds 1 and 2, as well as both ligand precursors, were also characterized in DMSO-d6 for comparative purposes. For compound 4, the presence of the hydroxyl groups can be confirmed by the appearance of three additional signals 5.50(dd), 5.46(d) and 5.23(d) ppm. The chemical shifts of the H−2′ and H−3′ vary significantly upfield (~1.5 ppm), with respect to 1. In the 13C{1H} NMR spectrum, the C−8 peak resonates at 147.5 ppm, similar to that of 1. 31P{1H} shows a slightly broader singlet at 17.93 ppm with a 1JP,Pt = 2820 Hz. The 195Pt NMR shows a triplet at −4470 ppm, with 1JPt,P ≈ 2812 Hz consistent with the value found in 31P{1H}.
As in the case of
1, compound
4 reacts in the presence of a proton or methyl source leading to the formation of compounds
5 (97% yield) and
6 (70% yield), respectively (
Scheme 5). Both compounds were characterized by NMR. In the
1H NMR, the new NH group in
5 can be detected at 14.03 ppm, a downfield shift of 1.54 ppm with respect to
2, probably a result of hydrogen bonding with the chloride. For
6, the new methyl group resonates as a singlet at 3.30 ppm. In both complexes, the NH
2 group undergoes significant variations, with downfield shifts of approximately 0.9 ppm for both
5 and
6. In the
13C{
1H} NMR, the carbenic C8 in
5 resonates at 156.7 ppm as triplet with a
2JC,P = 9.0 Hz, while for
6, the triplet resonates at 161.0 ppm (
2JC,P = 10.0 Hz). As in the case of
2 and
3, the variations of C8 in
5 (9 ppm) and
6 (14 ppm), with respect to
4, reflect the formation of the NHC. In the
31P{
1H} NMR, we observe an upfield shift of the
31P signals upon quaternization of N7. Again, an AB quartet due to the coupling between the two phosphorus nuclei is detected, at 16.79, 16.10 (
2JP,P = 418 Hz) for
5, and 15.70, 15.06 (
2JP,P = 415 Hz) for
6, and
1JP,Pt values of 2493 Hz and 2456 Hz, respectively. In the
195Pt, the corresponding triplets can be detected at −4490 (
5) and −4504 ppm (
6), and calculated values of
1JP,Pt are in agreement with those calculated for
31P NMR.
It should be noted that attempts to deprotect complexes
2 and
3, where the NHC is already formed, were not successful. Thus, the synthesis of the complexes requires the deprotection of
1 and subsequent protonation/methylation. This methodology has the advantage of allowing the synthesis of complex
4, the deprotected complex with an anionic adenosinyl ligand. For guanosine, the synthesis of the analogous compound was not possible thus far, since deprotection is performed under acidic conditions, which always lead to the formation of the corresponding protic NHC [
6].
2.3. Anion Exchange
We have noted that, when in DMSO-
d6 solutions, compound
5 is not stable. After a few days, the concentration of
5 decreases and another compound starts to form. An additional set of signals becomes very evident by
1H NMR after 6 days (
Figure 1). This new compound is characterized by a new NH signal at 13.65 ppm and a doublet at 6.49 ppm, close to the H−1′ signal in
5 (6.52 ppm). The remaining
1H NMR signals for the sugar moiety are also indicative of the formation of the new compound in DMSO-
d6 solutions, although they are mostly overlapped with those of
5. The
13C{
1H} NMR spectrum of the mixture after 6 days is not very informative, with only one clear extra signal at 94.4 ppm, close to the C−1′ signal of compound
5 (94.2 ppm).
31P{
1H} NMR analysis of the solution confirms the presence of a second compound, with two singlets observed at 18.10, 18.24 ppm (
Figure 2). Additionally, the
195Pt NMR spectrum also shows a second triplet at −4337 ppm (
Figure 3), which is a shift of 153 ppm with respect to
5.
The lability found for 5 is specific for DMSO, since solutions of 5 in CDCl3 (prepared in situ by addition of HCl) showed only the presence 5 and no further changes were observed, even after 2 weeks (see Supporting Information).
We monitored the evolution of
5 by
1H NMR for several days (
Figure 4). The concentration of the new compound increases very slowly, from circa 30% after 6 days to 70% after 5 weeks, after which the mixture remains essentially stable. During this time, the NH signals and the NH
2 undergo an upfield shift for both compounds. For the remaining signals of
5, no variation is noted, while new signals evolve with time, confirming the formation of a new compound (
7).
We hypothesized that this new compound could be the result of either a DMSO derivative of
5, whereby the bromide is replaced by DMSO, or an exchange of halides between bromide and chloride, the counterion (
Scheme 6).
To determine which hypothesis was correct, we prepared a solution of
5 in non-deuterated DMSO and examined this solution after 5 days by mass spectrometry (ESI-HRMS). The mass spectrum displayed a set of peaks at 1066.1608, corresponding to compound
5 (M + H), and 1022.2123, which can be attributed to a compound bearing a chloride instead of a bromide as co-ligand (M + H adduct). These values agree with the calculated theoretical values and also with the expected isotopic pattern. Additionally, to the solutions of
5 in DMSO-
d6 that already had reached maximum conversion to
7 (3:7), 10 equivalents of NaCl were added. Analysis by
1H NMR showed that the amount of the new compound
7 increases (
Figure 5), albeit very slowly, and after 17 days, it was the only compound detected. It should be noted that, in the absence of an external chloride source, the mixture remains unchanged for months. Upon the addition of NaCl, the NH signals change to lower field, probably due to hydrogen bonding.
Compound
7 was fully characterized by NMR. Apart from the changes in the NH and H
1′ group already highlighted, the carbenic C8 resonates at 155.2 ppm, a very slight shift with respect to
5. The
31P{
1H} NMR shows an AB quartet at 18.27, 18.14 ppm,
2JP,P coupling of 420 Hz and
195Pt satellites with a
1JPt,P of 2530 Hz. In
195Pt NMR, a triplet is detected at −4340 ppm, with the coupling constant similar to that determined by
31P NMR. Anion exchange was also noted for DMSO solutions of compound
6, with iodide as counterion. When dissolved in DMSO-
d6, it shows the presence of two new minor species, around 6% each. The mixture remains stable and the ratio of the compounds does not increase with time nor with the addition of an iodide source. Mass spectrometry of compound
6 in DMSO solutions allowed to identify one of the compounds as the iodide adduct (
8), but we were unable to identify the second complex. In
195Pt NMR, only the triplet corresponding to compound
6 is detected.
31P{
1H} NMR shows two pairs of singlets at 17, 28, 17.15 (
1JPt,P of 2482 Hz) and 11.47, 11.40 (
1JPt,P of 2420 Hz), but we cannot confidently distinguish which one is compound
8. Most probably, the singlets located at higher field correspond to compound
8, being similar to other trans-[Pt(PPh
3)
2(NHC)I] [
19]
, but we emphasize that this assignment is purely speculative.
It should be noted that the substitutions have only been observed for the NHCs
5 and
6. Probably, this is a consequence of the trans effect exerted by the NHC, which can accelerate [
20] the dissociation of bromide to form a vacant site/solvento complex that would then lead to the formation of the halide complexes, to an extent that is most probably dependent on the stability of the final compound. The lability of compound
5 and
6 is indicative of the propensity of these complexes to undergo exchange of the ligand trans to the NHC moiety. This lability is suggestive of the capacity of adenosine complexes to coordinate to DNA and of their potential use as antiproliferative compounds which could complement the well-known activity of adenosine as an anticancer agent [
21].
2.4. Comments on the 31P and 195Pt NMR
The coupling constant of the Pt–P bond can be used as an estimate of the bond strength in compounds
1–
7. Indeed, one bond coupling constants can be used as a measure of the bond strength when the bond involves some s character, as is the case of Pt–P [
22]. Elegant work from Manassero and Pasini showed that in complexes of the type trans-[PtXY(PPh
3)
2], in which the mutual trans influence of the two PPh
3 remains constant throughout, any variation on the properties of the Pt–P bond results from changes in X and Y, and thus reflect the cis influence of these ligands [
23]. For these complexes, Pt(II) back donation to phosphorus is not of relevance [
24], and the Pt–P bonds are formed mainly by sigma donation from P to Pt. Therefore, Pt–P bonds become weaker as the central platinum becomes less electrophilic [
25]. In line with this, cis influence can be defined as the capacity of a given ligand to lower the positive charge on Pt [
20,
26]. For these reasons, we considered that the
1JPt,P obtained for complexes
1–
7 should be diagnostic of the influence of the formation of the NHC and the corresponding σ-donor properties on the strength of Pt–P. Indeed, in complexes
1–
7, the trans effect for the phosphines remains constant. Compounds
1 and
4 are neutral, while
2,
3,
5,
6 and
7 are salts, with a cationic platinum centre. The data for
195Pt,
31P and
1JPt,P is compiled in
Table 1. All complexes were measured in DMSO-
d6, except complex
3, which was measured only in CDCl
3.
When inspecting the values of
1JPt,P for complexes
1,
2 and
3, and
4,
5 and
6, it is evident that upon NHC formation,
1JPt,P decreases considerably, more than 300 Hz. This decrease is consistent in protic and methyl NHCs, both in protected and deprotected complexes. The decrease is higher for methyl NHCs
3 and
6 than for protic NHCs
2 and
5, perhaps reflecting a slightly higher electron donation due to the methyl group [
27]. This is demonstrative of the cis influence of the NHC as a consequence of its σ-donor capacity, which lowers the electrophilicity of the metal centre. Hence, we can conclude that formation of the NHC leads to a decrease in Pt–P bond strength. It is also possible to observe an upfield shift in the
31P signals when going from
1 to
2/
3 and
4 to
5/
6, which agrees well with the reduction of electrophilic character of Pt [
28,
29]. As for the
195Pt values, an upfield shift is observed upon carbene formation, consistent with the increase of electronic density at the metal centre [
30]. Although complexes bearing the NHCs are cationic, the overall charge of the cation seems to exert little effect on
1JPt,P values, being largely compensated by the increase of electron density due to the σ donation of the NHC. For compound
7,
1JPt,P increases when changing from bromide (
6) to chloride. It has been reported that for halogens, the cis influence decreases in the order I > Cl > F [
23]. The cis influence for bromide was not computed in the aforementioned study, but the value of
1JPt,P for
7 agrees with a higher cis influence of bromide versus chloride. Although it is tempting to include in this discussion compound
8, bearing an iodide coordinated to platinum, we cannot state, in rigor, which of the signals in
31P{
1H} spectra correspond to
8. Two minor compounds are present, one with chemical shifts of 17.28, 17.15 and
1JPt,P of 2482 Hz and the other with chemical shifts of 11.47, 11.40 and
1JPt,P of 2420 Hz. The latter values agree well with the presence of an iodide [
19], and fit the trend previously reported. Still, our data is insufficient to state confidently that
8 corresponds to that specific peak. As indicated previously, we were unable to increase the concentration of
8 with the addition of an excess iodide source.