Effects of Combination Treatments with Astaxanthin-Loaded Microparticles and Pentoxifylline on Intracellular ROS and Radiosensitivity of J774A.1 Macrophages
Abstract
:1. Introduction
2. Results
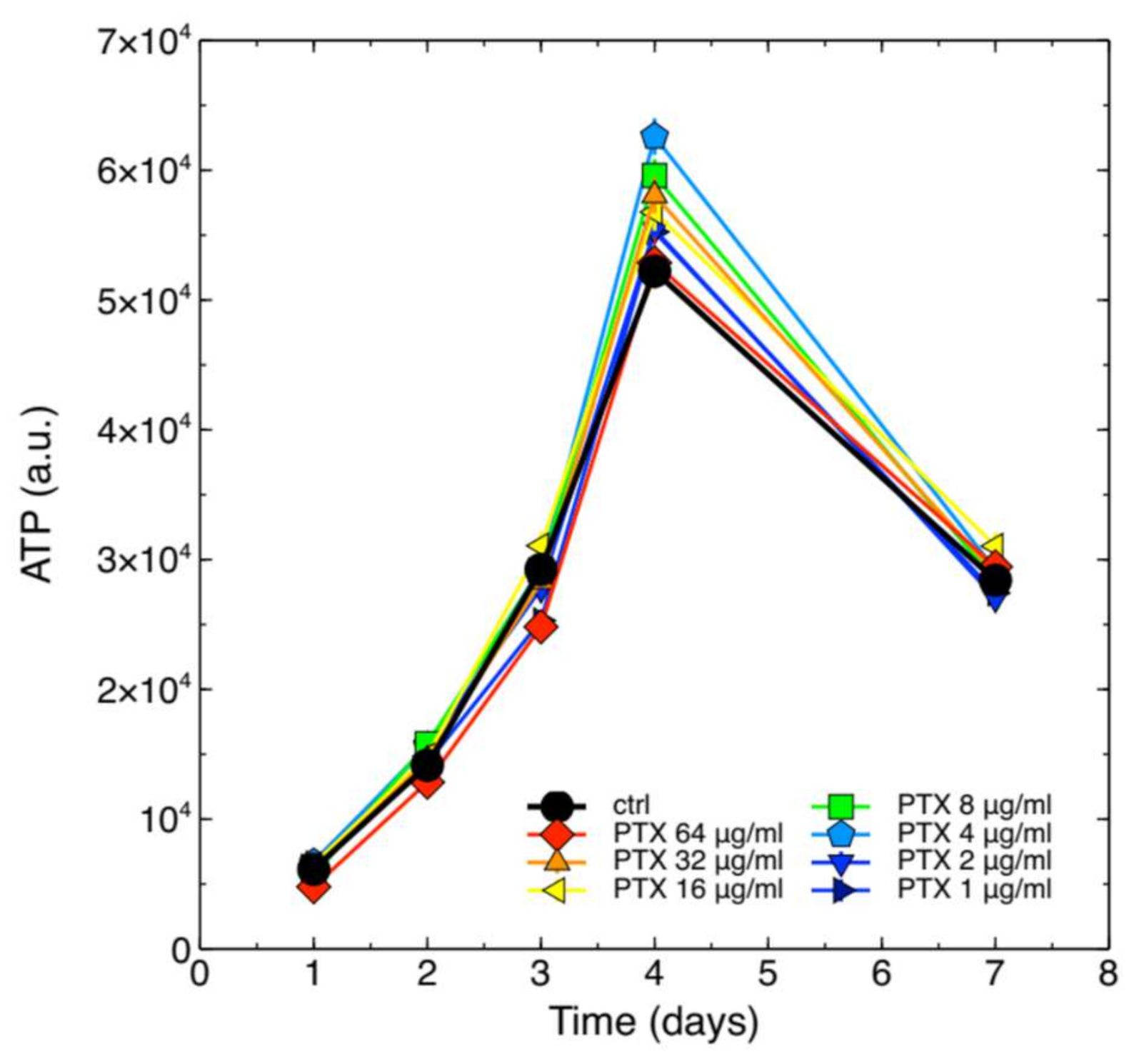
2.1. Cytotoxicity of PTX on J774A.1 Macrophages
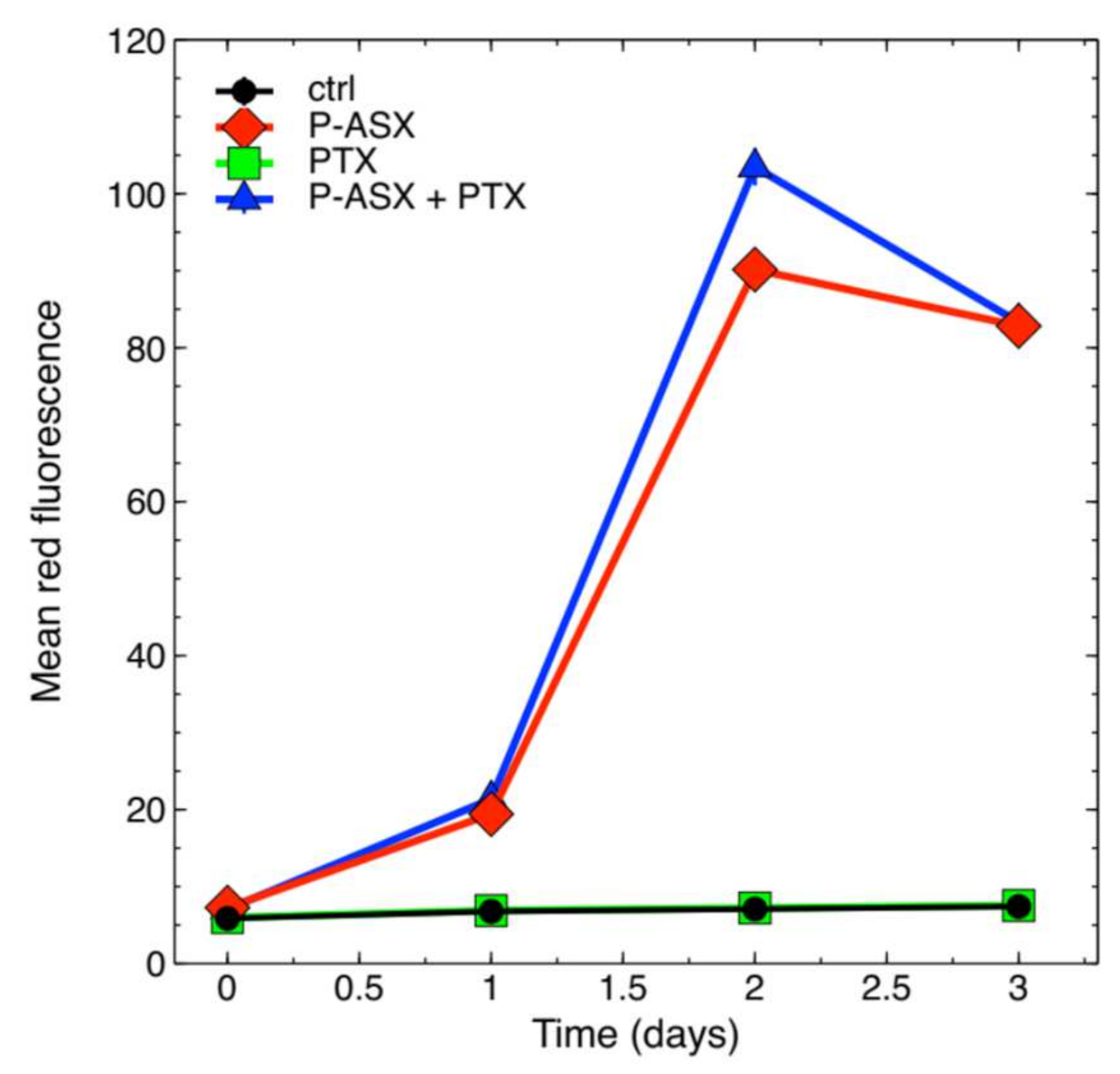
2.2. PTX Does Not Alter Phagocytosis of ASX-Loaded Microparticles
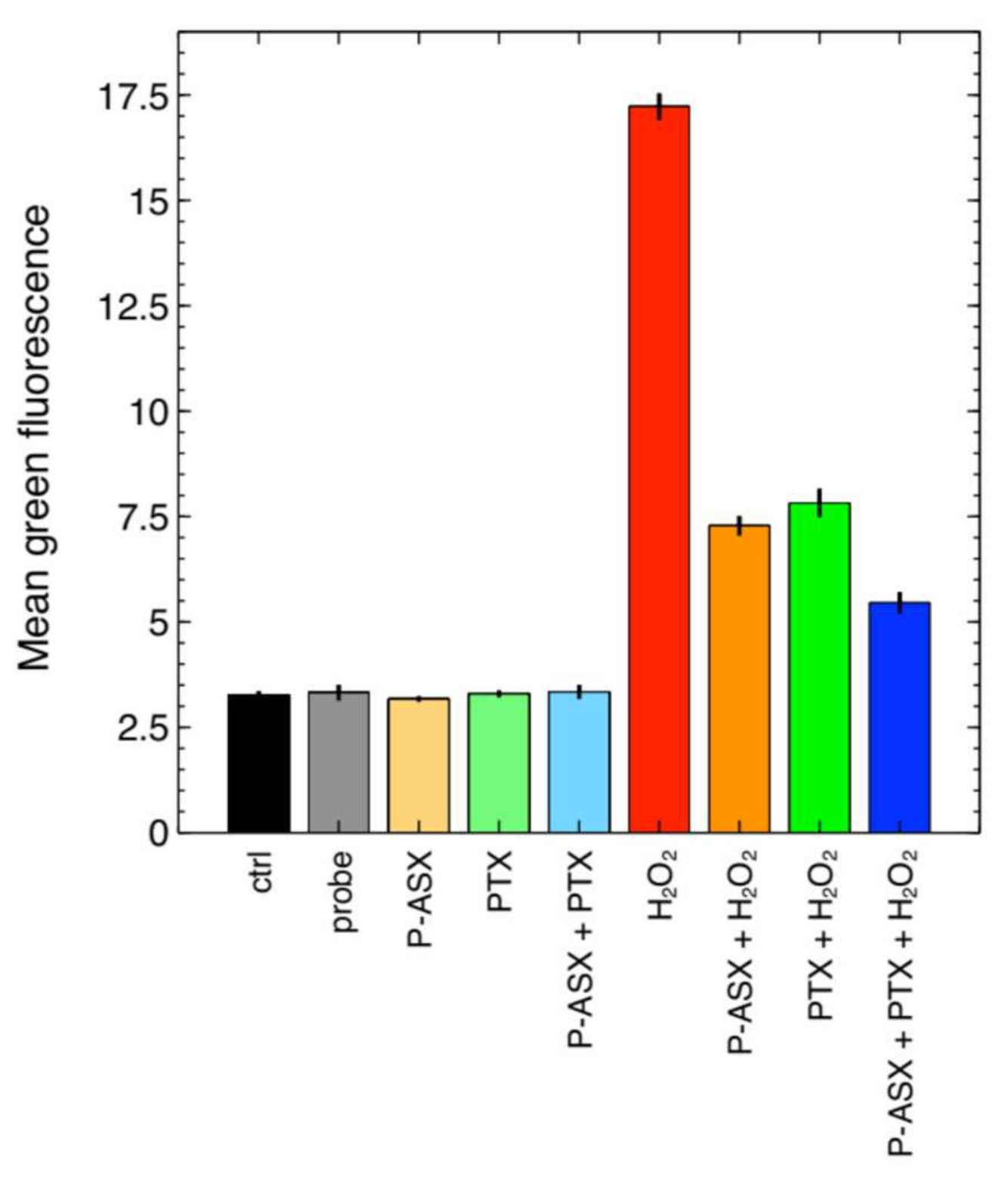
2.3. Effects of PTX and ASX Microparticles on Intracellular ROS Levels
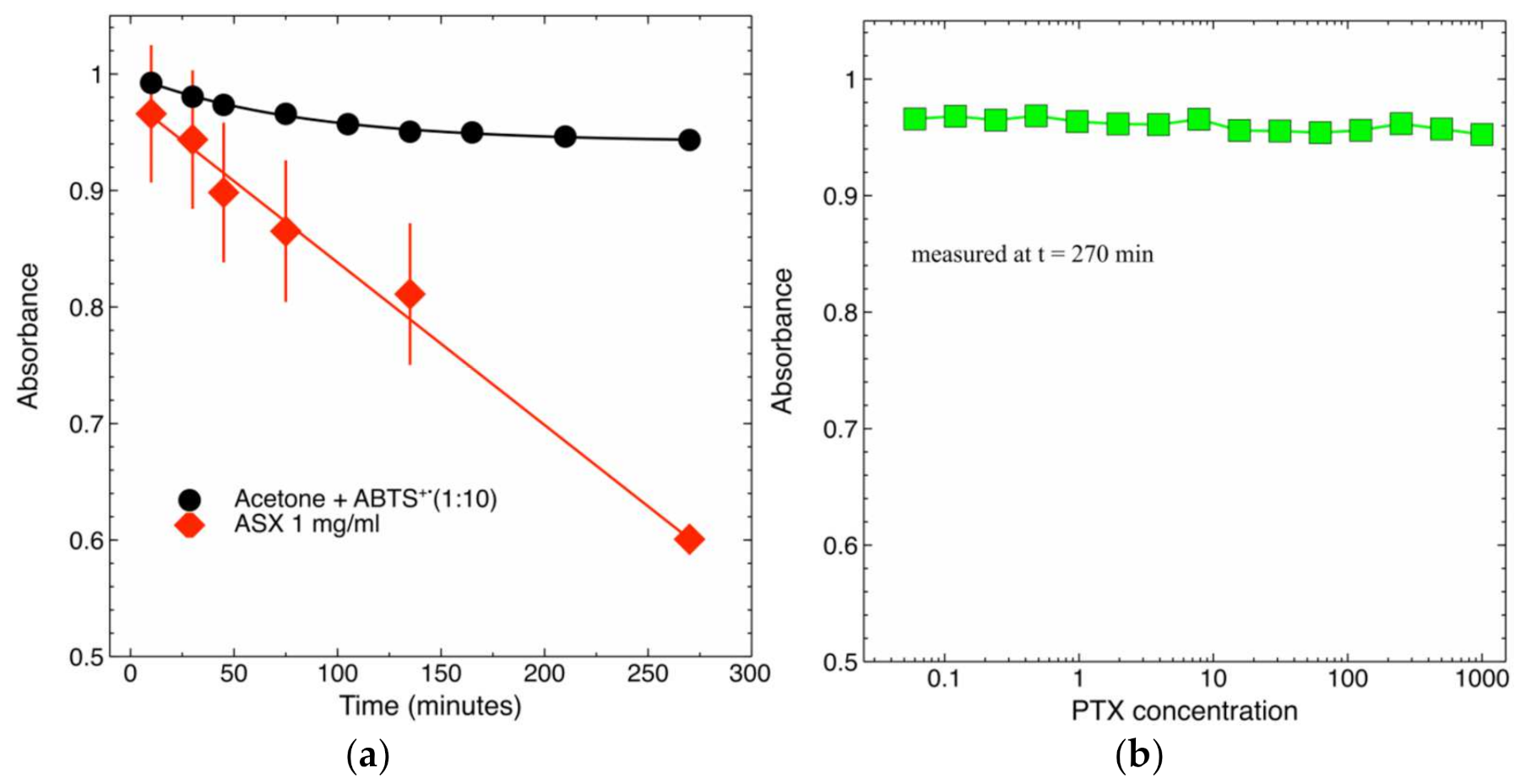
2.4. Radical-Scavenging Activity of PTX and ASX-Containing Extract in a Cell-Free Assay
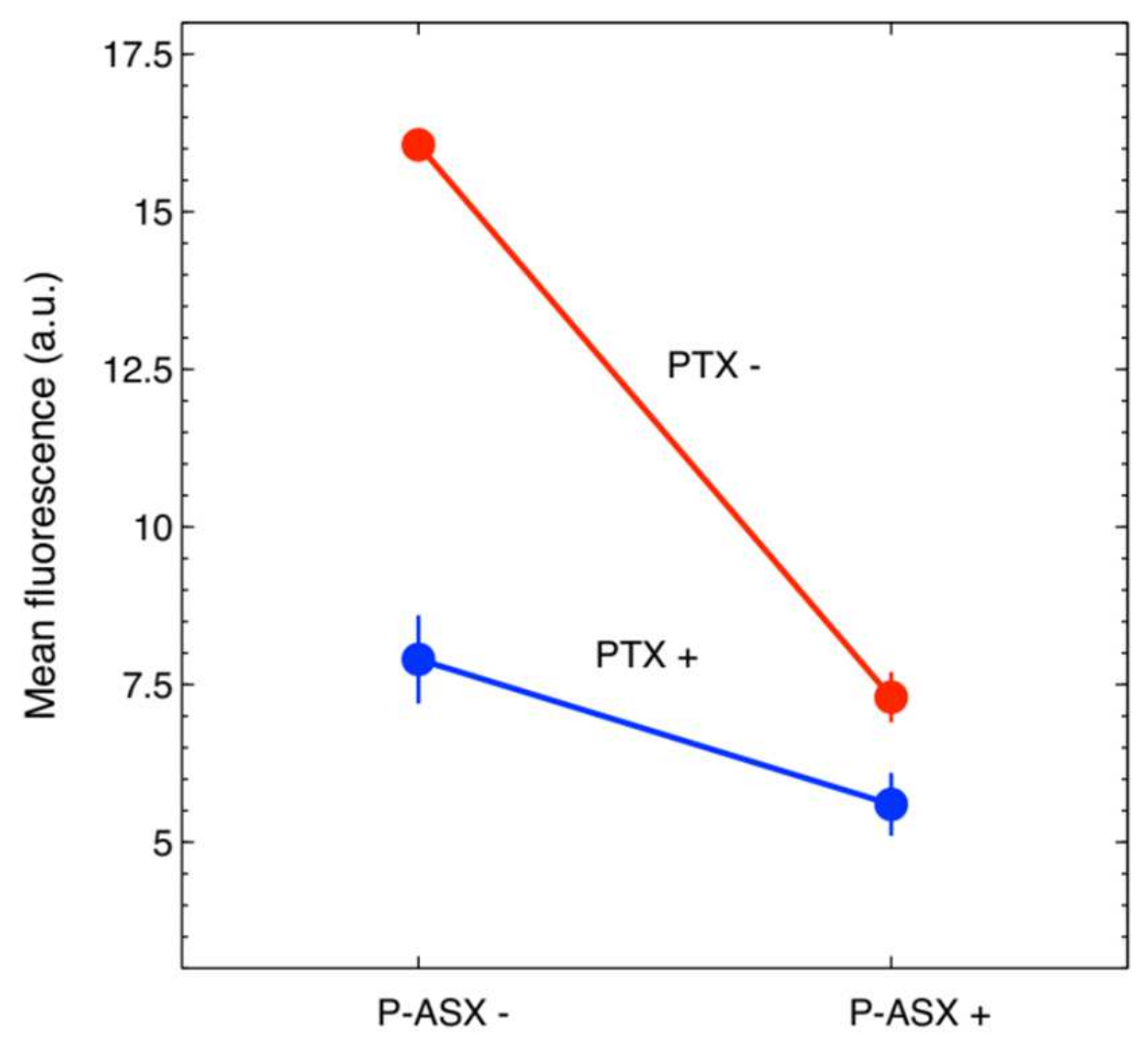
2.5. Effects of PTX and ASX Microparticles on Irradiated Cells
3. Discussion
4. Materials and Methods
4.1. Pentoxifylline and Astaxanthin Loaded Microparticles
4.2. Cells and Cell Culture
4.3. Cytotoxicity Assay
4.4. Flow Cytometry
4.5. Phagocytosis Kinetics
4.6. Intracellular ROS Detection
4.7. Free Radical Scavenging: ABTS Test
4.8. Irradiation of Cell Samples
4.9. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Hall, S.; Rudrawar, S.; Zunk, M.; Bernaitis, N.; Arora, D.; McDermott, C.M.; Anoopkumar-dukie, S. Protection against radiotherapy-induced toxicity. Antioxidants 2016, 5, 22. [Google Scholar] [CrossRef] [Green Version]
- Chiao, T.B.; Lee, A.J. Role of pentoxifylline and vitamin E in attenuation of radiation-induced fibrosis. Ann. Pharmacother. 2005, 39, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Delanian, S.; Lefaix, J. Current management for late normal tissue injury: Radiation-induced fibrosis and necrosis. Semin. Radiat. Oncol. 2006, 17, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Delanian, S.; Lefaix, J. The radiation-induced fibroatrophic process: Therapeutic perspective via the antioxidant pathway. Radiother. Oncol. 2004, 73, 119–131. [Google Scholar] [CrossRef]
- Lech, M.; Anders, H. Macrophages and fibrosis: How resident and infiltrating mononuclear phagocytes orchestrate all phases of tissue injury and repair. Biochim. Biophys. Acta 2012, 1832, 989–997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pareek, P.; Samdariya, S.; Sharma, A.; Gupta, N.; Shekhar, S.; Kirubakaran, R. Pentoxifylline and vitamin E alone or in combination for preventing and treating side effects of radiation therapy and concomitant chemoradiotherapy. Cochrane Database Syst. Rev. 2016, 2016, CD012117. [Google Scholar] [CrossRef]
- Wen, W.X.; Lee, S.Y.; Siang, R.; Koh, R.Y. Repurposing pentoxifylline for the treatment of fibrosis: An overwiew. Adv. Ther. 2017, 34, 1245–1269. [Google Scholar] [CrossRef]
- Okunieff, P.; Augustine, E.; Hicks, J.E.; Cornelison, T.L.; Altemus, R.M.; Naydich, B.G.; Ding, I.; Huser, A.K.; Abraham, E.H.; Smith, J.J.; et al. Pentoxifylline in the treatment of radiation-induced fibrosis. J. Clin. Oncol. 2004, 22, 2207–2213. [Google Scholar] [CrossRef] [PubMed]
- Delanian, S.; Porcher, R.; Balla-Mekias, S.; Lefaix, J. Randomised, placebo-controlled trial of combined pentoxifylline and tocopherol for regression of superficial radiation-induced fibrosis. J. Clin. Oncol. 2003, 21, 2545–2550. [Google Scholar] [CrossRef]
- Fakhria, S.; Abbaszadehb, F.; Dargahic, L.; Jorjania, M. Astaxanhin: A mechanistic review on its biological activities and health benefits. Pharmacol. Res. 2018, 136, 1–20. [Google Scholar] [CrossRef]
- Binatti, E.; Zoccatelli, G.; Zanoni, F.; Donà, G.; Mainente, F.; Chignola, R. Phagocytosis of astaxanthin-loaded microparticles modulates TGFβ production and intracellular ROS levels in J774A.1 macrophages. Mar. Drugs 2021, 19, 163. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.M.; Desai, L.P. Reciprocal regulation of TGF-β and reactive oxygen species: A perverse cycle for fibrosis. Redox. Biol. 2015, 6, 565–577. [Google Scholar] [CrossRef] [Green Version]
- Bessler, H.; Gilgal, R.; Djaldetti, M.; Zahavi, I. Effect of pentoxifylline on the phagocytic activity, cAMP levels, and superoxide anion production by monocytes and polymorphonuclear cells. J. Leukoc Biol. 1986, 40, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Kim, S.M.; Cho, Y.J.; Eo, M.Y.; Lee, S.K.; Woo, K.M. New approach for the treatment of osteoradionecrosis with pentoxifylline and tocopherol. Biomater. Res. 2014, 18, 13. [Google Scholar] [CrossRef] [Green Version]
- Slinker, B.K. The statistics of synergism. J. Mol. Cell. Cardiol. 1998, 30, 723–731. [Google Scholar] [CrossRef]
- Kobayashi, M.; Takizono, T.; Nishio, N.; Nagai, S.; Kurimura, Y.; Tsuji, Y. Antioxidant role of astaxanthin in the green alga Haematococcus pluvialis. Appl. Microbiol. 1997, 48, 351–356. [Google Scholar] [CrossRef]
- Mishra, K.; Ojha, H.; Chaudhury, N.K. Estimation of antiradical properties of antioxidants using DPPH assay: A critical review and results. Food Chem. 2012, 130, 1036–1043. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects–A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Azzam, E.; Jay Gerin, J.P.; Pain, D. Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer Lett. 2012, 327, 48–60. [Google Scholar] [CrossRef] [Green Version]
- Franken, N.A.P.; Rodermond, H.M.; Stap, J.; Haveman, J.; Van Bree, C. Clonogenic assay of cells in vitro. Nat. Protoc. 2006, 1, 2315–2319. [Google Scholar] [CrossRef] [PubMed]
- Chignola, R.; Sega, M.; Molesini, B.; Baruzzi, A.; Stella, S.; Milotti, E. Collective radioresistance of T47D breast carcinoma cells is mediated by a Syncytin-1 homologous protein. PLoS ONE 2019, 14, e0206713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, V.; Altavilla, D.; Bitto, A. Oxidative stress: Harms and benefits for human health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Pouget, J.; Georgakilas, A.G.; Ravanat, J. Targeted and off-target (bystander and abscopal) effects of radiation therapy: Redox mechanisms and risk/benefit analysis. Antioxid. Redox Signal. 2018, 29, 1447–1487. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ranjbar, A.; Ghahremani, M.H.; Sharifzadeh, M.; Golestani, A.; Ghazi-Khansari, M.; Baeeri, M.; Abdollahi, M. Protection by pentoxifylline of malathion-induced toxic stress and mithocondrial damage in rat brain. Hum. Exp. Toxicol. 2010, 29, 851–864. [Google Scholar] [CrossRef] [PubMed]

| Treatment | df 1 | SS | MS | F | p |
|---|---|---|---|---|---|
| P-ASX | 1 | 97.4 | 97.4 | 381.9 | 1.8 × 10−10 |
| PTX | 1 | 122.1 | 122.1 | 478.9 | 4.9 × 10−11 |
| PTX and ASX | 1 | 41.2 | 41.2 | 161.4 | 2.6 × 10−8 |
| Error | 12 | 3.1 | 0.255 | ||
| Total | 15 | 263.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Binatti, E.; Zoccatelli, G.; Zanoni, F.; Donà, G.; Mainente, F.; Chignola, R. Effects of Combination Treatments with Astaxanthin-Loaded Microparticles and Pentoxifylline on Intracellular ROS and Radiosensitivity of J774A.1 Macrophages. Molecules 2021, 26, 5152. https://doi.org/10.3390/molecules26175152
Binatti E, Zoccatelli G, Zanoni F, Donà G, Mainente F, Chignola R. Effects of Combination Treatments with Astaxanthin-Loaded Microparticles and Pentoxifylline on Intracellular ROS and Radiosensitivity of J774A.1 Macrophages. Molecules. 2021; 26(17):5152. https://doi.org/10.3390/molecules26175152
Chicago/Turabian StyleBinatti, Eleonora, Gianni Zoccatelli, Francesca Zanoni, Giulia Donà, Federica Mainente, and Roberto Chignola. 2021. "Effects of Combination Treatments with Astaxanthin-Loaded Microparticles and Pentoxifylline on Intracellular ROS and Radiosensitivity of J774A.1 Macrophages" Molecules 26, no. 17: 5152. https://doi.org/10.3390/molecules26175152
APA StyleBinatti, E., Zoccatelli, G., Zanoni, F., Donà, G., Mainente, F., & Chignola, R. (2021). Effects of Combination Treatments with Astaxanthin-Loaded Microparticles and Pentoxifylline on Intracellular ROS and Radiosensitivity of J774A.1 Macrophages. Molecules, 26(17), 5152. https://doi.org/10.3390/molecules26175152







