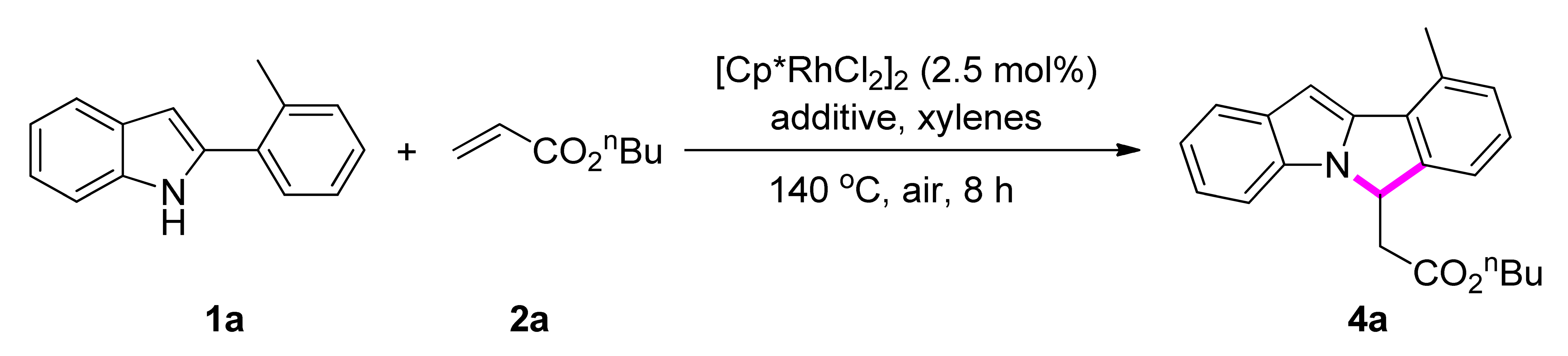Abstract
Developing an efficient catalytic system using molecular oxygen as the oxidant for rhodium-catalyzed cross-dehydrogenative coupling remains highly desirable. Herein, rhodium-catalyzed oxidative annulation of 2- or 7-phenyl-1H-indoles with alkenes or alkynes to assemble valuable 6H-isoindolo[2,1-a]indoles, pyrrolo[3,2,1-de]phenanthridines, or indolo[2,1-a]isoquinolines using the atmospheric pressure of air as the sole oxidant enabled by quaternary ammonium salt has been accomplished. Mechanistic studies provided evidence for the fast intramolecular aza-Michael reaction and aerobic reoxidation of Rh(I)/Rh(III), facilitated by the addition of quaternary ammonium salt.
1. Introduction
C-H functionalization, including the reaction of a C-H bond with a (pseudo)halide, the reaction of a C-H bond with an organometallic reagent, and cross-coupling between two C-H bonds (CDC reaction), has gained tremendous popularity in recent years as a methodology for the construction of C-C bonds or C-heteroatom bonds [1,2,3,4,5,6,7,8,9,10,11,12]. Among these reactions, the CDC reaction is especially noteworthy because this reaction precludes both coupling partners from pre-functionalization, and as a result has high step economy and atom economy [13,14,15,16,17,18,19,20,21]. In 2007, Miura and Satoh reported on [RhCp*Cl2]2-catalyzed oxidative coupling of benzoic acids with alkynes [22]. Since then, rhodium-catalyzed oxidative C-H coupling has drawn increasing attention, and many important organic building blocks have been produced [23,24,25,26,27,28,29,30]. However, despite indisputable advances, all rhodium-catalyzed C-H oxidative coupling reactions are extremely limited to hazardous and stoichiometric oxidants such as AgOAc [31,32,33,34,35,36,37] and Cu(OAc)2 [38,39,40,41,42,43,44,45,46,47]. The use of molecular oxygen is advantageous over other oxidants because only water is generated as a by-product [48,49,50,51,52,53,54]. So far, in sharp contrast to aerobic palladium-catalyzed CDC reactions [55,56,57,58,59,60,61,62,63,64,65], only very limited examples of rhodium-catalyzed CDC reaction utilizing molecular oxygen as the sole oxidant have been reported to date [66,67,68,69,70]. Therefore, the development of protocols using molecular oxygen as the oxidant is highly desirable. In continuation of our research on transition metal-catalyzed aerobic CDC reactions [71,72,73], herein we report on the rhodium-catalyzed oxidative annulation of 2-arylindoles or 7-arylindoles with alkenes or alkynes using molecular oxygen as the sole oxidant enabled by quaternary ammonium salt (Scheme 1).
Scheme 1.
Rhodium-catalyzed oxidative annulation of 2- or 7-phenyl-1H-indoles with alkenes or alkynes using molecular oxygen as the sole oxidant enabled by quaternary ammonium salt.
2. Results and Discussion
Our investigation on the aerobic rhodium-catalyzed CDC reaction began with the NH-indole-directed ortho-C-H alkenylation of 2-phenyl-1H-indole (1a) with n-butyl acrylate. The catalytic system consisting of [Cp*RhCl2]2 (2.5 mol%) and n-Bu4NOAc (1 equiv.) promoted the reaction at 140 °C under air atmosphere in xylenes to afford 6H-isoindolo[2,1-a]indole (4a) in 93% yield (Table 1, entry 2), derived from ortho-C-H olefination and the subsequent intramolecular aza-Michael addition. The addition of n-Bu4NOAc was indispensable as the reaction became very sluggish in its absence in various solvents such as xylenes, DMF, THF, EtOAc, and 1,4-dioxane (entry 1). A similar yield was obtained when Me4NOAc (1 equiv.) was added (entry 3), while other quaternary ammonium salts gave inferior results (entries 4–11). Control experiments have shown that no reaction occurred in the absence of rhodium catalyst or molecular oxygen (entry 12).

Table 1.
Optimization of the reaction conditions a.
To gain further insights into the impact of quaternary ammonium salts in the present transformation, we conducted several kinetic studies via 1H NMR spectroscopy. The time study shown in Figure 1 revealed that the one-pot C-H olefination/aza-Michael reaction under air atmosphere afforded 50% yield of 4a after 30 min and was completed within 2 h by adding n-Bu4NOAc. It must be pointed out that the C-H olefinated product was not detected during monitoring period. Without n-Bu4NOAc, 4a was not obtained at all, and nor was the C-H olefinated product (3a) formed. Quaternary ammonium salts have always been considered to be an effective catalyst for Michael reactions [74,75,76,77,78,79]. As one can see from Figure 2, the intramolecular aza-Michael reaction of ortho-alkenylated-2-phenyl-1H-indole could indeed be improved by the addition of n-Bu4NOAc. 1 equiv. of n-Bu4NOAc, and provided complete conversion and quantitative yield of 4a after just 3 min. In the absence of n-Bu4NOAc, no reaction occurred, and the ortho-alkenylated-2-phenyl-1H-indole was totally recovered. The further kinetic experiments were carried out using Cu(OAc)2 instead of O2 as the terminal oxidant. As seen in Figure 3, the C-H olefination of 2-phenylindole with with n-butyl acrylate completed within 2 h in the absence of n-Bu4NOAc, affording 90% yield of 3a. By adding n-Bu4NOAc, the C-H olefinated product (3a) was totally transformed into aza-Michael product 4a within 2 h (Figure 4). In order to illustrate the impact of n-Bu4NOAc in the C-H olefination step, styrene was chosen as the coupling partner because it is not a Michael acceptor, and the reaction can stop after C-H olefination. As shown in Figure 5, no significant differences were observed between experiments performed with or without n-Bu4NOAc. These observations suggest that quaternary ammonium salt plays at least two roles in the oxidative annuation of 2-phenyl-1H-indole with with alkenes: (a) It promotes the intramolecular aza-Michael reaction of the C-H olefinated product; and (b) It promotes aerobic reoxidation of Rh(I) to Rh(III). The second role was partly validated by the fact that the current catalytic system ([Cp*RhCl2]2/n-Bu4NOAc/O2) was also effective for the oxidative annulation of 2-phenylindoles with alkynes to assemble indolo[2,1-a]isoquinoline skeletons. One reason why quaternary ammonium salt can speed up aerobic reoxidation is probably due to the increased dissolved quantity of O2 from adding quaternary ammonium salt [80,81,82].
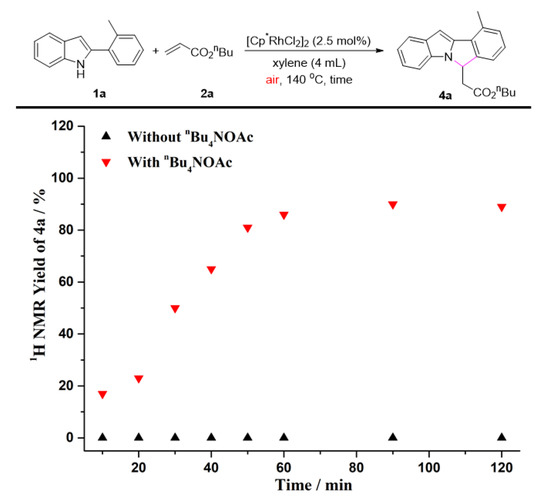
Figure 1.
The one-pot C-H olefination/aza-Michael reaction of 1a with 2a under air.
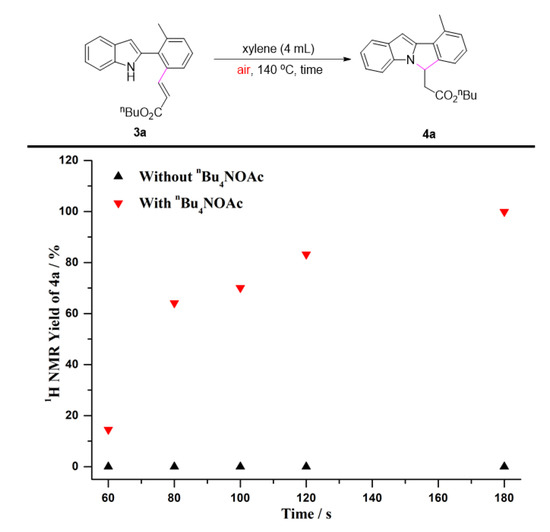
Figure 2.
The intramolecular aza-Michael reaction of 3a under air.
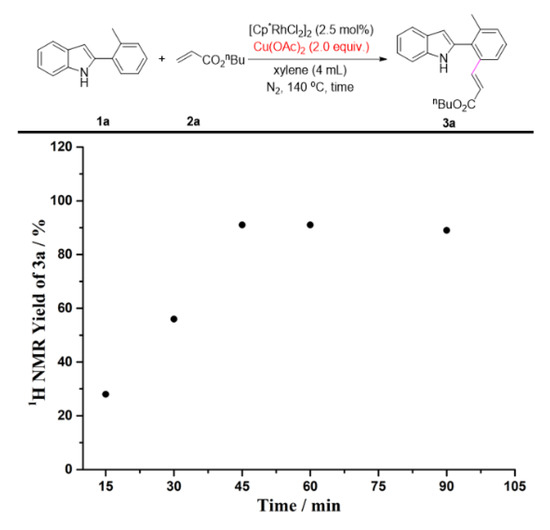
Figure 3.
The C-H olefination of 1a with 2a using Cu(OAc)2 instead of O2 as the terminal oxidant.
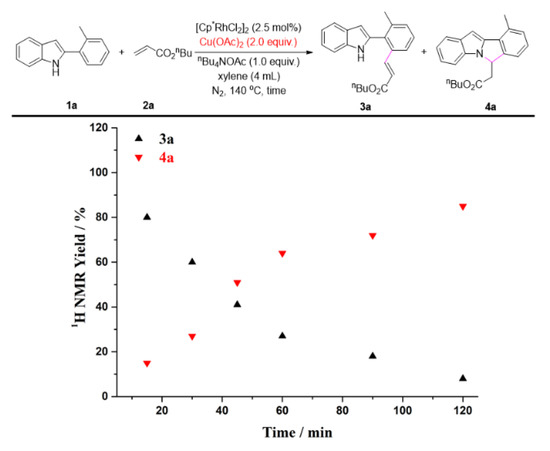
Figure 4.
The C-H olefination/aza-Michael reaction of 1a with 2a using Cu(OAc)2 as the terminal oxidant.
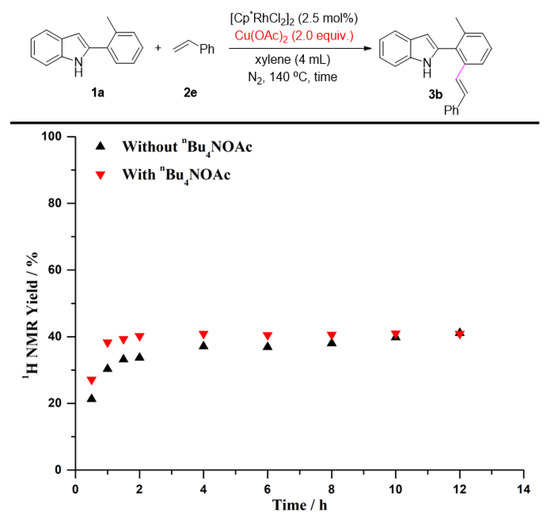
Figure 5.
The C-H olefination of 1a with 2e using Cu(OAc)2 as the terminal oxidant.
Reaction condition: Figure 1. A solution of 1a (0.2 mmol), 2a (0.4 mmol), [Cp*RhCl2]2 (2.5 mol%), and n-Bu4NOAc (1.0 equiv.) in xylenes (4 mL) at 140 °C under air. Figure 2. A solution of 3a (0.2 mmol) and n-Bu4NOAc (1.0 equiv.) in xylenes (4 mL) at 140 °C under air. Figure 3. A solution of 1a (0.2 mmol), 2a (0.4 mmol), [Cp*RhCl2]2 (2.5 mol%), and Cu(OAc)2 (2.0 equiv.) in xylenes (4 mL) at 140 °C under N2. Figure 4. A solution of 1a (0.2 mmol), 2a (0.4 mmol), [Cp*RhCl2]2 (2.5 mol%), n-Bu4NOAc (1.0 equiv.), and Cu(OAc)2 (2.0 equiv.) in xylenes (4 mL) at 140 °C under N2. Figure 5. A solution of 1a (0.2 mmol), 2e (0.4 mmol), [Cp*RhCl2]2 (2.5 mol%), n-Bu4NOAc (1.0 equiv.), and Cu(OAc)2 (2.0 equiv.) in xylenes (4 mL) at 140 °C under N2. The yields were determined by the 1H NMR yield using CH2Br2 (0.3 M, 0.2 mmol, 14 mg) as an internal standard.
With the optimized conditions in hand, the generality of the rhodium-catalyzed aerobic C-H olefination/aza-Michael reaction was then explored (Scheme 2). The reaction of 2-phenyl-1H-indole, which contains two ortho-C-H bonds with n-butyl acrylate, provided the desired annulated product 4b in low yield (30%) with recovered starting material (65%). Therefore, blocking one of the ortho-C-H bonds with methyl or chloro is essential for full conversion. 2-phenyl-1H-indole derivatives with substituents at the benzene ring or indole ring were delivered the corresponding products in good to excellent yields, showing very limited effect on the reaction efficiency (4c–4f). As expected, other acrylates bearing methyl, ethyl, or tert-butyl all well reacted with 1a to afford the desired product 4g–4i in good yields. The C-H olefination/aza-Michael reaction of 7-phenyl-1H-indoles with ethyl acrylate afforded the corresponding pyrrolo[3,2,1-de]phenanthridine derivatives under the reaction conditions by changing n-Bu4NOAc with Me4NOAc. By contrast, only one ortho-C-H bond was cleaved, showing good chemoselectivity (4j–4q). 7-phenylindoles and acrylates bearing various substituents, such as chloro (4l), ketone (4m), CN (4n), NO2 (4o), naphthyl (4p), and n-butyl (4q) coupled well with ethyl acrylate or ethyl acrylate, showing good functional group tolerance. The experiment results also showed no electronic effect on the reaction efficiency.
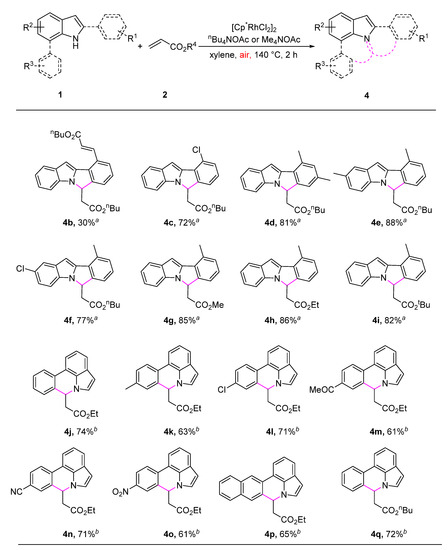
Scheme 2.
Substrate scope of oxidative annulation of 2- or 7-phenyl-1H-indoles with alkenes. a: Reaction condition: 1a (0.2 mmol), 2 (0.4 mmol), [Cp*RhCl2]2 (2.5 mol%), and n-Bu4NOAc (1 equiv.) in xylenes (4 mL) at 140 °C under air atmosphere for 2 h. Isolated yield. b: Me4NOAc was used.
Next, the scope of oxidative annulation of 2-phenyl-1H-indoles with alkynes was briefly investigated. As shown in Scheme 3, the reaction of 2-phenyl-1H-indoles 1 bearing an electron-rich or electron-deficient group at the phenyl ring or indole ring proceeded smoothly to give the corresponding products 6a–6c, 6f–6g in 39–81% yields. For 2-(2-chlorophenyl)-1H-indole or 2-(2-bromophenyl)-1H-indole, both C-H and C-Cl (or C-Br) cleavage occurred. The corresponding C-H oxidative annulation product is difficult to separate from the mixture (6d + 6a or 6e + 6a). In the present [4 + 2] oxidative annulation, when an unsymmetrical diarylalkyne was employed, the formation of two possible regioisomers was observed as expected (6h). Again, valuable functional groups were well accommodated.
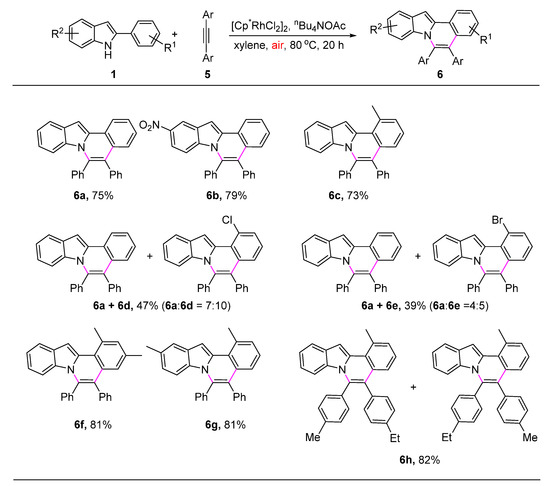
Scheme 3.
Substrate scope of oxidative annulation of 2-phenyl-1H-indoles with alkynes a. a: Reaction condition: 1a (0.2 mmol), 5 (0.4 mmol), [Cp*RhCl2]2 (2.5 mol%), and n-Bu4NOAc (1 equiv.) in xylenes (4 mL) at 80 °C under air atmosphere for 20 h. Isolated yield.
Based on the experimental results obtained above and precedent reports [31,32,34,44], a plausible mechanism for the aerobic rhodium-catalyzed oxidative annulation of 2-phenylindole with alkene or alkyne is postulated in Scheme 4. Coordination of N atom of phenylindole to Rh(III) and the subsequent ortho-C-H activation produced the five-membered rhodacycle B. B inserted into the alkene or alkyne affording the intermediate C1 or C2, and the subsequent β-H elimination/reductive elimination provided Rh(I) sandwich complex D1 or D2. Then D1 or D2 was oxidized by oxygen to regenerate the active Rh(III) species and released the corresponding product 3 or 5. The C-H olefinated product (3) can be transformed into aza-Michael product 4 efficiently, and the oxidation step by molecular oxygen will be sped up substantially by adding quaternary ammonium salts.
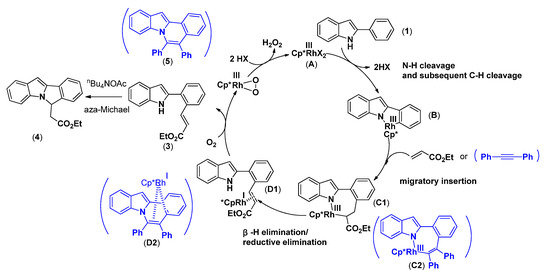
Scheme 4.
Plausible mechanism for the aerobic rhodium-catalyzed oxidative annulation of 2-phenylindole with alkene or alkyne.
3. Materials and Methods
3.1. General Information
Unless otherwise noted, the reagents (chemicals) were purchased from commercial sources and were used without further purification. 2-phenyl-1H-indole is commercially available. The other 2-arylindoles were synthesized from phenylhydrazine hydrochlorides via Fisher indole synthesis [44]. 7-phenyl-1H-indoles were synthesized from 7-bromo-1H-indoles and phenylboronic acid via Suzuki coupling [34,35]. Quaternary ammonium salts were purchased from commercial sources. Their purity was more than 99.0% and they were stored in a glovebox. 1H NMR spectra were recorded at 400 MHz and 13C NMR spectra at 100 MHz, respectively (Supplementary material). 1H chemical shifts (δ) were referenced to TMS, and 13C NMR chemical shifts (δ) were referenced to internal solvent resonance. ESI-HRMS spectra were recorded by using a Q-TOF mass spectrometer.
3.2. General Procedure for Rhodium-Catalyzed Oxidative Annulation of 2- or 7-Arylindoles with Alkenes/Alkynes
Under air atmosphere, 2- or 7-arylindoles (0.2 mmol), alkenes or alkynes (0.4 mmol), [Cp*RhCl2]2 (3.2 mg, 0.005 mmol, 2.5 mol%), n-Bu4NOAc or Me4NOAc (0.2 mmol, 1 equiv.), and xylenes (4 mL) were placed in a 25 mL tube. The mixture was heated in oil bath at 140 °C for 2 h or 80 °C for 20 h. After the reaction mixture cooled to room temperature, the crude reaction mixture was diluted with EtOAc to 5 mL, filtered through a celite pad, and then washed with 10 mL EtOAc. The combined mixture was washed with saturated aqueous Na2CO3 and dried over anhydrous MgSO4. After filtration, the volatiles were removed under reduced pressure, and the residue was subjected to silica gel column chromatography (eluting with petroleum ether/dichloromethane = 1/1 or petroleum ether/ethyl acetate = 100/1) to afford the corresponding product.
3.3. Analytical Characterization Data of Products
Butyl 3-(2-(1H-indol-2-yl)-3-methylphenyl)acrylate (3a), 57.3 mg, 85% yield, yellow oil. 1H NMR (400 MHz, CDCl3) δ 8.22 (s, 1H), 7.68 (d, J = 7.6 Hz, 1H), 7.60–7.55 (m, 2H), 7.39–7.32 (m, 3H), 7.24–7.15 (m, 2H), 6.48 (dd, J = 2.0, 1.2 Hz, 1H), 6.33 (d, J = 16.0 Hz, 1H), 4.06 (t, J = 6.4 Hz, 2H), 2.23 (s, 3H), 1.59–1.51 (m, 2H), 1.31–1.25 (m, 2H), 0.86 (t, J = 7.2 Hz, 3H). 13C NMR and HRMS data for the desired product were in agreement with the previously reported literature data [44].
2-(2-Methyl-6-styrylphenyl)-1H-indole (3b), 25.3 mg, 41% yield, yellow oil. 1H NMR (400 MHz, CDCl3) δ 7.98 (s, 1H), 7.73–7.71 (m, 1H), 7.67 (d, J = 8.0 Hz, 1H), 7.42–7.36 (m, 2H), 7.32–7.25 (m, 4H), 7.24–7.18 (m, 4H), 7.03 (d, J = 8.4 Hz, 2H), 6.54 (dd, J = 2.0, 0.8 Hz, 1H), 2.26 (s, 3H). 13C NMR and HRMS data for the desired product were in agreement with the previously reported literature data [44].
Butyl 2-(10-methyl-6H-isoindolo[2,1-a]indol-6-yl)acetate (4a), 61.4 mg, 93% yield, yellow oil. 1H NMR (400 MHz, CDCl3) δ 7.69 (d, J = 8.0 Hz, 1H), 7.40 (dd, J = 8.4, 0.8 Hz, 1H), 7.30 (t, J = 4.4 Hz, 1H), 7.23–7.19 (m, 3H), 7.13 (td, J = 8.0, 0.8 Hz, 1H), 6.61 (s, 1H), 5.75 (dd, J = 8.0, 4.4 Hz, 1H), 4.21–4.14 (m, 2H), 3.30 (dd, J = 16.4, 4.8 Hz, 1H), 2.76 (dd, J = 16.4, 8.0 Hz, 1H), 2.63 (s, 3H), 1.62–1.55 (m, 2H), 1.35–1.30 (m, 2H), 0.92 (t, J = 7.2 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 171.1, 145.8, 143.1, 133.5, 133.2, 132.5, 131.3, 129.9, 127.5, 121.9, 120.7, 119.8, 109.6, 94.2, 65.2, 56.8, 39.9, 30.6, 19.5, 19.2, 13.8. HRMS (ESI) calcd for C22H24NO2 [M + H]+: 334.1807, found: 334.1808.
Butyl 3-(6-(2-butoxy-2-oxoethyl)-6H-isoindolo[2,1-a]indol-10-yl)acrylate (4b), 29.7 mg, 30% yield, red solid. 1H NMR (400 MHz, CDCl3) δ 8.32 (d, J = 16.0 Hz, 1H), 7.70 (dt, J = 8.0, 0.8 Hz, 1H), 7.64 (d, J = 7.6 Hz, 1H), 7.46 (dt, J = 7.2, 1.2 Hz, 1H), 7.39 (dd, J = 8.0, 0.8 Hz, 1H), 7.30 (t, J = 7.6 Hz, 1H), 7.23 (td, J = 7.2, 1.2 Hz, 1H), 7.16–7.12 (m, 1H), 6.79 (s, 1H), 6.56 (d, J = 15.6 Hz, 1H), 5.74 (dd, J = 8.0, 4.4 Hz, 1H), 4.29 (t, J = 6.8 Hz, 2H), 4.19–4.12 (m, 2H), 3.31 (dd, J = 16.4, 4.4 Hz, 1H), 2.77 (dd, J = 16.0, 8.0 Hz, 1H), 1.80–1.73 (m, 2H), 1.56–1.48 (m, 4H), 1.33–1.26 (m, 2H), 1.02 (t, J = 7.6 Hz, 3H), 0.90 (t, J = 7.6 Hz, 3H). 13C NMR and HRMS data for the desired product were in agreement with the previously reported literature data [44].
Butyl 2-(10-chloro-6H-isoindolo[2,1-a]indol-6-yl)acetate (4c), 50.6 mg, 72% yield, yellow oil. 1H NMR (400 MHz, CDCl3) δ 7.71 (dt, J = 8.0, 0.8 Hz, 1H), 7.40–7.36 (m, 3H), 7.23 (td, J = 8.0, 1.2 Hz, 2H), 7.16–7.12 (m, 1H), 6.91 (s, 1H), 5.77 (dd, J = 8.0, 4.4 Hz, 1H), 4.19–4.13 (m, 2H), 3.31 (dd, J = 16.4, 4.4 Hz, 1H), 2.77 (dd, J = 16.4, 8.4 Hz, 1H), 1.59–1.55 (m, 2H), 1.34–1.28 (m, 2H), 0.91 (t, J = 7.6 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 170.7, 147.4, 140.6, 133.3, 133.2, 131.0, 129.4, 128.3, 128.0, 122.5, 121.8, 120.1, 109.6, 96.0, 65.3, 57.0, 39.6, 30.6, 19.2, 13.8. HRMS (ESI) calcd for C21H21NO2Cl [M + H]+: 354.1261, found: 354.1257.
Butyl 2-(8,10-dimethyl-6H-isoindolo[2,1-a]indol-6-yl)acetate (4d), 56.2 mg, 81% yield, yellow oil. 1H NMR (400 MHz, CDCl3) δ 7.69 (dt, J = 7.6, 1.2 Hz, 1H), 7.39 (d, J = 8.0 Hz, 1H), 7.23–7.19 (m, 1H), 7.15–7.11 (m, 2H), 7.05 (s, 1H), 6.56 (s, 1H), 5.70 (dd, J = 8.0, 4.4 Hz, 1H), 4.25–4.16 (m, 2H), 3.29 (dd, J = 16.0, 4.4 Hz, 1H), 2.76 (dd, J = 16.0, 8.0 Hz, 1H), 2.59 (s, 3H), 2.40 (s, 3H), 1.63–1.59 (m, 2H), 1.38–1.33 (m, 2H), 0.94 (t, J = 7.6 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 171.1, 146.1, 143.2, 137.6, 133.6, 133.1, 132.2, 130.8, 128.6, 121.7, 121.6, 121.4, 119.6, 109.4, 93.4, 65.1, 56.7, 39.9, 30.7, 21.7, 19.4, 19.2, 13.8. HRMS (ESI) calcd for C23H26NO2 [M + H]+: 348.1964, found: 348.1960.
Butyl 2-(2,10-dimethyl-6H-isoindolo[2,1-a]indol-6-yl)acetate (4e), 60.7 mg, 88% yield, yellow oil. 1H NMR (400 MHz, CDCl3) δ 7.47 (s, 1H), 7.30–7.27 (m, 2H), 7.22–7.20 (m, 2H), 7.04 (dd, J = 8.4, 1.2 Hz, 1H), 6.52 (s, 1H), 5.72 (dd, J = 8.0, 4.8 Hz, 1H), 4.21–4.14 (m, 2H), 3.27 (dd, J = 16.4, 4.8 Hz, 1H), 2.74 (dd, J = 16.0, 8.0 Hz, 1H), 2.62 (s, 3H), 2.47 (s, 3H), 1.61–1.57 (m, 2H), 1.36–1.30 (m, 2H), 0.92 (t, J = 7.6 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 171.1, 145.8, 143.1, 133.8, 132.4, 131.6, 131.5, 129.9, 129.0, 127.3, 123.5, 121.6, 120.7, 109.2, 93.7, 65.1, 56.8, 39.9, 30.7, 21.6, 19.5, 19.2, 13.8. HRMS (ESI) calcd for C23H26NO2 [M + H]+: 348.1964, found: 348.1964.
Butyl 2-(2-chloro-10-methyl-6H-isoindolo[2,1-a]indol-6-yl)acetate (4f), 57.2 mg, 77% yield, yellow oil. 1H NMR (400 MHz, CDCl3) δ 7.63 (dd, J = 2.0, 0.4 Hz, 1H), 7.31–7.28 (m, 2H), 7.23–7.22 (m, 2H), 7.14 (dd, J = 8.4, 2.0 Hz, 1H), 6.53 (s, 1H), 5.71 (dd, J = 7.6, 4.8 Hz, 1H), 4.18–4.12 (m, 2H), 3.19 (dd, J = 16.4, 4.8 Hz, 1H), 2.79 (dd, J = 16.0, 7.6 Hz, 1H), 2.61 (s, 3H), 1.58–1.54 (m, 2H), 1.33–1.27 (m, 2H), 0.91 (t, J = 7.2 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 170.8, 145.7, 144.4, 134.5, 132.7, 131.6, 130.9, 130.1, 127.9, 125.4, 122.0, 121.1, 120.7, 110.4, 93.8, 65.2, 57.1, 39.9, 30.6, 19.5, 19.2, 13.8. HRMS (ESI) calcd for C22H23NO2Cl [M + H]+: 368.1417, found: 368.1412.
Methyl 2-(10-methyl-6H-isoindolo[2,1-a]indol-6-yl)acetate (4g), 49.4 mg, 85% yield, yellow oil. 1H NMR (400 MHz, CDCl3) δ 7.74 (dd, J = 7.6, 0.8 Hz, 1H), 7.41 (d, J = 8.0 Hz, 1H), 7.33–7.30 (m, 1H), 7.28–7.24 (m, 3H), 7.19–7.15 (m, 1H), 6.64 (s, 1H), 5.74 (dd, J = 8.4, 4.8 Hz, 1H), 3.82 (s, 3H), 3.32 (dd, J = 16.4, 4.8 Hz, 1H), 2.73 (dd, J = 16.0, 8.0 Hz, 1H), 2.64 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 171.4, 145.7, 142.9, 133.5, 133.1, 132.5, 131.1, 129.9, 127.4, 121.8, 120.7, 119.7, 109.5, 94.2, 56.7, 52.2, 39.6, 19.5. HRMS (ESI) calcd for C19H18NO2 [M + H]+: 292.1338, found: 292.1340.
Ethyl 2-(10-methyl-6H-isoindolo[2,1-a]indol-6-yl)acetate (4h), 51.9 mg, 86% yield, yellow oil. 1H NMR (400 MHz, CDCl3) δ 7.69 (dt, J = 7.6, 1.2 Hz, 1H), 7.40 (dq, J = 8.0, 0.8 Hz, 1H), 7.32–7.29 (m, 1H), 7.23–7.19 (m, 3H), 7.14–7.10 (m, 1H), 6.61 (s, 1H), 5.75 (dd, J = 8.0, 4.4 Hz, 1H), 4.24 (qd, J = 7.2, 2.4 Hz, 2H), 3.29 (dd, J = 16.4, 4.8 Hz, 1H), 2.74 (dd, J = 16.0, 8.0 Hz, 1H), 2.62 (s, 3H), 1.25 (t, J = 6.8 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 171.0, 145.8, 143.1, 133.5, 133.2, 132.5, 131.3, 129.9, 127.5, 121.9, 120.8, 119.8, 109.6, 94.2, 61.2, 56.8, 39.9, 19.5, 14.2. HRMS (ESI) calcd for C20H20NO2 [M + H]+: 306.1494, found: 306.1493.
Tert-butyl 2-(10-methyl-6H-isoindolo[2,1-a]indol-6-yl)acetate (4i), 53.6 mg, 81% yield, yellow oil. 1H NMR (400 MHz, CDCl3) δ 7.69 (dt, J = 8.0, 0.8 Hz, 1H), 7.44 (dq, J = 8.0, 0.8 Hz, 1H), 7.35–7.32 (m, 1H), 7.24–7.19 (m, 3H), 7.15–7.10 (m, 1H), 6.61 (s, 1H), 5.71 (dd, J = 7.6, 4.4 Hz, 1H), 3.21 (dd, J = 16.0, 4.4 Hz, 1H), 2.77 (dd, J = 16.0, 7.6 Hz, 1H), 2.63 (s, 3H), 1.39 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 170.0, 145.9, 143.2, 133.5, 133.2, 132.4, 131.4, 129.8, 127.4, 121.8, 120.8, 119.7, 109.7, 105.1, 94.0, 81.6, 57.0, 40.8, 28.0, 19.5. HRMS (ESI) calcd for C22H24NO2 [M + H]+: 334.1807, found: 334.1804.
Ethyl 2-(7H-pyrrolo[3,2,1-de]phenanthridin-7-yl)acetate (4j), 43.5 mg, 74% yield, yellow oil. 1H NMR (400 MHz, CDCl3) δ 7.97 (d, J = 7.6 Hz, 1H), 7.59 (d, J = 7.6 Hz, 1H), 7.55 (dd, J = 7.6, 0.4 Hz, 1H), 7.41–7.37 (m, 1H), 7.32–7.30 (m, 2H), 7.25 (d, J = 3.2 Hz, 1H), 7.16 (t, J = 7.6 Hz, 1H), 6.56 (d, J = 3.2 Hz, 1H), 6.14 (dd, J = 7.2, 5.2 Hz, 1H), 4.19–4.03 (m, 2H), 2.77 (dd, J = 7.2, 4.8 Hz, 2H), 1.16 (t, J = 7.2 Hz, 3H). 13C NMR and HRMS data for the desired product were in agreement with the previously reported literature data [35].
Ethyl 2-(9-methyl-7H-pyrrolo[3,2,1-de]phenanthridin-7-yl)acetate (4k), 39.1 mg, 63% yield, yellow soild. 1H NMR (400 MHz, CDCl3) δ 7.85 (d, J = 7.6 Hz, 1H), 7.54 (d, J = 7.2 Hz, 1H), 7.51 (dd, J = 8.0, 0.8 Hz, 1H), 7.23 (d, J = 3.2 Hz, 1H), 7.21–7.11 (m, 3H), 6.54 (d, J = 3.2 Hz, 1H), 6.08 (dd, J = 6.8, 5.6 Hz, 1H), 4.15–4.07 (m, 2H), 2.76 (d, J = 1.6 Hz, 1H), 2.75 (s, 1H), 2.38 (s, 3H), 1.16 (t, J = 7.2 Hz, 3H). 13C NMR and HRMS data for the desired product were in agreement with the previously reported literature data [35].
Ethyl 2-(9-chloro-7H-pyrrolo[3,2,1-de]phenanthridin-7-yl)acetate (4l), 48.4 mg, 71% yield, yellow oil. 1H NMR (400 MHz, CDCl3) δ 7.87 (d, J = 8.4 Hz, 1H), 7.55–7.52 (m, 2H), 7.35 (dd, J = 8.4, 2.0 Hz, 1H), 7.30 (d, J = 2.0 Hz, 1H), 7.23 (d, J = 3.2 Hz, 1H), 7.14 (t, J = 7.6 Hz, 1H), 6.56 (d, J = 3.2 Hz, 1H), 6.08 (dd, J = 6.8, 5.2 Hz, 1H), 4.11 (q, J = 7.2 Hz, 2H), 2.76 (dd, J = 7.2, 4.8 Hz, 2H), 1.17 (t, J = 6.8 Hz, 3H). 13C NMR and HRMS data for the desired product were in agreement with the previously reported literature data [35].
Ethyl 2-(9-acetyl-7H-pyrrolo[3,2,1-de]phenanthridin-7-yl)acetate (4m), 40.9 mg, 61% yield, yellow oil. 1H NMR (400 MHz, CDCl3) δ 8.00–7.91 (m, 3H), 7.60 (t, J = 6.4 Hz, 2H), 7.25 (s, 1H), 7.16 (t, J = 8.0 Hz, 1H), 6.57 (d, J = 3.2 Hz, 1H), 6.15 (dd, J = 7.6, 5.2 Hz, 1H), 4.14–4.05 (m, 2H), 2.82–2.74 (m, 2H), 2.62 (s, 3H), 1.15 (t, J = 7.2 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 197.1, 170.4, 136.2, 134.9, 134.0, 133.3, 128.6, 127.7, 127.1, 126.4, 122.9, 122.4, 120.9, 117.0, 115.1, 103.8, 61.2, 55.4, 46.4, 26.7, 14.1. HRMS (ESI) calcd for C21H20NO3 [M + H]+: 334.1443, found: 334.1446.
Ethyl 2-(9-cyano-7H-pyrrolo[3,2,1-de]phenanthridin-7-yl)acetate (4n), 44.7 mg, 71% yield, yellow solid. 1H NMR (400 MHz, CDCl3) δ 8.02 (d, J = 8.0 Hz, 1H), 7.66 (dd, J = 8.0, 1.6 Hz, 1H), 7.63–7.58 (m, 3H), 7.25 (d, J = 3.2 Hz, 1H), 7.18 (t, J = 7.6 Hz, 1H), 6.59 (d, J = 3.2 Hz, 1H), 6.13 (dd, J = 7.6, 5.2 Hz, 1H), 4.10 (q, J = 7.2 Hz, 2H), 2.77 (qd, J = 16.0, 7.6 Hz, 2H), 1.16 (t, J = 7.2 Hz, 3H). 13C NMR and HRMS data for the desired product were in agreement with the previously reported literature data [35].
Ethyl 2-(9-nitro-7H-pyrrolo[3,2,1-de]phenanthridin-7-yl)acetate (4o), 41.3 mg, 61% yield, yellow oil. 1H NMR (400 MHz, CDCl3) δ 8.24 (dd, J = 8.8, 2.4 Hz, 1H), 8.20 (d, J = 2.0 Hz, 1H), 8.06 (d, J = 8.4 Hz, 1H), 7.64 (d, J = 3.2 Hz, 1H), 7.62 (d, J = 2.4 Hz, 1H), 7.27 (d, J = 3.2 Hz, 1H), 7.19 (t, J = 7.6 Hz, 1H), 6.60 (d, J = 3.2 Hz, 1H), 6.20 (dd, J = 7.2, 4.8 Hz, 1H), 4.10 (qd, J = 7.2, 2.0 Hz, 2H), 2.82 (qd, J = 16.0, 7.2 Hz, 2H), 1.16 (t, J = 7.2 Hz, 3H). 13C NMR and HRMS data for the desired product were in agreement with the previously reported literature data [35].
Ethyl 2-(7H-benzo[j]pyrrolo[3,2,1-de]phenanthridin-7-yl)acetate (4p), 44.5 mg, 65% yield, yellow solid. 1H NMR (400 MHz, CDCl3) δ 8.40 (s, 1H), 7.90 (d, J = 7.6 Hz, 1H), 7.80 (s, 1H), 7.78 (d, J = 2.8 Hz, 2H), 7.59 (dd, J = 8.0, 0.8 Hz, 1H), 7.55–7.44 (m, 2H), 7.30 (d, J = 3.2 Hz, 1H), 7.23 (t, J = 7.6 Hz, 1H), 6.60 (d, J = 3.2 Hz, 1H), 6.26 (t, J = 6.4 Hz, 1H), 4.14–4.06 (m, 2H), 2.82 (qd, J = 15.6, 7.2 Hz, 2H), 1.14 (t, J = 7.2 Hz, 3H). 13C NMR and HRMS data for the desired product were in agreement with the previously reported literature data [35].
Butyl 2-(7H-pyrrolo[3,2,1-de]phenanthridin-7-yl)acetate (4q), 47.5 mg, 72% yield, yellow oil. 1H NMR (400 MHz, CDCl3) δ 7.97 (d, J = 7.6 Hz, 1H), 7.58 (d, J = 7.6 Hz, 1H), 7.54 (dd, J = 8.0, 0.8 Hz, 1H), 7.41–7.37 (m, 1H), 7.32–7.30 (m, 2H), 7.24 (d, J = 3.2 Hz, 1H), 7.16 (t, J = 7.6 Hz, 1H), 6.55 (d, J = 3.2 Hz, 1H), 6.14 (dd, J = 7.2, 5.2 Hz, 1H), 4.11–4.00 (m, 2H), 2.78 (qd, J = 16.0, 7.6 Hz, 2H), 1.54–1.47 (m, 2H), 1.30–1.24 (m, 2H), 0.89 (t, J = 7.6 Hz, 3H). 13C NMR and HRMS data for the desired product were in agreement with the previously reported literature data [35].
5,6-Diphenylindolo[2,1-a]isoquinoline (6a), 55.8 mg, 75% yield, yellow solid. 1H NMR (400 MHz, CDCl3) δ 8.31 (d, J = 8.0 Hz, 1H), 7.81 (d, J = 8.0 Hz, 1H), 7.52 (t, J = 7.6 Hz, 1H), 7.43 (s, 1H), 7.41–7.25 (m, 7H), 7.26–7.13 (m, 6H), 6.83 (t, J = 8.0 Hz, 1H), 6.01 (d, J = 8.8 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ 136.9, 136.1, 135.5, 132.9, 132.0, 131.0, 130.4, 129.8, 128.8, 128.7, 128.0, 127.5, 127.2, 126.9, 126.3, 125.5, 123.4, 121.8, 120.3, 120.2, 114.7, 94.3. HRMS data for the desired product were in agreement with the previously reported literature data [40].
10-Nitro-5,6-diphenylindolo[2,1-a]isoquinoline (6b), 64.8 mg, 79% yield, orange solid. 1H NMR (400 MHz, CDCl3) δ 8.70 (d, J = 2.4 Hz, 1H), 8.32 (d, J = 8.0 Hz, 1H), 7.66 (dd, J = 9.6, 2.4 Hz, 1H), 7.59–7.54 (m, 2H), 7.44–7.36 (m, 4H), 7.31–7.27 (m, 3H), 7.25–7.17 (m, 5H), 5.99 (d, J = 9.6 Hz, 1H). 13C NMR and HRMS data for the desired product were in agreement with the previously reported literature data [40].
1-Methyl-5,6-diphenylindolo[2,1-a]isoquinoline (6c), 55.3 mg, 73% yield, yellow solid, m.p. 173.7–174.0 °C. 1H NMR (400 MHz, CDCl3) δ 7.84 (d, J = 8.0 Hz, 1H), 7.56 (s, 1H), 7.39 (d, J = 7.6 Hz, 1H), 7.36–7.26 (m, 6H), 7.25–7.16 (m, 6H), 7.04 (d, J = 8.0 Hz, 1H), 6.86–6.81 (m, 1H), 6.00 (d, J = 8.4 Hz, 1H), 3.03 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 137.6, 135.9, 135.2, 132.0, 131.9, 131.0, 130.3, 129.6, 128.7, 128.0, 126.8, 126.6, 125.1, 124.5, 121.6, 120.6, 120.5, 114.8, 100.7, 25.4. HRMS (ESI) calcd for C29H22N [M + H]+: 384.1752, found: 384.1751.
1-Chloro-5,6-diphenylindolo[2,1-a]isoquinoline (6d) and 5,6-diphenylindolo[2,1-a]isoquinoline (6a), 37.5 mg, 47% yield, yellow solid, m.p. 201.1–201.6 °C. 1H NMR (400 MHz, CDCl3) δ 8.40 (6d, d, J = 0.8 Hz, 1H), 8.30 (6a, dt, J = 8.0, 0.8 Hz, 1H), 7.85 (6d, dt, J = 8.0, 1.2 Hz, 1H), 7.79 (6a, dt, J = 8.0, 1.2 Hz, 1H), 7.57 (6d, dd, J = 8.0, 1.2 Hz, 1H), 7.53–7.49 (6a, m, 1H), 7.42 (6a, d, J = 0.4 Hz, 1H), 7.36–7.28 (6d + 6a, m, 11H), 7,24–7.13 (6d + 6a, m, 14H), 7.05 (6d, dd, J = 8.0, 1.2 Hz, 1H), 6.87–6.79 (6d + 6a, m, 2H), 6.00–5.95 (6d + 6a, m, 2H). 13C NMR (100 MHz, CDCl3) δ 137.0, 135.4, 132.9, 132.0, 131.0, 130.8, 129.7, 128.9, 128.8, 128.7, 128.1, 128.0, 127.5, 127.2, 127.1, 126.9, 126.3, 125.1, 123.6, 123.4, 121.8, 121.8, 121.3, 121.1, 120.2, 114.7, 102.5, 94.3. HRMS (ESI) calcd for 6d C29H19NCl [M + H]+: 404.1206, found: 404.1209.
1-Bromo-5,6-diphenylindolo[2,1-a]isoquinoline (6e) and 5,6-diphenylindolo[2,1-a]isoquinoline (6a), 34.9 mg, 39% yield, yellow solid, m.p. 188.5–188.9 °C. 1H NMR (400 MHz, CDCl3) δ 8.67 (6e, s, 1H), 8.31 (6a, d, J = 8.0 Hz, 1H), 7.85 (6e, d, J = 8.0 Hz, 1H), 7.82–7.79 (6e + 6a, m, 2H), 7.53–7.49 (6a, m, 1H), 7.43–7.27 (6e + 6a, m, 13H), 7.25–7.10 (6e + 6a, m, 14H), 6.87–6.79 (6e + 6a, m, 2H), 6.00 (6a, d, J = 8.8 Hz, 1H), 5.97 (6e, d, J = 8.8 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ 133.7, 132.0, 132.0, 131.0, 130.8, 128.9, 128.9, 128.8, 128.8, 128.2, 128.0, 127.5, 127.3, 127.2, 127.1, 127.1, 126.9, 126.3, 125.9, 121.3, 121.1, 120.3, 120.2, 119.7, 114.7, 102.2, 94.3. HRMS (ESI) calcd for 6e C29H19NBr [M + H]+: 448.0701, found: 448.0705.
1,3-Dimethyl-5,6-diphenylindolo[2,1-a]isoquinoline (6f), 64.4 mg, 81% yield, orange solid, m.p. 183.2–183.7 °C. 1H NMR (400 MHz, CDCl3) δ 7.82 (dt, J = 8.0, 1.2 Hz, 1H), 7.50 (s, 1H), 7.35–7.27 (m, 5H), 7.25–7.15 (m, 7H), 6.84–6.79 (m, 2H), 5.98 (d, J = 8.4 Hz, 1H), 2.99 (s, 3H), 2.31 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 137.7, 136.5, 136.0, 135.9, 135.7, 135.1, 132.1, 131.9, 131.7, 131.0, 129.7, 128.7, 128.7, 128.0, 126.7, 124.5, 122.7, 122.0, 121.5, 120.3, 120.3, 114.7, 99.9, 25.3, 21.5. HRMS (ESI) calcd for C30H24N [M + H]+: 398.1909, found: 398.1905.
1,10-Dimethyl-5,6-diphenylindolo[2,1-a]isoquinoline (6g), 64.2 mg, 81% yield, orange solid, m.p. 212.6–213.0 °C. 1H NMR (400 MHz, CDCl3) δ 7.61 (s, 1H), 7.38 (d, J = 7.2 Hz, 1H), 7.36–7.26 (m, 6H), 7.25–7.16 (m, 6H), 7.05 (dd, J = 8.0, 1.2 Hz, 1H), 6.68 (dd, J = 8.8, 2.0 Hz, 1H), 5.87 (d, J = 8.8 Hz, 1H), 3.02 (s, 3H), 2.44 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 137.7, 136.0, 135.9, 135.6, 135.1, 132.1, 131.9, 131.0, 130.3, 130.2, 130.0, 128.7, 128.0, 126.8, 126.5, 125.1, 124. 5, 122.4, 121.8, 120.0, 114.4, 100.3, 25.4, 21.5. HRMS (ESI) calcd for C30H24N [M + H]+: 398.1909, found: 398.1912.
5-(4-Ethylphenyl)-1-methyl-6-(p-tolyl)indolo[2,1-a]isoquinoline and 6-(4-ethylphenyl)-1-methyl-5-(p-tolyl)indolo[2,1-a]isoquinoline (6h), 68.6 mg, 82% yield, yellow solid, m.p. 151.6–151.9 °C. 1H NMR (400 MHz, CDCl3) δ 7.82 (dq, J = 8.0, 1.2 Hz, 1H), 7.54 (s, 1H), 7.37 (d, J = 6.8 Hz, 1H), 7.25–7.12 (m, 6H), 7.07 and 7.05 (a pair of s, 5H), 6.87–6.81 (m, 1H), 6.04 and 5.98 (a pair of dd, J = 8.8, 0.8 Hz, 1H), 3.02 (s, 3H), 2.69 and 2.62 (a pair of q, J = 7.6 Hz, 2H), 2.39 and 2.32 (a pair of s, 3H), 1.27 and 1.22 (a pair of t, J = 7.6 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 144.8, 142.5, 138.3, 136.1, 135.6, 135.1, 134.8, 134.6, 133.2, 132.0, 131.8, 130.8, 130.8, 130.1, 129.6, 129.4, 128.7, 128.2, 127.4, 126.5, 125.0, 124.6, 124.5, 122.1, 122.0, 121.4, 120.4, 120.4, 115.0, 100.6, 28.8, 28.6, 25.4, 21.6, 21.4, 15.6, 15.5. HRMS (ESI) calcd for C32H28N [M + H]+: 426.2222, found: 426.2224.
4. Conclusions
In conclusion, we have reported on the rhodium-catalyzed oxidative annulation of 2- or 7-phenyl-1H-indoles with alkenes or alkynes to assemble valuable 6H-isoindolo[2,1-a]indoles, pyrrolo[3,2,1-de]phenanthridines, or indolo[2,1-a]isoquinolines using molecular oxygen as the sole oxidant enable by quaternary ammonium salt. Salient features of present catalytic system comprise (a) the atmospheric pressure of air as the sole oxidant, (b) one catalytic system for three discrete reactions, and (c) mechanistic insights. Mechanistic studies provided support for fast intramolecular aza-Michael reaction and aerobic reoxidation of Rh(I) to Rh(III) by adding quaternary ammonium salt. Additional mechanistic/computational studies will be needed to fully elucidate the unique influence of quaternary ammonium salt on the catalytic cycle, and are in progress in our laboratory.
Supplementary Materials
The following are available online. Figure S1: Copies of the 1H NMR, 13C NMR charts for compounds.
Author Contributions
Conceptualization, W.Z. and Q.H.; experiments and analyses, W.Z., Y.Z., and J.Z.; writing—original draft preparation, W.Z.; writing—review and editing, Q.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Science Foundation of China (Grant No. 21872028), the Natural Science Foundation of Fujian Province (Grant No. 2020J01149), the Fujian Province University Fund for New Century Excellent Talents, and the National College Students’ innovation and entrepreneurship training program (Y.Z.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
References
- Lyons, T.W.; Sanford, M.S. Palladium-Catalyzed Ligand-Directed C-H Functionalization Reactions. Chem. Rev. 2010, 110, 1147–1169. [Google Scholar] [CrossRef] [PubMed]
- Arockiam, P.B.; Bruneau, C.; Dixneuf, P.H. Ruthenium (II)-catalyzed C-H bond activation and functionalization. Chem. Rev. 2012, 112, 5879–5918. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Huang, H. Transition-metal-catalyzed direct addition of unactivated C-H bonds to polar unsaturated bonds. Chem. Rev. 2015, 115, 3468–3517. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Chang, S. Transition-Metal-Mediated Direct C–H Amination of Hydrocarbons with Amine Reactants: The Most Desirable but Challenging C–N Bond-Formation Approach. ACS Catal. 2016, 6, 2341–2351. [Google Scholar] [CrossRef]
- He, J.; Wasa, M.; Chan, K.S.L.; Shao, Q.; Yu, J.-Q. Palladium-Catalyzed Transformations of Alkyl C-H Bonds. Chem. Rev. 2017, 117, 8754–8786. [Google Scholar] [CrossRef] [PubMed]
- Murakami, K.; Yamada, S.; Kaneda, T.; Itami, K. C-H Functionalization of Azines. Chem. Rev. 2017, 117, 9302–9332. [Google Scholar] [CrossRef]
- Newton, C.G.; Wang, S.-G.; Oliveira, C.C.; Cramer, N. Catalytic Enantioselective Transformations Involving C-H Bond Cleavage by Transition-Metal Complexes. Chem. Rev. 2017, 117, 8908–8976. [Google Scholar] [CrossRef]
- Karimov, R.R.; Hartwig, J.F. Transition-Metal-Catalyzed Selective Functionalization of C(sp3)-H Bonds in Natural Products. Angew. Chem. Int. Ed. 2018, 57, 4234–4241. [Google Scholar] [CrossRef]
- Zheng, L.; Hua, R. C-H Activation and Alkyne Annulation via Automatic or Intrinsic Directing Groups: Towards High Step Economy. Chem. Rec. 2018, 18, 556–569. [Google Scholar] [CrossRef]
- Kalepu, J.; Pilarski, L.T. Weinreb Amides as Directing Groups for Transition Metal-Catalyzed C-H Functionalizations. Molecules 2019, 24, 830. [Google Scholar] [CrossRef]
- Niu, B.; Yang, K.; Lawrence, B.; Ge, H. Transient Ligand-Enabled Transition Metal-Catalyzed C-H Functionalization. ChemSusChem 2019, 12, 2955–2969. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Yang, L.; Li, P.; Wang, F.; Li, X. Recent advances in transition metal-catalyzed olefinic C-H functionalization. Org. Chem. Front. 2021, 8, 1085–1101. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, H.; Shi, W.; Lei, A. Bond formations between two nucleophiles: Transition metal catalyzed oxidative cross-coupling reactions. Chem. Rev. 2011, 111, 1780–1824. [Google Scholar] [CrossRef] [PubMed]
- Yeung, C.S.; Dong, V.M. Catalytic dehydrogenative cross-coupling: Forming carbon-carbon bonds by oxidizing two carbon-hydrogen bonds. Chem. Rev. 2011, 111, 1215–1292. [Google Scholar] [CrossRef] [PubMed]
- Girard, S.A.; Knauber, T.; Li, C.J. The cross-dehydrogenative coupling of C(sp3)-H bonds: A versatile strategy for C-C bond formations. Angew. Chem. Int. Ed. 2014, 53, 74–100. [Google Scholar] [CrossRef]
- Liu, C.; Yuan, J.; Gao, M.; Tang, S.; Li, W.; Shi, R.; Lei, A. Oxidative Coupling between Two Hydrocarbons: An Update of Recent C-H Functionalizations. Chem. Rev. 2015, 115, 12138–12204. [Google Scholar] [CrossRef]
- Ruiz-Castillo, P.; Buchwald, S.L. Applications of Palladium-Catalyzed C-N Cross-Coupling Reactions. Chem. Rev. 2016, 116, 12564–12649. [Google Scholar] [CrossRef]
- Yang, Y.; Lan, J.; You, J. Oxidative C-H/C-H Coupling Reactions between Two (Hetero)arenes. Chem. Rev. 2017, 117, 8787–8863. [Google Scholar] [CrossRef]
- Tang, S.; Zeng, L.; Lei, A. Oxidative R(1)-H/R(2)-H Cross-Coupling with Hydrogen Evolution. J. Am. Chem. Soc. 2018, 140, 13128–13135. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Gao, X.; Lv, Z.; Abdelilah, T.; Lei, A. Recent Advances in Oxidative R(1)-H/R(2)-H Cross-Coupling with Hydrogen Evolution via Photo-/Electrochemistry. Chem. Rev. 2019, 119, 6769–6787. [Google Scholar] [CrossRef]
- Zhuang, W.; Zhang, X.; Huang, Q. Recent Advances in Cross-Dehydrogenative-Coupling Reactions Using Molecular Oxygen as the Sole Oxidant. Chin. J. Org. Chem. 2021, 41, 529–542. [Google Scholar] [CrossRef]
- Ueura, K.; Satoh, T.; Miura, M. An efficient waste-free oxidative coupling via regioselective C-H bond cleavage: Rh/Cu-catalyzed reaction of benzoic acids with alkynes and acrylates under air. Org. Lett. 2007, 9, 1407–1409. [Google Scholar] [CrossRef] [PubMed]
- Colby, D.A.; Bergman, R.G.; Ellman, J.A. Rhodium-Catalyzed C-C Bond Formation via Heteroatom-Directed C-H Bond Activation. Chem. Rev. 2010, 110, 624–655. [Google Scholar] [CrossRef] [PubMed]
- Colby, D.A.; Tsai, A.S.; Bergman, R.G.; Ellman, J.A. Rhodium Catalyzed Chelation-Assisted C–H Bond Functionalization Reactions. Acc. Chem. Res. 2012, 45, 814–825. [Google Scholar] [CrossRef]
- Song, G.; Li, X. Substrate activation strategies in rhodium(III)-catalyzed selective functionalization of arenes. Acc. Chem. Res. 2015, 48, 1007–1020. [Google Scholar] [CrossRef]
- Bera, M.; Agasti, S.; Chowdhury, R.; Mondal, R.; Pal, D.; Maiti, D. Rhodium-Catalyzed meta-C-H Functionalization of Arenes. Angew. Chem. Int. Ed. 2017, 56, 5272–5276. [Google Scholar] [CrossRef] [PubMed]
- Vásquez-Céspedes, S.; Wang, X.; Glorius, F. Plausible Rh(V) Intermediates in Catalytic C–H Activation Reactions. ACS Catal. 2017, 8, 242–257. [Google Scholar] [CrossRef]
- Rej, S.; Chatani, N. Rh-Catalyzed Removable Directing Group Assisted sp2 or sp3-C-H Bond Functionalization. Angew. Chem. Int. Ed. 2019, 58, 8304–8329. [Google Scholar] [CrossRef]
- Zhu, W.; Gunnoe, T.B. Advances in Rhodium-Catalyzed Oxidative Arene Alkenylation. Acc. Chem. Res. 2020, 53, 920–936. [Google Scholar] [CrossRef]
- Dongbang, S.; Confair, D.N.; Ellman, J.A. Rhodium-Catalyzed C-H Alkenylation/Electrocyclization Cascade Provides Dihydropyridines That Serve as Versatile Intermediates to Diverse Nitrogen Heterocycles. Acc. Chem. Res. 2021, 54, 1766–1778. [Google Scholar] [CrossRef]
- Huang, Q.; Han, Q.; Fu, S.; Yao, Z.; Su, L.; Zhang, X.; Lin, S.; Xiang, S. Rhodium-Catalyzed NH-Indole-Directed C–H Carbonylation with Carbon Monoxide: Synthesis of 6H-Isoindolo[2,1-a]indol-6-ones. J. Org. Chem. 2016, 81, 12135–12142. [Google Scholar] [CrossRef]
- Guo, X.; Han, Q.; Tang, Z.; Su, L.; Zhang, X.; Zhang, X.; Lin, S.; Huang, Q. Rhodium-catalyzed NH-indole-directed ortho C-H coupling of 2-arylindoles with diazo compounds via metal carbene migratory insertion. Tetrahedron Lett. 2018, 59, 1568–1572. [Google Scholar] [CrossRef]
- Liu, A.; Han, Q.; Zhang, X.; Li, B.; Huang, Q. Transition-Metal-Controlled Synthesis of 11H-Benzo[a]carbazoles and 6-Alkylidene-6H-isoindo[2,1-a]indoles via Sequential Intermolecular/Intramolecular Cross-Dehydrogenative Coupling from 2-Phenylindoles. Org. Lett. 2019, 21, 6839–6843. [Google Scholar] [CrossRef]
- Yuan, Y.; Pan, G.; Zhang, X.; Li, B.; Xiang, S.; Huang, Q. Synthesis of Seven-Membered Azepino[3,2,1-hi]indoles via Rhodium-Catalyzed Regioselective C-H Activation/1,8-Diazabicyclo[5.4.0]undec-7-ene-Catalyzed Intramolecular Amidation of 7-Phenylindoles in One Pot. J. Org. Chem. 2019, 84, 14701–14711. [Google Scholar] [CrossRef]
- Yuan, Y.; Pan, G.; Zhang, X.; Huang, Q. One pot synthesis of pyrrolo[3,2,1-de]phenanthridines from 7-phenylindoles via tandem C–H olefination/aza-Michael addition. Org. Chem. Front. 2020, 7, 53–63. [Google Scholar] [CrossRef]
- Yuan, Y.; Guo, X.; Zhang, X.; Li, B.; Huang, Q. Access to 5H-benzo[a]carbazol-6-ols and benzo[6,7]cyclohepta[1,2-b]indol-6-ols via rhodium-catalyzed C–H activation/carbenoid insertion/aldol-type cyclization. Org. Chem. Front. 2020, 7, 3146–3159. [Google Scholar] [CrossRef]
- Lou, J.; Han, W.; Liu, Z.; Xiao, J. Rhodium-catalyzed enone carbonyl directed C–H activation for the synthesis of indanones containing all-carbon quaternary centers. Org. Chem. Front. 2021, 8, 1447–1453. [Google Scholar] [CrossRef]
- Patureau, F.W.; Besset, T.; Glorius, F. Rhodium-catalyzed oxidative olefination of C-H bonds in acetophenones and benzamides. Angew. Chem. Int. Ed. 2011, 50, 1064–1067. [Google Scholar] [CrossRef]
- Jayakumar, J.; Parthasarathy, K.; Cheng, C.-H. One-pot synthesis of isoquinolinium salts by rhodium-catalyzed C-H bond activation: Application to the total synthesis of oxychelerythrine. Angew. Chem. Int. Ed. 2012, 51, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, L.; Wang, L.; Lygin, A.V. Ruthenium-catalyzed aerobic oxidative coupling of alkynes with 2-aryl-substituted pyrrole. Chem. Sci. 2012, 3, 177–180. [Google Scholar] [CrossRef]
- Peng, S.; Liu, S.; Zhang, S.; Cao, S.; Sun, J. Synthesis of Polyheteroaromatic Compounds via Rhodium-Catalyzed Multiple C-H Bond Activation and Oxidative Annulation. Org. Lett. 2015, 17, 5032–5035. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yu, X.; Ji, D.; Yamamoto, Y.; Almansour, A.I.; Arumugam, N.; Kumar, R.S.; Bao, M. Rhodium-Catalyzed Oxidative Benzannulation of N-Adamantyl-1-naphthylamines with Internal Alkynes via Dual C-H Bond Activation: Synthesis of Substituted Anthracenes. Org. Lett. 2016, 18, 4246–4249. [Google Scholar] [CrossRef]
- Li, B.; Zhang, B.; Zhang, X.; Fan, X. Regio-selective synthesis of diversely substituted benzo[a]carbazoles through Rh(III)-catalyzed annulation of 2-arylindoles with alpha-diazo carbonyl compounds. Chem. Commun. 2017, 53, 1297–1300. [Google Scholar] [CrossRef]
- Han, Q.; Guo, X.; Tang, Z.; Su, L.; Yao, Z.; Zhang, X.; Lin, S.; Xiang, S.; Huang, Q. Rhodium-Catalyzed Regioselective Ortho C-H Olefination of 2-Arylindoles via NH-Indole-Directed C-H Bond Cleavage. Adv. Synth. Catal. 2018, 360, 972–984. [Google Scholar] [CrossRef]
- Yan, X.; Ye, R.; Sun, H.; Zhong, J.; Xiang, H.; Zhou, X. Synthesis of 2-Arylindoles by Rhodium-Catalyzed/Copper-Mediated Annulative Coupling of N-Aryl-2-aminopyridines and Propargyl Alcohols via Selective C-H/C-C Activation. Org. Lett. 2019, 21, 7455–7459. [Google Scholar] [CrossRef] [PubMed]
- Mihara, G.; Noguchi, T.; Nishii, Y.; Hayashi, Y.; Kawauchi, S.; Miura, M. Rhodium-Catalyzed Annulative Coupling of Isothiazoles with Alkynes through N-S Bond Cleavage. Org. Lett. 2020, 22, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.T.; Kang, E.; Kim, M.; Joo, J.M. Synthesis of Bidentate Nitrogen Ligands by Rh-Catalyzed C-H Annulation and Their Application to Pd-Catalyzed Aerobic C-H Alkenylation. Org. Lett. 2021, 23, 3657–3662. [Google Scholar] [CrossRef]
- Kawashita, Y.; Hayashi, M. Synthesis of heteroaromatic compounds by oxidative aromatization using an activated carbon/molecular oxygen system. Molecules 2009, 14, 3073–3093. [Google Scholar] [CrossRef]
- Campbell, A.N.; Stahl, S.S. Overcoming the “Oxidant Problem”: Strategies to Use O2 as the Oxidant in Organometallic C–H Oxidation Reactions Catalyzed by Pd (and Cu). Acc. Chem. Res. 2012, 45, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Zhang, C.; Tang, C.; Jiao, N. Recent advances in transition-metal catalyzed reactions using molecular oxygen as the oxidant. Chem. Soc. Rev. 2012, 41, 3381–3430. [Google Scholar] [CrossRef]
- McCann, S.D.; Stahl, S.S. Copper-Catalyzed Aerobic Oxidations of Organic Molecules: Pathways for Two-Electron Oxidation with a Four-Electron Oxidant and a One-Electron Redox-Active Catalyst. Acc. Chem. Res. 2015, 48, 1756–1766. [Google Scholar] [CrossRef]
- Liang, Y.F.; Jiao, N. Oxygenation via C-H/C-C Bond Activation with Molecular Oxygen. Acc. Chem. Res. 2017, 50, 1640–1653. [Google Scholar] [CrossRef]
- Wang, D.; Weinstein, A.B.; White, P.B.; Stahl, S.S. Ligand-Promoted Palladium-Catalyzed Aerobic Oxidation Reactions. Chem. Rev. 2018, 118, 2636–2679. [Google Scholar] [CrossRef]
- Tang, C.; Qiu, X.; Cheng, Z.; Jiao, N. Molecular oxygen-mediated oxygenation reactions involving radicals. Chem. Soc. Rev. 2021, 50, 8067–8101. [Google Scholar] [CrossRef]
- Shi, Z.; Ding, S.; Cui, Y.; Jiao, N. A palladium-catalyzed oxidative cycloaromatization of biaryls with alkynes using molecular oxygen as the oxidant. Angew. Chem. Int. Ed. 2009, 48, 7895–7898. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-H.; Shi, B.-F.; Yu, J.-Q. Pd(II)-Catalyzed Olefination of Electron-Deficient Arenes Using 2,6-Dialkylpyridine Ligands. J. Am. Chem. Soc. 2009, 131, 5072–5074. [Google Scholar] [CrossRef]
- Engle, K.M.; Wang, D.-H.; Yu, J.-Q. Constructing multiply substituted arenes using sequential palladium(II)-catalyzed C-H olefination. Angew. Chem. Int. Ed. 2010, 49, 6169–6173. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.-H.; Chok, Y.K.; Loh, T.-P. Synthesis and characterization of a cyclic vinylpalladium(II) complex: Vinylpalladium species as the possible intermediate in the catalytic direct olefination reaction of enamide. Chem. Sci. 2011, 2, 1822–1825. [Google Scholar] [CrossRef]
- Wei, Y.; Deb, I.; Yoshikai, N. Palladium-Catalyzed Aerobic Oxidative Cyclization of N-Aryl Imines: Indole Synthesis from Anilines and Ketones. J. Am. Chem. Soc. 2012, 134, 9098–9101. [Google Scholar] [CrossRef] [PubMed]
- Piotrowicz, M.; Zakrzewski, J. Aerobic Dehydrogenative Heck Reaction of Ferrocene with a Pd(OAc)2/4,5-Diazafluoren-9-one Catalyst. Organometallics 2013, 32, 5709–5712. [Google Scholar] [CrossRef]
- Li, N.-N.; Zhang, Y.-L.; Mao, S.; Gao, Y.-R.; Guo, D.-D.; Wang, Y.-Q. Palladium-catalyzed C-H homocoupling of furans and thiophenes using oxygen as the oxidant. Org. Lett. 2014, 16, 2732–2735. [Google Scholar] [CrossRef]
- Kim, N.; Min, M.; Hong, S. Regioselective palladium(II)-catalyzed aerobic oxidative Heck-type C3 alkenylation of sulfocoumarins. Org. Chem. Front. 2015, 2, 1621–1624. [Google Scholar] [CrossRef]
- Kumar, K.S.; Meesa, S.R.; Naikawadi, P.K. Palladium-Catalyzed [2 + 2 + 2] Annulation via Transformations of Multiple C-H Bonds: One-Pot Synthesis of Diverse Indolo[3,2-a]carbazoles. Org. Lett. 2018, 20, 6079–6083. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-B.; Xie, D.; Zang, Z.-L.; Zhou, C.-H.; Cai, G.-X. Palladium-catalyzed aerobic regio- and stereo-selective olefination reactions of phenols and acrylates via direct dehydrogenative C(sp2)-O cross-coupling. Chem. Commun. 2018, 54, 4437–4440. [Google Scholar] [CrossRef]
- Beckers, I.; Krasniqi, B.; Kumar, P.; Escudero, D.; De Vos, D. Ligand-Controlled Selectivity in the Pd-Catalyzed C–H/C–H Cross-Coupling of Indoles with Molecular Oxygen. ACS Catal. 2021, 11, 2435–2444. [Google Scholar] [CrossRef]
- Zhang, G.; Yang, L.; Wang, Y.; Xie, Y.; Huang, H. An efficient Rh/O2 catalytic system for oxidative C-H activation/annulation: Evidence for Rh(I) to Rh(III) oxidation by molecular oxygen. J. Am. Chem. Soc. 2013, 135, 8850–8853. [Google Scholar] [CrossRef]
- Zhang, G.; Yu, H.; Qin, G.; Huang, H. Rh-catalyzed oxidative C-H activation/annulation: Converting anilines to indoles using molecular oxygen as the sole oxidant. Chem. Commun. 2014, 50, 4331–4334. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wang, H.-W.; Spangler, J.E.; Chen, K.; Cui, P.-P.; Zhao, Y.; Sun, W.-Y.; Yu, J.-Q. Rh(III)-catalyzed C-H olefination of N-pentafluoroaryl benzamides using air as the sole oxidant. Chem. Sci. 2015, 6, 1923–1927. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tang, J.; Li, S.; Liu, Z.; Zhao, Y.; She, Z.; Kadam, V.D.; Gao, G.; Lan, J.; You, J. Cascade C-H Annulation of Aldoximes with Alkynes Using O2 as the Sole Oxidant: One-Pot Access to Multisubstituted Protoberberine Skeletons. Org. Lett. 2017, 19, 604–607. [Google Scholar] [CrossRef]
- Jambu, S.; Sivasakthikumaran, R.; Jeganmohan, M. Aerobic Oxidative Alkenylation of Weak O-Coordinating Arylacetamides with Alkenes via a Rh(III)-Catalyzed C-H Activation. Org. Lett. 2019, 21, 1320–1324. [Google Scholar] [CrossRef]
- Huang, Q.; Zhang, X.; Qiu, L.; Wu, J.; Xiao, H.; Zhang, X.; Lin, S. Palladium-Catalyzed Olefination and Arylation of Polyfluoroarenes Using Molecular Oxygen as the Sole Oxidant. Adv. Synth. Catal. 2015, 357, 3753–3757. [Google Scholar] [CrossRef]
- Zhang, X.; Su, L.; Qiu, L.; Fan, Z.; Zhang, X.; Lin, S.; Huang, Q. Palladium-catalyzed C-H olefination of uracils and caffeines using molecular oxygen as the sole oxidant. Org. Biomol. Chem. 2017, 15, 3499–3506. [Google Scholar] [CrossRef] [PubMed]
- Pan, G.; Wu, K.; Deng, Z.; Zhang, X.; Zhang, X.; Lin, S.; Huang, Q. Palladium-Catalyzed C-H Direct Arylation of Uracils and Caffeines with Arenes Using Molecular Oxygen as the Sole Oxidant. Chin. J. Org. Chem. 2018, 38, 2076–2084. [Google Scholar] [CrossRef]
- Duce, S.; Mateo, A.; Alonso, I.; Garcia Ruano, J.L.; Cid, M.B. Role of quaternary ammonium salts as new additives in the enantioselective organocatalytic beta-benzylation of enals. Chem. Commun. 2012, 48, 5184–5186. [Google Scholar] [CrossRef]
- Ferrary, T.; David, E.; Milanole, G.E.; Besset, T.; Jubault, P.; Pannecoucke, X. A Straightforward and Highly Diastereoselective Access to Functionalized Monofluorinated Cyclopropanes via a Michael Initiated Ring Closure Reaction. Org. Lett. 2013, 15, 5598–5601. [Google Scholar] [CrossRef]
- Sheshenev, A.E.; Boltukhina, E.V.; White, A.J.; Hii, K.K. Methylene-bridged bis(imidazoline)-derived 2-oxopyrimidinium salts as catalysts for asymmetric Michael reactions. Angew. Chem. Int. Ed. 2013, 52, 6988–6991. [Google Scholar] [CrossRef] [PubMed]
- Ding, R.; Zheng, B.; Wang, Y.; Peng, Y.G. A Cation-Directed Enantioselective Sulfur-Mediated Michael/Mannich Three-Component Domino Reaction involving Chalcones as Michael Acceptors. Org. Lett. 2015, 17, 4128–4131. [Google Scholar] [CrossRef]
- Zhang, J.-X.; Wang, H.-Y.; Jin, Q.-W.; Zheng, C.-W.; Zhao, G.; Shang, Y.-J. Thiourea-Quaternary Ammonium Salt Catalyzed Asymmetric 1, 3-Dipolar Cycloaddition of Imino Esters To Construct Spiro[pyrrolidin-3,3′-oxindoles]. Org. Lett. 2016, 18, 4774–4777. [Google Scholar] [CrossRef]
- Jayakumar, J.; Vedarethinam, G.; Hsiao, H.-C.; Sun, S.-Y.; Chuang, S.-C. Cascade One-Pot Synthesis of Orange-Red-Fluorescent Polycyclic Cinnolino[2,3-f]phenanthridin-9-ium Salts by Palladium(II)-Catalyzed C-H Bond Activation of 2-Azobiaryl Compounds and Alkenes. Angew. Chem. Int. Ed. 2020, 59, 689–694. [Google Scholar] [CrossRef]
- Kenichi, F.; Katsutoshi, O.; Tokio, Y. The Catalytic Activity of Onium Compounds in the Homogeneous Liquid Phase Oxidation of Cumene and α-Pinene. Bull. Chem. Soc. Jpn. 1969, 42, 312–318. [Google Scholar]
- Csányi, L.J.; Jáky, K.; Kóta, Z.; Páli, T. Oxidation of hydrocarbons by O2 in the presence of onium salts and onium ion-pair complexes as catalysts. J. Mol. Catal. A Chem. 2004, 209, 59–68. [Google Scholar] [CrossRef]
- Santos, V.P.; Bakker, J.J.W.; Kreutzer, M.T.; Kapteijn, F.; Gascon, J. Transport Limitations during Phase Transfer Catalyzed Ethyl-Benzene Oxidation: Facts and Fictions of “Halide Catalysis”. ACS Catal. 2012, 2, 1421–1424. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).