Lepidium graminifolium L.: Glucosinolate Profile and Antiproliferative Potential of Volatile Isolates
Abstract
:1. Introduction
2. Results and Discussion
2.1. Glucosinolates and Volatile Constituents
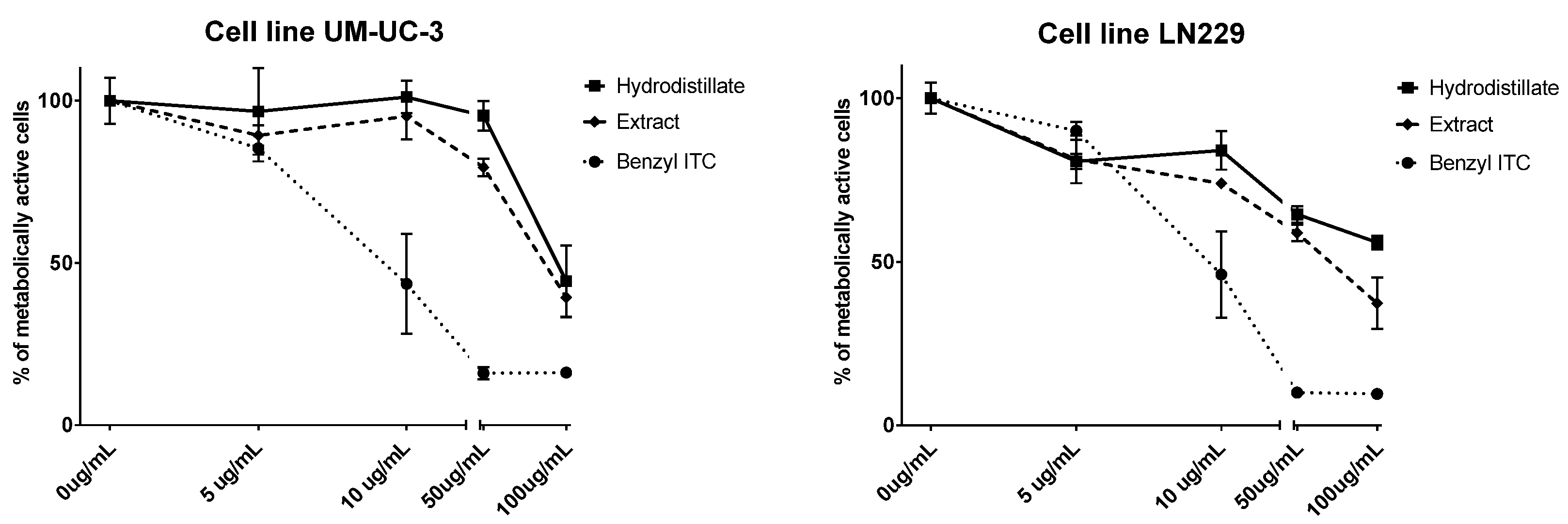
2.2. Antiproliferative Activity
3. Materials and Methods
3.1. Materials and Reagents
3.2. Isolation and Chemical Analysis
3.2.1. Isolation of Desulfoglucosinolates
3.2.2. UHPLC-DAD-MS/MS Analysis
3.2.3. Isolation of Volatiles
3.2.4. GC-MS Analysis
3.3. Cell Viability Assay (MTT)
3.4. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Blažević, I.; Montaut, S.; Burčul, F.; Olsen, C.E.; Burow, M.; Rollin, P.; Agerbirk, N. Glucosinolate structural diversity, identification, chemical synthesis and metabolism in plants. Phytochemistry 2020, 169, 112100. [Google Scholar] [CrossRef]
- Montaut, S.; Read, S.; Blažević, I.; Nuzillard, J.-M.; Roje, M.; Harakat, D.; Rollin, P. Investigation of the glucosinolates in Hesperis matronalis L. and Hesperis laciniata All.: Unveiling 4′-O-β-D-apiofuranosylglucomatronalin. Carbohydr. Res. 2020, 488, 107898. [Google Scholar] [CrossRef] [PubMed]
- Trabelcy, B.; Chinkov, N.; Samuni-Blank, M.; Merav, M.; Izhaki, I.; Carmeli, S.; Gerchman, Y. Investigation of glucosinolates in the desert plant Ochradenus baccatus (Brassicales: Resedaceae). Unveiling glucoochradenin, a new arabinosylated glucosinolate. Phytochemistry 2021, 187, 112760. [Google Scholar] [CrossRef]
- Pagnotta, E.; Agerbirk, N.; Olsen, C.E.; Ugolini, L.; Cinti, S.; Lazzeri, L. Hydroxyl and methoxyl derivatives of benzylglucosinolate in Lepidium densiflorum with hydrolysis to isothiocyanates and non-isothiocyanate products: Substitution governs product type and mass spectral fragmentation. J. Agric. Food Chem. 2017, 65, 3167–3178. [Google Scholar] [CrossRef]
- Blažević, I.; Montaut, S.; Burčul, F.; Rollin, P. Glucosinolates: Novel sources and biological potential. In Glucosinolates, Reference Series in Phytochemistry; Mérillon, J.-M., Ramawat, K.G., Eds.; Springer: Cham, Switzerland, 2017; pp. 3–60. [Google Scholar] [CrossRef]
- Radulović, N.S.; Dekić, M.S.; Stojanović-Radić, Z.Z. Antimicrobial volatile glucosinolate autolysis products from Hornungia petraea (L.) Rchb. (Brassicaceae). Phytochem. Lett. 2012, 5, 351–357. [Google Scholar] [CrossRef]
- De Nicola, G.R.; Nyegue, M.; Montaut, S.; Iori, R.; Menut, C.; Tatibouët, A.; Rollin, P.; Ndoyé, C.; Zollo, P.-H.A. Profile and quantification of glucosinolates in Pentadiplandra brazzeana Baillon. Phytochemistry 2012, 73, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Blažević, I.; Đulović, A.; Čikeš Čulić, V.; Burčul, F.; Ljubenkov, I.; Ruščić, M.; Generalić Mekinić, I. Bunias erucago L.: Glucosinolate profile and in vitro biological potential. Molecules 2019, 24, 741. [Google Scholar] [CrossRef] [Green Version]
- De Nicola, G.R.; Montaut, S.; Rollin, P.; Nyegue, M.; Menut, C.; Iori, R.; Tatibouët, A. Stability of benzylic-type isothiocyanates in hydrodistillation-mimicking conditions. J. Agric. Food Chem. 2013, 61, 137–142. [Google Scholar] [CrossRef]
- Nikolić, T. (2005-Onwards): Flora Croatica Database. Available online: http://hirc.botanic.hr/fcd (accessed on 14 August 2021).
- Stevens, P.F. Angiosperm Phylogeny Website.Version 14. Available online: http://www.mobot.org/MOBOT/research/APweb/ (accessed on 22 July 2021).
- Friis, P.; Kjær, A. Glucolepigramin, a new thioglucoside, present in Lepidium graminifolium L. Acta Chem. Scand. 1963, 17, 1515–1520. [Google Scholar] [CrossRef]
- Bäuerle, R.; Wagner, H.; Schraudolf, H. Distribution of 4-methoxy-3-indolylmethyl-glucosinolate (4-methoxy-glucobrassicin) in Brassicaceae. Experientia 1986, 42, 86. [Google Scholar] [CrossRef]
- Cole, R.A. Isothiocyanates, nitriles and thiocyanates as products of autolysis of glucosinolates in Cruciferae. Phytochemistry 1976, 15, 759–762. [Google Scholar] [CrossRef]
- Daxenbichler, M.E.; Spencer, G.F.; Carlson, D.G.; Rose, G.B.; Brinker, A.M.; Powell, R.G. Glucosinolate composition of seeds from 297 species of wild plants. Phytochemistry 1991, 30, 2623. [Google Scholar] [CrossRef]
- Montaut, S.; Read, S.; Blažević, I.; Nuzillard, J.-M.; Harakat, D.; Rollin, P. Glucosinolates of Lepidium graminifolium L. (Brassicaceae) from Croatia. Nat. Prod. Res. 2019, 33, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Bennett, R.N.; Mellon, F.A.; Kroon, P.A. Screening crucifer seeds as sources of specific intact glucosinolates using ion-pair high-performance liquid chromatography negative ion electrospray mass spectrometry. J. Agric. Food Chem. 2004, 52, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Agerbirk, N.; Warwick, S.I.; Hansen, P.R.; Olsen, C.E. Sinapis phylogeny and evolution of glucosinolates and specific nitrile degrading enzymes. Phytochemistry 2008, 69, 2937–2949. [Google Scholar] [CrossRef]
- Kjær, A.; Schuster, A.; Park, R.J. Glucosinolates in Lepidium species from Queensland. Phytochemistry 1971, 10, 455. [Google Scholar] [CrossRef]
- Kjær, A.; Wagnieres, M. 3,4,5-Trimethoxybenzylglucosinolate: A constituent of Lepidium sordidum. Phytochemistry 1971, 10, 2195. [Google Scholar] [CrossRef]
- Radulović, N.; Zlatković, B.; Skropeta, D.; Palić, R. Chemotaxonomy of the peppergrass Lepidium coronopus (L.) Al-Shehbaz (syn. Coronopus squamatus) based on its volatile glucosinolate autolysis products. Biochem. Syst. Ecol. 2008, 36, 807–811. [Google Scholar] [CrossRef]
- Sønderby, I.E.; Geu-Flores, F.; Halkier, B.A. Biosynthesis of glucosinolates—Gene discovery and beyond. Trends Plant. Sci. 2010, 15, 283–290. [Google Scholar] [CrossRef]
- Mastuo, T.; Miyata, Y.; Yuno, T.; Mukae, Y.; Otsubo, A.; Mitsunari, K.; Ohba, K.; Sakai, H. Molecular mechanisms of the anti-cancer effects of isothiocyanates from cruciferous vegetables in bladder cancer. Molecules 2020, 25, 575. [Google Scholar] [CrossRef] [Green Version]
- Tang, L.; Zhang, Y. Mitochondria are the primary target in isothiocyanate-induced apoptosis in human bladder cancer cells. Mol. Cancer. Ther. 2005, 4, 1250–1259. [Google Scholar] [CrossRef] [Green Version]
- Tang, L.; Li, G.; Song, L.; Zhang, Y. The principal urinary metabolites of dietary isothiocyanates, N-acetylcysteine conjugates, elicit the same anti-proliferative response as their parent compounds in human bladder cancer cells. Anti-Cancer Drugs 2006, 17, 297–305. [Google Scholar] [CrossRef]
- Dinh, T.N.; Parat, M.-O.; Ong, Y.S.; Khaw, K.Y. Anticancer activities of dietary benzyl isothiocyanate: A comprehensive review. Pharmacol. Res. 2021, 169, 105666. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhuang, J.-X.; Wang, Q.; Zhang, H.-Y.; Yang, P. Inhibitory effect of benzyl isothiocyanate on proliferation in vitro of human glioma cells. Asian Pac. J. Cancer Prev. 2013, 14, 2607–2610. [Google Scholar] [CrossRef] [Green Version]
- Tang, N.Y.; Chueh, F.S.; Yu, C.-C.; Liao, C.-L.; Lin, J.-J.; Hsia, T.-C.; Wu, K.-C.; Liu, H.-C.; Lu, K.-W.; Chung, J.-G. Benzyl isothiocyanate alters the gene expression with cell cycle regulation and cell death in human brain glioblastoma GBM 8401 cells. Oncol. Rep. 2016, 35, 2089–2096. [Google Scholar] [CrossRef]
- Shang, H.-S.; Shih, Y.-L.; Lu, T.-J.; Lee, C.-H.; Hsueh, S.-C.; Chou, Y.-C.; Lu, H.-F.; Liao, N.-C.; Chung, J.-G. Benzyl isothiocyanate (BITC) induces apoptosis of GBM 8401 human brain glioblastoma multiforms cells via activation of caspase-8/bid and the reactive oxygen species-dependent mitochondrial pathway. Environ. Toxicol. 2016, 31, 1751–1760. [Google Scholar] [CrossRef] [PubMed]
- Su, J.-C.; Lin, K.; Wang, Y.; Sui, S.-H.; Gao, Z.-Y.; Wang, Z.-G. In vitro studies of phenethyl isothiocyanate against the growth of LN229 human glioma cells. Int. J. Clin. Exp. Pathol. 2015, 8, 4269–4276. [Google Scholar] [PubMed]
- Blažević, I.; Đulović, A.; Čikeš Čulić, V.; Popović, M.; Guillot, X.; Burčul, F.; Rollin, P. Microwave-assisted versus conventional isolation of glucosinolate degradation products from Lunaria annua L. and their cytotoxic activity. Biomolecules 2020, 10, 215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popović, M.; Maravić, A.; Čikeš Čulić, V.; Đulović, A.; Burčul, F.; Blažević, I. Biological effects of glucosinolate degradation products from horseradish: A horse that wins the race. Biomolecules 2020, 10, 343. [Google Scholar] [CrossRef] [Green Version]
- Grosser, K.; van Dam, N.M.A. Straightforward method for glucosinolate extraction and analysis with high-pressure liquid chromatography (HPLC). J. Vis. Exp. 2017, 121, e55425. [Google Scholar] [CrossRef] [Green Version]
- Wathelet, J.-P.; Iori, R.; Leoni, O.; Quinsac, A.; Palmieri, S.; Rollin, P. Guidelines for glucosinolate analysis in green tissues used for biofumigation. Agroindustria. 2004, 3, 257–344. [Google Scholar]
- Al-Gendy, A.A.; Nematallah, K.A.; Zaghloul, S.S.; Ayoub, N.A. Glucosinolates profile, volatile constituents, antimicrobial, and cytotoxic activities of Lobularia libyca. Pharm. Biol. 2016, 54, 3257–3263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Šimat, V.; Vlahović, J.; Soldo, B.; Generalić Mekinić, I.; Čagalj, M.; Hamed, I.; Skroza, D. Production and characterization of crude oils from seafood processing by-products. Food Biosci. 2020, 33, 100484. [Google Scholar] [CrossRef]
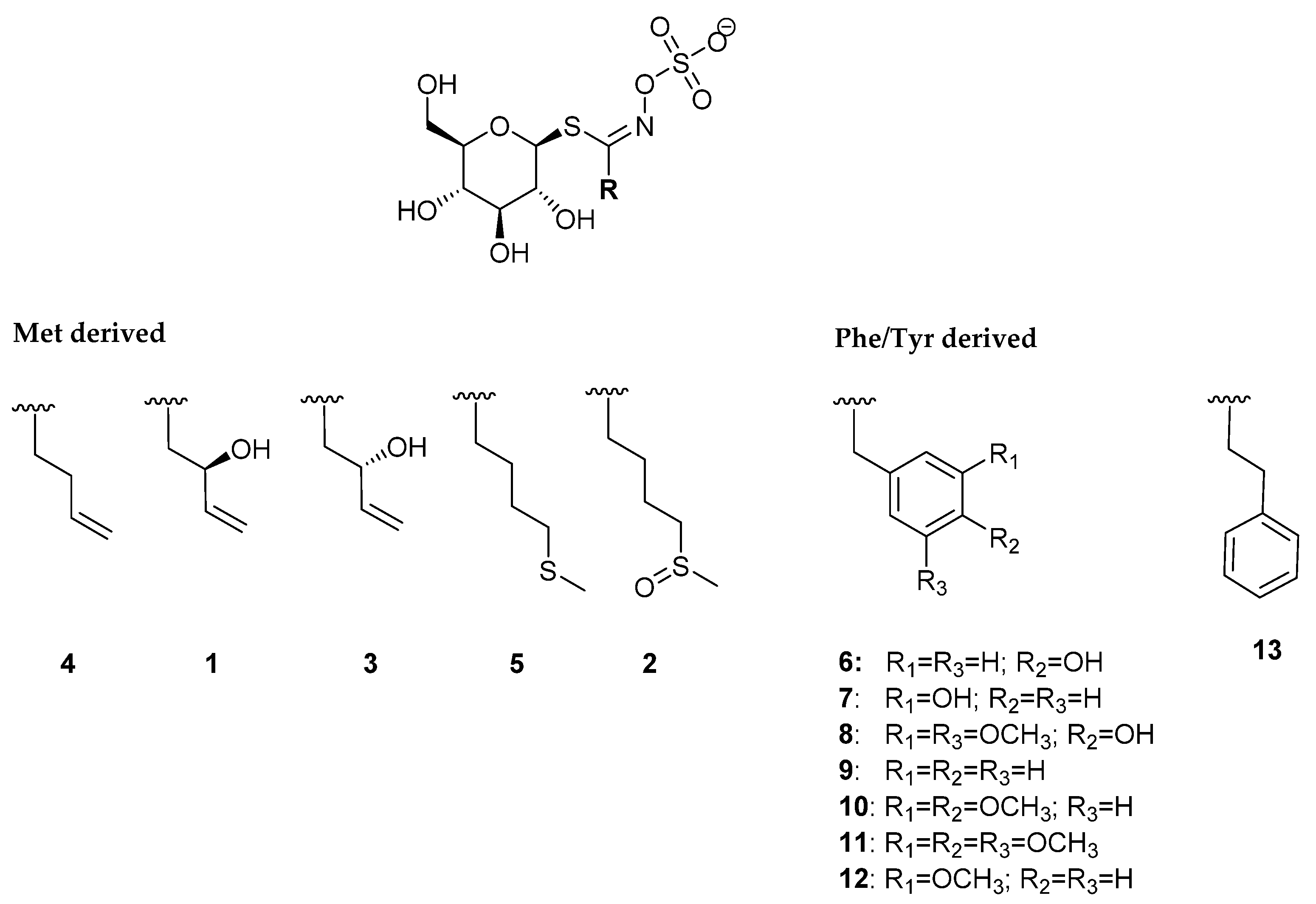
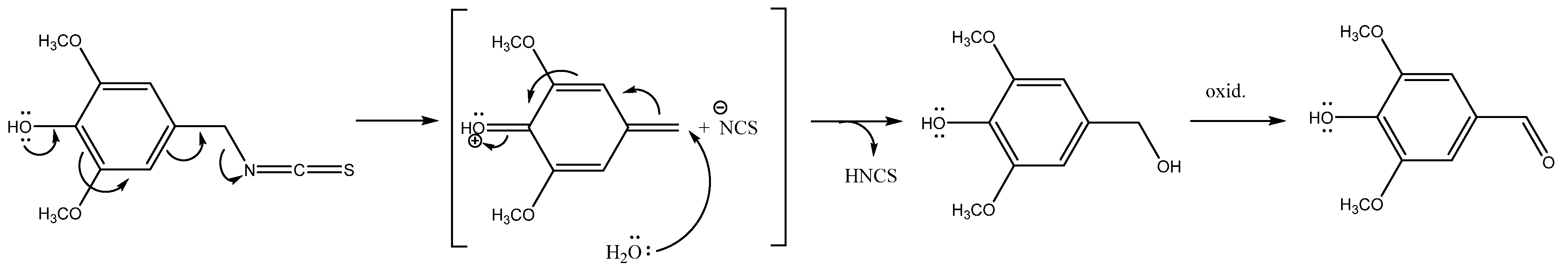
| Glucosinolate (GSL) (Trivial Name) | [M + Na]+ | Glucosinolates µmol/g DW | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Rab Island | Split | ||||||||
| Arial Part | Flower | Leaf | Stem | Siliquae | Root | ||||
| Met derived | |||||||||
| 1 | (2R)-Hydroxybut-3-enyl GSL (progoitrin) | 332 | 0.26 ± 0.02 A | - | - | - | - | - | |
| 2 | 4-(Methylsulfinyl)butyl GSL (glucoraphanin) | 380 | - | 7.32 ± 1.67 A, V | 3.49 ± 0.34 A, W | - | 4.00 ± 0.50 A, X | - | |
| 3 | (2S)-Hydroxybut-3-enyl GSL (epiprogoitrin) | 332 | 1.56 ± 0.11 B | - | - | - | - | - | |
| 4 | But-3-enyl GSL (gluconapin) | 316 | tr | - | - | - | - | - | |
| 5 | 4-(Methylsulfanyl)butyl GSL (glucoerucin) | 364 | - | - | - | - | - | tr | |
| Phe/Tyr derived | 6 | 4-Hydroxybenzyl GSL (glucosinalbin) | 368 | 0.19 ± 0.06 A, D, V | 2.15 ± 0.33 B, W | tr | tr | 3.92 ± 0.38 A, X | 1.62 ± 0.20 A, Y |
| 7 | 3-Hydroxybenzyl GSL (glucolepigramin) | 368 | 3.03 ± 0.23 C, V | 68.60 ± 7.30 C, W | 19.30 ± 0.20 B, X | 6.43 ± 1.33 A, V | 75.82 ± 8.06 B, W | 20.56 ± 1.78 B, X | |
| 8 | 4-Hydroxy-3,5-dimethoxybenzyl GSL (3,5-dimethoxysinalbin) | 428 | tr | 11.12 ± 1.86 A, D, V | 14.88 ± 0.05 C, W | 3.04 ± 0.85 B, X | 12.04 ± 0.21 C, V | 12.49 ± 1.48 C, V | |
| 9 | Benzyl GSL (glucotropaeolin) | 352 | 0.12 ± 0.00 D, V | 1.80 ± 0.44 B, V, W | tr | 0.47 ± 0.08 C, V | 5.14 ± 0.23 A, W | 61.69 ± 5.82 D, X | |
| 10 | 3,4-Dimethoxybenzyl GSL | 412 | tr | - | - | - | - | tr | |
| 11 | 3,4,5-Trimethoxybenzyl GSL | 442 | tr | 13.17 ± 2.64 D, V | 3.42 ± 0.08 A, W | 0.87 ± 0.00 C, X | 11.03 ± 0.31 C, V | 35.20 ± 4.33 E, Y | |
| 12 | 3-Methoxybenzyl GSL (glucolimnanthin) | 382 | 1.24 ± 0.10 E, V | - | - | tr | 11.95 ± 0.29 C, W | 18.18 ± 1.51 B, X | |
| 13 | 2-Phenylethyl GSL (gluconasturtiin) | 366 | tr | - | - | - | - | - | |
| Total (µmol/g DW) | 6.40 ± 0.52 V | 104.16 ± 14.24 W | 41.09 ± 0.67 X | 10.81 ± 2.26 V | 123.90 ± 9.98 Y | 149.74 ± 15.12 Z | |||
| Parent Glucosinolate Identified Breakdown Compound | RI | Rab Island | Split | |||||
|---|---|---|---|---|---|---|---|---|
| HD | EXT | EXT | ||||||
| Aerial Part | Aerial Part | Leaf | Stem | Siliquae | Root | |||
| Met derived | 4-(Methylsulfinyl)butyl GSL (glucoraphanin, 2) 5-(Methylsulfinyl)pentanenitrile a, b 4-(Methylsulfinyl)butyl ITC (sulforaphane) a, b | |||||||
| 1512 | - | - | 1.68 | 2.34 | - | - | ||
| 1760 | - | - | 71.74 | 52.70 | 30.34 | 0.83 | ||
| 4-(Methylsulfanyl)butyl GSL (glucoerucin, 5) | ||||||||
| 4-(Methylsulfanyl)butyl ITC (erucin) a, b | 1433 | - | - | - | - | - | 2.29 | |
| Phe/Tyr derived | 3-Hydroxybenzyl GSL (glucolepigramin, 7) | |||||||
| 3-Hydroxyphenylacetonitrile b, c | 1483 | - | - | tr | 4.71 | - | - | |
| 3-Hydroxybenzyl ITC b, c | 1701 | - | - | 3.19 | 5.14 | 20.15 | 8.61 | |
| 4-Hydroxy-3,5-dimethoxybenzyl GSL (3,5-dimethoxysinalbin, 8) | ||||||||
| 4-Hydroxy-3,5- dimethoxyphenylacetonitrilec | 1790 | - | - | 2.94 | 8.20 | - | - | |
| 4-Hydroxy-3,5-dimethoxybenzyl ITC c | 2017 | - | - | 0.98 | - | 0.43 | - | |
| Benzyl GSL (glucotropaeolin, 9) | ||||||||
| Phenylacetonitrile a, b | 1141 | 85.72 | 18.69 | tr | 2.52 | - | 0.31 | |
| Benzyl ITC a, b | 1365 | 6.04 | 0.53 | - | - | tr | 27.88 | |
| 3,4-Dimethoxybenzyl GSL (10) | ||||||||
| 3,4-Dimethoxybenzyl ITC b, c | 1822 | - | - | - | - | - | tr | |
| 3,4,5-Trimethoxybenzyl GSL (11) | ||||||||
| 3,4,5-Trimethoxybenzyl ITC b, c | 1961 | - | - | 1.80 | 3.06 | 4.95 | 18.71 | |
| 3-Methoxybenzyl GSL (glucolimnanthin, 12) | ||||||||
| 3-Methoxyphenylacetonitrile b, c | 1377 | 1.69 | 29.05 | - | 0.96 | - | tr | |
| 3-Methoxybenzyl ITC b, c | 1601 | 1.07 | 3.83 | - | - | 0.90 | 13.92 | |
| 2-Phenylethyl GSL (gluconasturtiin, 13) | ||||||||
| 3-Phenylpropanenitrile a, b | 1242 | - | 1.02 | - | - | - | - | |
| 2-Phenylethyl ITC a, b | 1466 | tr | 1.46 | - | - | - | - | |
| Other volatiles | ||||||||
| Benzaldehyde a, b | 963 | 1.97 | - | tr | 0.71 | 0.53 | 0.32 | |
| Benzyl alcohol a, b | 1036 | - | - | - | 0.96 | 0.80 | 0.58 | |
| 3-Methoxybenzaldehyde b | 1198 | - | 4.57 | - | - | - | - | |
| 2-Methoxy-4-allylphenol (eugenol) a, b | 1358 | - | - | 1.60 | 8.28 | 8.04 | 5.48 | |
| β-Selinene a, b | 1486 | - | 14.16 | - | - | - | - | |
| 4-Hydroxy-3,5-dimethoxybenzaldehydeb | 1659 | - | - | 1.44 | 1.70 | - | tr | |
| Caffeine a, b | 1839 | - | - | 7.96 | 8.20 | 30.55 | 18.71 | |
| 6,10,14-Trimethylpentadecan-2-one a, b | 1846 | - | 17.46 | - | - | - | - | |
| Hexadecanoic acid a, b | 1964 | - | 5.67 | - | - | - | - | |
| Ethylhexadecanoate a, b | 1995 | - | 1.87 | - | - | - | - | |
| Total (%) | 96.49 | 98.31 | 93.33 | 99.48 | 96.69 | 97.64 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Đulović, A.; Burčul, F.; Čulić, V.Č.; Ruščić, M.; Brzović, P.; Montaut, S.; Rollin, P.; Blažević, I. Lepidium graminifolium L.: Glucosinolate Profile and Antiproliferative Potential of Volatile Isolates. Molecules 2021, 26, 5183. https://doi.org/10.3390/molecules26175183
Đulović A, Burčul F, Čulić VČ, Ruščić M, Brzović P, Montaut S, Rollin P, Blažević I. Lepidium graminifolium L.: Glucosinolate Profile and Antiproliferative Potential of Volatile Isolates. Molecules. 2021; 26(17):5183. https://doi.org/10.3390/molecules26175183
Chicago/Turabian StyleĐulović, Azra, Franko Burčul, Vedrana Čikeš Čulić, Mirko Ruščić, Petra Brzović, Sabine Montaut, Patrick Rollin, and Ivica Blažević. 2021. "Lepidium graminifolium L.: Glucosinolate Profile and Antiproliferative Potential of Volatile Isolates" Molecules 26, no. 17: 5183. https://doi.org/10.3390/molecules26175183
APA StyleĐulović, A., Burčul, F., Čulić, V. Č., Ruščić, M., Brzović, P., Montaut, S., Rollin, P., & Blažević, I. (2021). Lepidium graminifolium L.: Glucosinolate Profile and Antiproliferative Potential of Volatile Isolates. Molecules, 26(17), 5183. https://doi.org/10.3390/molecules26175183











