High-Voltage Polyanion Positive Electrode Materials
Abstract
1. Introduction
2. Toward Higher Voltage
2.1. Inductive Effect in Polyanionic Compounds
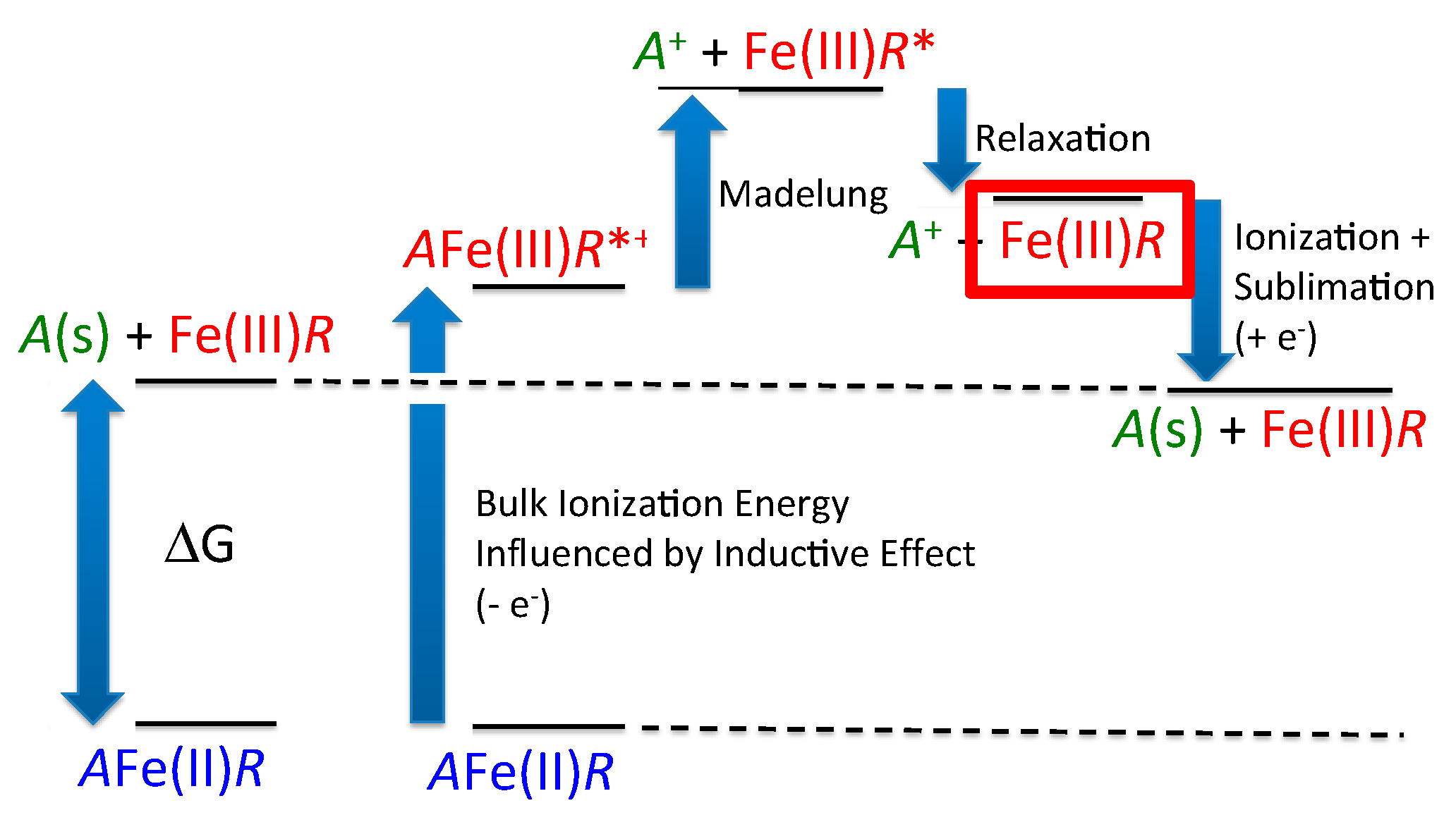
2.2. Thermodynamic Modification
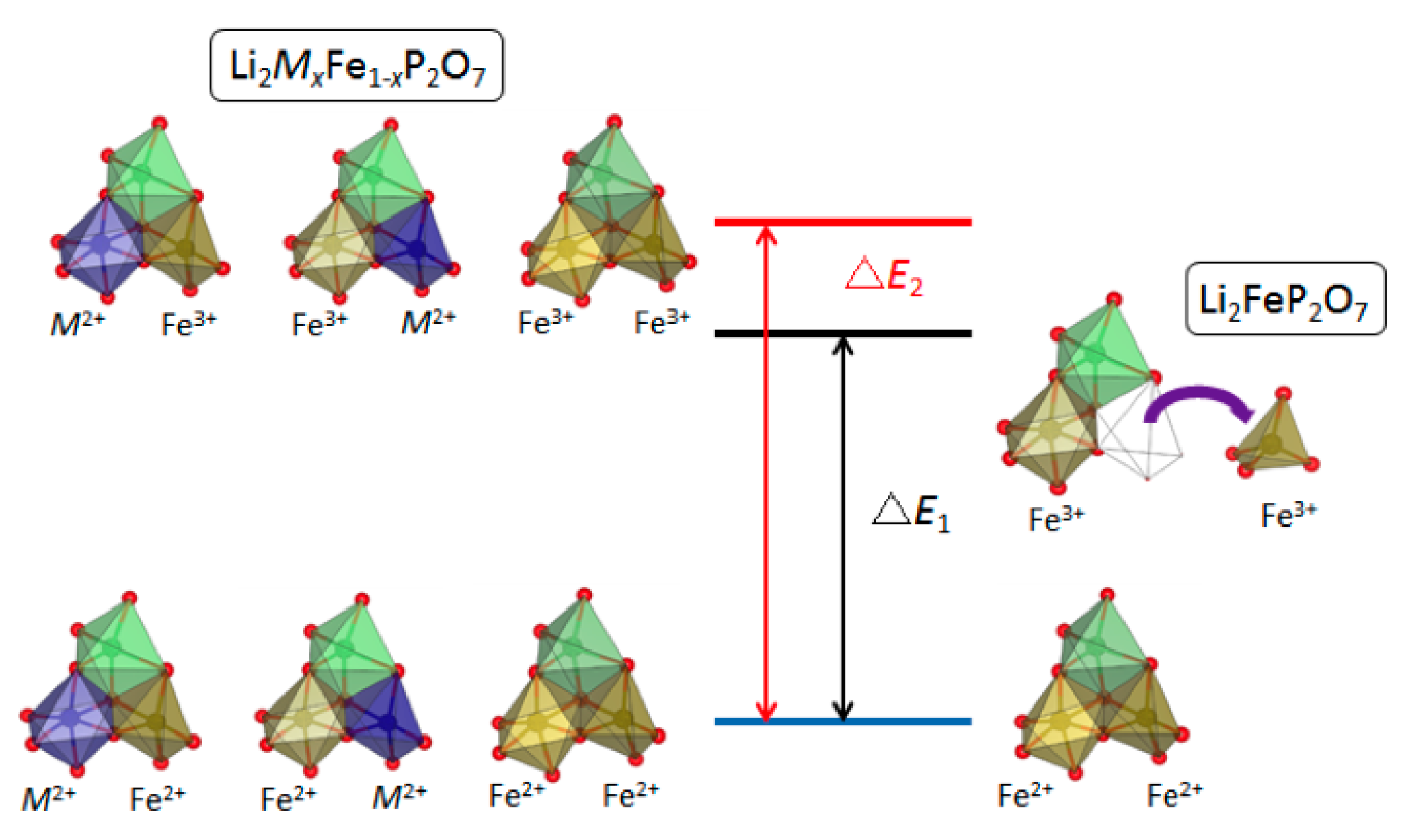
2.3. Choice of Transition Metals
3. High-Voltage System with Fe3+/Fe2+ and Cr4+/Cr3+
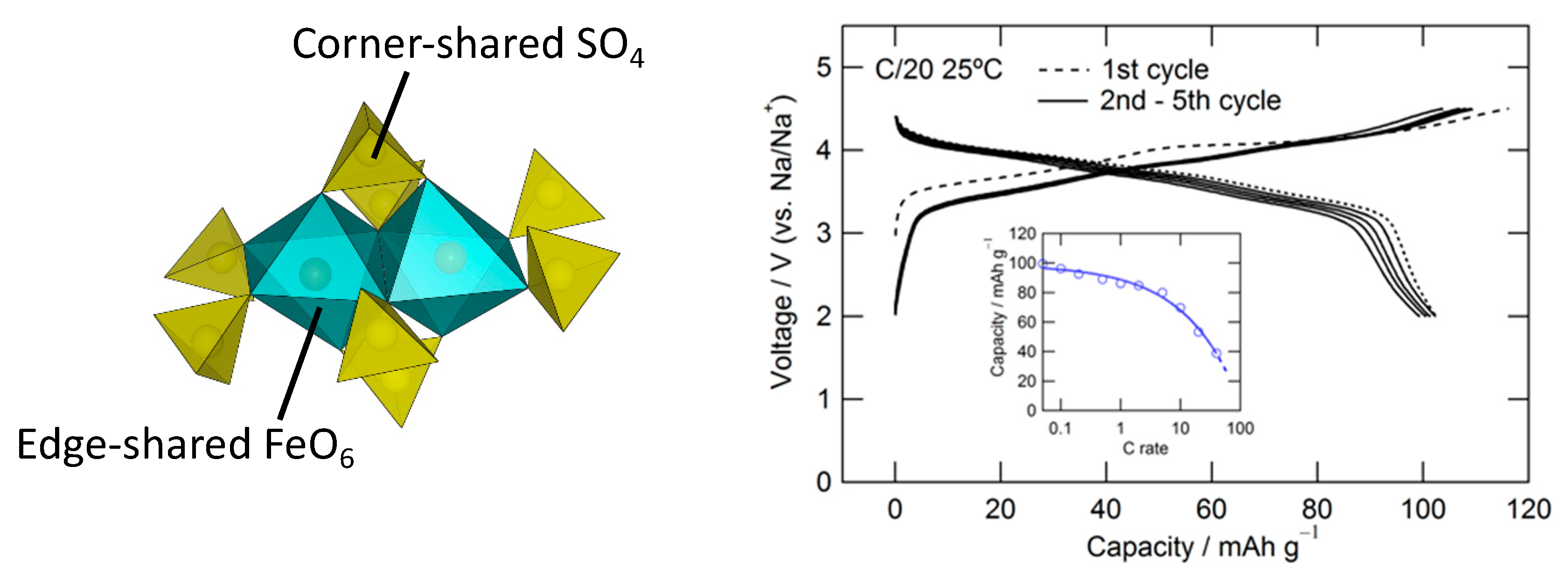
3.1. Pyrophosphates
3.2. Alluaudites
3.3. β1-Na3Al2(PO4)2F3-Type Fluoride Phosphates
4. Summary and Perspective
Funding
Conflicts of Interest
Sample Availability
References
- Nadiri, A.; Delmas, C.; Salmon, R.; Hagenmuller, P. Chemical and electrochemical alkali metal intercalation in the 3D framework of Fe2(MoO4)3. Rev. Chim. Minérale 1984, 21, 537–544. [Google Scholar]
- Manthiram, A.; Goodenough, J.B. Lithium insertion into Fe2(MO4)3 frameworks: Comparison of M = W with M. J. Solid State Chem. 1987, 71, 349–360. [Google Scholar] [CrossRef]
- Delmas, C.; Cherkaoui, F.; Nadiri, A.; Hagenmuller, P. A Nasicon-type phase as intercalation electrode: NaTi2(PO4)3. Mat. Res. Bull. 1987, 22, 631–639. [Google Scholar] [CrossRef]
- Padhi, A.K.; Nanjundaswamy, K.S.; Goodenough, J.B. Phospho-olivines as positive-electrode materials for rechargeable lithium batteries. J. Electrochem. Soc. 1997, 144, 1188–1194. [Google Scholar] [CrossRef]
- Yamada, A.; Chung, S.-C.; Hinokuma, K. Optimized LiFePO4 for lithium battery cathodes. J. Electrochem. Soc. 2001, 148, A224–A229. [Google Scholar] [CrossRef]
- Yonemura, M.; Yamada, A.; Takei, Y.; Sonoyama, N.; Kanno, R. Comparative kinetic study of olivine Lix MPO 4 (M=Fe, Mn). J. Electrochem. Soc. 2004, 151, A1352–A1356. [Google Scholar] [CrossRef]
- Delacourt, C.; Laffont, L.; Bouchet, R.; Wurm, C.; Leriche, J.-B.; Morcrette, M.; Tarasco, J.-M.; Masqueliera, C. Toward understanding of electrical limitations (electronic, ionic) in LiMPO4 (M = Fe, Mn) electrode materials. J. Electrochem. Soc. 2005, 152, A913–A921. [Google Scholar] [CrossRef]
- Yamada, A.; Takei, Y.; Koizumi, H.; Sonoyama, N.; Kanno, R.; Ito, K.; Yonemura, M.; Kamiyama, T. Electrochemical, magnetic, and structural investigation of the lix(MnyFe1-y)PO4 olivine phases. Chem. Mater. 2006, 18, 804–813. [Google Scholar] [CrossRef]
- Barker, J.; Saidi, M.Y.; Swoyer, J.L. Lithium Iron(II) Phospho-olivines prepared by a novel carbothermal reduction method. Electrochem. Solid State Lett. 2003, 6, A53–A55. [Google Scholar] [CrossRef]
- Ellis, B.L.; Ramesh, T.N.; Davis, L.J.M.; Goward, G.R.; Nazar, L.F. Structure and electrochemistry of two-electron redox couples in lithium metal fluorophosphates based on the tavorite structure. Chem. Mater. 2011, 23, 5138–5148. [Google Scholar] [CrossRef]
- Chayambuka, K.; Mulder, G.; Danilov, D.L.; Notten, P.H.L. From Li-ion batteries toward Na-ion chemistries: Challenges and opportunities. Adv. Energy Mater. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Amine, K.; Yasuda, H.; Yamachi, M. Olivine LiCoPO4 as 4.8 V electrode material for lithium batteries. Electrochem. Solid State Lett. 2000, 3, 178–179. [Google Scholar] [CrossRef]
- Wolfenstine, J.; Allen, J. Ni3+/Ni2+ redox potential in LiNiPO4. J. Power Sources 2005, 142, 389–390. [Google Scholar] [CrossRef]
- Masquelier, C.; Croguennec, L. Polyanionic (phosphates, silicates, sulfates) frameworks as electrode materials for rechargeable Li (or Na) batteries. J. Am. Chem. Soc. 2013, 113, 6552–6591. [Google Scholar]
- Bianchini, M.; Fauth, F.; Brisset, N.; Weill, F.; Suard, E.; Masquelier, C.; Croguennec, L. Comprehensive investigation of the Na3V2(PO4)2F3–NaV2(PO4)2F3 system by operando high resolution synchrotron X-ray diffraction. J. Am. Chem. Soc. 2015, 27, 3009–3020. [Google Scholar] [CrossRef]
- Nose, M.; Nakayama, H.; Nobuhara, K.; Yamaguchi, H.; Nakanishi, S.; Iba, H. Na4Co3(PO4)2P2O7: A novel storage material for sodium-ion batteries. J. Power Sources 2013, 234, 175–179. [Google Scholar] [CrossRef]
- Gezović, A.; Vujković, M.J.; Milović, M.; Grudić, V.; Dominko, R.; Mentus, S. Recent developments of Na4M3(PO4)2(P2O7) as the cathode material for alkaline-ion rechargeable batteries: Challenges and outlook. Energy Storage Mater. 2021, 37, 243–273. [Google Scholar] [CrossRef]
- Barpanda, P.; Oyama, G.; Nishimura, S.; Chung, S.-C.; Yamada, A. A 3.8-V earth-abundant sodium battery electrode. Nat. Commun. 2014, 5, 1–6. [Google Scholar] [CrossRef]
- Kawai, K.; Asakura, D.; Nishimura, S.; Yamada, A. 4.7 V Operation of the Cr4+/Cr3+ redox couple in Na3Cr2(PO4)2F3. Chem. Mater. 2021, 33, 1373–1379. [Google Scholar] [CrossRef]
- Padhi, A.K.; Nanjundaswamy, K.S.; Masquelier, C.; Okada, S.; Goodenough, J.B. Effect of structure on the Fe3+/Fe2+ redox couple in iron phosphates. J. Electrochem. Soc. 1997, 144, 1609–1613. [Google Scholar] [CrossRef]
- Yamada, A. Systematic studies on “abundant” battery materials: Identification and reaction mechanisms. Electrochemistry 2016, 84, 654–661. [Google Scholar] [CrossRef][Green Version]
- You, Y.; Manthiram, A. Progress in high-voltage cathode materials for rechargeable sodium-ion batteries. Adv. Energy Mater. 2018, 8, 1701785. [Google Scholar] [CrossRef]
- Chayambuka, K.; Mulder, G.; Danilov, D.L.; Notten, P.H.L. Sodium-ion battery materials and electrochemical properties reviewed. Adv. Energy Mater. 2018, 8, 1–49. [Google Scholar] [CrossRef]
- Kubota, K.; Yokoh, K.; Yabuuchi, N.; Komaba, S. Na2CoPO4F as a high-voltage electrode material for Na-ion batteries. Electrochemistry 2014, 82, 909–911. [Google Scholar] [CrossRef]
- Barpanda, P.; Ati, M.; Melot, B.; Rousse, G. A 3.90V iron-based fluorosulphate material for lithium-ion batteries crystallizing in the triplite structure. Nat. Mater. 2011, 10, 772–779. [Google Scholar] [CrossRef] [PubMed]
- Furuta, N.; Nishimura, S.; Barpanda, P.; Yamada, A. Fe3+/Fe2+ redox couple approaching 4 V in Li2-x(Fe1-yMny)P2O7 pyro-phosphate cathodes. Chem. Mater. 2012, 24, 1055–1061. [Google Scholar] [CrossRef]
- Ye, T.; Barpanda, P.; Nishimura, S.; Furuta, N.; Chung, S.-C.; Yamada, A. General observation of Fe3+/Fe2+ redox couple close to 4 V in partially substituted Li2FeP2O7 pyrophosphate solid-solution cathodes. Chem. Mater. 2013, 25, 3623–3629. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamada, A. High-Voltage Polyanion Positive Electrode Materials. Molecules 2021, 26, 5143. https://doi.org/10.3390/molecules26175143
Yamada A. High-Voltage Polyanion Positive Electrode Materials. Molecules. 2021; 26(17):5143. https://doi.org/10.3390/molecules26175143
Chicago/Turabian StyleYamada, Atsuo. 2021. "High-Voltage Polyanion Positive Electrode Materials" Molecules 26, no. 17: 5143. https://doi.org/10.3390/molecules26175143
APA StyleYamada, A. (2021). High-Voltage Polyanion Positive Electrode Materials. Molecules, 26(17), 5143. https://doi.org/10.3390/molecules26175143




