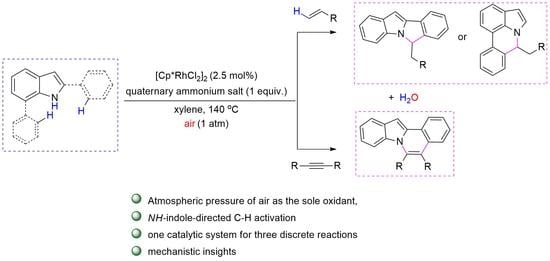
Rhodium-Catalyzed Oxidative Annulation of 2- or 7-Arylindoles with Alkenes/Alkynes Using Molecular Oxygen as the Sole Oxidant Enabled by Quaternary Ammonium Salt
Abstract
1. Introduction
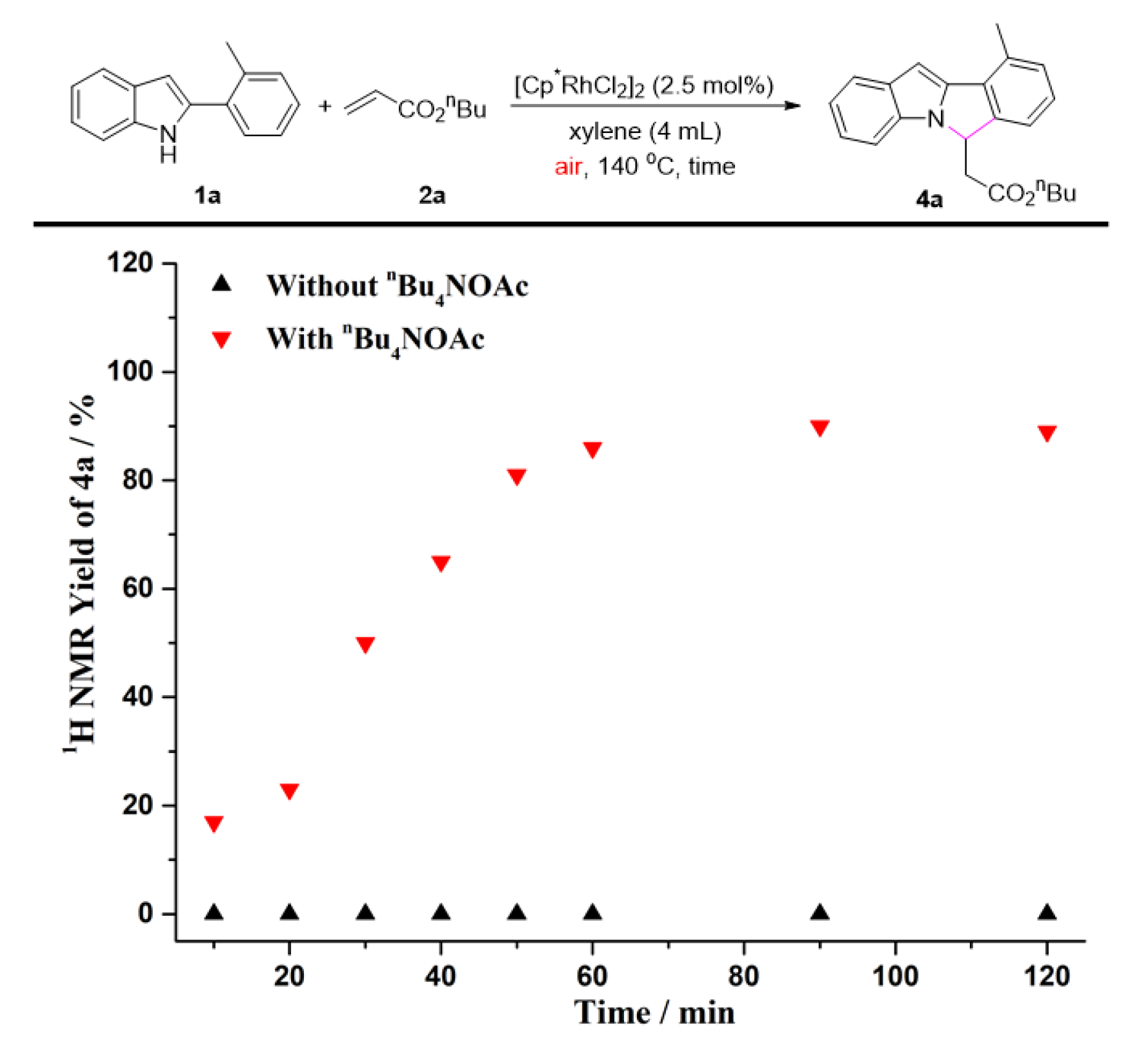
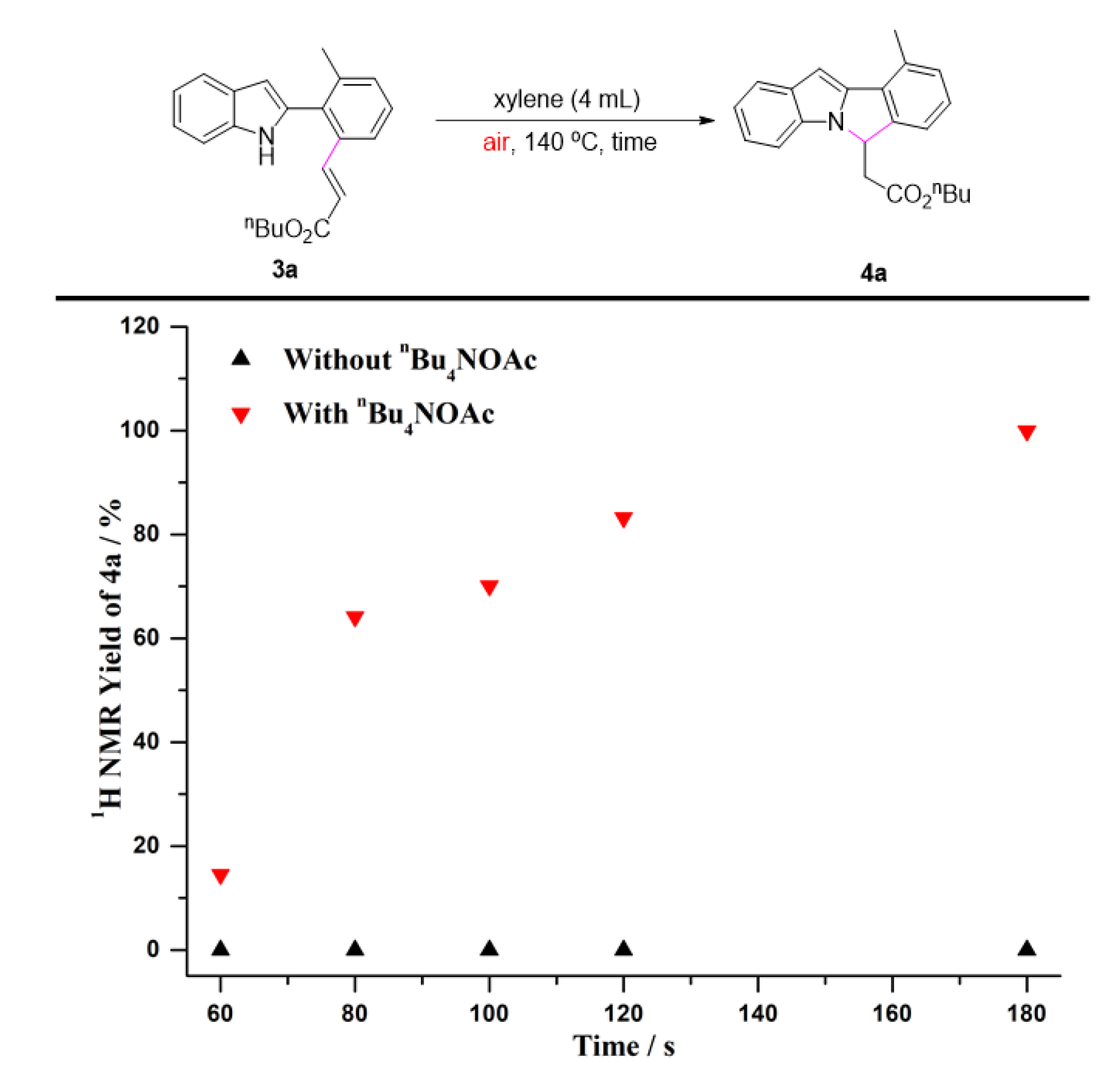
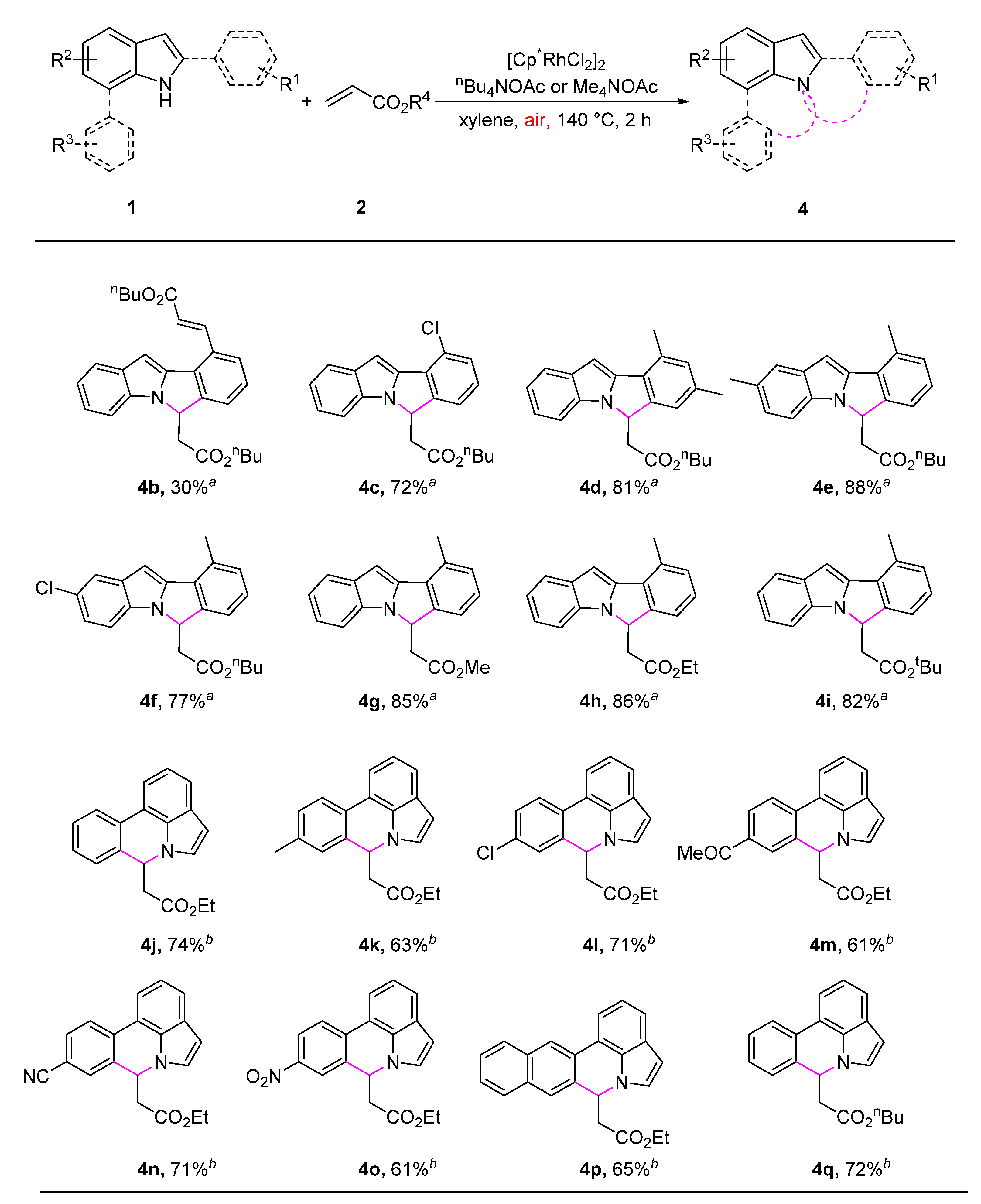
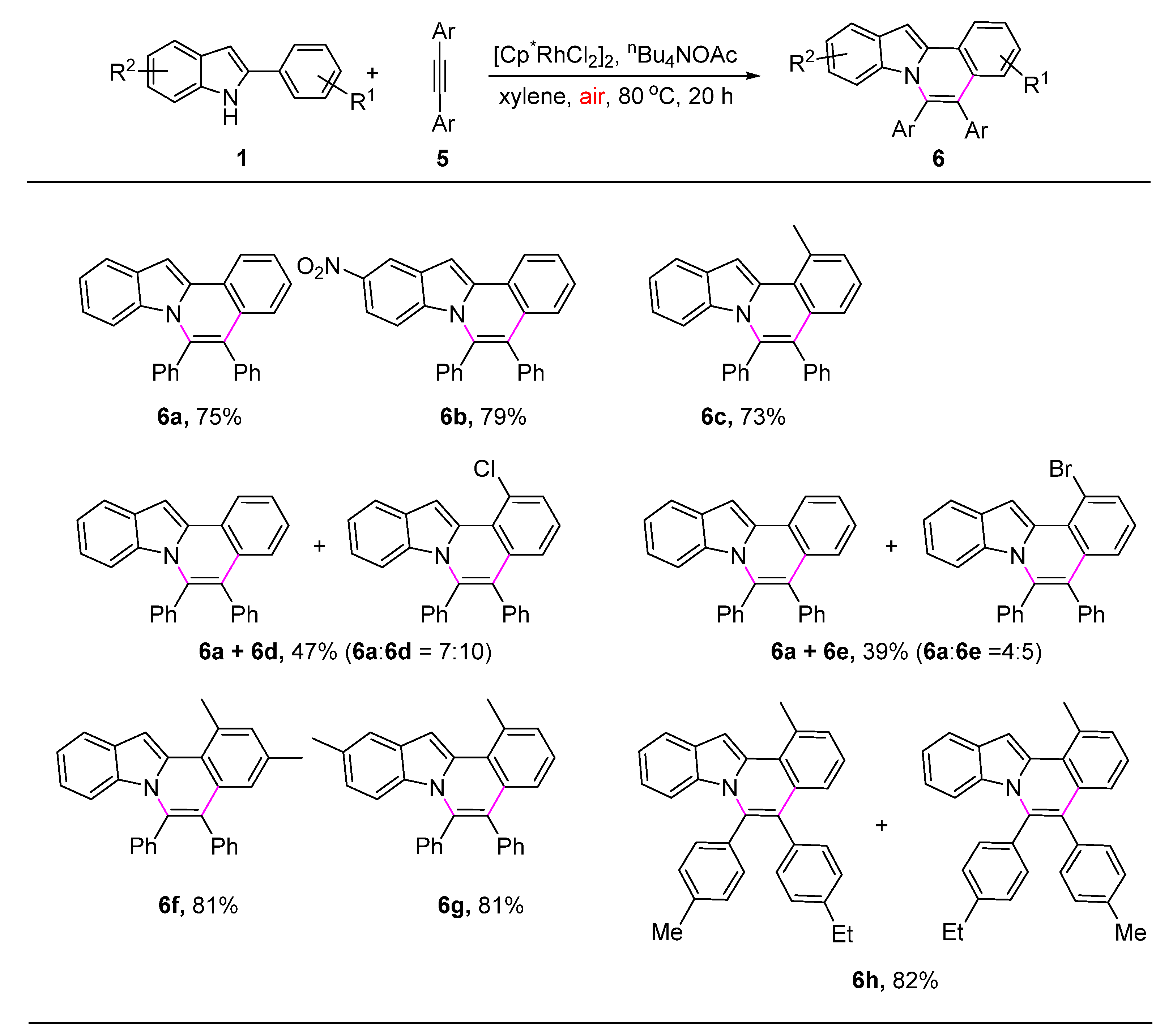
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. General Procedure for Rhodium-Catalyzed Oxidative Annulation of 2- or 7-Arylindoles with Alkenes/Alkynes
3.3. Analytical Characterization Data of Products
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Lyons, T.W.; Sanford, M.S. Palladium-Catalyzed Ligand-Directed C-H Functionalization Reactions. Chem. Rev. 2010, 110, 1147–1169. [Google Scholar] [CrossRef] [PubMed]
- Arockiam, P.B.; Bruneau, C.; Dixneuf, P.H. Ruthenium (II)-catalyzed C-H bond activation and functionalization. Chem. Rev. 2012, 112, 5879–5918. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Huang, H. Transition-metal-catalyzed direct addition of unactivated C-H bonds to polar unsaturated bonds. Chem. Rev. 2015, 115, 3468–3517. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Chang, S. Transition-Metal-Mediated Direct C–H Amination of Hydrocarbons with Amine Reactants: The Most Desirable but Challenging C–N Bond-Formation Approach. ACS Catal. 2016, 6, 2341–2351. [Google Scholar] [CrossRef]
- He, J.; Wasa, M.; Chan, K.S.L.; Shao, Q.; Yu, J.-Q. Palladium-Catalyzed Transformations of Alkyl C-H Bonds. Chem. Rev. 2017, 117, 8754–8786. [Google Scholar] [CrossRef] [PubMed]
- Murakami, K.; Yamada, S.; Kaneda, T.; Itami, K. C-H Functionalization of Azines. Chem. Rev. 2017, 117, 9302–9332. [Google Scholar] [CrossRef]
- Newton, C.G.; Wang, S.-G.; Oliveira, C.C.; Cramer, N. Catalytic Enantioselective Transformations Involving C-H Bond Cleavage by Transition-Metal Complexes. Chem. Rev. 2017, 117, 8908–8976. [Google Scholar] [CrossRef]
- Karimov, R.R.; Hartwig, J.F. Transition-Metal-Catalyzed Selective Functionalization of C(sp3)-H Bonds in Natural Products. Angew. Chem. Int. Ed. 2018, 57, 4234–4241. [Google Scholar] [CrossRef]
- Zheng, L.; Hua, R. C-H Activation and Alkyne Annulation via Automatic or Intrinsic Directing Groups: Towards High Step Economy. Chem. Rec. 2018, 18, 556–569. [Google Scholar] [CrossRef]
- Kalepu, J.; Pilarski, L.T. Weinreb Amides as Directing Groups for Transition Metal-Catalyzed C-H Functionalizations. Molecules 2019, 24, 830. [Google Scholar] [CrossRef]
- Niu, B.; Yang, K.; Lawrence, B.; Ge, H. Transient Ligand-Enabled Transition Metal-Catalyzed C-H Functionalization. ChemSusChem 2019, 12, 2955–2969. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Yang, L.; Li, P.; Wang, F.; Li, X. Recent advances in transition metal-catalyzed olefinic C-H functionalization. Org. Chem. Front. 2021, 8, 1085–1101. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, H.; Shi, W.; Lei, A. Bond formations between two nucleophiles: Transition metal catalyzed oxidative cross-coupling reactions. Chem. Rev. 2011, 111, 1780–1824. [Google Scholar] [CrossRef] [PubMed]
- Yeung, C.S.; Dong, V.M. Catalytic dehydrogenative cross-coupling: Forming carbon-carbon bonds by oxidizing two carbon-hydrogen bonds. Chem. Rev. 2011, 111, 1215–1292. [Google Scholar] [CrossRef] [PubMed]
- Girard, S.A.; Knauber, T.; Li, C.J. The cross-dehydrogenative coupling of C(sp3)-H bonds: A versatile strategy for C-C bond formations. Angew. Chem. Int. Ed. 2014, 53, 74–100. [Google Scholar] [CrossRef]
- Liu, C.; Yuan, J.; Gao, M.; Tang, S.; Li, W.; Shi, R.; Lei, A. Oxidative Coupling between Two Hydrocarbons: An Update of Recent C-H Functionalizations. Chem. Rev. 2015, 115, 12138–12204. [Google Scholar] [CrossRef]
- Ruiz-Castillo, P.; Buchwald, S.L. Applications of Palladium-Catalyzed C-N Cross-Coupling Reactions. Chem. Rev. 2016, 116, 12564–12649. [Google Scholar] [CrossRef]
- Yang, Y.; Lan, J.; You, J. Oxidative C-H/C-H Coupling Reactions between Two (Hetero)arenes. Chem. Rev. 2017, 117, 8787–8863. [Google Scholar] [CrossRef]
- Tang, S.; Zeng, L.; Lei, A. Oxidative R(1)-H/R(2)-H Cross-Coupling with Hydrogen Evolution. J. Am. Chem. Soc. 2018, 140, 13128–13135. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Gao, X.; Lv, Z.; Abdelilah, T.; Lei, A. Recent Advances in Oxidative R(1)-H/R(2)-H Cross-Coupling with Hydrogen Evolution via Photo-/Electrochemistry. Chem. Rev. 2019, 119, 6769–6787. [Google Scholar] [CrossRef]
- Zhuang, W.; Zhang, X.; Huang, Q. Recent Advances in Cross-Dehydrogenative-Coupling Reactions Using Molecular Oxygen as the Sole Oxidant. Chin. J. Org. Chem. 2021, 41, 529–542. [Google Scholar] [CrossRef]
- Ueura, K.; Satoh, T.; Miura, M. An efficient waste-free oxidative coupling via regioselective C-H bond cleavage: Rh/Cu-catalyzed reaction of benzoic acids with alkynes and acrylates under air. Org. Lett. 2007, 9, 1407–1409. [Google Scholar] [CrossRef] [PubMed]
- Colby, D.A.; Bergman, R.G.; Ellman, J.A. Rhodium-Catalyzed C-C Bond Formation via Heteroatom-Directed C-H Bond Activation. Chem. Rev. 2010, 110, 624–655. [Google Scholar] [CrossRef] [PubMed]
- Colby, D.A.; Tsai, A.S.; Bergman, R.G.; Ellman, J.A. Rhodium Catalyzed Chelation-Assisted C–H Bond Functionalization Reactions. Acc. Chem. Res. 2012, 45, 814–825. [Google Scholar] [CrossRef]
- Song, G.; Li, X. Substrate activation strategies in rhodium(III)-catalyzed selective functionalization of arenes. Acc. Chem. Res. 2015, 48, 1007–1020. [Google Scholar] [CrossRef]
- Bera, M.; Agasti, S.; Chowdhury, R.; Mondal, R.; Pal, D.; Maiti, D. Rhodium-Catalyzed meta-C-H Functionalization of Arenes. Angew. Chem. Int. Ed. 2017, 56, 5272–5276. [Google Scholar] [CrossRef] [PubMed]
- Vásquez-Céspedes, S.; Wang, X.; Glorius, F. Plausible Rh(V) Intermediates in Catalytic C–H Activation Reactions. ACS Catal. 2017, 8, 242–257. [Google Scholar] [CrossRef]
- Rej, S.; Chatani, N. Rh-Catalyzed Removable Directing Group Assisted sp2 or sp3-C-H Bond Functionalization. Angew. Chem. Int. Ed. 2019, 58, 8304–8329. [Google Scholar] [CrossRef]
- Zhu, W.; Gunnoe, T.B. Advances in Rhodium-Catalyzed Oxidative Arene Alkenylation. Acc. Chem. Res. 2020, 53, 920–936. [Google Scholar] [CrossRef]
- Dongbang, S.; Confair, D.N.; Ellman, J.A. Rhodium-Catalyzed C-H Alkenylation/Electrocyclization Cascade Provides Dihydropyridines That Serve as Versatile Intermediates to Diverse Nitrogen Heterocycles. Acc. Chem. Res. 2021, 54, 1766–1778. [Google Scholar] [CrossRef]
- Huang, Q.; Han, Q.; Fu, S.; Yao, Z.; Su, L.; Zhang, X.; Lin, S.; Xiang, S. Rhodium-Catalyzed NH-Indole-Directed C–H Carbonylation with Carbon Monoxide: Synthesis of 6H-Isoindolo[2,1-a]indol-6-ones. J. Org. Chem. 2016, 81, 12135–12142. [Google Scholar] [CrossRef]
- Guo, X.; Han, Q.; Tang, Z.; Su, L.; Zhang, X.; Zhang, X.; Lin, S.; Huang, Q. Rhodium-catalyzed NH-indole-directed ortho C-H coupling of 2-arylindoles with diazo compounds via metal carbene migratory insertion. Tetrahedron Lett. 2018, 59, 1568–1572. [Google Scholar] [CrossRef]
- Liu, A.; Han, Q.; Zhang, X.; Li, B.; Huang, Q. Transition-Metal-Controlled Synthesis of 11H-Benzo[a]carbazoles and 6-Alkylidene-6H-isoindo[2,1-a]indoles via Sequential Intermolecular/Intramolecular Cross-Dehydrogenative Coupling from 2-Phenylindoles. Org. Lett. 2019, 21, 6839–6843. [Google Scholar] [CrossRef]
- Yuan, Y.; Pan, G.; Zhang, X.; Li, B.; Xiang, S.; Huang, Q. Synthesis of Seven-Membered Azepino[3,2,1-hi]indoles via Rhodium-Catalyzed Regioselective C-H Activation/1,8-Diazabicyclo[5.4.0]undec-7-ene-Catalyzed Intramolecular Amidation of 7-Phenylindoles in One Pot. J. Org. Chem. 2019, 84, 14701–14711. [Google Scholar] [CrossRef]
- Yuan, Y.; Pan, G.; Zhang, X.; Huang, Q. One pot synthesis of pyrrolo[3,2,1-de]phenanthridines from 7-phenylindoles via tandem C–H olefination/aza-Michael addition. Org. Chem. Front. 2020, 7, 53–63. [Google Scholar] [CrossRef]
- Yuan, Y.; Guo, X.; Zhang, X.; Li, B.; Huang, Q. Access to 5H-benzo[a]carbazol-6-ols and benzo[6,7]cyclohepta[1,2-b]indol-6-ols via rhodium-catalyzed C–H activation/carbenoid insertion/aldol-type cyclization. Org. Chem. Front. 2020, 7, 3146–3159. [Google Scholar] [CrossRef]
- Lou, J.; Han, W.; Liu, Z.; Xiao, J. Rhodium-catalyzed enone carbonyl directed C–H activation for the synthesis of indanones containing all-carbon quaternary centers. Org. Chem. Front. 2021, 8, 1447–1453. [Google Scholar] [CrossRef]
- Patureau, F.W.; Besset, T.; Glorius, F. Rhodium-catalyzed oxidative olefination of C-H bonds in acetophenones and benzamides. Angew. Chem. Int. Ed. 2011, 50, 1064–1067. [Google Scholar] [CrossRef]
- Jayakumar, J.; Parthasarathy, K.; Cheng, C.-H. One-pot synthesis of isoquinolinium salts by rhodium-catalyzed C-H bond activation: Application to the total synthesis of oxychelerythrine. Angew. Chem. Int. Ed. 2012, 51, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, L.; Wang, L.; Lygin, A.V. Ruthenium-catalyzed aerobic oxidative coupling of alkynes with 2-aryl-substituted pyrrole. Chem. Sci. 2012, 3, 177–180. [Google Scholar] [CrossRef]
- Peng, S.; Liu, S.; Zhang, S.; Cao, S.; Sun, J. Synthesis of Polyheteroaromatic Compounds via Rhodium-Catalyzed Multiple C-H Bond Activation and Oxidative Annulation. Org. Lett. 2015, 17, 5032–5035. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yu, X.; Ji, D.; Yamamoto, Y.; Almansour, A.I.; Arumugam, N.; Kumar, R.S.; Bao, M. Rhodium-Catalyzed Oxidative Benzannulation of N-Adamantyl-1-naphthylamines with Internal Alkynes via Dual C-H Bond Activation: Synthesis of Substituted Anthracenes. Org. Lett. 2016, 18, 4246–4249. [Google Scholar] [CrossRef]
- Li, B.; Zhang, B.; Zhang, X.; Fan, X. Regio-selective synthesis of diversely substituted benzo[a]carbazoles through Rh(III)-catalyzed annulation of 2-arylindoles with alpha-diazo carbonyl compounds. Chem. Commun. 2017, 53, 1297–1300. [Google Scholar] [CrossRef]
- Han, Q.; Guo, X.; Tang, Z.; Su, L.; Yao, Z.; Zhang, X.; Lin, S.; Xiang, S.; Huang, Q. Rhodium-Catalyzed Regioselective Ortho C-H Olefination of 2-Arylindoles via NH-Indole-Directed C-H Bond Cleavage. Adv. Synth. Catal. 2018, 360, 972–984. [Google Scholar] [CrossRef]
- Yan, X.; Ye, R.; Sun, H.; Zhong, J.; Xiang, H.; Zhou, X. Synthesis of 2-Arylindoles by Rhodium-Catalyzed/Copper-Mediated Annulative Coupling of N-Aryl-2-aminopyridines and Propargyl Alcohols via Selective C-H/C-C Activation. Org. Lett. 2019, 21, 7455–7459. [Google Scholar] [CrossRef] [PubMed]
- Mihara, G.; Noguchi, T.; Nishii, Y.; Hayashi, Y.; Kawauchi, S.; Miura, M. Rhodium-Catalyzed Annulative Coupling of Isothiazoles with Alkynes through N-S Bond Cleavage. Org. Lett. 2020, 22, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.T.; Kang, E.; Kim, M.; Joo, J.M. Synthesis of Bidentate Nitrogen Ligands by Rh-Catalyzed C-H Annulation and Their Application to Pd-Catalyzed Aerobic C-H Alkenylation. Org. Lett. 2021, 23, 3657–3662. [Google Scholar] [CrossRef]
- Kawashita, Y.; Hayashi, M. Synthesis of heteroaromatic compounds by oxidative aromatization using an activated carbon/molecular oxygen system. Molecules 2009, 14, 3073–3093. [Google Scholar] [CrossRef]
- Campbell, A.N.; Stahl, S.S. Overcoming the “Oxidant Problem”: Strategies to Use O2 as the Oxidant in Organometallic C–H Oxidation Reactions Catalyzed by Pd (and Cu). Acc. Chem. Res. 2012, 45, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Zhang, C.; Tang, C.; Jiao, N. Recent advances in transition-metal catalyzed reactions using molecular oxygen as the oxidant. Chem. Soc. Rev. 2012, 41, 3381–3430. [Google Scholar] [CrossRef]
- McCann, S.D.; Stahl, S.S. Copper-Catalyzed Aerobic Oxidations of Organic Molecules: Pathways for Two-Electron Oxidation with a Four-Electron Oxidant and a One-Electron Redox-Active Catalyst. Acc. Chem. Res. 2015, 48, 1756–1766. [Google Scholar] [CrossRef]
- Liang, Y.F.; Jiao, N. Oxygenation via C-H/C-C Bond Activation with Molecular Oxygen. Acc. Chem. Res. 2017, 50, 1640–1653. [Google Scholar] [CrossRef]
- Wang, D.; Weinstein, A.B.; White, P.B.; Stahl, S.S. Ligand-Promoted Palladium-Catalyzed Aerobic Oxidation Reactions. Chem. Rev. 2018, 118, 2636–2679. [Google Scholar] [CrossRef]
- Tang, C.; Qiu, X.; Cheng, Z.; Jiao, N. Molecular oxygen-mediated oxygenation reactions involving radicals. Chem. Soc. Rev. 2021, 50, 8067–8101. [Google Scholar] [CrossRef]
- Shi, Z.; Ding, S.; Cui, Y.; Jiao, N. A palladium-catalyzed oxidative cycloaromatization of biaryls with alkynes using molecular oxygen as the oxidant. Angew. Chem. Int. Ed. 2009, 48, 7895–7898. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-H.; Shi, B.-F.; Yu, J.-Q. Pd(II)-Catalyzed Olefination of Electron-Deficient Arenes Using 2,6-Dialkylpyridine Ligands. J. Am. Chem. Soc. 2009, 131, 5072–5074. [Google Scholar] [CrossRef]
- Engle, K.M.; Wang, D.-H.; Yu, J.-Q. Constructing multiply substituted arenes using sequential palladium(II)-catalyzed C-H olefination. Angew. Chem. Int. Ed. 2010, 49, 6169–6173. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.-H.; Chok, Y.K.; Loh, T.-P. Synthesis and characterization of a cyclic vinylpalladium(II) complex: Vinylpalladium species as the possible intermediate in the catalytic direct olefination reaction of enamide. Chem. Sci. 2011, 2, 1822–1825. [Google Scholar] [CrossRef]
- Wei, Y.; Deb, I.; Yoshikai, N. Palladium-Catalyzed Aerobic Oxidative Cyclization of N-Aryl Imines: Indole Synthesis from Anilines and Ketones. J. Am. Chem. Soc. 2012, 134, 9098–9101. [Google Scholar] [CrossRef] [PubMed]
- Piotrowicz, M.; Zakrzewski, J. Aerobic Dehydrogenative Heck Reaction of Ferrocene with a Pd(OAc)2/4,5-Diazafluoren-9-one Catalyst. Organometallics 2013, 32, 5709–5712. [Google Scholar] [CrossRef]
- Li, N.-N.; Zhang, Y.-L.; Mao, S.; Gao, Y.-R.; Guo, D.-D.; Wang, Y.-Q. Palladium-catalyzed C-H homocoupling of furans and thiophenes using oxygen as the oxidant. Org. Lett. 2014, 16, 2732–2735. [Google Scholar] [CrossRef]
- Kim, N.; Min, M.; Hong, S. Regioselective palladium(II)-catalyzed aerobic oxidative Heck-type C3 alkenylation of sulfocoumarins. Org. Chem. Front. 2015, 2, 1621–1624. [Google Scholar] [CrossRef]
- Kumar, K.S.; Meesa, S.R.; Naikawadi, P.K. Palladium-Catalyzed [2 + 2 + 2] Annulation via Transformations of Multiple C-H Bonds: One-Pot Synthesis of Diverse Indolo[3,2-a]carbazoles. Org. Lett. 2018, 20, 6079–6083. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-B.; Xie, D.; Zang, Z.-L.; Zhou, C.-H.; Cai, G.-X. Palladium-catalyzed aerobic regio- and stereo-selective olefination reactions of phenols and acrylates via direct dehydrogenative C(sp2)-O cross-coupling. Chem. Commun. 2018, 54, 4437–4440. [Google Scholar] [CrossRef]
- Beckers, I.; Krasniqi, B.; Kumar, P.; Escudero, D.; De Vos, D. Ligand-Controlled Selectivity in the Pd-Catalyzed C–H/C–H Cross-Coupling of Indoles with Molecular Oxygen. ACS Catal. 2021, 11, 2435–2444. [Google Scholar] [CrossRef]
- Zhang, G.; Yang, L.; Wang, Y.; Xie, Y.; Huang, H. An efficient Rh/O2 catalytic system for oxidative C-H activation/annulation: Evidence for Rh(I) to Rh(III) oxidation by molecular oxygen. J. Am. Chem. Soc. 2013, 135, 8850–8853. [Google Scholar] [CrossRef]
- Zhang, G.; Yu, H.; Qin, G.; Huang, H. Rh-catalyzed oxidative C-H activation/annulation: Converting anilines to indoles using molecular oxygen as the sole oxidant. Chem. Commun. 2014, 50, 4331–4334. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wang, H.-W.; Spangler, J.E.; Chen, K.; Cui, P.-P.; Zhao, Y.; Sun, W.-Y.; Yu, J.-Q. Rh(III)-catalyzed C-H olefination of N-pentafluoroaryl benzamides using air as the sole oxidant. Chem. Sci. 2015, 6, 1923–1927. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tang, J.; Li, S.; Liu, Z.; Zhao, Y.; She, Z.; Kadam, V.D.; Gao, G.; Lan, J.; You, J. Cascade C-H Annulation of Aldoximes with Alkynes Using O2 as the Sole Oxidant: One-Pot Access to Multisubstituted Protoberberine Skeletons. Org. Lett. 2017, 19, 604–607. [Google Scholar] [CrossRef]
- Jambu, S.; Sivasakthikumaran, R.; Jeganmohan, M. Aerobic Oxidative Alkenylation of Weak O-Coordinating Arylacetamides with Alkenes via a Rh(III)-Catalyzed C-H Activation. Org. Lett. 2019, 21, 1320–1324. [Google Scholar] [CrossRef]
- Huang, Q.; Zhang, X.; Qiu, L.; Wu, J.; Xiao, H.; Zhang, X.; Lin, S. Palladium-Catalyzed Olefination and Arylation of Polyfluoroarenes Using Molecular Oxygen as the Sole Oxidant. Adv. Synth. Catal. 2015, 357, 3753–3757. [Google Scholar] [CrossRef]
- Zhang, X.; Su, L.; Qiu, L.; Fan, Z.; Zhang, X.; Lin, S.; Huang, Q. Palladium-catalyzed C-H olefination of uracils and caffeines using molecular oxygen as the sole oxidant. Org. Biomol. Chem. 2017, 15, 3499–3506. [Google Scholar] [CrossRef] [PubMed]
- Pan, G.; Wu, K.; Deng, Z.; Zhang, X.; Zhang, X.; Lin, S.; Huang, Q. Palladium-Catalyzed C-H Direct Arylation of Uracils and Caffeines with Arenes Using Molecular Oxygen as the Sole Oxidant. Chin. J. Org. Chem. 2018, 38, 2076–2084. [Google Scholar] [CrossRef]
- Duce, S.; Mateo, A.; Alonso, I.; Garcia Ruano, J.L.; Cid, M.B. Role of quaternary ammonium salts as new additives in the enantioselective organocatalytic beta-benzylation of enals. Chem. Commun. 2012, 48, 5184–5186. [Google Scholar] [CrossRef]
- Ferrary, T.; David, E.; Milanole, G.E.; Besset, T.; Jubault, P.; Pannecoucke, X. A Straightforward and Highly Diastereoselective Access to Functionalized Monofluorinated Cyclopropanes via a Michael Initiated Ring Closure Reaction. Org. Lett. 2013, 15, 5598–5601. [Google Scholar] [CrossRef]
- Sheshenev, A.E.; Boltukhina, E.V.; White, A.J.; Hii, K.K. Methylene-bridged bis(imidazoline)-derived 2-oxopyrimidinium salts as catalysts for asymmetric Michael reactions. Angew. Chem. Int. Ed. 2013, 52, 6988–6991. [Google Scholar] [CrossRef] [PubMed]
- Ding, R.; Zheng, B.; Wang, Y.; Peng, Y.G. A Cation-Directed Enantioselective Sulfur-Mediated Michael/Mannich Three-Component Domino Reaction involving Chalcones as Michael Acceptors. Org. Lett. 2015, 17, 4128–4131. [Google Scholar] [CrossRef]
- Zhang, J.-X.; Wang, H.-Y.; Jin, Q.-W.; Zheng, C.-W.; Zhao, G.; Shang, Y.-J. Thiourea-Quaternary Ammonium Salt Catalyzed Asymmetric 1, 3-Dipolar Cycloaddition of Imino Esters To Construct Spiro[pyrrolidin-3,3′-oxindoles]. Org. Lett. 2016, 18, 4774–4777. [Google Scholar] [CrossRef]
- Jayakumar, J.; Vedarethinam, G.; Hsiao, H.-C.; Sun, S.-Y.; Chuang, S.-C. Cascade One-Pot Synthesis of Orange-Red-Fluorescent Polycyclic Cinnolino[2,3-f]phenanthridin-9-ium Salts by Palladium(II)-Catalyzed C-H Bond Activation of 2-Azobiaryl Compounds and Alkenes. Angew. Chem. Int. Ed. 2020, 59, 689–694. [Google Scholar] [CrossRef]
- Kenichi, F.; Katsutoshi, O.; Tokio, Y. The Catalytic Activity of Onium Compounds in the Homogeneous Liquid Phase Oxidation of Cumene and α-Pinene. Bull. Chem. Soc. Jpn. 1969, 42, 312–318. [Google Scholar]
- Csányi, L.J.; Jáky, K.; Kóta, Z.; Páli, T. Oxidation of hydrocarbons by O2 in the presence of onium salts and onium ion-pair complexes as catalysts. J. Mol. Catal. A Chem. 2004, 209, 59–68. [Google Scholar] [CrossRef]
- Santos, V.P.; Bakker, J.J.W.; Kreutzer, M.T.; Kapteijn, F.; Gascon, J. Transport Limitations during Phase Transfer Catalyzed Ethyl-Benzene Oxidation: Facts and Fictions of “Halide Catalysis”. ACS Catal. 2012, 2, 1421–1424. [Google Scholar] [CrossRef]





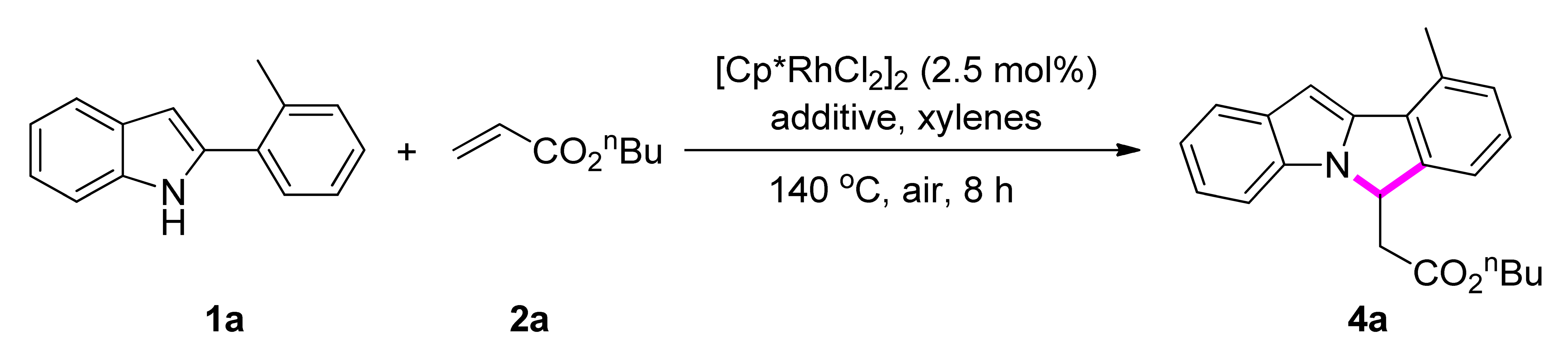
| Entry | Additive | Yield (%) (3a) b |
|---|---|---|
| 1 c | - | 0 |
| 2 | n-Bu4NOAc | 93 (91 d) |
| 3 | Me4NOAc | 88 |
| 4 | n-Bu4NPF6 | trace |
| 5 | n-Bu4NBF4 | 0 |
| 6 | n-Bu4NHSO4 | 0 |
| 7 | n-Bu4NCl | trace |
| 8 | n-Bu4NI | 0 |
| 9 | NH4Cl | 0 |
| 10 | NH4PF6 | trace |
| 11 | Et4NBr | 0 |
| 12 e | n-Bu4NOAc | trace |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhuang, W.; Zhang, J.; Zheng, Y.; Huang, Q. Rhodium-Catalyzed Oxidative Annulation of 2- or 7-Arylindoles with Alkenes/Alkynes Using Molecular Oxygen as the Sole Oxidant Enabled by Quaternary Ammonium Salt. Molecules 2021, 26, 5329. https://doi.org/10.3390/molecules26175329
Zhuang W, Zhang J, Zheng Y, Huang Q. Rhodium-Catalyzed Oxidative Annulation of 2- or 7-Arylindoles with Alkenes/Alkynes Using Molecular Oxygen as the Sole Oxidant Enabled by Quaternary Ammonium Salt. Molecules. 2021; 26(17):5329. https://doi.org/10.3390/molecules26175329
Chicago/Turabian StyleZhuang, Weihui, Jiaqi Zhang, Yanping Zheng, and Qiufeng Huang. 2021. "Rhodium-Catalyzed Oxidative Annulation of 2- or 7-Arylindoles with Alkenes/Alkynes Using Molecular Oxygen as the Sole Oxidant Enabled by Quaternary Ammonium Salt" Molecules 26, no. 17: 5329. https://doi.org/10.3390/molecules26175329
APA StyleZhuang, W., Zhang, J., Zheng, Y., & Huang, Q. (2021). Rhodium-Catalyzed Oxidative Annulation of 2- or 7-Arylindoles with Alkenes/Alkynes Using Molecular Oxygen as the Sole Oxidant Enabled by Quaternary Ammonium Salt. Molecules, 26(17), 5329. https://doi.org/10.3390/molecules26175329