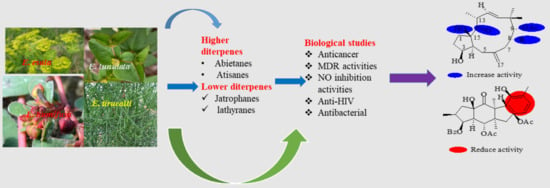
Euphorbia Diterpenes: An Update of Isolation, Structure, Pharmacological Activities and Structure–Activity Relationship
Abstract
:1. Introduction
2. Literature Sources and Search Strategy
3. Occurrence of Euphorbia Diterpenes
4. Higher Diterpenes
4.1. Abietanes, Atisane, Cembranes, Ent-Abietanes, Ent-Labdanes and Ent-Isopimaranes
4.2. Abietane and Ent-Abietanes
4.3. Meroterpenoids
4.4. Ent-Atisanes, Ent-Isopimaranes, Cembranes and Labdanes
5. Lower Diterpenes
5.1. Ingenanes
5.2. Jatrophanes and Modified Jatrophanes
5.3. Lathyranes Diterpenes
5.4. Meroterpenoids
5.5. Tiglianes
5.6. Other Euphorbia Diterpenes
| No | Species Name | Compound Name | Plant Part, Extraction Solvent | Pharmacological Effect (Cell Type, Reported Value and Control) | Reference |
|---|---|---|---|---|---|
| Abietane | |||||
| 1 | E. peplus | 11,12-didehydro-8α,14-dihydro-7-oxo-helioscopinolide A | Whole plant, CH3OH | Cytotoxic (HL-60, A-549, SMMC-7721, MCF-7, SW480). Inactive at 40 µM. Control (pactlitaxel and cisplatin) | [38] |
| 2 | 7α-hydroxy-8α,14-dihydro jolkinolide E | Whole plant, CH3OH | Cytotoxic (HL-60, A-549, SMMC-7721, MCF-7, SW480). Inactive at 40 µM. Control (pactlitaxel and cisplatin). | [38] | |
| 3 | E. stracheyi | ent-11β-hydroxyabieta-8(14),13(15)-dien-16,12-olide | Roots, MeOH | Cytotoxic (HGC-27, MV4-11, BaF3 SKvo3, IC50 > 50.00 µM) compared to IC50 of 0.015, 0.53 µM, respectively of taxol, the positive control | [32] |
| 4 | E.neriifolia | 1α,9β-dihydroxy-ent-abieta-8(14),13(15)-dien-16,12-olide | Aerial, EtOH | Antiangiogenic activity (HUVECs migration); no activity (IC50 > 50.00 µg/mL) | [68] |
| 5 | 1α-hydroxy-14-oxo-ent-abieta-8,13(15)-dien-16,12-olide | Aerial, EtOH | Antiangiogenic activity (HUVECs migration); no activity (IC50 > 50.00 µg/mL) | [68] | |
| Atisane | |||||
| 6 | E. kansuensis | atisane-3-oxo-16α,17-diol | Roots, EtOH | Inhibition of NO (IC50 > 50 µM; quercetin (IC50 = 10.80 µM) | [35] |
| Cembrane | |||||
| 7 | E. pekinensis | euphopane C | Roots, EtOH | Cytotoxic (C4-24B; C4-2B/ENZR, MDA-MB-231, IC50 = 32.30, 29.30 and >50 µM respectively). Doxorubicin (0.53, 1.06 and 0.78 µM respectively) | [35] |
| 8 | E. royleana | euphoroylean A | Whole plant, EtOH | Chemoreversal, combination abilities on Hep-G2/DOX; IC50 > 50 µM, Controls: verapamil (Vrp) (10.65 µM), tariquidar (Tar) (2.31 µM) | [33] |
| 9 | euphoroylean B | Whole plant, EtOH | Chemoreversal, combination abilities on Hep-G2/DOX; IC50 > 50 µM, Controls: Vrp (10.65 µM), tar (2.31 µM) | [33] | |
| ent-abietane | |||||
| 10 | E. peplus | 11-hydroxy-ent-abieta-8,11,13-trien-15-one | Whole plant, CH3OH | Cytotoxic (HL-60, A-549, SMMC-7721, MCF-7, SW480). Inactive at 40 µM, using pactlitaxel and cisplatin as control. | [38] |
| 11 | E. wallichii | 11β-hydroxy-14-oxo-17-al-ent-abieta-8(9), 13(15)-dien-16,12β-olide | Roots, EtOH | Antibacterial (T25-17; MIC = 37.00 µg/L, C159-6; MIC = 45.00 µg/L, 8152; MIC = 56.00 µg/L) using gentamicin as positive control | [47] |
| 12 | 11β, 17-dihydroxy-12-methoxy-ent-abieta-8(14), 13(15)-dien-16,12A-olide | Roots, EtOH | Antibacterial (T25-17; MIC = 41.00 µg/L, C159-6; MIC = 49.00 µg/L, 8152; MIC = 60.00 µg/L) using gentamicin as positive control | [47] | |
| 13 | 14α-hydroxy-17-al-entabieta-7(8), 1 1(12), 13(15)-trien-16, 12-olide | Roots, EtOH | Antibacterial (T25-17; MIC = 35.00 µg/L, C159-6; MIC = 51.00 µg/L, 8152; MIC = 59.00 µg/L) using gentamicin as positive control | [47] | |
| 14 | E. fischeriana | euphoractone | Roots, EtOH | Cytotoxic (H23; IC50 = 21.07 mmol/L, H460; IC50 = 20.91 mmol/L) using cisplatin the positive control | [60] |
| 15 | E. antiquorum | euphonoid F | Aerial, EtOH | Melanin synthesis (B16 cells) No activity | [34] |
| 16 | E. pekinensis | euphopane B | Roots, EtOH | Cytotoxic (C4-24B; C4-2B/ENZR, MDA-MB-231, IC50 = 16.90, 36.80 and > 50 µM respectively). Doxorubicin (0.53, 1.06 and 0.78 µM respectively) | [35,44] |
| 17 | E. neriifolia | eupneria A | Stem barks, C3H6O: H2O (7:3) | Anti-infammatory (RAW 264.7) and anti-influenza (A/WSN/33/2009 (H1N1). Inactive, using oseltamivir positive control | [69] |
| 18 | eupneria B | Stem barks, C3H6O: H2O (7:3) | Anti-infammatory (RAW 264.7) and anti-influenza (A/WSN/33/2009 (H1N1). Inactive, using oseltamivir positive control | [69] | |
| 19 | eupneria C | Stem barks, C3H6O: H2O (7:3) | Anti-infammatory (RAW 264.7) and anti-influenza (A/WSN/33/2009 (H1N1). Inactive, using oseltamivir positive control | [69] | |
| 20 | eupneria D | Stem barks, C3H6O: H2O (7:3) | Anti-infammatory (RAW 264.7) and anti-influenza (A/WSN/33/2009 (H1N1). Inactive, using oseltamivir positive control | [69] | |
| 21 | eupneria E | Stem barks, C3H6O: H2O (7:3) | Anti-infammatory (RAW 264.7) and anti-influenza (A/WSN/33/2009 (H1N1). Inactive, using oseltamivir positive control | [69] | |
| 22 | eupneria F | Stem barks, C3H6O: H2O (7:3) | Anti-infammatory (RAW 264.7) and anti-influenza (A/WSN/33/2009 (H1N1). Inactive, using oseltamivir positive control | [69] | |
| 23 | eupneria G | Stem barks, C3H6O: H2O (7:3) | Anti-HIV (inactive, EC50 > 25 µg/mL), Cytotoxic (Hep-G2; IC50 = 13.70 µM; adriamycin (IC50 = 7.03 µM) | [70] | |
| 24 | eupneria H. | Stem barks, C3H6O: H2O (7:3) | Anti-HIV (inactive, EC50 > 25 µg/mL), Cytotoxic (Hep-G2; IC50 = 13.70 µM; adriamycin (IC50 = 7.03 µM) | [70] | |
| 25 | eupneria I | Stem barks, C3H6O: H2O (7:3) | Anti-HIV (inactive, EC50 > 25 µg/mL), Cytotoxic (Hep-G2; IC50 = 13.70 µM; adriamycin (IC50 = 7.03 µM) | [70] | |
| 26 | E.fischeriana | fischerianoids A | Roots, C3H6O | Cytotoxic (HL-60; no activity, MM-231; IC50 = 12.10 µM, A549; no activity, SMMC-7721; IC50 = 32.58 µM, Hep-3B; IC50 = 15.95 µM), cisplatin; 1.60, 3.82, 2.81, 2.78 and 2.97 µM respectively) | [61] |
| 27 | fischerianoids B | Roots, C3H6O | Cytotoxic (HL-60; IC50 = 28.78 µM, MM-231; IC50 = 9.12 µM, A549; no activity, SMMC-7721; no activity, Hep-3B; IC50 = 8.50 µM), cisplatin; 1.60, 3.82, 2.81, 2.78 and 2.97 µM respectively) | [61] | |
| 28 | fischerianoids C | Roots, C3H6O | Cytotoxic (HL-60; no activity, MM-231; IC50 = 25.45 µM, A549; no activity, SMMC-7721; no activity, Hep-3B; IC50 = 27.34 µM), cisplatin; 1.60, 3.82, 2.81, 2.78 and 2.97 µM respectively) | [61] | |
| ent-atisane | |||||
| 29 | E. stracheyi | ent-atis-16-ene-3,14-dione | Roots, MeOH | Cytotoxic (HGC-27, MV4-11, BaF3 SKvo3, IC50 > 50.00) compared to IC50 of 0.015, 0.53 µM, respectively of taxol, the positive control | [32] |
| 30 | E. royleana | euphoroylean F | Whole plant, EtOH | Chemoreversal, combination abilities on Hep-G2/DOX; IC50 > 100 (10.65 µM), tar (2.31 µM) | [33] |
| 31 | euphoroylean G | Whole plant, EtOH | Chemoreversal, combination abilities on Hep-G2/DOX; IC50 > 100 (10.65 µM), tar (2.31 µM) | [33] | |
| 32 | E. antiquorum | ent-3α-acetoxy-16β,17,18-trihydroxyatisane | Stems, MeOH | Inhibitory (α-glucosidase); IC50 = 119.90 µM. Cytotoxicity (K562; no activity). Acarbose (IC50 = 162.50 µM) | [54] |
| 33 | E. antiquorum | ent-3α,14,16b,17-tetrahydroxyatisane | Stems, MeOH | Inhibitory (α-glucosidase); IC50 > 200.00 µM. Cytotoxicity (K562; no activity). Acarbose (IC50 = 162.50 µM) | [54] |
| 34 | ent-14[S],16α,17-trihydroxyatisan-3-one | Stems, MeOH | Inhibitory (α-glucosidase); IC50 = 135.50 µM. Cytotoxicity (K562; no activity). Acarbose (IC50 = 162.50 µM) | [54] | |
| 35 | gallochaol C | Stems, MeOH | Inhibitory (α-glucosidase); IC50 = 134.30 µM. Cytotoxicity (K562; no activity). Acarbose (IC50 = 162.50 µM) | [54] | |
| 36 | E. kansuensis | ent-atisane-3β,16α,17-triol | Roots, EtOH | Inhibition of NO (IC50 > 50 µM; quercetin (IC50 = 10.80 µM) | [35] |
| 37 | E.antiquorum | euphorin A | Stems, MeOH | Inhibitory (NO production in BV-2; IC50 = 35.80 µM); 2-methyl-2-thiopseudourea, sulfate (SMT) (4.2 µM) | [56] |
| 38 | euphorin B | Stems, MeOH | Inhibitory (NO production in BV-2; IC50 = 41.40 SMT (4.2 µM); SMT (4.2 SMT (4.2 µM) | [56] | |
| 39 | E. royleana | (4R,5S,8S,9R,10S,12S,16S)-ent-19-acetoyloxy-16α,17-dihydroxyatisan-3-one | Stems, MeOH | Inhibitory (NO production in BV-2; IC50 > 50 µM); SMT (3.7 µM) | [75] |
| 40 | (4R,5R,8S,9R, -10R,12S,16S)-ent-16α,17-dihydroxy-19-noratisan-3-one | Stems, MeOH | Inhibitory (NO production in BV-2; IC50 > 50 µM); SMT (3.7 µM) | [75] | |
| 41 | E. antiquorum | ent-(3α,5β,8α,9β,10α,12α)-3-hydroxyatis-16-en-14-one | Stems, MeOH | Inhibitory (NO production in BV-2; IC50 = 71.0 SMT (4.2 µM); SMT (4.2 SMT (4.2 µM) | [56] |
| ent-isopimarane | |||||
| 42 | E. neriifolia | eupneria J. | Stem barks, EtOH | Anti-HIV (HIV-1 NL4-3; 0.31 μg/mL), AZT; 0.0043 μg/mL | [73] |
| 43 | eupneria K. | Stem barks, EtOH | Anti-HIV (HIV-1 NL4-3), inactive (IC50 > 25.00 µg/mL), AZT; 0.0043 μg/mL | [73] | |
| 44 | eupneria L | Stem barks, EtOH | Anti-HIV (HIV-1 NL4-3), inactive (IC50 > 25.00 µg/mL), AZT; 0.0043 μg/mL | [73] | |
| 45 | eupneria M | Stem barks, EtOH | Anti-HIV (HIV-1 NL4-3), inactive (IC50 > 25.00 µg/mL), AZT; 0.0043 μg/mL | [73] | |
| 46 | eupneria N | Stem barks, EtOH | Anti-HIV (HIV-1 NL4-3), inactive (IC50 > 25.00 µg/mL), AZT; 0.0043 μg/mL | [73] | |
| 47 | eupneria O | Stem barks, EtOH | Anti-HIV (HIV-1 NL4-3), inactive (IC50 > 25.00 µg/mL), AZT; 0.0043 μg/mL | [73] | |
| 48 | eupneria P | Stem barks, EtOH | Anti-HIV (HIV-1 NL4-3), inactive (IC50 > 25.00 µg/mL), AZT; 0.0043 μg/mL | [73] | |
| 49 | eurifoloid I | Stem barks, EtOH | Anti-HIV (HIV-1 NL4-3), inactive (IC50 > 25.00 µg/mL), AZT; 0.0043 μg/mL | [73] | |
| 50 | oryzalexin F | Stem barks, EtOH | Anti-HIV (HIV-1 NL4-3), inactive (IC50 > 25.00 µg/mL), AZT; 0.0043 μg/mL | [73] | |
| 51 | eurifoloid H | Stem barks, EtOH | Anti-HIV (HIV-1 NL4-3; 6.70 μg/mL), MDCK, AZT; 0.0043 μg/mL | [73] | |
| 52 | ent-isopimara-8(14),15-dien-3β,12β-diol | Stem barks, EtOH | Anti-HIV (HIV-1 NL4-3), MDCK; 3.86 μg/mL. AZT; 0.0043 μg/mL | [73] | |
| 53 | 3α,12α-dihydroxy-ent-8(14),15-isopimaradien-18-al | Stem barks, EtOH | Anti-HIV (HIV-1 NL4-3), inactive (IC50 > 25.00 µg/mL), AZT; 0.0043 μg/mL | [73] | |
| 54 | E. royleana | (1S,5R,9R,10R,12R)-1α-acetoyloxy-ent-abieta-8(14),13-(15)-dien-12α,l6- | Stems, MeOH | Inhibitory (NO production in BV-2; IC50 = 12.0 µM); SMT (3.7 µM) | [75] |
| 55 | (1S,4S,5R,9R,10S,12R)-1α,18-dihydroxy-ent-abieta-8(14),13(15)-dien-12α,l6-olide | Stems, MeOH | Inhibitory (NO production in BV-2; IC50 > 50 µM); SMT (3.7 µM) | [75] | |
| 56 | E. hylonoma | (2R,3S,12S)-2,3,12-trihydroxy-ent-isopimara-7,15-diene | Roots, EtOH | Inhibitory (NO in RAW264.7; IC50 = 45.48 µM; indomethacin (IC50 = 41.41 µM) | [45] |
| 57 | (2R,3S,11R,12S)-2,3-dihydroxy-11,12-epoxy-ent-isopimara-7,15-diene | Roots, EtOH | Inhibitory (NO in RAW264.7; not evaluated, indomethacin (IC50 = 41.41 µM) | [45] | |
| 58 | (1R,2S,3S,12S)-1,2-epoxy-3,12-dihydroxy-ent-isopimara-7,15-diene | Roots, EtOH | Inhibitory (NO in RAW264.7; not evaluated, indomethacin (IC50 = 41.41 µM) | [45] | |
| 59 | (1R,2S,3R,12S)-1,2-epoxy-3,12-dihydroxy-ent-isopimara-7,15-diene | Roots, EtOH | Inhibitory (NO in RAW264.7; IC50 = 57.51 µM; indomethacin (IC50 = 41.41 µM) | [45] | |
| 60 | (1R,2S,3S,12R)-1,2,3,12-tetrahydroxy-ent-isopimara-7,15-diene | Roots, EtOH | Inhibitory (NO in RAW264.7; not active; indomethacin (IC50 = 41.41 µM) | [45] | |
| 61 | (1R,2S,3R,12R)-1,2,3,12-tetrahydroxy-ent-isopimara-7,15-diene | Roots, EtOH | Inhibitory (NO in RAW264.7; not active µM; indomethacin (IC50 = 41.41 µM) | [45] | |
| 62 | (2S,12R)-2,12-dihydroxy-ent-isopimara-7,15-dien-3-one | Roots, EtOH | Inhibitory (NO in RAW264.7; not active; indomethacin (IC50 = 41.41 µM) | [45] | |
| 63 | 3α,12β-dihydroxy-ent-isopimara-8,15-dien-11-one | Roots, EtOH | Inhibitory (NO in RAW264.7; IC50 > 100 µM; indomethacin (IC50 = 41.41 µM) | [45] | |
| 64 | (12R,13R,15R)-2,15-dihydroxy-12,16-epoxy-12-methoxy-ent-isopimara-1,7-dien-3-one | Roots, EtOH | Inhibitory (NO in RAW264.7; not active; indomethacin (IC50 = 41.41 µM) | [45] | |
| ent-kaurane | |||||
| 65 | E. royleana | euphoroylean H | Whole plant, EtOH | Chemoreversal, combination abilities on Hep-G2/DOX; IC50 > 100 (10.65 µM), tar (2.31 µM) | [33] |
| 66 | (4R,5S,8S,9R,10S,13R,16S)-ent-16α,17-dihydroxy-19-(2β-methylbutanoyloxy)kauran-3-one | Stems, MeOH | Inhibitory (NO production in BV-2; IC50 = 32.60 µM); SMT (3.7 µM) | [75] | |
| 67 | (4R,5S,8S,9R,10S,13R,16S)-ent-16α,17-dihydroxy-19-tigloyloxykauran-3-one | Stems, MeOH | Inhibitory (NO production in BV-2; IC50 = 19.30 µM); SMT (3.7 µM) | [75] | |
| ent-labdane | |||||
| 68 | E. peplus | helioscopinolide A | Whole plant, CH3OH | Cytotoxic (HL-60, A-549, SMMC-7721, MCF-7, SW480). Inactive at 40 µM, using paclitaxel and cisplatin as control. | [38] |
| 69 | E. kansuensis | helioscopinolide A | Roots, EtOH | Inhibition of NO (IC50 = 47.0 µM; quercetin (IC50 = 10.80 µM) | [35] |
| 70 | neriifolene | Roots, EtOH | Inhibition of NO (IC50 > 50 µM; quercetin (IC50 = 10.80 µM) | [35] | |
| 71 | E. yinshanica | ent-3α,16-dihydroxylabda-8(17),12(E),14-triene | Roots, EtOH | Cytotoxic (HL-60, SMMC-7721, A-549, MCF-7, SW-480); not active (IC50 > 40 µM) using cisplatin control | [51] |
| 72 | ent-14(S),15-dihydroxylabda-8(17)-12(E)-dien-18-oic acid | Roots, EtOH | Cytotoxic (HL-60, SMMC-7721, A-549, MCF-7, SW-480); not active (IC50 > 40 µM) using cisplatin control | [51] | |
| ent-rosane | |||||
| 73 | E. neriifolia | euphominoid E | Stems, MeOH | Not evaluated | [71] |
| 74 | E. hylonoma | ent-rosa-1(10),15-dien-2-one | Roots, EtOH | Inhibitory (NO in RAW264.7; IC50 = 48.40 µM; Indomethacin (IC50 = 41.41 µM) | [45] |
| 75 | E. milii | euphominoid A | Aerial, C3H6O | Inhibitory (anti-EBV lytic replication; EC50 = 13.20 µM) compared to (+)-rutamarin (EC50 = 5.40 µM) | [46] |
| 76 | euphominoid B | Aerial, C3H6O | Inhibitory (anti-EBV lytic replication; EC50 = 5.40 µM) compared to (+)-rutamarin (EC50 = 5.40 µM) | [46] | |
| 77 | euphominoid C | Aerial, C3H6O | Inhibitory (anti-EBV lytic replication; EC50 = 24.40 µM) compared to (+)-rutamarin (EC50 = 5.40 µM) | [46] | |
| 78 | euphominoid D | Aerial, C3H6O | Inhibitory (anti-EBV lytic replication; EC50 > 50 µM) compared to (+)-rutamarin (EC50 = 5.40 µM) | [46] | |
| 79 | euphominoid E | Aerial, C3H6O | Inhibitory (anti-EBV lytic replication; EC50 > 50 µM) compared to (+)-rutamarin (EC50 = 5.40 µM) | [46] | |
| 80 | euphominoid F | Aerial, C3H6O | Inhibitory (anti-EBV lytic replication; EC50 > 50 µM) compared to (+)-rutamarin (EC50 = 5.40 µM) | [46] | |
| 81 | euphominoid G | Aerial, C3H6O | Inhibitory (anti-EBV lytic replication; EC50 > 50 µM) compared to (+)-rutamarin (EC50 = 5.40 µM) | [46] | |
| 82 | euphominoid H | Aerial, C3H6O | Inhibitory (anti-EBV lytic replication; EC50 > 50 µM) compared to (+)-rutamarin (EC50 = 5.40 µM) | [46] | |
| 83 | euphominoid I | Aerial, C3H6O | Inhibitory (anti-EBV lytic replication; EC50 > 50 µM) compared to (+)-rutamarin (EC50 = 5.40 µM) | [46] | |
| 84 | euphominoid J | Aerial, C3H6O | Inhibitory (anti-EBV lytic replication; EC50 = 29.21 µM) compared to (+)-rutamarin (EC50 = 5.40 µM) | [46] | |
| 85 | 5-epi-euphominoid J | Aerial, C3H6O | Inhibitory (anti-EBV lytic replication; EC50 > 50 µM) compared to (+)-rutamarin (EC50 = 5.40 µM) | [46] | |
| 86 | euphominoid K | Aerial, C3H6O | Inhibitory (anti-EBV lytic replication; EC50 > 50 µM) compared to (+)-rutamarin (EC50 = 5.40 µM) | [46] | |
| 87 | euphominoid L | Aerial, C3H6O | Inhibitory (anti-EBV lytic replication; EC50 > 50 µM) compared to (+)-rutamarin (EC50 = 5.40 µM) | [46] | |
| Gaditanone | |||||
| 88 | E. gaditana | gaditanone | Whole plant, MeOH | Not evaluated | [27] |
| Ingenane | |||||
| 89 | E. stracheyi | 3β, 20-diacetoxy-5β-deca-2′′E, 4′′E, 6′′E-trien-4β-hydroxyl-1-one | Roots, MeOH | Cytotoxic (HGC-27; IC50 = 23.76 µM; taxol (0.015 µM), MV4-11; IC50 = 7.92 µM; taxol (0.055 µM), BaF3; IC50 > 20.00 µM compared to IC50 of 0.015, 0.53 µM, respectively of taxol | [32] |
| 90 | ingenane | Roots, MeOH | Cytotoxic (HGC-27; IC50 = 48.81; taxol (0.015 µM), MV4-11; 7.92; taxol (0.055 µM) BaF3; IC50 > 20.00) compared to IC50 of 0.015, 0.53 µM, respectively of taxol | [32] | |
| 91 | 20-O-acetyl-[3-O-(2′E, 4′Z)-decadienoyl]-ingenol | Roots, MeOH | Cytotoxic (HGC-27; IC50 = 41.51; taxol (0.015 µM) MV4-11; IC50 = 3.18; taxol (0.055 µM) BaF3, compared to IC50 of 0.015, 0.53 µM, respectively of taxol | [32] | |
| 92 | 3-O-(2′E, 4′Z)-decadienoylingenol | Roots, MeOH | Cytotoxic (HGC-27; IC50 = 48.51; taxol (0.015 µM); MV4-11; IC50 = 10.80; taxol (0.055 µM) compared to IC50 of 0.015, 0.53 µM, respectively of taxol | [32] | |
| 93 | E. kansui | 3-O-(2′E, 4′Z-decadienoyl)-20-O-acetylingenol | Roots, EtOH | Antiproliferative (MCF-7; IC50 > 30 µM, Hep-G2; IC50 > 30 µM, DU145; IC50 = 24.49 µM) | [65] |
| 94 | 5-O-(2′E, 4′Z-decadienoyl)-20-O-acetylingenol | Roots, EtOH | Antiproliferative (MCF-7; IC50 > 30 µM, Hep-G2; IC50 > 30 µM, DU145; IC50 > 30 µM) | [65] | |
| 95 | 3-O-(2′E, 4′E-decadienoyl)-20-O-acetylingenol | Roots, EtOH | Antiproliferative (MCF-7; IC50 > 30 µM, Hep-G2; IC50 = 24.07 µM, DU145; IC50 = 8.20 µM) | [65] | |
| 96 | 5-O-(2′E, 4′E-decadienoyl)-20-O-acetylingenol | Roots, EtOH | Antiproliferative (MCF-7; IC50 = 25.76 µM, Hep-G2; IC50 = 26.96 µM, DU145; IC50 = 16.24 µM) | [65] | |
| 97 | 20-O-(2′E, 4′Z-decadienoyl) ingenol | Roots, EtOH | Antiproliferative (MCF-7; IC50 = 30.48 µM, Hep-G2; IC50 = 12.79 µM, DU145; IC50 = 8.86 µM) | [65] | |
| 98 | 20-O-(2′E, 4′E-decadienoyl) ingenol | Roots, EtOH | Antiproliferative (MCF-7; IC50 > 30 µM, Hep-G2; IC50 > 30 µM, DU145; IC50 > 30 µM) | [65] | |
| 99 | 20-O-acetylingenol | Roots, EtOH | Antiproliferative (MCF-7; IC50 > 30 µM, Hep-G2; IC50 > 30 µM, DU145; IC50 > 30 µM) | [65] | |
| 100 | 5-O-benzoyl-20-deoxyingenol | Roots, EtOH | Antiproliferative (MCF-7; IC50 = 28.35 µM, Hep-G2; IC50 = 24.56 µM, DU145; IC50 = 15.55 µM) | [65] | |
| 101 | 3-O-benzoyl-20-deoxyingenol | Roots, EtOH | Antiproliferative (MCF-7; IC50 = 25.56 µM, Hep-G2; IC50 = 23.75 µM, DU145; IC50 = 9.91 µM) | [65] | |
| 102 | kansuiphorin C | Roots, EtOH | Antiproliferative (MCF-7; IC50 = 12.58 µM, Hep-G2; IC50 = 25.00 µM, DU145; IC50 = 7.38 µM) | [65] | |
| 103 | 20-deoxyingenol | Roots, EtOH | Antiproliferative (MCF-7; IC50 > 30 µM, Hep-G2; IC50 > 30 µM, DU145; IC50 > 30 µM) | [65] | |
| 104 | kansuinin D | Roots, EtOH | Antiproliferative (MCF-7; IC50 > 30 µM, Hep-G2; IC50 > 30 µM, DU145; IC50 > 30 µM) | [65] | |
| 105 | kansuinins A | Roots, EtOH | Antiproliferative (MCF-7; IC50 > 30 µM, Hep-G2; IC50 > 30 µM, DU145; IC50 > 30 µM) | [65] | |
| 106 | kansuinin E | Roots, EtOH | Antiproliferative (MCF-7; IC50 > 30 µM, Hep-G2; IC50 > 30 µM, DU145; IC50 > 30 µM) | [65] | |
| 107 | kansuinin B | Roots, EtOH | Antiproliferative (MCF-7; IC50 > 30 µM, Hep-G2; IC50 > 30 µM, DU145; IC50 > 30 µM) | [59,65] | |
| 108 | 3,5,7,15-tetraacetoxy-9-nicotinoyloxy- 14-oxojatropha-6(17),11-diene | Roots, EtOH | Antiproliferative (MCF-7; IC50 > 30 µM, Hep-G2; IC50 > 30 µM, DU145; IC50 > 30 µM) | [65] | |
| 109 | E. royleana | euphoroylean C | Whole plant, EtOH | Chemoreversal, combination abilities on Hep-G2/DOX; IC50 > 50 (10.65 µM), tar (2.31 µM) | [33] |
| 110 | euphoroylean D | Whole plant, EtOH | Chemoreversal, combination abilities on Hep-G2/DOX; IC50 > 50 (10.65 µM), tar (2.31 µM) | [33] | |
| 111 | euphoroylean E | Whole plant, EtOH | Chemoreversal, combination abilities on Hep-G2/DOX; IC50 > 50 (10.65 µM), tar (2.31 µM) | [33] | |
| 112 | E. antiquorum | 20-deoxy-16-hydroxyingenol | Stems, MeOH | α-glucosidase inhibitory; IC50 > 200.00 µM, cytotoxicity (K562; inactive) | [54,55] |
| 113 | E. lathyris | ingenol 6,7-epoxy | Seeds, EtOH | Not evaluated | [31] |
| 114 | E. kansuensis | euphorkanlide A | Roots, EtOH | Cytotoxic (C4-24B; C4-2B/ENZR, MDA-MB-231, IC50 = 14.30, 28.20 and > 50 µM respectively). Doxorubicin (0.53, 1.06 and 0.78 µM) | [36] |
| Ingenol | |||||
| 115 | E. saudiarabica | saudiarabicain A | Aerial, EtOH | Inhibitory (α-glucosidase; IC50 > 150.00 µM, P-glycoprotein; IC50 = 0.80 µM | [28] |
| 116 | saudiarabicain B | Aerial, EtOH | Inhibitory (α-glucosidase; IC50 > 150.00 µM. P-glycoprotein control; IC50 = 1.40 µM | [28] | |
| 117 | E. antiquorum | euphonoid A | Aerial, EtOH | Melanin synthesis (B16; 159.89% at 50.00 µM. 8-MOP; 124.38%) | [34] |
| 118 | 3,8,12-O-triacetylingol-7-benzoate | Aerial, EtOH | Melanin synthesis (B16) at 50.00 µM, no activity | [34] | |
| 119 | ingol-3,8,12-O-triacetate-7-tiglate | Aerial, EtOH | Melanin synthesis (B16) at 50.00 µM, no activity | [34] | |
| 120 | 3,12-O-diacetylingol-7-benzoate-8-methoxyl | Aerial, EtOH | Melanin synthesis (B16) at 50.00 µM, no activity | [34] | |
| 121 | 3,12-diacetyl-7-angeloyl-8-methoxyingol | Aerial, EtOH | Melanin synthesis (B16) at 50.00 µM, no activity | [34] | |
| 122 | 3,12-diacetyl-7-tigloyl-8-methoxyingol | Aerial, EtOH | Melanin synthesis (B16) at 50.00 µM, no activity | [34] | |
| 123 | euphorantin I | Aerial, EtOH | Melanin synthesis (B16; 203.11% at 50.00 µM. 8-MOP; 124.38%) | [34] | |
| 124 | 12-acetyl-7-angeloyl-8-methoxyingol | Aerial, EtOH | Melanin synthesis (B16) at 50.00 µM, no activity | [34] | |
| 125 | 3,12-diacetyl-ingol-7-tigliate | Aerial, EtOH | Melanin synthesis (B16) at 50.00 µM, No activity | [34] | |
| 126 | 3,12-diacetyl-7-angolyl-8-hydroxyingol | Aerial, EtOH | Melanin synthesis (B16) at 50.00 µM, no activity | [34] | |
| 127 | euphorantin J | Aerial, EtOH | Melanin synthesis (B16; 177.43% at 50.00 µM. 8-MOP; 124.38%) | [34] | |
| 128 | tirucalicine | Aerial, EtOH | Melanin synthesis (B16) at 50.00 µM, no activity | [34] | |
| 129 | eurifoloid A | Aerial, EtOH | Melanin synthesis (B16) at 50.00 µM, no activity | [34] | |
| 130 | 3-O-[(Z)-2-methyl-2-butenoyl]-20-O-acetylingenol | Aerial, EtOH | Melanin synthesis (B16) at 50.00 µM, no activity | [34] | |
| 131 | eurifoloid L | Aerial, EtOH | Melanin synthesis (B16) at 50.00 µM, no activity | [34] | |
| 132 | E. antiquorum | antiquorine A | Aerial, EtOH | Melanin synthesis (B16) at 50.00 µM, no activity | [34] |
| Ingol | |||||
| 133 | E. saudiarabica | saudiarabicain C | Aerial, EtOH | Inhibitory (α-glucosidase; IC50 = 9.10 µM. P-glycoprotein IC50 = 0.10 µM | [28] |
| 134 | saudiarabicain D | Aerial, EtOH | Inhibitory (α-glucosidase; IC50 = 8.00 µM. P-glycoprotein; IC50 = 0.10 µM | [28] | |
| 135 | saudiarabicain E | Aerial, EtOH | Inhibitory (α-glucosidase; IC50 = 1.80 µM. P-glycoprotein; IC50 = 0.60 µM | [28] | |
| 136 | E. royleana | ingol | Whole plant, EtOH | Chemoreversal, combination abilities on Hep-G2/DOX; IC50 > 100 (10.65 µM), tar (2.31 µM) | [33] |
| 137 | quorumolide C | Whole plant, EtOH | Chemoreversal, combination abilities on Hep-G2/DOX; IC50 > 100 (10.65 µM), tar (2.31 µM) | [33] | |
| 138 | (3S,4S,5R,8S,10S,11R,13R,14R,15R)-3β-O-angeloyl-17-tigloyloxy-20- deoxyingenol | Whole plant, EtOH | Chemoreversal, combination abilities on Hep-G2/DOX; IC50 > 100 (10.65 µM), tar (2.31 µM) | [33,75] | |
| 139 | 20-acetyl-ingenol-3-angelate | Whole plant, EtOH | Chemoreversal, combination abilities on Hep-G2/DOX; IC50 > 100 (10.65 µM), tar (2.31 µM) | [33] | |
| 140 | 3-angelate- 20-hydroxyl-ingenol | whole plant, EtOH | Chemoreversal, combination abilities on Hep-G2/DOX; IC50 > 100 (10.65 µM), tar (2.31 µM) | [33] | |
| 141 | (3S,4S,5R,8S,10S,11R,13R,14R,15R) -3β-O-angeloyl-17-benzoyloxy-20- deoxyingenol | Whole plant, EtOH | Chemoreversal, combination abilities on Hep-G2/DOX; IC50 > 100 (10.65 µM), tar (2.31 µM) | [33,75] | |
| 142 | E. marginata | euphornan A | Seeds, EtOH | Multidrug reversal activity (Hep-G2/ADR; IC50 > 100 µM at 5 µM). Adriamycin control | [41] |
| 143 | euphornan B | Seeds, EtOH | Multidrug reversal activity (Hep-G2/ADR; IC50 > 100 µM at 5 µM). Adriamycin control | [41] | |
| 144 | euphornan C | Seeds, EtOH | Multidrug reversal activity (Hep-G2/ADR; IC50 > 100 µM at 5 µM). Adriamycin control | [41] | |
| 145 | euphornan D | Seeds, EtOH | Multidrug reversal activity (Hep-G2/ADR; IC50 > 50 µM at 5 µM). Adriamycin control | [41] | |
| 146 | euphornan E | Seeds, EtOH | Multidrug reversal activity (Hep-G2/ADR; IC50 > 25 µM at 5 µM). Adriamycin control | [41] | |
| 147 | euphornan F | Seeds, EtOH | Multidrug reversal activity (Hep-G2/ADR; IC50 > 25 µM at 5 µM). Adriamycin control | [41] | |
| 148 | euphornan G | Seeds, EtOH | Multidrug reversal activity (Hep-G2/ADR; IC50 > 100 µM at 5 µM). Adriamycin control | [41] | |
| 149 | euphornan H | Seeds, EtOH | Multidrug reversal activity (Hep-G2/ADR; IC50 > 100 µM at 5 µM). Adriamycin control | [41] | |
| 150 | euphornan I | Seeds, EtOH | Multidrug reversal activity (Hep-G2/ADR; IC50 > 25 µM at 5 µM). Adriamycin control | [41] | |
| 151 | euphornan J | Seeds, EtOH | Multidrug reversal activity (Hep-G2/ADR; IC50 > 25 µM at 5 µM). Adriamycin control | [41] | |
| 152 | euphornan K | Seeds, EtOH | Multidrug reversal activity (Hep-G2/ADR; IC50 > 25 µM at 5 µM). Adriamycin control | [41] | |
| 153 | euphornan L | Seeds, EtOH | Multidrug reversal activity (Hep-G2/ADR; IC50 > 25 µM at 5 µM). Adriamycin control | [41] | |
| 154 | euphornan M | Seeds, EtOH | Multidrug reversal activity (Hep-G2/ADR; IC50 > 25 µM at 5 µM). Adriamycin control | [41] | |
| 155 | euphornan N | Seeds, EtOH | Multidrug reversal activity (Hep-G2/ADR; IC50 > 25 µM at 5 µM). Adriamycin control | [41] | |
| 156 | euphornan O | Seeds, EtOH | Multidrug reversal activity (Hep-G2/ADR; IC50 > 25 µM at 5 µM). Adriamycin control | [41] | |
| 157 | euphornan P | Seeds, EtOH | Multidrug reversal activity (Hep-G2/ADR; IC50 > 100 µM at 5 µM). Adriamycin control | [41] | |
| 158 | euphornan Q | Seeds, EtOH | Multidrug reversal activity (Hep-G2/ADR; IC50 > 25 µM at 5 µM). Adriamycin control | [41] | |
| 159 | euphornan R | Seeds, EtOH | Multidrug reversal activity (Hep-G2/ADR; IC50 > 25 µM at 5 µM). Adriamycin control | [41] | |
| 160 | euphornan S | Seeds, EtOH | Multidrug reversal activity (Hep-G2/ADR; IC50 > 100 µM at 5 µM). Adriamycin control | [41] | |
| 161 | euphornan T | Seeds, EtOH | Multidrug reversal activity (Hep-G2/ADR; IC50 > 100 µM at 5 µM). Adriamycin control | [41] | |
| 162 | E. resinifera | euphoresins A | Latex, MeOH | Cytotoxic (MCF-7; IC50 = 85.87 µM, C6; IC50 = 8.31 µM) compared to taxol; 5.48, 6.79 and 8.31 µM respectively | [43] |
| 163 | euphoresins B | Latex, MeOH | Cytotoxic (MCF-7; IC50 = 87.36 µM, C6; IC50 = 94.89 µM) compared to taxol; 5.48, 6.79 and 8.31 µM respectively | [43] | |
| 164 | euphorantin S | Stem barks, C3H6O | Anti-HIV-1 (EC50 > 44 µM) compared to zidovudine (AZT); EC50 = 0.0019 µM | [42] | |
| 165 | E. neriifolia | euphorantin T | Stem barks, C3H6O | Anti-HIV-1 (EC50 > 44 µM) compared to zidovudine (AZT); EC50 = 0.0019 µM | [42] |
| 166 | euphorneroid A | Stem barks, C3H6O | Anti-HIV-1 (EC50 > 44 µM) compared to zidovudine (AZT); EC50 = 0.0019 µM | [42] | |
| 167 | euphorneroid B | Stem barks, C3H6O | Anti-HIV-1 (EC50 > 44 µM) compared to zidovudine (AZT); EC50 = 0.0019 µM | [42] | |
| 168 | euphorneroid C | Stem barks, C3H6O | Anti-HIV-1 (EC50 > 44 µM) compared to zidovudine (AZT); EC50 = 0.0019 µM | [42] | |
| 169 | euphorneroid D | Stem barks, C3H6O | Anti-HIV-1 (EC50 = 34 µM) compared to zidovudine (AZT); EC50 = 0.0019 µM | [42] | |
| Isopimarane | |||||
| 170 | E. pekinensis | euphopane A | Roots, EtOH | Cytotoxic (C4-24B; C4-2B/ENZR, MDA-MB-231, IC50 = 32.30, 29.30 and > 50 µM respectively) compared to doxorubicin (0.53, 1.06 and 0.78 µM) | [44] |
| 171 | (12β)-2,12-dihydroxyisopimara-1,7,15-trien-3-one | Roots, EtOH | Cytotoxic (C4-24B; C4-2B/ENZR, MDA-MB-231, IC50 = 32.30, > 50 and > 50 µM respectively) compared to doxorubicin (0.53, 1.06 and 0.78 µM) | [44] | |
| 172 | yuexiandajisu C | Roots, EtOH | Cytotoxic (C4-24B; C4-2B/ENZR, MDA-MB-231, IC50 = 23.10, 30.0 and > 50 µM respectively) compared to doxorubicin (0.53, 1.06 and 0.78 µM) | [44] | |
| 173 | (3β,12α,13α)-3,12-dihydroxypimara-7,15-dien-2-one | Roots, EtOH | Cytotoxic (C4-24B; C4-2B/ENZR, MDA-MB-231, IC50 = 32.60, > 50 and > 50 µM respectively) compared to doxorubicin (0.53, 1.06 and 0.78 µM) | [44] | |
| Jatrophane | |||||
| 174 | E. kansui | kansuingenol A | Roots, EtOH | Antiproliferative (MCF-7; IC50 = 20.86 µM, Hep-G2; IC50 = 14.20 µM, DU145; IC50 = 6.19 µM) | [65] |
| 175 | kansuingenol B | Roots, EtOH | Antiproliferative (MCF-7; IC50 = 15.82 µM, Hep-G2; IC50 = 29.16 µM, DU145; IC50 = 9.27 µM) | [65] | |
| 176 | kansuingenol C | Roots, EtOH | Antiproliferative (MCF-7; IC50 = 10.26 µM, Hep-G2; IC50 = 23.09 µM, DU145; IC50 = 26.06 µM) | [65] | |
| 177 | kansuijatrophanol A | Roots, EtOH | Antiproliferative (MCF-7; IC50 = 21.64 µM, Hep-G2; IC50 = 20.19 µM, DU145; IC50 = 7.21 µM) | [65] | |
| 178 | kansuijatrophanol B | Roots, EtOH | Antiproliferative (MCF-7; IC50 = 15.25 µM, Hep-G2; IC50 = 13.24 µM, DU145; IC50 = 7.24 µM) | [65] | |
| 179 | kansuijatrophanol C | Roots, EtOH | Antiproliferative (MCF-7; IC50 = 11.25 µM, Hep-G2; IC50 = 9.47 µM, DU145; IC50 = 8.29 µM) | [65] | |
| 180 | kansuijatrophanol D | Roots, EtOH | Antiproliferative (MCF-7; IC50 = 6.29 µM, Hep-G2; IC50 = 10.07 µM, DU145; IC50 = 4.19 µM) | [65] | |
| 181 | E. helioscopia | euphoheliphane A | Aerial, EtOH | Cytotoxic (OS-RC-2; IC50 = 47.00 µM, Ketr-3; IC50 = 45.00 µM, 769-P; IC50 = 43.00 µM, G401; IC50 = 38.00 µM, GRC-1; IC50 = 41.00 µM, ACHN; IC50 = 40.00 µM compared to doxorubicin (DOX); 5, 4, 3, 5, 4, and 3 µM respectively | [50] |
| 182 | euphoheliphane B | Aerial, EtOH | Cytotoxic (OS-RC-2; IC50 = 31.00 µM, Ketr-3; IC50 = 32.00 µM, 769-P; IC50 = 30.00 µM, G401; IC50 = 34.00 µM, GRC-1; IC50 = 33.00 µM, ACHN; IC50 = 35.00 µM compared to doxorubicin (DOX); 5, 4, 3, 5, 4, and 3 µM respectively | [50] | |
| 183 | euphoheliphane C | Aerial, EtOH | Cytotoxic (OS-RC-2; IC50 = 35.00 µM, Ketr-3; IC50 = 41.00 µM, 769-P; IC50 = 39.00 µM, G401; IC50 = 32.00 µM, GRC-1; IC50 = 38.00 µM, ACHN; IC50 = 36.00 µM compared to doxorubicin (DOX); 5, 4, 3, 5, 4, and 3 µM respectively | [50] | |
| 184 | E. esula | euphoesulatin A | Whole plant, EtOH | Ostiosteoporotic activity (BMM; IC50 = 1.20 µM) compared to RANKL control | [100] |
| 185 | euphoesulatin B | Whole plant, EtOH | Ostiosteoporotic activity (BMM; IC50 > 10 µM) compared to RANKL control | [100] | |
| 186 | euphoesulatin C | Whole plant, EtOH | Ostiosteoporotic activity (BMM; IC50 > 10 µM) compared to RANKL control | [100] | |
| 187 | euphoesulatin D | Whole plant, EtOH | Inhibitory (BMM; IC50 = 6.60 µM) compared to RANKL control | [100] | |
| 188 | euphoesulatin E | Whole plant, EtOH | Ostiosteoporotic activity (BMM; IC50 = 5.90 µM) compared to RANKL control | [100] | |
| 189 | euphoesulatin F | Whole plant, EtOH | Ostiosteoporotic activity (BMM; IC50 = 6.10 µM) compared to RANKL control | [100] | |
| 190 | euphoesulatin G | Whole plant, EtOH | Ostiosteoporotic activity (BMM; IC50 = 10.00 µM) compared to RANKL control | [100] | |
| 191 | euphoesulatin H | Whole plant, EtOH | Ostiosteoporotic activity (BMM; IC50 = 3.50 µM) compared to RANKL control | [100] | |
| 192 | euphoesulatin I | Whole plant, EtOH | Ostiosteoporotic activity (BMM; IC50 > 10 µM) compared to RANKL control | [100] | |
| 193 | euphoesulatin J | Whole plant, EtOH | Ostiosteoporotic activity (BMM; IC50 = 2.30 µM) compared to RANKL control | [100] | |
| 194 | euphoesulatin K | Whole plant, EtOH | Ostiosteoporotic activity (BMM; IC50 > 10 µM) compared to RANKL control | [100] | |
| 195 | euphoesulatin L | Whole plant, EtOH | Ostiosteoporotic activity (BMM; IC50 > 10 µM) compared to RANKL control | [100] | |
| 196 | euphoesulatin M | Whole plant, EtOH | Ostiosteoporotic activity (BMM; IC50 = 7.60 µM) compared to RANKL control | [100] | |
| 197 | euphoesulatin N | Whole plant, EtOH | Ostiosteoporotic activity (BMM; not active, compared to RANKL control | [100] | |
| 198 | euphoesulatin O | Whole plant, EtOH | Ostiosteoporotic activity (BMM; IC50 = 5.90 µM), compared to RANKL control | [100] | |
| 199 | euphoesulatin P | Whole plant, EtOH | Ostiosteoporotic activity (BMM; IC50 > 10 µM) compared to RANKL control | [100] | |
| 200 | euphoesulatin Q | Whole plant, EtOH | Ostiosteoporotic activity (BMM; IC50 > 10 µM) compared to RANKL control | [100] | |
| 201 | euphoesulatin R | Whole plant, EtOH | Ostiosteoporotic activity (BMM; IC50 > 10 µM) compared to RANKL control | [100] | |
| 202 | esulone B | Whole plant, EtOH | Ostiosteoporotic activity (BMM; No activity) compared to RANKL control | [100] | |
| 203 | kansuinine B | Whole plant, EtOH | Ostiosteoporotic activity (BMM; not active, compared to RANKL control | [59,100] | |
| 204 | esulone A | Whole plant, EtOH | Ostiosteoporotic activity (BMM; not active, compared to RANKL control | [59,100] | |
| 205 | euphoresulane A | Whole plant, EtOH | MDR-chemoreversal (Hep-G2; IC50 > 100 µM), adriamycin (ADR); IC50 = 284.50 µM | [59] | |
| 206 | euphoresulane B | Whole plant, EtOH | MDR-chemoreversal (Hep-G2; IC50 > 25 µM), ADR; IC50 = 284.50 µM | [59] | |
| 207 | euphoresulane C | Whole plant, EtOH | MDR-chemoreversal (Hep-G2; IC50 > 100 µM), ADR; IC50 = 284.50 µM | [59] | |
| 208 | euphoresulane D | Whole plant, EtOH | MDR-chemoreversal (Hep-G2; IC50 > 100 µM), ADR; IC50 = 284.50 µM | [59] | |
| 209 | euphoresulane E | Whole plant, EtOH | MDR-chemoreversal (Hep-G2; IC50 > 100 µM), ADR; IC50 = 284.50 µM | [59] | |
| 210 | euphoresulane F | Whole plant, EtOH | MDR-chemoreversal (Hep-G2; IC50 > 50 µM), ADR; IC50 = 284.50 µM | [59] | |
| 211 | euphoresulane G | Whole plant, EtOH | MDR-chemoreversal (Hep-G2; IC50 > 100 µM), ADR; IC50 = 284.50 µM | [59] | |
| 212 | euphoresulane H | Whole plant, EtOH | MDR-chemoreversal (Hep-G2; IC50 = 165.30 µM, ADR; IC50 = 284.50 µM | [59] | |
| 213 | euphoresulane I | Whole plant, EtOH | MDR-chemoreversal (Hep-G2; IC50 > 100 µM), ADR; IC50 = 284.50 µM | [59] | |
| 214 | euphoresulane J | Whole plant, EtOH | MDR-chemoreversal (Hep-G2; IC50 > 100 µM), ADR; IC50 = 284.50 µM | [59] | |
| 215 | euphoresulane K | Whole plant, EtOH | MDR-chemoreversal (Hep-G2; IC50 > 100 µM), ADR; IC50 = 284.50 µM | [59] | |
| 216 | euphoresulane L | Whole plant, EtOH | MDR-chemoreversal (Hep-G2; IC50 > 100 µM), ADR; IC50 = 284.50 µM | [59] | |
| 217 | euphoresulane M | Whole plant, EtOH | MDR-chemoreversal (Hep-G2; IC50 > 100 µM), ADR; IC50 = 284.50 µM | [59] | |
| 218 | kanesulone A | Whole plant, EtOH | MDR-chemoreversal (Hep-G2; IC50 > 100 µM), ADR; IC50 = 284.50 µM | [59] | |
| 219 | 3β,7β,8α,15β-tetraacetoxy-5α-benzoyloxyjatropha-6(17), 11E-dien-9,14-dione | Whole plant, EtOH | MDR-chemoreversal (Hep-G2; IC50 > 100 µM), ADR; IC50 = 284.50 µM | [59] | |
| 220 | kanesulone B | Whole plant, EtOH | MDR-chemoreversal (Hep-G2; IC50 > 100 µM), ADR; IC50 = 284.50 µM | [59] | |
| 221 | (2S,3S,4R,5R,7S,8R,13R,15R)−3,5,7,8,15-pentaacetoxy-9,14-dioxojatropha-6(17),11E-diene | Whole plant, EtOH | MDR-chemoreversal (Hep-G2; IC50 > 100 µM), ADR; IC50 = 284.50 µM | [59] | |
| 222 | kansuinin C | Whole plant, EtOH | MDR-chemoreversal (Hep-G2; IC50 > 100 µM), ADR; IC50 = 284.50 µM | [59] | |
| 223 | E. glomerulans | euphoglomeruphane A | Whole plant, C3H6O | MDR-chemoreversal (MCF-7/ADR IC50 > 100 µM), verapamil; IC50 = 4.70 µM | [29] |
| 224 | euphoglomeruphane B | Whole plant, C3H6O | MDR-chemoreversal (MCF-7/ADR IC50 > 100 µM), verapamil; IC50 = 4.70 µM | [29] | |
| 225 | euphoglomeruphane C | Whole plant, C3H6O | MDR-chemoreversal (MCF-7/ADR IC50 > 100 µM), verapamil; IC50 = 4.70 µM | [29] | |
| 226 | euphoglomeruphane D | Whole plant, C3H6O | MDR-chemoreversal (MCF-7/ADR IC50 > 100 µM), verapamil; IC50 = 4.70 µM | [29] | |
| 227 | euphoglomeruphane E | Whole plant, C3H6O | MDR-chemoreversal (MCF-7/ADR IC50 > 100 µM), verapamil; IC50 = 4.70 µM | [29] | |
| 228 | euphoglomeruphane F | Whole plant, C3H6O | MDR-chemoreversal (MCF-7/ADR IC50 > 100 µM), verapamil; IC50 = 4.70 µM | [29] | |
| 229 | euphoglomeruphane G | Whole plant, C3H6O | MDR-chemoreversal (MCF-7/ADR IC50 > 100 µM), verapamil; IC50 = 4.70 µM | [29] | |
| 230 | euphoglomeruphane H | Whole plant, C3H6O | MDR-chemoreversal (MCF-7/ADR IC50 = 39.30 µM), verapamil; IC50 = 4.70 µM | [29] | |
| 231 | euphoglomeruphane I | Whole plant, C3H6O | MDR-chemoreversal (MCF-7/ADR IC50 > 100 µM), verapamil; IC50 = 4.70 µM | [29] | |
| 232 | euphoglomeruphane J | Whole plant, C3H6O | MDR-chemoreversal (MCF-7/ADR IC50 > 100 µM), verapamil; IC50 = 4.70 µM | [29] | |
| 233 | euphoglomeruphane K | Whole plant, C3H6O | MDR-chemoreversal (MCF-7/ADR IC50 > 100 µM), verapamil; IC50 = 4.70 µM | [29] | |
| 234 | euphoglomeruphane L | Whole plant, C3H6O | MDR-chemoreversal (MCF-7/ADR IC50 = 50.20 µM), verapamil; IC50 = 4.70 µM | [29] | |
| 235 | euphoglomeruphane M | Whole plant, C3H6O | MDR-chemoreversal (MCF-7/ADR IC50 > 100 µM), verapamil; IC50 = 4.70 µM | [29] | |
| 236 | euphoglomeruphane N | Whole plant, C3H6O | MDR-chemoreversal (MCF-7/ADR IC50 > 100 µM), verapamil; IC50 = 4.70 µM | [29] | |
| 237 | euphoglomeruphane O | Whole plant, C3H6O | MDR-chemoreversal (MCF-7/ADR IC50 > 100 µM), verapamil; IC50 = 4.70 µM | [29] | |
| 238 | euphoglomeruphane P | Whole plant, C3H6O | MDR-chemoreversal (MCF-7/ADR IC50 > 100 µM), verapamil; IC50 = 4.70 µM | [29] | |
| 239 | euphoglomeruphane Q | Whole plant, C3H6O | MDR-chemoreversal (MCF-7/ADR IC50 > 100 µM), verapamil; IC50 = 4.70 µM | [29] | |
| 240 | E.helioscopia | heliojatrone C | Aerial, EtOH | Inhibitory (nitric oxide (NO) in RAW 264.7; IC50 = 7.40 μM) compared to dexamethasone (Dex) | [64] |
| 241 | heliojatrone D | Aerial, EtOH | Inhibitory (nitric oxide (NO) in RAW 264.7; not active, compared to Dex | [64] | |
| 242 | euphoscopoid E | Aerial, EtOH | Inhibitory (nitric oxide (NO) in RAW 264.7; not active, compared to Dex | [64] | |
| 243 | euphoscopoid F | Aerial, EtOH | Inhibitory (nitric oxide (NO) in RAW 264.7; IC50 > 50 μM) compared to Dex | [64] | |
| 244 | euphorhelipanes A | Whole plant, EtOH | Triglyceride lowering effect (HuH7) in range of 1–50 μM compared to rosiglitazone positive control | [99] | |
| 245 | euphorhelipanes B | Whole plant, EtOH | Triglyceride lowering effect (HuH7) in range of 1–50 μM compared to rosiglitazone positive control | [99] | |
| Kaurane | |||||
| 246 | E. kansuensis | abbeokutone | Roots, EtOH | Inhibition of NO (IC50 = 43.60 µM; quercetin (IC50 = 10.80 µM) | [35] |
| Lathyrane | |||||
| 247 | E. lathyris | euphorbia factor L2 | Seeds, EtOH | Not evaluated | [66,105] |
| 248 | euphorbia factor L3 | Seeds, EtOH | Not evaluated | [66,105] | |
| 249 | E. stracheyi | euphstrachenol A | Roots, MeOH | Cytotoxic (HGC-27; IC50 > 50; taxol (0.015 µM) MV4-11; IC50 = 12.29; (0.055 µM) BaF3; IC50 > 20.00, compared to IC50 of 0.015, 0.53 µM, respectively for taxol | [32] |
| 250 | euphstrachenol B | Roots, MeOH | Cytotoxic (HGC-27; IC50 = 49.90; taxol (0.015 µM); MV4-11; IC50 = 14.80; (0.055 µM), BaF3; IC50 > 20.00, compared to IC50 of 0.015, 0.53 µM, respectively for taxol | [32] | |
| 251 | euphstrachenol C | Roots, MeOH | Cytotoxic (HGC-27, MV4-11, BaF3 SKvo3, IC50 > 50.00) compared to IC50 of 0.015, 0.53 µM, respectively for taxol | [32] | |
| 252 | (2R, 3S, 4R, 5R, 9S, 11S, 15R)-3, 5, 15-triacetoxy-14-oxolathyr- 6(17), 12E-diene | Roots, MeOH | Cytotoxic (HGC-27; IC50 > 50.00; taxol (0.015 µM) MV4-11; IC50 = 30.02; taxol (0.055 µM), BaF3; IC50 = 19.20, | [32] | |
| 253 | jolkinol B | Roots, MeOH | Cytotoxic (HGC-27; IC50 = 39.00; taxol (0.015 µM) MV4-11; IC50 = 9.82; (0.055 µM), BaF3; IC50 = 11.20, compared to IC50 of 0.015, 0.53 µM, respectively for taxol | [32] | |
| 254 | jolkinol A | Roots, MeOH | Cytotoxic (HGC-27, MV4-11, BaF3 SKvo3, IC50 > 50.00) compared to IC50 of 0.015, 0.53 µM, respectively for taxol | [32] | |
| 255 | jolkinoate C | Roots, MeOH | Cytotoxic (HGC-27; IC50 = 32.54; taxol (0.015 µM) MV4-11; IC50 = 15.37; (0.055 µM), BaF3; 18.80, SKvo3) compared to IC50 of 0.015, 0.53 µM, respectively for taxol | [32] | |
| 256 | jolkinol D | Roots, MeOH | Cytotoxic (HGC-27, MV4-11, BaF3 SKvo3, IC50 > 50.00) compared to IC50 of 0.015, 0.53 µM, respectively for taxol | [32] | |
| 257 | jolkinoate | Roots, MeOH | Cytotoxic (HGC-27; IC50 > 50.00; taxol (0.015 µM), MV4-11; IC50 = 5.96; (0.055 µM), BaF3; IC50 = 13.40 compared to IC50 of 0.015, 0.53 µM, respectively for taxol | [32] | |
| 258 | 3β, 5α, 20-trihydroxy-15β-cinnamoyloxy-14-oxolathyra-6Z, 12E-diene | Roots, MeOH | Cytotoxic (HGC-27, MV4-11, BaF3 SKvo3, IC50 > 50.00), taxol (0.015 µM) | [32] | |
| 259 | yuexiandajisu C | Roots, MeOH | Cytotoxic (HGC-27; IC50 > 50.00; taxol (0.015 µM), MV4-11; IC50 = 12.24; (0.055 µM), BaF3; IC50 = 13.40 µM compared to IC50 of 0.015, 0.53 µM, respectively for taxol | [32] | |
| 260 | jolkinolide E | Roots, MeOH | Cytotoxic (HGC-27, MV4-11, BaF3 SKvo3, IC50 > 50.00), (0.015 µM compared to IC50 of 0.015, 0.53 µM, respectively for taxol | [32] | |
| 261 | stracheyioid C | Roots, MeOH | Cytotoxic (HGC-27, MV4-11, BaF3 SKvo3, IC50 > 50.00), (0.015 µM compared to IC50 of 0.015, 0.53 µM, respectively for taxol | [32] | |
| 262 | E. royleana | ingol-3,7,12-triacetate-8-benzoate | Whole plant, EtOH | MDR-chemoreversal (Hep-G2/DOX; IC50 = 4.76 µM, Dox; 499.88 µM | [33] |
| 263 | ingol-3,8,12-triacetate-7-tiglate | Whole plant, EtOH | MDR-chemoreversal (Hep-G2/DOX; IC50 = 27.29 µM, dox; 499.88 µM | [33] | |
| 264 | 3,7,12-O-triacetyl-8-O-(2-methylbutanoyl)-ingol | Whole plant, EtOH | MDR-chemoreversal (Hep-G2/DOX; IC50 = 18.98 µM, dox; 499.88 µM | [33] | |
| 265 | euphorantin M | Whole plant, EtOH | MDR-chemoreversal (Hep-G2/DOX; IC50 = 20.81 µM, dox; 499.88 µM | [33] | |
| 266 | 3,12-di-O-acetyl-8-O-tigloyl-ingol | Whole plant, EtOH | Chemoreversal, combination abilities on Hep-G2/DOX; IC50 > 100 (10.65 µM), tar (2.31 µM) | [33] | |
| 267 | 8-O-methyl-ingol-3,12-diacetate-7-benzoate | Whole plant, EtOH | Chemoreversal, combination abilities on Hep-G2/DOX; IC50 > 100 (10.65 µM), tar (2.31 µM) | [33] | |
| 268 | 3,8,12-O-triacetylingol-7-benzoate | Whole plant, EtOH | MDR-chemoreversal (Hep-G2/DOX; IC50 = 11.18 µM, dox; 499.88 µM | [33] | |
| 269 | 8-O-methylingol-3,8,12-triacetate-7-angelate | Whole plant, EtOH | MDR-chemoreversal (Hep-G2/DOX; IC50 = 17.83 µM, dox; 499.88 µM | [33] | |
| 270 | 3,12-diacetyl-8-benzoylingol | Whole plant, EtOH | MDR-chemoreversal (Hep-G2/DOX; IC50 = 17.83 µM, dox; 499.88 µM | [33] | |
| 271 | 8-O-methylingol-12-acetate-7-angelate | Whole plant, EtOH | Chemoreversal, combination abilities on Hep-G2/DOX; IC50 > 100 (10.65 µM), tar (2.31 µM) | [33] | |
| 272 | ent-atis-16-ene-3,14-dione | Whole plant, EtOH | Chemoreversal, combination abilities on Hep-G2/DOX; IC50 > 100 (10.65 µM), tar (2.31 µM) | [33] | |
| 273 | eurifoloid L | Whole plant, EtOH | Chemoreversal, combination abilities on Hep-G2/DOX; IC50 > 100 (10.65 µM), tar (2.31 µM) | [33] | |
| 274 | eurifoloid J | Whole plant, EtOH | Chemoreversal, combination abilities on Hep-G2/DOX; IC50 > 100 (10.65 µM), tar (2.31 µM) | [33] | |
| 275 | eurifoloid G | Whole plant, EtOH | Chemoreversal, combination abilities on Hep-G2/DOX; IC50 > 100 (10.65 µM), tar (2.31 µM) | [33] | |
| 276 | eurifoloid E | Whole plant, EtOH | Chemoreversal, combination abilities on Hep-G2/DOX; IC50 > 100 (10.65 µM), tar (2.31 µM) | [33] | |
| 277 | antiquorine A | Whole plant, EtOH | Chemoreversal, combination abilities on Hep-G2/DOX; IC50 > 100 (10.65 µM), tar (2.31 µM) | [33] | |
| 278 | 5, 15-di-O-acetoxy-3-nicotinoyllathyol-6, 13(20)-diene-12-ol-14-one | Seeds, EtOH | MDR-chemoreversal (Hep-G2; IC50 > 100.00 µM, ADR; IC50 = 28.00 µM | [106] | |
| 279 | 5, 15,17-O-tri- acetyl-3-O-nicotinoyllathyol-6,12-diene -14-one | Seeds, EtOH | MDR-chemoreversal (Hep-G2; IC50 = 37.25 µM, ADR; IC50 = 14.81 | [106] | |
| 280 | 15-O-acetoxy-3,7-di-O-benzoyllathyra-6(17),12-diene-5-ol-14-one | Seeds, EtOH | MDR-chemoreversal (Hep-G2; IC50 = 66.05 µM, ADR; IC50 = 27.09 µM | [106] | |
| 281 | 15-O-acetyl-3-O-phenlacetate-6, 17-epoxylathyra-5-ol-14-one | Seeds, EtOH | MDR-chemoreversal (Hep-G2; IC50 > 100.00 µM, ADR; IC50 > 100.00 µM | [106] | |
| 282 | euphorbia factor L9 | Seeds, EtOH | Ant-HIV-1; inactive compared to zidovudine positive control | [75] | |
| 283 | euphorbia factor L15 | Seeds, EtOH | Ant-HIV-1; inactive compared to zidovudine control | [75] | |
| 284 | euphorbia factor L8 | Seeds, EtOH | Ant-HIV-1; inactive compared to zidovudine control | [75] | |
| 285 | 15-O-acetyl-3-O-nicotinoyljolkinol-5β,6β-oxide | Seeds, EtOH | Ant-HIV-1; inactive compared to zidovudine control | [75] | |
| 286 | 15,17-di-O-acetyl-3-O-hexanoyl-17-hydroxyjolkinol | Seeds, EtOH | Ant-HIV-1; inactive compared to zidovudine control | [75] | |
| 287 | 15,17-di-O-acetyl-3-O-benzoyl-17-hydroxyjolkinol | Seeds, EtOH | Ant-HIV-1; inactive compared to zidovudine control | [75] | |
| 288 | 15,17-di-O-acetyl-3-O-cinnamoyl-17-hydroxyjolkinol | Seeds, EtOH | Ant-HIV-1; inactive compared to zidovudine control | [75] | |
| 289 | 15-O-acetyl-3-O-cinnamoyl-17-hydroxyjolkinol | Seeds, EtOH | Ant-HIV-1; inactive compared to zidovudine control | [75] | |
| 290 | 15-O-acetyl-3-O-phenylacetyl-17-hydroxyjolkinol | Seeds, EtOH | Ant-HIV-1; inactive compared to zidovudine control | [75] | |
| 291 | 15-acetoxy-3-O-hexanoyl-17-hydroxyjolkinol-12-en-17-ol-14-one | Seeds, EtOH | Ant-HIV-1; inactive compared to zidovudine control | [75] | |
| 292 | 5,15,17-tri-O-acetyl-3-O-benzoyl-17-hydroxyisolathyrol | Seeds, EtOH | Ant-HIV-1; inactive compared to zidovudine control | [75] | |
| 293 | 5,15-di-O-acetyl-3-O-benzoyl-17-hydroxyisolathyrol | Seeds, EtOH | Ant-HIV-1; inactive compared to zidovudine control | [75] | |
| 294 | 5,15-di-acetoxy-3-nicotinoyloxy-6,17-epoxylathyra-12-en-14-one | Seeds, EtOH | Ant-HIV-1; inactive compared to zidovudine control | [75] | |
| 295 | ingenol-20-O-decanoyl | Seeds, EtOH | Ant-HIV-1; inactive compared to zidovudine control | [75] | |
| 296 | E. antiquorum | euphonoid B | Aerial, EtOH | Melanin synthesis (B16) at 50.00 µM, inactive | [34] |
| 297 | euphonoid C | Aerial, EtOH | Melanin synthesis (B16) at 50.00 µM, inactive | [34] | |
| 298 | euphonoid D | Aerial, EtOH | Melanin synthesis (B16) at 50.00 µM, inactive | [34] | |
| 299 | euphonoid E | Aerial, EtOH | Melanin synthesis (B16) at 50.00 µM, inactive | [34] | |
| 300 | E. kansuensis | euphanoid A | Roots, EtOH | Inhibitory (NO in RAW264.7; IC50 = 4.70 µM), quercetin (IC50 = 10.80 µM) | [35] |
| 301 | euphanoid B | Roots, EtOH | Inhibitory (NO in RAW264.7; IC50 = 9.50 µM), quercetin (IC50 = 10.80 µM) | [35] | |
| 302 | E. lathyris | (2S,3S,4S,5R,9S,11R,15R)-15-acetoxy-3-cinnamoyloxy-5-hydroxy-14-oxolathyra-6(17),12E-diene | Seeds, EtOH | Inhibitory (NO in RAW264.7; IC50 = 3.00 µM compared to dexamethasone (7.9 µM) | [103] |
| 303 | (2S,3S,4S,5R,7R,9S,11R,15R)-7,15-diacetoxy-3-benzoyloxy-5-hydroxy-14-oxolathyra 6(17),12E-diene | Seeds, EtOH | Inhibitory (NO in RAW264.7; IC50 = 4.00 µM compared to dexamethasone (7.9 µM) | [103] | |
| 304 | (2S,3S,4R,5R,7R,9S,11R,15R)-5,15-diacetoxy-3-benzoyloxy-7-hydroxy-14-oxolathyra-6(17),12E-diene | Seeds, EtOH | Inhibitory (NO in RAW264.7; IC50 = 5.00 µM compared to dexamethasone (7.9 µM) | [103] | |
| 305 | (2S,3S,4R,9S,11R,15R)-15,17-diacetoxy-3-hydroxy-14-oxolathyra-5E,12E-diene | Seeds, EtOH | Inhibitory (NO in RAW264.7; IC50 > 50.0 µM compared to dexamethasone (7.9 µM) | [103] | |
| 306 | (2S,3S,4R,5R,7R,9S,11R,15R)-5,15-diacetoxy-3-benzoyloxy-7-hydroxy-14-oxolathyra-6(17),12E-diene | Seeds, EtOH | Inhibitory (NO in RAW264.7; IC50 > 50.0 µM compared to dexamethasone (7.9 µM) | [103] | |
| 307 | (2S,3S,4R,5R,9S,11R,15R)-3-benzoyloxy-5,17-diacetoxy-15-hydroxy-14-oxolathyra-6E,12E-diene | Seeds, EtOH | Inhibitory (NO in RAW264.7; IC50 > 100.0 µM compared to dexamethasone (7.9 µM) | [103] | |
| 308 | E.antiquorum | euphorin C | Stems, MeOH | Inhibitory (NO production in BV-2; inactive | [56] |
| 309 | euphorin D | Stems, MeOH | Inhibitory (NO production in BV-2; IC50 = 32.00 µM); SMT (4.2 µM) | [56] | |
| 310 | euphorin E | Stems, MeOH | Inhibitory (NO production in BV-2; IC50 = 40.70 µM), SMT (4.2 µM) | [56] | |
| Meroterpenoid | |||||
| 311 | E. fischeriana | fischernolide A | Roots, EtOH: H2O (95:5) | Cytotoxic (Bel-7402; IC50 = 27.30, HT; IC50 = 49.61 µM, A549; IC50 = 20.53 µM, MCF-7; IC50 = 33.70 µM, HeLa; IC50 = 35.65 µM) compared to cisplatin; 11.9, 33.48, 12.02, 12.78, 8.65 µM respectively | [30] |
| 312 | fischernolide B | Roots, EtOH: H2O (95:5) | Cytotoxic (Bel-7402; IC50 = 5.04 µM, HT; IC50 = 7.59 µM, A549; IC50 = 8.69 µM, MCF-7; IC50 = 4.95 µM, HeLa; IC50 = 7.53 µM) compared to cisplatin; 11.9, 33.48, 12.02, 12.78, 8.65 µM respectively | [30] | |
| 313 | fischernolide C | Roots, EtOH: H2O (95:5) | Cytotoxic (Bel-7402; IC50 = 3.30 µM, HT; IC50 = 4.21 µM, A549; IC50 = 3.27, µM MCF-7; IC50 = 2.04 µM, HeLa; IC50 = 4.22 µM) compared to cisplatin; 11.9, 33.48, 12.02, 12.78, 8.65 µM respectively | [30] | |
| 314 | fischernolide D | Roots, EtOH: H2O (95:5) | Cytotoxic (Bel-7402; IC50 = 11.96 µM, HT; IC50 = 33.48 µM, A549; IC50 = 9.57 µM, MCF-7; IC50 = 14.98 µM, HeLa; IC50 = 10.22 µM) compared to cisplatin; 11.9, 33.48, 12.02, 12.78, 8.65 µM respectively | [30] | |
| 315 | fischeriana A | Roots, EtOH | Not evaluated | [47] | |
| Mysrinane | |||||
| 316 | E. prolifera | 5α,10β,14β,15β-O-tetraacetyl-8β-O-benzoyl-3β-O-nicotinoylcyclomyrsinol | Roots, MeOH | Lipid-lowering activity in 3T3-L1 adipocytemodel using R17 control | [37] |
| 317 | 5α,10β,14β,15β-O-tetraacetyl-8β-O-isobutyryl-3β-O-nicotinoylcyclomyrsinol | Roots, MeOH | Lipid-lowering activity in 3T3-L1 adipocytemodel using R17 control | [37] | |
| 318 | 5α,7β,10,14β,15β-O-pentaacetyl-3β-O-butyryl-14-desoxo-10,18-dihydromyrsinol | Roots, MeOH | Lipid-lowering activity in 3T3-L1 adipocytemodel using R17 control | [37] | |
| 319 | 5α,7β,10,14β,15β-O-pentaacetyl-14-desoxo-10,18-dihydro-3β-O-propionylmyrsinol | Roots, MeOH | Lipid-lowering activity in 3T3-L1 adipocytemodel using R17 control | [37] | |
| 320 | 5α,7β,10,15β-O-tetraacetyl-3β-O-benzoyl-14-desoxo-10,18-dihydro-14α-O-nicotinoylmyrsinol | Roots, MeOH | Lipid-lowering activity in 3T3-L1 adipocytemodel using R17 control | [37] | |
| 321 | 3β,5α,7β,10,15β-O-pentaacetyl-14α-O-benzoyl-14-desoxo-10,18-dihydro-2α-hydroxylmyrsinol | Roots, MeOH | Lipid-lowering activity in 3T3-L1 adipocytemodel using R17 control | [37] | |
| 322 | 7β,13β,17-O-triacetyl-5-O-benzoyl-3β-O-nicotinoylpremyrsinol | Roots, MeOH | Lipid-lowering activity in 3T3-L1 adipocytemodel using R17 control | [37] | |
| Paralianone | |||||
| 323 | E. peplus | 8β-acetyl- paralianone D | Whole plant, CH3OH | Cytotoxic (HL-60, A-549, SMMC-7721, MCF-7, SW480). Inactive at 40 µM, using paclitaxel and cisplatin as control. Enhanced the LysoTracker intensity of 132.6% at 3.20 µM using DMSO as the control | [38] |
| 324 | paralianone | Whole plant, CH3OH | Cytotoxic (HL-60, A-549, SMMC-7721, MCF-7, SW480). Inactive at 40 µM, using paclitaxel and cisplatin as control. | [38] | |
| 325 | paralianone D | Whole plant, CH3OH | Cytotoxic (HL-60, A-549, SMMC-7721, MCF-7, SW480). Inactive at 40 µM, using paclitaxel and cisplatin as control. | [38] | |
| Pimarane | |||||
| 326 | E. stracheyi | (3β, 12α, 13α)-3, 12-dihydrxypiar-7, 15-dien-2-one | Roots, MeOH | Not evaluated | [32] |
| 327 | (5β, 9β, 10α)-2-hydroxypimara-1, 7, 15-trien-3-one | Roots, MeOH | Not evaluated | [32] | |
| 328 | (3α, 5β, 8α, 9β, 10α, 12α)-3-hydroxytis-16-en-14-one | Roots, MeOH | Not evaluated | [32] | |
| Premyrsinane | |||||
| 329 | E. sanctae-catharinae | euphosantianane E | Aerial, CH2CI2: MeOH | Not evaluated | [26] |
| 330 | euphosantianane F | Aerial, CH2CI2: MeOH | Not evaluated | [26] | |
| 331 | euphosantianane G | Aerial, CH2CI2: MeOH | Not evaluated | [26] | |
| Rosane | |||||
| 332 | E. ebracteolata | ebraphenol A | Roots, EtOH (n-BuOH, EtOAc) | Lipase inhibitory (IC50 = 1.00 µM) compared to lovastatin positive control; IC50 = 0.24 µM | [48] |
| 333 | ebraphenol B | Roots, EtOH (n-BuOH, EtOAc) | Lipase inhibitory (IC50 = 0.24 µM) compared to lovastatin positive control; IC50 = 0.24 µM | [48] | |
| 334 | ebraphenol C | Roots, EtOH (n-BuOH, EtOAc) | Lipase inhibitory (IC50 = 0.24 µM) compared to lovastatin positive control; IC50 = 0.24 µM | [48] | |
| 335 | ebraphenol D | Roots, EtOH (n-BuOH, EtOAc) | Lipase inhibitory (IC50 = 0.24 µM) compared to lovastatin positive control; IC50 = 0.24 µM | [48] | |
| 336 | ebralactone A | Roots, EtOH (n-BuOH, EtOAc) | Lipase inhibitory (IC50 = 0.24 µM) compared to lovastatin positive control; IC50 = 0.24 µM | [48] | |
| 337 | E. neriifolia | euphnerin A | Stems, MeOH | NO inhibitory (BV-2, IC50 = 22.00 µM) compared to SMT positive control 2.00 µM | [71] |
| 338 | euphnerin B | Stems, MeOH | NO inhibitory (BV-2, IC50 = 30.00 µM) compared to SMT positive control 2.00 µM | [71] | |
| Tigliane | |||||
| 339 | E. fischeriana | prostratin 20-O-(6′-acetate)-β-D-glucopyranoside | Roots, EtOH | Cytotoxic (AGS; IC50 = 40.56 µM, Hep-G2; IC50 = 27.97 µM) compared to oxaliplatin; IC50 of 17.06 and 24.26 µM respectively | [63] |
| 340 | fischeroside A | Roots, EtOH | Cytotoxic (AGS; IC50 = 27.97 µM, Hep-G2; IC50 = 17.59 µM) compared to oxaliplatin; IC50 of 17.06 and 24.26 µM respectively | [63] | |
| 341 | 12-deoxyphorbol-13-dimethylpentadecanoate | Roots, MeOH | Lysosomal biogenesis activity (183.21%) using blank control | [102] | |
| 342 | 17-hydroxy,11α, 8(14) epoxy-ent-abieta-13(15)-ene-11,12-dioxide | Roots, MeOH | Lysosomal biogenesis activity (181.95%) using blank control | [102] | |
| 343 | E. lathyris | eupholathone | Seeds, EtOH | Not evaluated | [66] |
| 344 | E. grandicornis | 16-angeloyloxy-13α -isobutanoyloxy-4β, 9α-dihydroxytiglia-1, 6- dien-3-one. | Aerial, MeOH | Protein kinase C activation and platelet stimulation abilities | [52] |
| 345 | 20-acetoxy-13α-isobutanoyloxy-4β, 9α, 16-trihydroxytiglia-1, 6-dien-3-one. | Aerial, MeOH | Protein kinase C activation and platelet stimulation abilities | [52] | |
| 346 | E. dracunculoides | 4-deoxy-4(β)H-8-hydroperoxyphorbol-12-benzoate-13-isobutyrate | Whole plant, EtOH | Not evaluated | [57] |
| Myrsinol | |||||
| 347 | E. prolifera | euphorbialoid K | Roots, MeOH | Not evaluated | [74] |
| 348 | euphorbialoid L | Roots, MeOH | Not evaluated | [74] | |
| 349 | euphorbialoid M | Roots, MeOH | Not evaluated | [74] | |
| 350 | euphorbialoid N | Roots, MeOH | Not evaluated | [74] | |
| 351 | E. dracunculoides | euphordracunculin A | Aerial, EtOH | Cytotoxic (HL-60, SMMMC-7721, A-549, MCF-7, SW-480); Inactive (IC50 > 40 µM) | [107] |
| 352 | euphordracunculin B | Aerial, EtOH | Cytotoxic (HL-60, SMMMC-7721, A-549, MCF-7, SW-480); Inactive (IC50 > 40 µM) | [107] | |
| Paraliane | |||||
| 353 | E. esula | euphorbesulin D | Twigs, EtOH | Antimalarial (IC50 > 50 µM) compared to artemisinin (7.01 µM) as a positive control | [49] |
| 354 | E. peplus | pepluanol A | Whole plant, C3H6O | Inhibition of LPS-stimulated NO production in RAW264.7 cells (IC50 > 50 µM) compared to proteasome inhibitor (MG-132) with IC50 of 0.18 µM | [72] |
| 355 | pepluanol B | Whole plant, C3H6O | Inhibition of LPS-stimulated NO production in RAW264.7 cells (IC50 > 50 µM) compared to proteasome inhibitor (MG-132) with IC50 of 0.18 µM | [72] | |
| 356 | pepluanol C | Whole plant, C3H6O | Inhibition of LPS-stimulated NO production in RAW264.7 cells (IC50 > 50 µM) compared to proteasome inhibitor (MG-132) with IC50 of 0.18 µM | [72] | |
| 357 | pepluanol D | Whole plant, C3H6O | Inhibition of LPS-stimulated NO production in RAW264.7 cells (IC50 > 50 µM) compared to proteasome inhibitor (MG-132) with IC50 of 0.18 µM | [72] | |
| 358 | pepluanol E | Whole plant, C3H6O | Inhibition of LPS-stimulated NO production in RAW264.7 cells (IC50 > 50 µM) compared to proteasome inhibitor (MG-132) with IC50 of 0.18 µM | [72] | |
| 359 | pepluanol F | Whole plant, C3H6O | Inhibition of LPS-stimulated NO production in RAW264.7 cells (IC50 > 50 µM) compared to proteasome inhibitor (MG-132) with IC50 of 0.18 µM | [72] | |
| 360 | pepluanol G | Whole plant, C3H6O | Inhibition of LPS-stimulated NO production in RAW264.7 cells (IC50 = 36.6 µM) compared to proteasome inhibitor (MG-132) with IC50 of 0.18 µM | [72] | |
| 361 | pepluanol H | Whole plant, C3H6O | Inhibition of LPS-stimulated NO production in RAW264.7 cells (IC50 > 50 µM) compared to proteasome inhibitor (MG-132) with IC50 of 0.18 µM | [72] | |
| Pepluane | |||||
| 362 | E. peplus | paralianone A | Whole plant, C3H6O | Inhibition of LPS-stimulated NO production in RAW264.7 cells (IC50 = 43.2 µM) compared to proteasome inhibitor (MG-132) with IC50 of 0.18 µM | [72] |
| 363 | paralianone B | Whole plant, C3H6O | Inhibition of LPS-stimulated NO production in RAW264.7 cells (IC50 > 50 µM) compared to proteasome inhibitor (MG-132) with IC50 of 0.18 µM | [72] | |
| 364 | paralianone C | Whole plant, C3H6O | Inhibition of LPS-stimulated NO production in RAW264.7 cells (IC50 = 33.7 µM) compared to proteasome inhibitor (MG-132) with IC50 of 0.18 µM | [72] | |
| 365 | paralianone D | Whole plant, C3H6O | Inhibition of LPS-stimulated NO production in RAW264.7 cells (IC50 = 38.3 µM) compared to proteasome inhibitor (MG-132) with IC50 of 0.18 µM | [72] | |
| Presegetane | |||||
| 366 | E. esula | euphorbesulin A | Twigs, EtOH | Antimalarial (IC50 = 2.41 µM) compared to artemisinin (7.01 µM) as a positive control | [49] |
| 367 | euphorbesulin B | Twigs, EtOH | Antimalarial (IC50 > 5 µM) compared to artemisinin (7.01 µM) as a positive control | [49] | |
| 368 | euphorbesulin C | Twigs, EtOH | Antimalarial (IC50 > 5 µM) compared to artemisinin (7.01 µM) as a positive control | [49] | |
| Others | |||||
| 369 | E. aellenii | 3-nicotinyl-5,10,14,15- tetraacetyl-8-(20,30-dimethyl butanoyl)-cyclomyrsinol | Aerial, C3H6O: CHCl3 (1:2) | Lymphocytes proliferative effects (p > 0.05, at 50 µg/mL) using stimulated and unstimulated T cells in absence of the compound as the control | [39] |
| 370 | 3,5,10,14,15-pentaacetyl-8-isobutanoyl cyclomyrsinol | Aerial, C3H6O: CHCl3 (1:2) | Lymphocytes proliferative effects (p > 0.05, at 50 µg/mL) using stimulated and unstimulated T cells in absence of the compound as the control | [39] | |
| 371 | E. pilosa | euphopiloside A | Whole plant, EtOH | Cytotoxic (HL-60, SMMMC-7721, A-549, MCF-7, SW-480); moderate activity compared to cisplatin with IC50 values of 3.29, 9.26, 9.98, 15.92 and 14.43 µM respectively | [40] |
| 372 | euphopiloside B | Whole plant, EtOH | Cytotoxic (HL-60, SMMMC-7721, A-549, MCF-7, SW-480); moderate activity compared to cisplatin with IC50 values of 3.29, 9.26, 9.98, 15.92 and 14.43 µM respectively | [40] | |
| 373 | E. kansui | euphorikanin A | Roots, EtOH | Cytotoxic effect (HeLa; IC50 = 20.89 µM, NCI-446; 28.83 µM compared to etoposide (IC50 of 26.23 and 30.68 µM respectively) | [53] |
| 374 | E. esula | euphorbesulin E | Twigs, EtOH | Antimalarial (IC50 > 50 µM) compared to artemisinin (7.01 µM) as a positive control | [49] |
| 375 | E. dracunculoides | euphordracunculin C | Aerial, C3H6O: H2O (7:3) | Not evaluated | [107] |
| 376 | E. peplus | pepluacetal | Roots, MeOH | Inhibition of Kv1.3 channel with IC50 value of 24.9 µM | [72] |
| 377 | pepluanol A | Roots, MeOH | Inhibition of Kv1.3 channel with IC50 value of 46.0 µM | [72] | |
| 378 | pepluanol B | Roots, MeOH | Inhibition of Kv1.3 channel with IC50 value of 9.50 µM | [72] | |
| 379 | E. micractina | secoeuphoractin | Roots, EtOH | Anti-HIV-1 replication ability (IC50 = 1.76 µmol/L) compared to zidovudine (0.005 µmol/L) as positive control | [67] |
| 380 | euphorbactin | Roots, EtOH | Anti-HIV-1 replication ability (IC50 = 28.6 µM) compared to zidovudine (0.005 µM) as positive control | [104] | |
| 381 | E. kopetdaghi | kopetdaghinane A | Aerial, CH2CI2: C3H6O (2:1) | Cytotoxic (MCF-7; IC50 = 38.10 µM, OCVAR-3; IC50 = 51.23 µM) compared to taxol (44.61 and 52.3 µM respectively) | [25] |
| 382 | kopetdaghinane B | Aerial, CH2CI2: C3H6O (2:1) | Cytotoxic (MCF-7; IC50 =38.10 µM, OCVAR-3; IC50 = 51.23 µM) compared to taxol (44.61 and 52.3 µM respectively) | [25] | |
6. Pharmacological Activities and Structure–Activity Relationship (SAR)
6.1. Anticancer Activities
6.2. Multidrug Resistance Activities
6.3. Inhibition Activities
6.4. Anti-HIV Activities
6.5. Anti-Influenza
6.6. Melanin Synthesis
6.7. Antibacterial and Antimalarial Activities
7. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AA | Arachidonic acid |
| A-549 | Human lung cancer cells |
| AGS | Human gastric cancer cell lines |
| BaF3 | Human murine cell line lymphocyte |
| BMM | Osteoclastogenesis cells |
| C4-2B | Human prostate cancer |
| C4-2B/ENZR | Enzalutamide-resistant C4-2B cell line |
| DU145 | Human prostate cancer cells |
| GGPP | Geranyl geranyl pyrophosphate |
| H460 | Human lung carcinoma |
| Hep-G2 | Human liver cancer cells |
| Hep-G2/ADR | Hepatocellular carcinoma |
| HGC-27 | Human stomach cancer cell lines |
| HL-60 | Human leukemia cells |
| MDA-MB-231 | Human breast cancer cells |
| MDCK | Madin-Darby canine kidney cells |
| MV4-11 | Human leukemia cells |
| RANKL | Receptor activator of nuclear factor kappa B ligand |
| RAW264.7 | Macrophages cells |
| Skvo3 | Human ovarian carcinoma |
| SMMC-7721 | Liver cancer cells |
| SW480 | Colon cancer cells |
References
- De Montellano, B.O. Empirical Aztec medicine. Science 1975, 188, 215–220. [Google Scholar] [CrossRef] [Green Version]
- Hooper, M. Major Herbs of Ayurveda; Elsevier Health Sciences, Elsevier: Amsterdam, The Netherlands, 2002; pp. 340–345. [Google Scholar]
- Hargreaves, B.J. The spurges of Botswana. Botsw. Notes Rec. 1991, 23, 115–158. [Google Scholar]
- Lai, X.Z.; Yang, Y.B.; Shan, X.U.L. The investigation of Euphorbiaceus medicinal plants in Southern China. Econ. Bot. 2004, 58, S307–S320. [Google Scholar] [CrossRef]
- Ernst, M.; Grace, O.M.; Saslis-Lagoudakis, C.H.; Haris, S.L.; Niclas, N.; Henrik, T.; Nina, R. Global medicinal uses of Euphorbia L. (Euphorbiaceae). J. Ethnopharmacol. 2015, 76, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Mwine, J.; Van Damme, P. Why do Euphorbiaceae tick as medicinal plants? A review of the Euphorbiaceae family and its medicinal features. J. Med. Plant. Res. 2011, 5, 652–662. [Google Scholar]
- Zeghad, F.; Djilani, S.E.; Djilani, A.; Dicko, A. Antimicrobial and antioxidant activities of three Euphorbia species. Turk. J. Pharm. Sci. 2016, 13, 47–56. [Google Scholar] [CrossRef]
- Vasas, A.; Rédei, D.; Csupor, D.; Molnár, J.; Hohmann, J. Diterpenes from European Euphorbia species serving as prototypes for natural-product-based drug discovery. Eur. J. Org. Chem. 2012, 5115–5130. [Google Scholar] [CrossRef]
- Vasas, A.; Hohmann, J. Euphorbia diterpenes: Isolation, structure, biological activity, and synthesis (2008−2012). Chem. Rev. 2014, 114, 8579–8612. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.T.; Mata, O.; Aguiar, R.S.; Paz, M.C.J.; Alencar, M.B.; Melo-cavalcante, A.C. Therapeutic potential of essential oils focusing on diterpenes. Phytother. Res. 2016, 30, 1420–1444. [Google Scholar] [CrossRef]
- Scholz, A. Euphorbiaceae. In Syllabus der Planzenfamilien; Engler, A., Ed.; Gebrüder Bornträger: Berlin, Germany, 1964; pp. 255–261. [Google Scholar]
- Webster, G.L. Classification of the Euphorbiaceae. Ann. Mol. Bot. Gar. 1994, 81, 3–32. [Google Scholar] [CrossRef]
- Shi, Q.W.; Su, X.H.; Kiyota, H. Chemical and pharmacological research of the plants in genus Euphorbia. Chem. Rev. 2008, 108, 4295–4327. [Google Scholar] [CrossRef]
- Jin, Y.X.; Shi, L.L.; Zhang, D.P.; Wei, H.Y.; Si, Y.; Ma, G.X.; Zhang, J. A review on daphnane-type diterpenoids and their bioactive studies. Molecules 2019, 24, 1842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jury, L.; Reynolds, T.; Cutler, F.; Evans, F. The Euphorbiales: Chemistry, Taxonomy, and Economic Botany; Academic Press: London, UK, 1987. [Google Scholar]
- Gras, J. Ingenol mebutate: A new option for actinic keratosis treatment. Drugs Today 2013, 49, 15–22. [Google Scholar] [CrossRef]
- Hanson, J.R. Diterpenoids: Methods in Plant Biochemistry; Charlwood, B.V., Banthorpe, D.V., Eds.; Academic Press: London, UK, 1991; pp. 263–288. [Google Scholar]
- Cheng, A.; Lou, Y.; Mao, Y.; Lu, S.; Wang, L.; Chen, X. Plant terpenoids: Biosynthesis and ecological functions. J. Integr. Plant. Biol. 2007, 49, 179–186. [Google Scholar] [CrossRef]
- Dewick, M. Medicinal Natural Products: A Biosynthetic Approach, 3rd ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2006; ISBN 978-0-470-74168-9. [Google Scholar]
- Goel, G.; Makkar, S.; Francis, G.; Becker, K. Phorbol esters: Structure, biological activity, and toxicity in animals. Int. J. Toxicol. 2007, 26, 279. [Google Scholar] [CrossRef]
- Wang, H.B.; Wang, X.Y.; Liu, L.P.; Qin, G.W.; Kang, T.G. Tigliane Diterpenoids from the Euphorbiaceae and Thymelaeaceae Families. Chem. Rev. 2015, 115, 2975–3011. [Google Scholar] [CrossRef]
- Wongrakpanich, W.; Purin, C. Induction of apoptosis in cancer cells by plants in the genus Euphorbia. Thai Bull. Pharm. Sci. 2018, 13, 1–11. [Google Scholar] [CrossRef]
- Salehi, B.; Iriti, M.; Vitalini, S.; Antolak, H.; Pawlikowska, E.; Kręgiel, D.; Sharifi-Rad, J.; Oyeleye, I.; Ademiluyi, O.; Czopek, K.; et al. Euphorbia-derived natural products with potential for use in health maintenance. Biomolecules 2019, 9, 337. [Google Scholar] [CrossRef] [Green Version]
- Kemboi, D.; Peter, X.; Langat, M.; Tembu, J. A review of the ethnomedicinal uses, biological activities, and Triterpenoids of Euphorbia Species. Molecules 2020, 25, 4019. [Google Scholar] [CrossRef] [PubMed]
- Riahi, F.; Dashti, N.; Ghanadian, M.; Aghaei, M.; Faez, F.; Jafari, S.M.; Zargar, N. Kopetdaghinanes, pro-apoptotic hemiacetialic cyclomyrsinanes from Euphorbia kopetdaghi. Fitoterapia 2020, 146, 104636. [Google Scholar] [CrossRef]
- Elshamy, A.I.; Mohamed, T.A.; Al-Rowaily, S.L.; Abd-ElGawad, A.M.; Dar, B.A.; Shahat, A.A.; Hegazy, M.E.F. Euphosantianane E-G: Three new premyrsinane type diterpenoids from Euphorbia sanctae-catharinae with the contribution to chemotaxonomy. Molecules 2019, 24, 2412. [Google Scholar] [CrossRef] [Green Version]
- Flores-Giubi, M.E.; Durán-Pena, M.J.; Botubol-Ares, J.M.; Escobar-Montano, F.; Zorrilla, D.; Macías-Sánchez, A.J.; Hernández-Galán, R. Gaditanone, a diterpenoid based on an unprecedented carbon skeleton isolated from Euphorbia gaditana. J. Nat. Prod. 2017, 80, 2161–2165. [Google Scholar] [CrossRef] [Green Version]
- Bin Muhsinah, A.; Eko Nugroho, A.; Li, H.; Lazzaro, S.; DaSilva, N.A.; Li, D.; Ma, H.; Alsayari, A.; Morita, H.; Liu, Y.; et al. Saudiarabicains A-E, bioactive 19-acetoxyingol diterpenoids from Euphorbia saudiarabica. Tetrahedron Lett. 2020, 61, 152203. [Google Scholar] [CrossRef]
- Hasan, A.; Liu, G.Y.; Hu, R.; Aisa, H.A. Jatrophane Diterpenoids from Euphorbia glomerulans. J. Nat. Prod. 2019, 82, 724–734. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; He, J.; Cheng, Y.C.; Zhang, P.C.; Yan, Y.; Zhang, H.J.; Zhang, W.K.; Xu, J.K. Fischernolides A-D, four novel diterpene-based meroterpenoid scaffolds with antitumor activities from: Euphorbia fischeriana. Org. Chem. Front. 2019, 6, 2312–2318. [Google Scholar] [CrossRef]
- Wang, S.; Li, H.; Liu, D.; Zhao, Q.; Yang, T.; Li, R.; Chen, X. Diterpenoids from the Seeds of Euphorbia lathyris and their in Vitro Anti-HIV Activity. Chem. Nat. Compd. 2020, 56, 78–85. [Google Scholar] [CrossRef]
- Ye, Y.; Liu, G.H.; Dawa, D.; Ding, L.S.; Cao, Z.X.; Zhou, Y. Cytotoxic diterpenoids from the roots of Euphorbia stracheyi. Phytochem. Lett. 2020, 36, 183–187. [Google Scholar] [CrossRef]
- Shaker, S.; Sang, J.; Yan, X.L.; Fan, R.Z.; Tang, G.H.; Xu, Y.K.; Yin, S. Diterpenoids from Euphorbia royleana reverse P-glycoprotein-mediated multidrug resistance in cancer cells. Phytochemistry 2020, 176, 112395. [Google Scholar] [CrossRef]
- Yuan, W.J.; Gao, W.F.; Zhao, J.Y.; Zhang, Y.; Chen, D.Z.; Li, S.L.; Di, Y.T.; Hao, X.J. Diterpenes with the potential treatment of vitiligo from the aerials parts of Euphorbia antiquorum L. Fitoterapia 2020, 144, 104583. [Google Scholar] [CrossRef]
- Yan, X.L.; Fan, R.Z.; Sang, J.; Xie, X.L.; Tang, G.H.; Yin, S. Euphanoids A, and B, two new lathyrane diterpenoids with nitric oxide (NO) inhibitory activity from Euphorbia kansuensis. Nat. Prod. Res. 2020, 1–7. [Google Scholar] [CrossRef]
- Yan, X.L.; Sang, J.; Chen, S.X.; Li, W.; Tang, G.H.; Gan, L.S.; Yin, S. Euphorkanlide A, a highly modified ingenane diterpenoid with a C24 appendage from Euphorbia kansuensis. Org. Lett. 2019, 21, 4128–4131. [Google Scholar] [CrossRef]
- Song, Q.Q.; Rao, Y.; Tang, G.H.; Sun, Z.H.; Zhang, J.S.; Huang, Z.S.; Yin, S. Tigliane diterpenoids as a new type of antiadipogenic agents inhibit GRα-Dexras1 axis in adipocytes. J. Med. Chem. 2019, 62, 2060–2075. [Google Scholar] [CrossRef]
- Chen, Y.N.; Lu, Q.Y.; Li, D.M.; Li, Y.Y.; Pu, X.X.; Li, B.T.; Tang, X.H.; Tang, H.Y.; Liu, S.; Yang, L.; et al. Three new diterpenoids from Euphorbia peplus. Nat. Prod. Res. 2020, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ghanadian, M.; Choudhary, M.I.; Ayatollahi, A.M.; Mesaik, M.A.; Abdalla, O.M.; Afsharypour, S. New cyclomyrsinol diterpenes from Euphorbia aellenii with their immunomodulatory effects. J. Asian Nat. Prod. Res. 2013, 15, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.D.; Ni, W.; Yan, H.; Li, G.T.; Zhong, H.M.; Li, Y.; Liu, H.Y. Daphnane-type diterpenoid glucosides and further constituents of Euphorbia pilosa. Chem. Biodivers. 2014, 11, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fan, R.Z.; Sang, J.; Tian, Y.J.; Chen, J.Q.; Tang, G.H.; Yin, S. Ingol diterpenoids as P-glycoprotein-dependent multidrug resistance (MDR) reversal agents from Euphorbia marginata. Bioorg. Chem. 2020, 95, 103546. [Google Scholar] [CrossRef]
- Yan, S.L.; Li, Y.H.; Chen, X.Q.; Liu, D.; Chen, C.H.; Li, R.T. Diterpenes from the stem bark of Euphorbia neriifolia and their in vitro anti-HIV activity. Phytochemistry 2018, 145, 40–47. [Google Scholar] [CrossRef]
- Wang, S.Y.; Li, G.Y.; Zhang, K.; Wang, H.Y.; Liang, H.G.; Huang, C.; Huang, J.; Wang, J.H.; Yang, B.F. New ingol-type diterpenes from the latex of Euphorbia resinifera. J. Asian Nat. Prod. Res. 2019, 21, 1075–1082. [Google Scholar] [CrossRef]
- Yan, X.L.; Huang, J.L.; Tang, Y.Q.; Tang, G.H.; Yin, S. Euphopanes A–C, three new diterpenoids from Euphorbia pekinensis. Nat. Prod. Res. 2020, 1–8. [Google Scholar] [CrossRef]
- Wei, W.J.; Qi, W.; Gao, X.M.; Feng, K.N.; Ma, K.L.; Li, H.Y.; Li, Y.; Gao, K. Anti-inflammatory evaluation and structure-activity relationships of diterpenoids isolated from Euphorbia hylonoma. Bioorg. Chem. 2019, 93, 103256. [Google Scholar] [CrossRef]
- Liu, S.N.; Hu, J.; Tan, S.H.; Wang, Q.; Xu, J.; Wang, Y.; Yuan, Y.; Gu, Q. Ent -rosane diterpenoids from Euphorbia milii showing an Epstein-Barr virus lytic replication assay. RSC Adv. 2017, 7, 46938–46947. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Yang, P.; Zhang, E.H.; Kong, L.M.; Meng, C.Y. Antimicrobial ent-abietane-type diterpenoids from the roots of Euphorbia wallichii. J. Asian Nat. Prod. Res. 2020, 23, 652–659. [Google Scholar] [CrossRef]
- Li, L.; Li, D.; Wang, C.; Feng, L.; Yu, Z.; Ning, J.; Zhang, B.; Zhang, H.; Wang, C.; Ma, X. Aromatic rosane diterpenoids from the roots of Euphorbia ebracteolata and their inhibitory effects against lipase. Bioorg. Chem. 2020, 94, 103360. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Wu, Y.; Dalal, S.; Cassera, M.B.; Yue, J.M. Euphorbesulins A-P, Structurally diverse diterpenoids from Euphorbia esula. J. Nat. Prod. 2016, 79, 1952–1961. [Google Scholar] [CrossRef]
- Zhou, M.; Ma, Q.; He, L.; Chen, Y.H.; Zhu, B.Y.; Wang, J.H.; Yang, Q.; Liu, S.; Ma, L.M. Cytotoxic jatrophane diterpenoids from the aerial parts of Euphorbia helioscopia. J. Asian Nat. Prod. Res. 2020, 1–7. [Google Scholar] [CrossRef]
- Zhang, B.Y.; Yin, H.X.; Zhang, D.J. Two New ent-labdane diterpenes from the roots of Euphorbia yinshanica. Chem. Nat. Compd. 2017, 53, 295–298. [Google Scholar] [CrossRef]
- Tsai, J.Y.; Rédei, D.; Forgo, P.; Li, Y.; Vasas, A.; Hohmann, J.; Wu, C.C. Isolation of phorbol esters from Euphorbia grandicornis and evaluation of protein kinase C- and human platelet-activating effects of Euphorbiaceae diterpenes. J. Nat. Prod. 2016, 79, 2658–2666. [Google Scholar] [CrossRef]
- Fei, D.Q.; Le Dong, L.; Qi, F.M.; Fan, G.X.; Li, H.H.; Li, Z.Y.; Zhang, Z.X. Euphorikanin A, a diterpenoid lactone with a fused 5/6/7/3 ring system from Euphorbia kansui. Org. Lett. 2016, 18, 2844–2847. [Google Scholar] [CrossRef]
- Tran, T.N.; Sichaem, J.; Nguyen, V.K.; Nguyen, H.H.; Cao, T.T.; Nguyen, T.P.; Vo, V.G.; Niamnont, N.; Nguyen, N.H.; Duong, T.H. New diterpenoids from the stems of Euphorbia antiquorum growing in Vietnam. Nat. Prod. Res. 2020, 1–8. [Google Scholar] [CrossRef]
- Tran, T.N.; Sichaem, J.; Nguyen, V.K.; Chavasiri, W.; Niamnont, N.; Jongaramruong, J.; Duong, T.H. A new ent-atisane diterpenoid from the aerial parts of Euphorbia antiquorum L. Nat. Prod. Res. 2021, 35, 312–317. [Google Scholar] [CrossRef]
- An, L.; Liang, Y.; Yang, X.; Wang, H.; Zhang, J.; Tuerhong, M.; Li, D.; Wang, C.; Lee, D.; Xu, J.; et al. NO inhibitory diterpenoids as potential anti-inflammatory agents from Euphorbia antiquoru. Bioorg. Chem. 2019, 92, 103237. [Google Scholar] [CrossRef]
- Dai, L.F.; Liang, Q.; Liu, T.; He, M.Y.; Zhao, P.; Xu, W.H. A new tigliane-type diterpene from Euphorbia dracunculoides Lam. Nat. Prod. Res. 2016, 30, 1639–1645. [Google Scholar] [CrossRef]
- Wang, L.; Zang, Z.; Wang, Y.F.; Huang, S.X.; Cao, P.; Zhao, Y. Two new myrinsol diterpenoids from Euphorbia dracunculoides Lam. Chin. Chem. Lett. 2015, 26, 121–123. [Google Scholar] [CrossRef]
- Xie, X.L.; Fan, R.Z.; Hu, R.; Luo, S.Y.; Tang, G.H.; Yin, S. Euphoresulanes A–M, structurally diverse jatrophane diterpenoids from Euphorbia esula. Bioorg. Chem. 2020, 98, 103763. [Google Scholar] [CrossRef]
- Xie, R.; Li, L.; Fan, X.; Zi, J. Euphoractone, a cytotoxic meroterpenoid with an unusual ent-abietane-phloroglucinol skeleton, from Euphorbia fischeriana Steud. Chin. Chem. Lett. 2020, 31, 431–433. [Google Scholar] [CrossRef]
- Li, M.; He, F.; Zhou, Y.; Wang, M.; Tao, P.; Tu, Q.; Lv, G.; Chen, X. Three new ent-abietane diterpenoids from the roots of Euphorbia fischeriana and their cytotoxicity in human tumor cell lines. Arch. Pharm. Res. 2019, 42, 512–518. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Xu, J.K.; Zhang, J.; Bai, H.J.; Ma, B.Z.; Cheng, Y.C.; Zhang, W.K. Fischeriana A, a meroterpenoid with an unusual 6/6/5/5/5/6/6 heptacyclic carbon skeleton from the roots of Euphorbia fischeriana. Org. Biomol. Chem. 2019, 17, 2721–2724. [Google Scholar] [CrossRef] [PubMed]
- Du, K.; Yang, X.; Li, J.; Meng, D. Antiproliferative diterpenoids and acetophenone glycoside from the roots of Euphorbia fischeriana. Phytochemistry 2020, 177, 112437. [Google Scholar] [CrossRef]
- Su, J.C.; Cheng, W.; Song, J.G.; Zhong, Y.L.; Huang, X.J.; Jiang, R.W.; Li, Y.L.; Li, M.M.; Ye, W.C.; Wang, Y. Macrocyclic diterpenoids from Euphorbia helioscopia and their potential anti-inflammatory activity. J. Nat. Prod. 2019, 82, 2818–2827. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.H.; Wang, K.; Chai, T.; Guo, Z.Y.; Zhao, M.; Yang, J.L. Ingenane and jatrophane diterpenoids from Euphorbia kansui and their antiproliferative effects. Phytochemistry 2020, 172, 112257. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.F.; Zhang, Y.; Zhang, X.; Chen, M.J.; Zhan, Z.J.; Shan, W.G. A new tetracyclic diterpenoid from the seeds of Euphorbia lathyris. J. Chem. Res. 2020, 44, 322–325. [Google Scholar] [CrossRef]
- Xu, W.D.; Tian, Y.; Guo, Q.L.; Yang, Y.C.; Shi, J.G. Secoeuphoractin, a minor diterpenoid with a new skeleton from Euphorbia micractina. Chin. Chem. Lett. 2014, 25, 1531–1534. [Google Scholar] [CrossRef]
- Qi, W.Y.; Gao, X.M.; Ma, Z.Y.; Xia, C.L.; Xu, H.M. Antiangiogenic activity of terpenoids from Euphorbia neriifolia Linn. Bioorg. Chem. 2020, 96, 103536. [Google Scholar] [CrossRef]
- Li, J.C.; Feng, X.Y.; Liu, D.; Zhang, Z.J.; Chen, X.Q.; Li, R.T.; Li, H.M. Diterpenoids from Euphorbia neriifolia and their related anti-HIV and cytotoxic activity. Chem. Biodivers. 2019, 16, 2–8. [Google Scholar] [CrossRef]
- Li, J.C.; Zhang, Z.J.; Yang, T.; Jiang, M.Y.; Liu, D.; Li, H.M.; Li, R.T. Six new ent-abietane-type diterpenoids from the stem bark of Euphorbia neriifolia. Phytochem. Lett. 2019, 34, 13–17. [Google Scholar] [CrossRef]
- Du, M.; An, L.; Xu, J.; Guo, Y. Euphnerins A, and B, diterpenoids with a 5/6/6 rearranged spirocyclic carbon skeleton from the stems of Euphorbia neriifolia. J. Nat. Prod. 2020, 83, 2592–2596. [Google Scholar] [CrossRef]
- Wan, L.S.; Chu, R.; Peng, X.R.; Zhu, G.L.; Yu, M.Y.; Li, L.; Zhou, L.; Lu, S.Y.; Dong, J.R.; Zhang, Z.R.; et al. Pepluane and paraliane diterpenoids from Euphorbia peplus with potential anti-inflammatory activity. J. Nat. Prod. 2016, 79, 1628–1634. [Google Scholar] [CrossRef]
- Li, J.-C.; Dai, W.-F.; Liu, D.; Jiang, M.-Y.; Zhang, Z.-J.; Chen, X.-Q.; Chen, C.-H.; Li, R.-T.; Li, H.-M. Bioactive ent-isopimarane diterpenoids from Euphorbia neriifolia. Phytochemistry 2020, 175, 112373. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yang, B.; Fang, L.; Wang, S.; Guo, Y.; Yamakuni, T.; Ohizumi, Y. Four new myrsinol diterpenes from Euphorbia prolifera. J. Nat. Med. 2013, 67, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Xie, C.; An, L.; Yang, X.; Xi, Y.; Yuan, S.; Zhang, C.; Tuerhong, M.; Jin, D.Q.; Lee, D.; et al. Bioactive diterpenoids from the stems of Euphorbia royleana. J. Nat. Prod. 2019, 82, 183–193. [Google Scholar] [CrossRef]
- Demetzos, C.; Dimas, K.S. Labdane-type diterpenes: Chemistry and biological activity. Stud. Nat. Prod. Chem. 2001, 25, 235–292. [Google Scholar]
- Liu, M.; Wang, W.G.; Sun, H.D.; Pu, J.X. Diterpenoids from Isodon species: An update. Nat. Prod. Rep. 2017, 34, 1090–1140. [Google Scholar] [CrossRef]
- Wu, Q.C.; Tang, Y.P.; Ding, A.W.; You, F.Q.; Zhang, L.; Duan, J.A. 13C-NMR data of three important diterpenes isolated from Euphorbia species. Molecules 2009, 14, 4454–4475. [Google Scholar] [CrossRef] [Green Version]
- Satti, N.K.; Suri, O.P.; Dhar, K.L.; Kawasaki, T.; Miyahara, K.; Noda, N. Diterpenes from Euphorbia fidjiana. J. Nat. Prod. 1987, 50, 790. [Google Scholar] [CrossRef]
- Lal, A.R.; Cambie, R.C.; Rutledge, P.S.; Woodgate, P.D. Ent-pimarane and ent-abietane diterpenes from Euphorbia fidjiana. Phytochemistry 1990, 29, 2239–2246. [Google Scholar] [CrossRef]
- Haba, H.; Lavaud, C.; Magid, A.A.; Benkhaled, M. Diterpenoids, and triterpenoids from Euphorbia retusa. J. Nat. Prod. 2009, 72, 1258–1264. [Google Scholar] [CrossRef]
- Haba, H.; Lavaud, C.; Marcourt, L.; Long, C.; Harkat, H.; Benkhaled, M. Ent-abietane diterpenoids from Euphorbia guyoniana Boiss. & Reut. Biochem. Syst. Ecol. 2009, 37, 504. [Google Scholar]
- Yu, C.C.; Hsieh, C.R.; Hsiao, G.; Chen, P.Y.; Chang, M.L.; Yin, H.W.; Lee, T.H.; Lee, C.K. Regulated expressions of MMP-2, -9 by diterpenoids from Euphorbia formosana hayata. Molecules 2012, 17, 2082–2090. [Google Scholar] [CrossRef] [Green Version]
- Geris, R.; Simpson, T.J. Meroterpenoids produced by fungi. Nat. Prod. Rep. 2009, 26, 1063–1094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baloch, I.B.; Baloch, M.K.; Baloch, A.K. Schistosomiasis suppressing deoxyphorbol esters from Euphorbia cauducifolia L. Latex. Planta Med. 2010, 76, 809–814. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.H.; Latif, A.; Ali, M.; Zhang, G.P.; Xiang, W.J.; Ma, L.; Arfan, M.; Hu, L.H. Diterpenoids from Euphorbia neriifolia. Phytochemistry 2012, 75, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Satti, N.K.; Suri, O.P.; Thaper, R.K.; Kachroo, P.L. Ent-atisane-3β, 16α, 17-triol, a diterpene from E. acaulis. Phytochemistry 1988, 27, 1530. [Google Scholar] [CrossRef]
- Mbwambo, Z.H.; Lee, S.K.; Mshiu, E.N.; Pezzuto, J.M.; Kinghorn, A.D. Constituents from the stem wood of Euphorbia quinquecostata with phorbol dibutyrate receptor-binding inhibitory activity. J. Nat. Prod. 1996, 16, 1051–1055. [Google Scholar] [CrossRef] [PubMed]
- Drummond, G.J.; Grant, P.S.; Brimble, M.A. ent-atisane diterpenoids: Isolation, structure, and bioactivity. Nat. Prod. Rep. 2020. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, X.; Zhou, Y.; Peng, S.; Zhou, D.; Ding, L. Two new diterpenes from Euphorbia kansuensis. Fitoterapia 2008, 79, 262. [Google Scholar] [CrossRef]
- Li, X.L.; Li, Y.; Wang, S.F.; Zhao, Y.L.; Liu, K.C.; Wang, X.M.; Yang, Y.P. Ingol and ingenol diterpenes from the aerial parts of Euphorbia royleana and their antiangiogenic activities. J. Nat. Prod. 2009, 72, 1001–1005. [Google Scholar] [CrossRef]
- Liang, Q.L.; Dai, C.C.; Jiang, J.H.; Tang, Y.P.; Duan, J.A. A new cytotoxic casbane diterpene from Euphorbia pekinensis. Fitoterapia 2009, 80, 514–516. [Google Scholar] [CrossRef]
- Su, B.N.; Park, E.J.; Mbwambo, Z.H.; Santarsiero, B.D.; Mesecar, A.D.; Fong, H.H.S.; Pezzuto, J.M.; Kinghorn, A.D. New chemical constituents of Euphorbia quinquecostata and absolute configuration assignment by a convenient Mosher ester procedure carried out in NMR tubes. J. Nat. Prod. 2002, 65, 1278. [Google Scholar] [CrossRef]
- Appendino, G.; Belloro, E.; Tron, G.S.C.; Jakupovic, J.; Ballero, M. Pentacyclic diterpenoids from Euphorbia characias. Fitoterapia 2002, 71, 134. [Google Scholar] [CrossRef]
- Tran, Q.T.N.; Wong, W.S.F.; Chai, C.L.L. Labdane diterpenoids as potential anti-inflammatory agents. Pharmacol. Res. 2017, 124, 43–63. [Google Scholar] [CrossRef]
- Kubo, I.; Xu, Y.; Shimizu, K. Antibacterial Activity of ent-kaurene diterpenoids from Rabdosia rosthornii. Phyther. Res. 2004, 18, 180–183. [Google Scholar] [CrossRef]
- Kusumoto, N.; Ashitani, T.; Murayama, T.; Ogiyama, K.; Takahashi, K. Antifungal abietane-type diterpenes from the cones of Taxodium distichum Rich. J. Chem. Ecol. 2010, 36, 1381–1386. [Google Scholar] [CrossRef]
- Schmid, T.R.J. The biosynthesis of tigliane and related diterpenoids; an intriguing problem. J. Linn. Soc. Bot. 1987, 94, 221–230. [Google Scholar] [CrossRef]
- Li, W.; Tang, Y.Q.; Chen, S.X.; Tang, G.H.; Gan, L.S.; Li, C.; Rao, Y.; Huang, Z.S.; Yin, S. Euphorhelipanes A, and B, triglyceride-lowering euphorbia diterpenoids with a bicycle [4.3.0] nonane core from Euphorbia helioscopia. J. Nat. Prod. 2019, 82, 412–416. [Google Scholar] [CrossRef]
- Yuan, S.; Hua, P.; Zhao, C.; Zhou, H.; Xu, J.; Xu, J.; Gu, Q. Jatrophane diterpenoids from Euphorbia esula as inhibitors of RANKL-induced osteoclastogenesis. J. Nat. Prod. 2020, 83, 1005–1017. [Google Scholar] [CrossRef]
- Devappa, R.K.; Makkar, H.P.S.; Becker, K. Jatropha diterpenes: A review, JAOCS, J. Am. Oil Chem. Soc. 2011, 88, 301–322. [Google Scholar] [CrossRef]
- Adelakun, T.A.; Ding, X.; Ombati, R.M.; Zhao, N.D.; Obodozie-Ofoegbu, O.O.; Di, Y.T.; Zhang, Y.; Hao, X.J. A new highly oxygenated abietane diterpenoid and a new lysosome generating phorbol ester from the roots of Euphorbia fischeriana Steud. Nat. Prod. Res. 2020, 34, 3027–3035. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Y.; Wu, Y.L.; Zhang, P.; Chen, Z.Z.; Li, H.; Chen, L.X. Anti-inflammatory lathyrane diterpenoids from Euphorbia lathyris. J. Nat. Prod. 2019, 82, 756–764. [Google Scholar] [CrossRef]
- Tian, Y.; Guo, Q.; Xu, W.; Zhu, C.; Yang, Y.; Shi, J. A minor diterpenoid with a new 6/5/7/3 fused-ring skeleton from Euphorbia micractina. Org. Lett. 2014, 16, 3950–3953. [Google Scholar] [CrossRef]
- Wang, W.; Wu, Y.; Li, C.; Yang, Y.; Li, X.; Li, H.; Chen, L. Synthesis of new lathyrane diterpenoid derivatives from Euphorbia lathyris and evaluation of their anti-inflammatory activities. Chem. Biodivers. 2020, 17, e1900531. [Google Scholar] [CrossRef]
- Yang, T.; Wang, S.; Li, H.; Zhao, Q.; Yan, S.; Dong, M.; Liu, D.; Chen, X.; Li, R. Lathyrane diterpenes from Euphorbia lathyris and the potential mechanism to reverse the multi-drug resistance in HepG2/ADR cells. Biomed. Pharmacother. 2020, 121, 109663. [Google Scholar] [CrossRef]
- Wang, L.; Ma, Y.T.; Sun, Q.Y.; Zang, Z.; Yang, F.M.; Liu, J.P.; Jiang, J.H.; Huang, S.X.; Zhao, Y. A new lathyrane diterpenoid ester from Euphorbia dracunculoides. Chem. Nat. Compd. 2016, 52, 1037–1040. [Google Scholar] [CrossRef]
- Aleksandrov, M.; Maksimova, V.; Koleva Gudeva, L. Review of the anticancer and cytotoxic activity of some species from genus Euphorbia. Agric. Conspec. Sci. 2019, 84, 1–5. [Google Scholar]
- Yan, S.S.; Li, Y.; Wang, Y.; Shen, S.S.; Gu, Y.; Wang, H.B.; Qin, G.W.; Yu, Q. 17-acetoxyjolkinolide B irreversibly inhibits IkappaB kinase and induces apoptosis of tumor cells. Mol. Cancer Ther. 2008, 7, 1523–1532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baloch, I.B.; Baloch, M.K.; Saqib, Q.N. Tumor-promoting diterpene esters from the latex of Euphorbia cauducifolia L. Helv. Chim. Acta 2005, 88, 3145–3150. [Google Scholar] [CrossRef]
- Kulig, J.; Sehl, T.; Mackfeld, U.; Wiechert, W.; Pohl, M.; Rother, D. An enzymatic 2-step cofactor and co-product recycling cascade towards a chiral 1,2-diol. Part I: Cascade design. Adv. Synth. Catal. 2019, 361, 2607–2615. [Google Scholar] [CrossRef]
- Krstić, G.; Jadranin, M.; Stanković, M.; Aljančić, I.; Vujisić, L.; Mandić, B.; Tešević, V. Jatrophane diterpenoids with a protective effect on human lymphocytes DNA. Nat. Prod. Commun. 2019, 14. [Google Scholar] [CrossRef]
- Zolfaghari, B.; Yazdiniapour, Z.; Ghanadian, M.; Lanzotti, V. Cyclomyrsinane and premyrsinane diterpenes from Euphorbia sogdiana Popov. Tetrahedron 2016, 72, 5394–5401. [Google Scholar] [CrossRef]
- Corea, G.; Di Pietro, A.; Dumontet, C.; Fattorusso, E.; Lanzotti, V. Jatrophane diterpenes from Euphorbia spp. as modulators of multidrug resistance in cancer therapy. Phytochem. Rev. 2009, 8, 431–447. [Google Scholar] [CrossRef]
- Barile, E.; Gabriella, C.; Virginia, L. Diterpenes from Euphorbia as potential leads for drug design. Nat. Prod. Commun. 2008, 6, 1005. [Google Scholar] [CrossRef] [Green Version]
- Bani, S.; Kaul, A.; Jaggi, B.S.; Suri, K.A.; Suri, O.P.; Sharma, O.P. Anti-inflammatory activity of the hydrosoluble fraction of Euphorbia royleana latex. Fitoterapia 2000, 71, 655–662. [Google Scholar] [CrossRef]
- Wang, B.; Wei, Y.; Zhao, X.; Tian, X.; Ning, J.; Zhang, B.; Deng, S.; Li, D.; Ma, X.; Wang, C. Unusual ent-atisane type diterpenoids with 2-oxopropyl skeleton from the roots of Euphorbia ebracteolata and their antiviral activity against human rhinovirus 3 and enterovirus. Bioorg. Chem. 2018, 81, 234–240. [Google Scholar] [CrossRef] [PubMed]
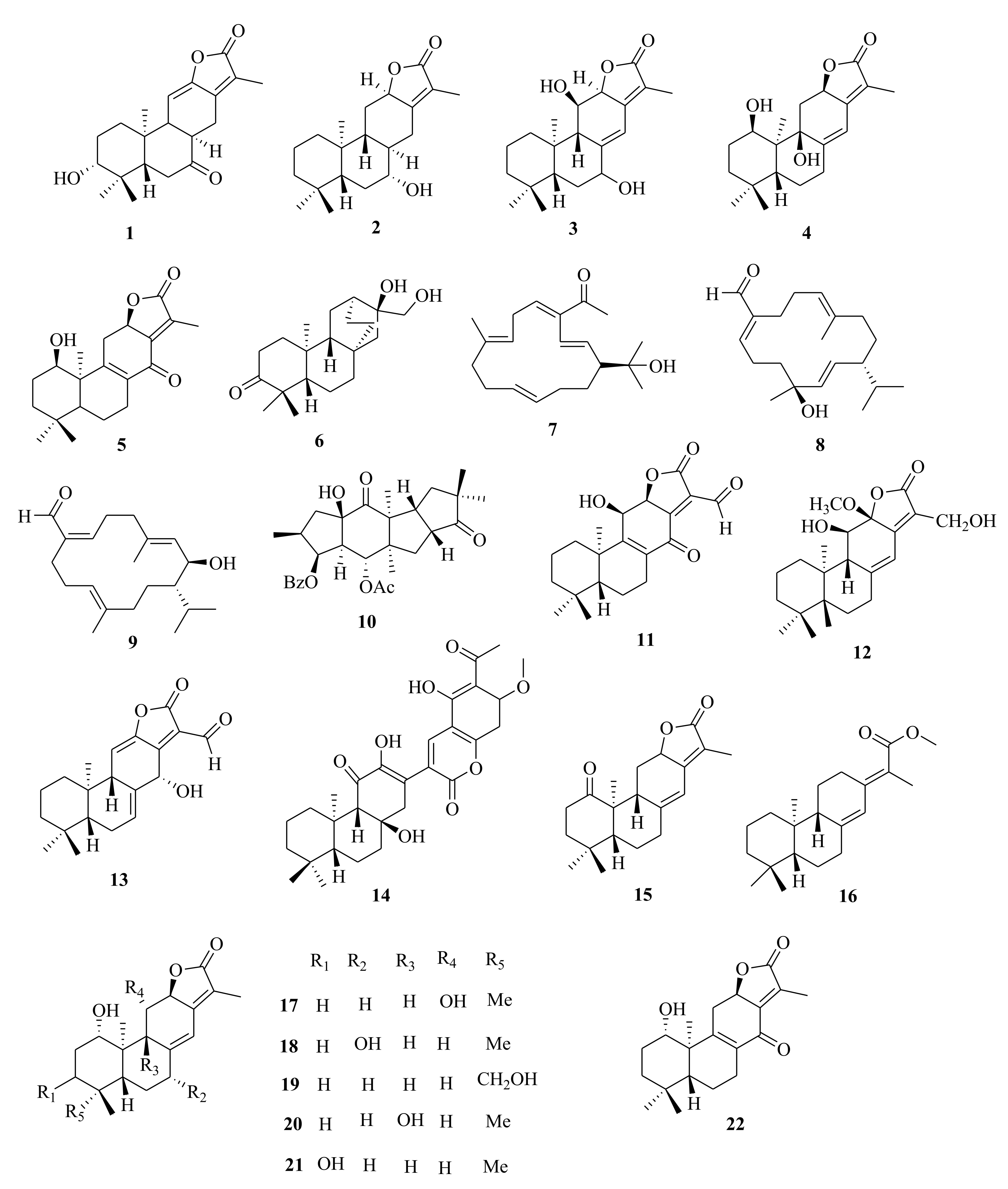
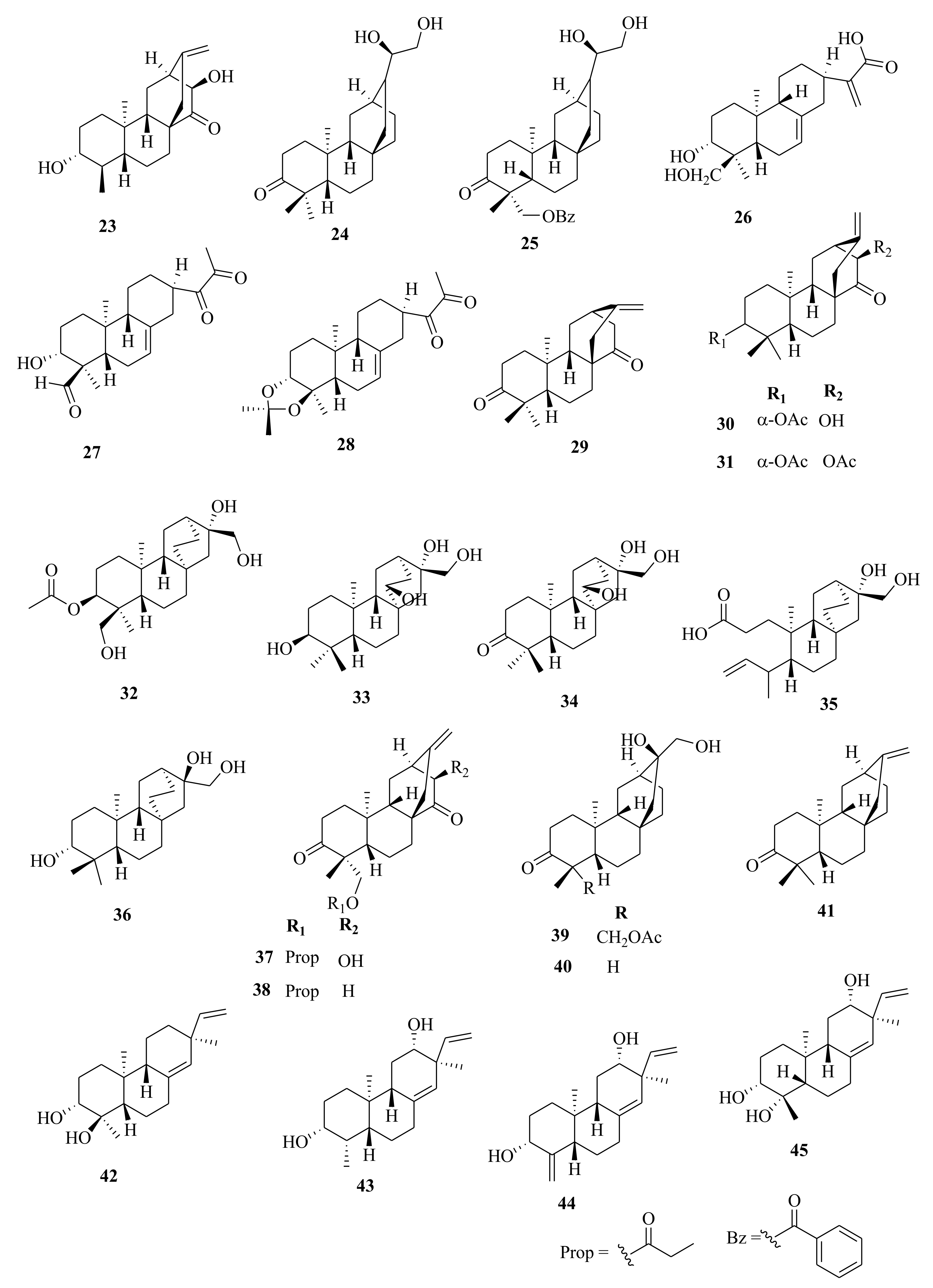
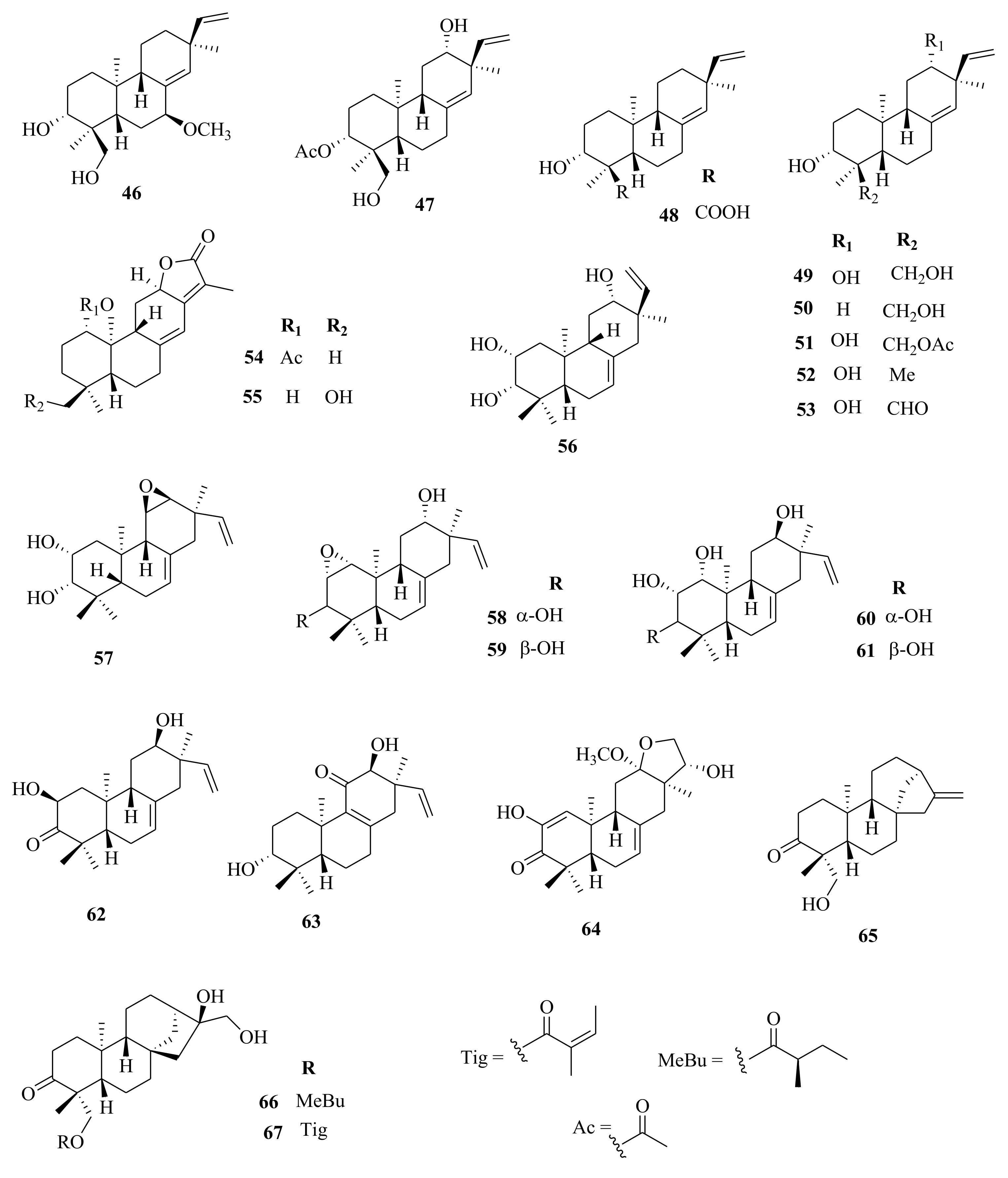

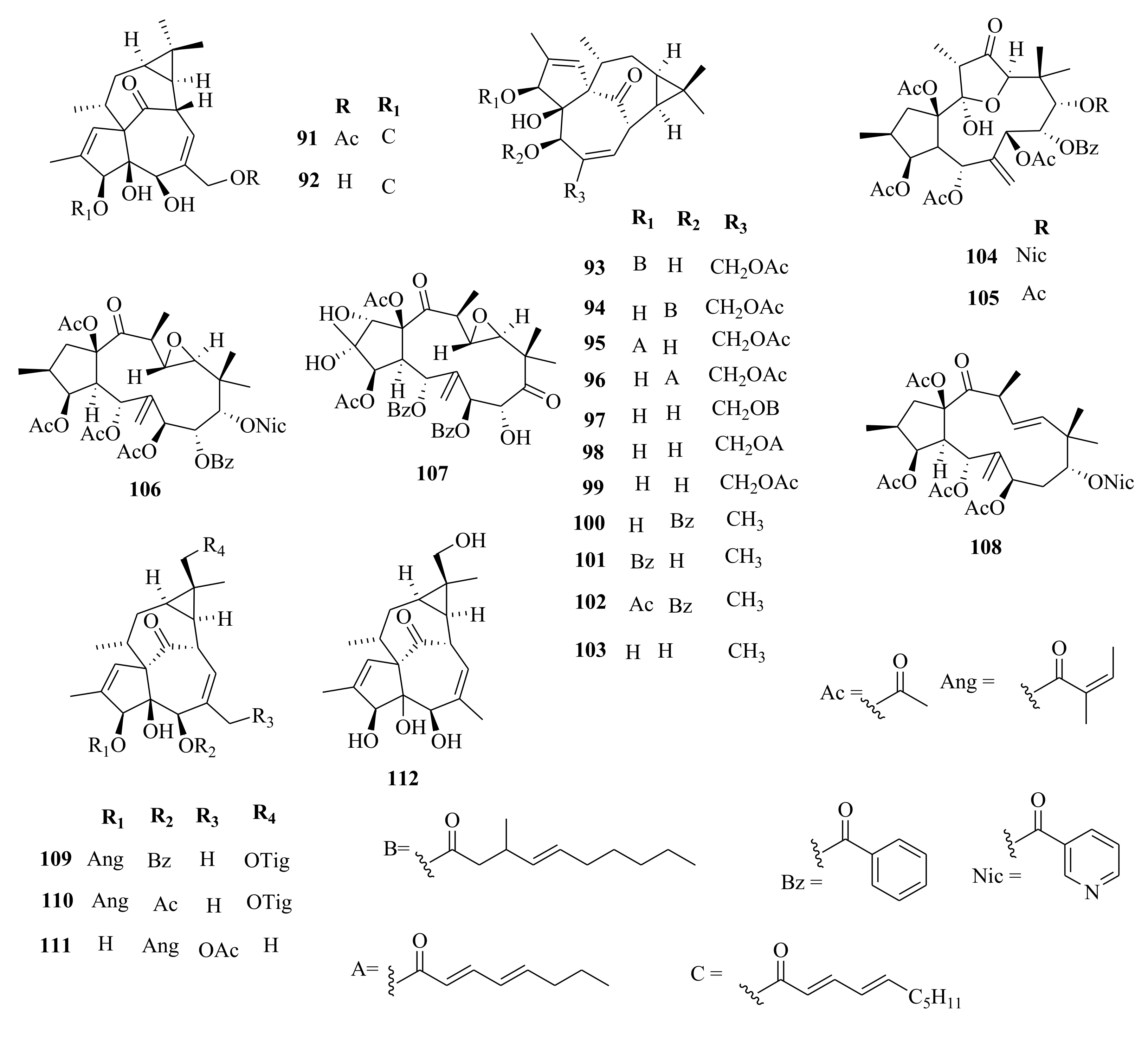


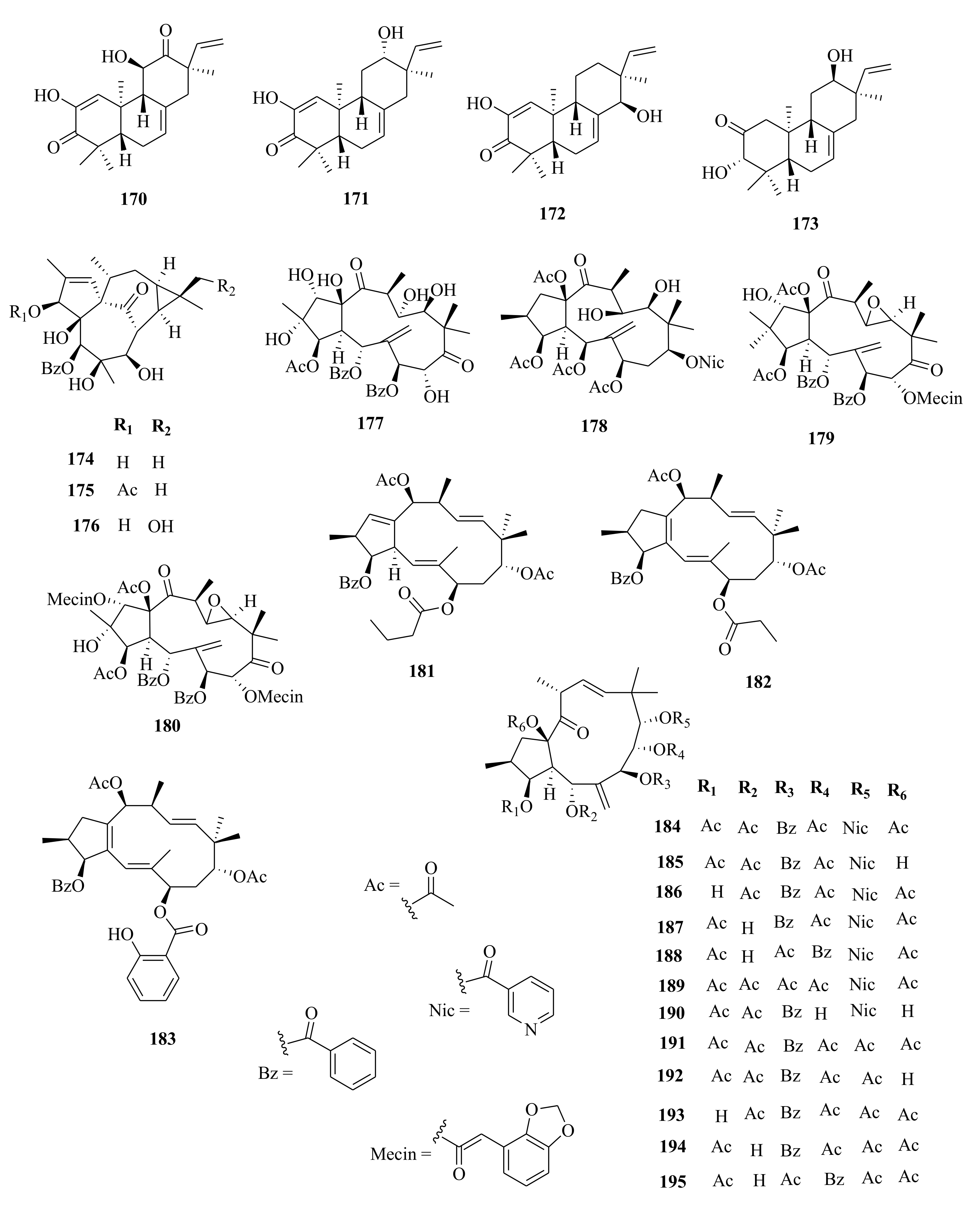
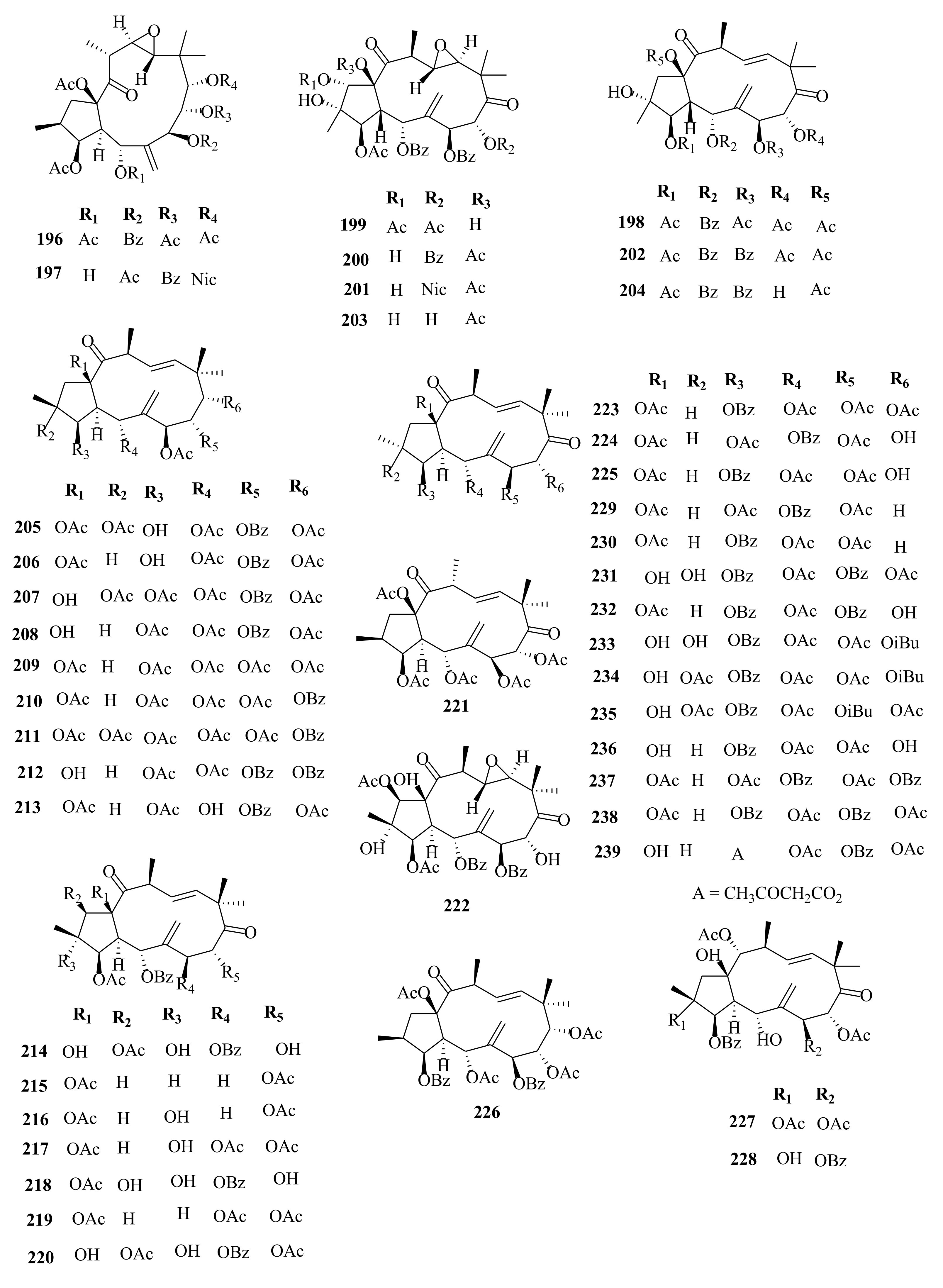
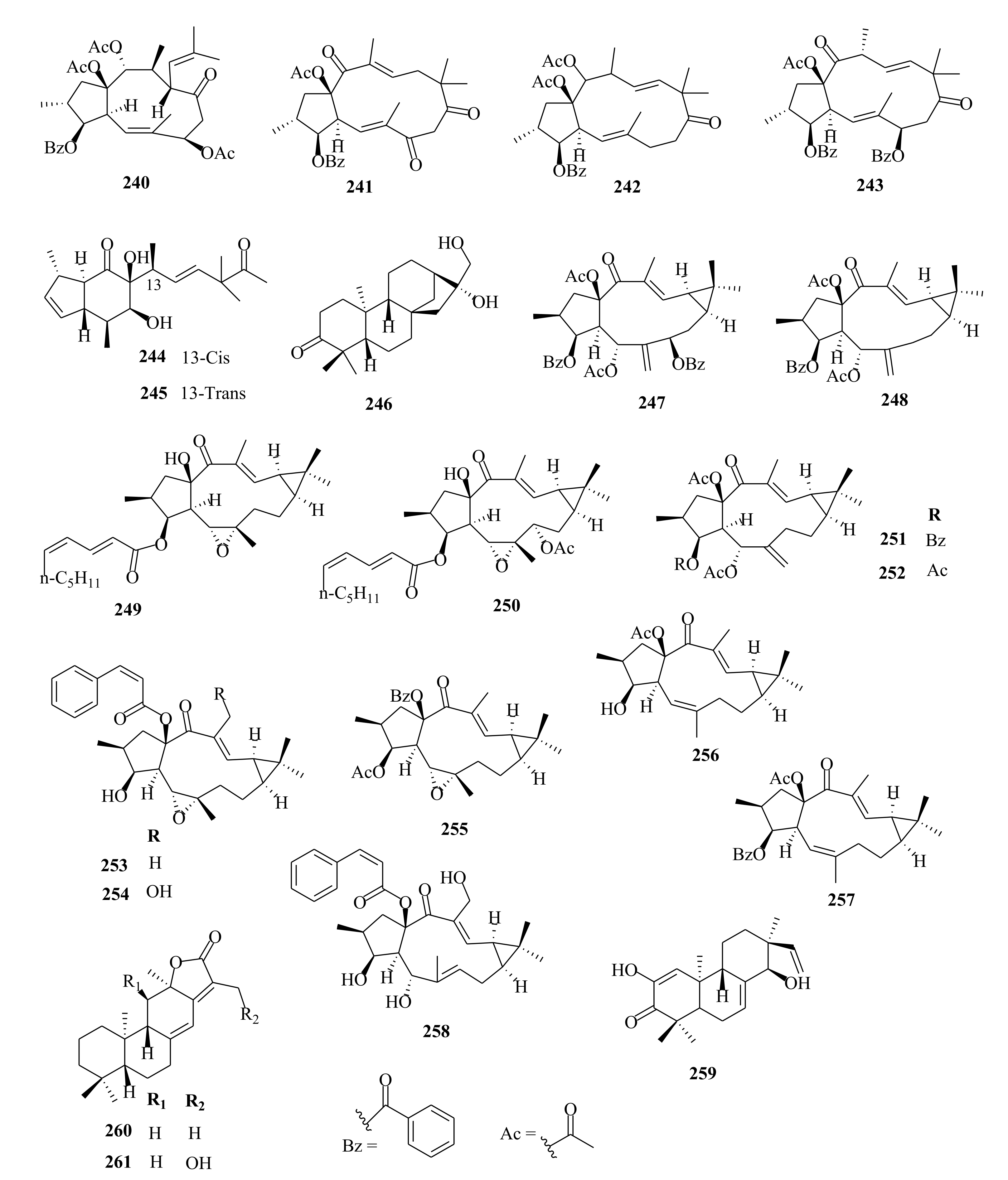



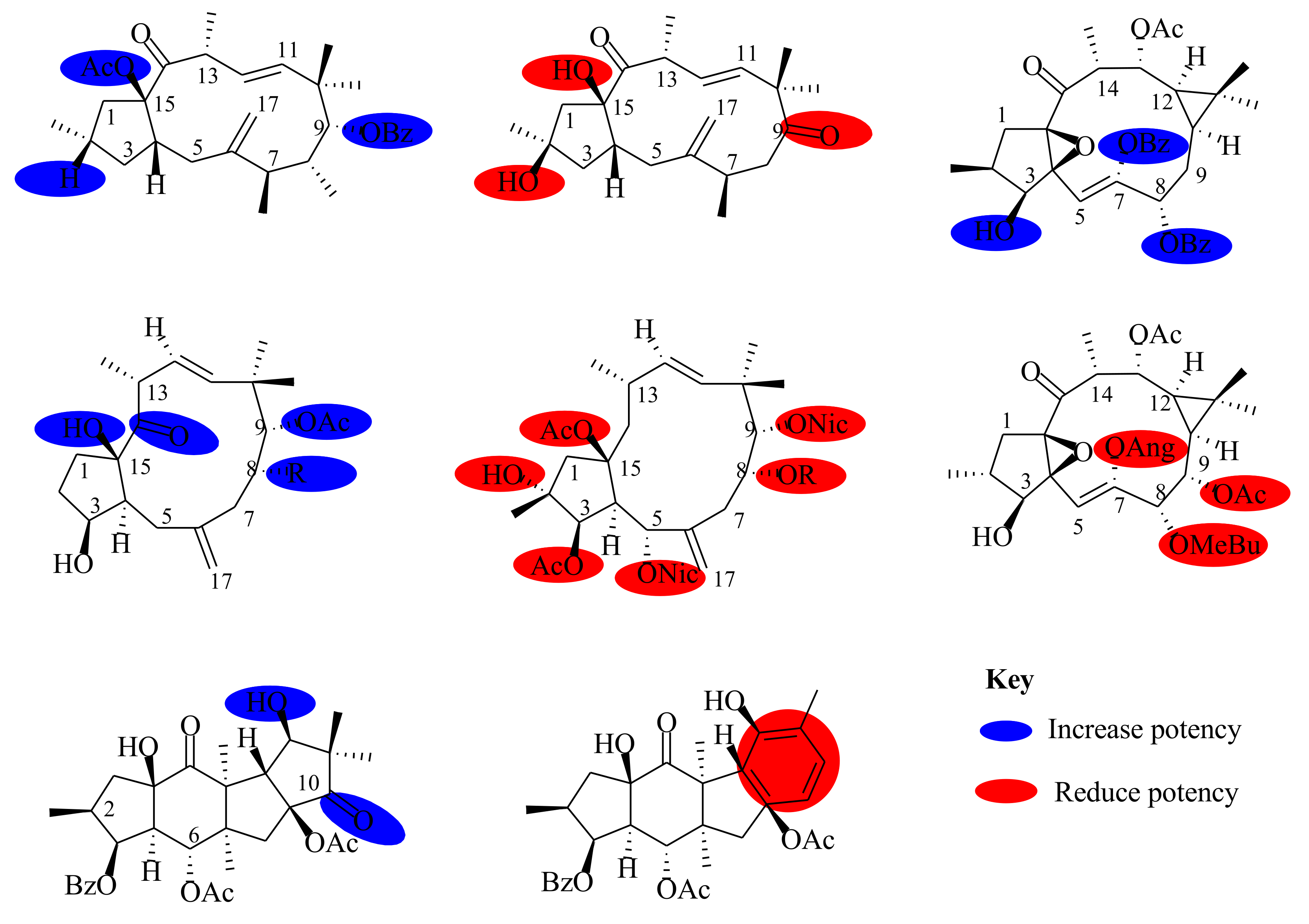
| Species Name | Class (n = Number of Isolated Compounds) | Biological Study | Reference |
|---|---|---|---|
| E. aellenii | jatrophane (n = 2) | Antiproliferative | [39] |
| E. antiquorum | ent-abietane n = 1), ent-atisane (n = 7), ingenol (n = 1), ingol (n = 16), ingol (n = 4), lathyrane (n = 3) | Melanin synthesis activity, inhibitory (α-glucosidase), inhibitory (NO production) | [34,54,55,56] |
| E. dracunculoides | tigliane (n = 1), myrsinol (n = 2) | Cytotoxic | [57,58] |
| E. ebracteolata | rosane (n = 5) | Lipase inhibitory | [48] |
| E. esula | jatrophane (n = 41) | Antimalarial | [34,59] |
| E. fischeriana | ent-abietane (n = 4), meroterpenoid (n = 5), tigliane (n = 5) | Cytotoxic | [30,60,61,62,63] |
| E. gaditana | gaditanone (n = 1) | Not evaluated | [27] |
| E. glomerulans | jatrophane (n = 17) | MDR-chemoreversal | [29] |
| E. grandicornis | tigliane (n = 2) | Protein kinase C activation and platelet stimulation abilities | [52] |
| E. helioscopia | jatrophane (n = 10) | Cytotoxic, inhibitory (nitric oxide (NO) | [50,64] |
| E. hylonoma | ent-isopimarane (n = 9), ent-rosane (n = 1) | Inhibitory (NO) | [45] |
| E. kansuensis | atisane (n = 1), ent-atisane (n = 1), ent-labdane (n = 2), ingenane (n = 1), kaurane (n = 1) | Cytotoxic, inhibition of NO | [35,36] |
| E. kansui | ingenane (n = 15), jatrophane (n = 8) | Cytotoxic effect, antiproliferative | [53,65] |
| E. kopetdaghi | Other | Cytotoxic | [25] |
| E. lathyris | ingenane (n = 1), lathyrane (n = 8), tigliane (n = 1) | Inhibitory of NO | [31,66] |
| E. marginata | ingol (n = 20), | Multidrug reversal activity | [41] |
| E. micractina | jatrophane (n = 2) | Anti-HIV-1 replication ability | [67] |
| E. milii | ent-rosane (n = 16) | Inhibitory (anti-EBV lytic replication | [46] |
| E.neriifolia | abietane n = 2), ent-abietane (n = 9), ent-isopimarane (n = 12), ent-rosane (n = 1), ingol (n = 5), rosane (n = 2) | Anti-HIV-1, antiangiogenic activity, anti-influenza, NO inhibitory effects | [42,68,69,70,71,72,73] |
| E. pekinensis | cembrane (n = 1), ent-abietane (n = 1), isopimarane (n = 4) | Cytotoxic | [44] |
| E. peplus | abietane (n = 2), ent-abietane (n = 1), ent-labdane (n = 1), paralianone (n = 3), paraliane (n = 8), pepluane (n = 7) | Cytotoxic, inhibition of LPS-stimulated NO production | [38,72] |
| E. pilosa | jatrophane (n = 2) | Cytotoxic | [40] |
| E. prolifera | mysrinane (n = 7), myrsinol (n = 4) | Lipid-lowering activity | [37,74] |
| E. resinifera | Ingol (n = 3), | Cytotoxic | [43] |
| E. royleana | cembrane (n = 2), ent-atisane (n = 4), ent-isopimarane (n = 2), ent-kaurane (n = 3), ingenane (n = 3), ingol (n = 5), lathyrane (n = 35) | MDR-chemoreversal, chemoreversal combination abilities, inhibitory (NO production) | [33,43,75] |
| E. sanctae-catharinae | premyrsinane (n = 3) | Not evaluated | [26] |
| E. saudiarabica | ingol (n = 2), ingenol (n = 2) | Inhibitory (α-glucosidase) | [28] |
| E. stracheyi | abietane (n = 2), ent-atisane (n = 2), ingenane (n = 4), lathyrane (n = 12), pimarane (n = 3), | Cytotoxic | [32] |
| E. wallichii | ent-abietane (n = 3) | Antibacterial | [47] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kemboi, D.; Siwe-Noundou, X.; Krause, R.W.M.; Langat, M.K.; Tembu, V.J. Euphorbia Diterpenes: An Update of Isolation, Structure, Pharmacological Activities and Structure–Activity Relationship. Molecules 2021, 26, 5055. https://doi.org/10.3390/molecules26165055
Kemboi D, Siwe-Noundou X, Krause RWM, Langat MK, Tembu VJ. Euphorbia Diterpenes: An Update of Isolation, Structure, Pharmacological Activities and Structure–Activity Relationship. Molecules. 2021; 26(16):5055. https://doi.org/10.3390/molecules26165055
Chicago/Turabian StyleKemboi, Douglas, Xavier Siwe-Noundou, Rui W. M. Krause, Moses K. Langat, and Vuyelwa Jacqueline Tembu. 2021. "Euphorbia Diterpenes: An Update of Isolation, Structure, Pharmacological Activities and Structure–Activity Relationship" Molecules 26, no. 16: 5055. https://doi.org/10.3390/molecules26165055
APA StyleKemboi, D., Siwe-Noundou, X., Krause, R. W. M., Langat, M. K., & Tembu, V. J. (2021). Euphorbia Diterpenes: An Update of Isolation, Structure, Pharmacological Activities and Structure–Activity Relationship. Molecules, 26(16), 5055. https://doi.org/10.3390/molecules26165055