Pseudosterins A–C, Three 1-Ethyl-3-formyl-β-carbolines from Pseudostellaria heterophylla and Their Cardioprotective Effects
Abstract
:1. Introduction
2. Results and Discussion
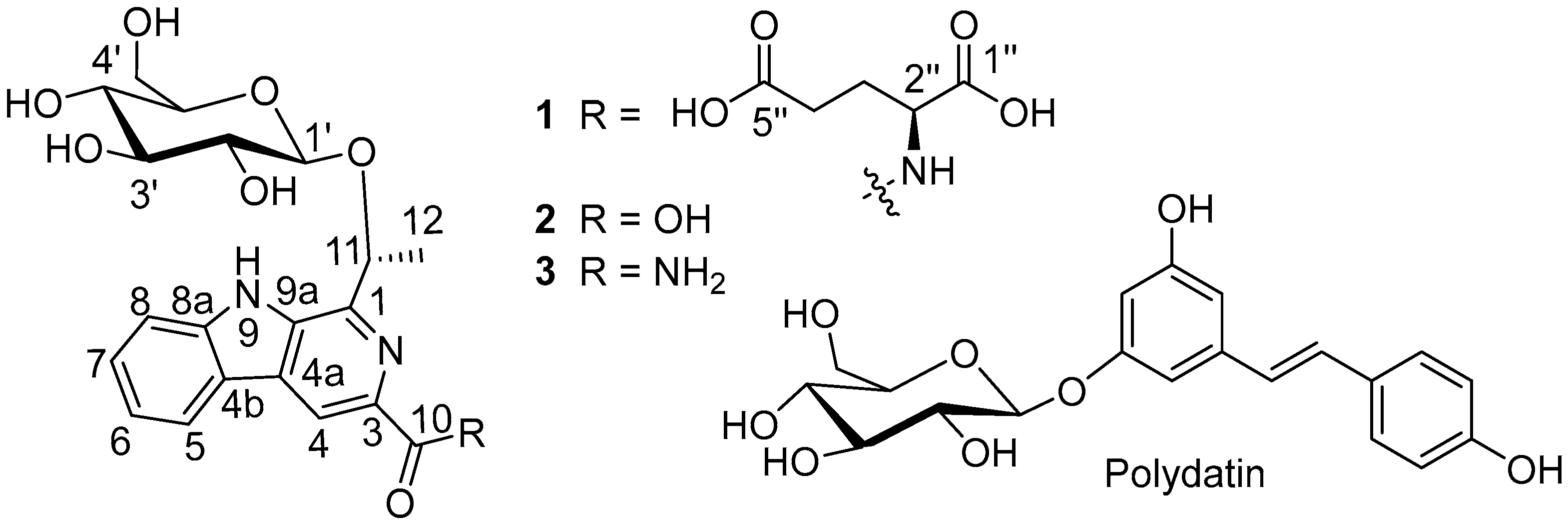
2.1. Structures Elucidation
2.2. Hypoxia/Reoxygenation Injury Protective Activity
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Acid Hydrolysis, Derivatization, and GC Analysis
3.4. Acid Hydrolysis, Derivatization, and LC-ESI MS Analysis
3.5. Hypoxia/Reoxygenation (H/R) Model and Experimental Protocols
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Cardiovascular Diseases. Available online: http://www.who.int/en/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 11 June 2021).
- Thygesen, K.; Alpert, J.S.; White, H.D. Universal definition of myocardial infarction. J. Am. Coll. Cardiol. 2007, 50, 2173–2195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Y.; Li, C.; Ma, Q.; Chen, S. Esculetin inhibits oxidative stress and apoptosis in H9c2 cardiomyocytes following hypoxia/reoxygenation injury. Biochem. Bioph. Res. Commun. 2018, 501, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Hausenloy, D.J.; Yellon, D.M. Myocardial ischemia-reperfusion injury: A neglected therapeutic target. J. Clin. Investig. 2013, 123, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.T.; Yang, T.L.; Gong, L.; Wu, P. Isorhamnetin protects against hypoxia/reoxygenation-induced injure by attenuating apoptosis and oxidative stress in H9c2 cardiomyocytes. Gene 2018, 666, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez, B.; Heusch, G.; Ovize, M.; Van de Werf, F. Evolving therapies for myocardial ischemia/reperfusion injury. J. Am. Coll. Cardiol. 2015, 65, 1454–1471. [Google Scholar] [CrossRef] [Green Version]
- Piot, C.; Croisille, P.; Staat, P.; Thibault, H.; Rioufol, G.; Mewton, N.; Elbelghiti, R.; Cung, T.T.; Bonnefoy, E.; Angoulvant, D.; et al. Effect of cyclosporine on reperfusion injury in acute myocardial infarction. N. Engl. J. Med. 2008, 359, 473–481. [Google Scholar] [CrossRef] [Green Version]
- Ibanez, B.; Cimmino, G.; Prat-González, S.; Vilahur, G.; Hutter, R.; Garcia, M.J.; Fuster, V.; Sanz, J.; Badimon, L.; Badimon, J.J. The cardioprotection granted by metoprolol is restricted to its administration prior to coronary reperfusion. Int. J. Cardiol. 2011, 147, 428–432. [Google Scholar] [CrossRef] [Green Version]
- Editorial Committee of Chinese Materia Medica, State Administration of Traditional Chinese Medicine. Chinese Materia Medica (Zhonghua Bencao); Shanghai Science and Technology Press: Shanghai, China, 1999; Volume 2, pp. 1428–1429. [Google Scholar]
- Wu, C.; Lin, Y. Advances in studies on Taizishen. J. Fujian Agri. Forest. Univ. (Nat. Sci. Edit.) 2004, 33, 426–430. [Google Scholar]
- Chen, J. Edible development and prospects on Pseudostellariae heterophylla. Food Nutr. China 2011, 17, 72–74. [Google Scholar]
- Zhang, J.; Yu, H.; You, Y. The edible and medicinal value of Zherong radix pseudostellariae. Trait Pharm. J. 2011, 23, 48–50. [Google Scholar]
- You, S.; Liu, X.; Xu, G.; Ye, M.; Bai, L.; Lin, R.; Sha, X.; Liang, L.; Huang, J.; Zhou, C.; et al. Identification of bioactive polysaccharide from Pseudostellaria heterophylla with its anti-inflammatory effects. J. Funct. Foods 2021, 78, 104353. [Google Scholar] [CrossRef]
- Tan, N.-H.; Zhou, J.; Chen, C.-X.; Zhao, S.-X. Cyclopeptide from the roots of Pseudostellaria hetetophylla. Phytochemistry 1993, 32, 1327–1330. [Google Scholar]
- Fang, Z.H.; Duan, X.C.; Zhao, J.D.; Wu, Y.J.; Liu, M.-M. Novel polysaccharide H-1-2 from Pseudostellaria heterophylla alleviates type 2 diabetes mellitus. Cell Physiol. Biochem. 2018, 49, 996–1006. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.C.; Tao, L.; Peng, J.; Fang, T.H. Effects of radix pseudostellariae on cardiac function and matrix metalloproteinases in rat chronic heart failure induced by coronary artery ligation. Chin. J. Pathophys. 2008, 24, 1694–1698. [Google Scholar]
- Xiao, T.T.; Peng, J.; Tao, L.; Shen, X.C. Protection of effective fractions from pseudostellariae radix on primary cultured cardiac myocytes injury induced by norepinephreine in vitro. Chin. J. Exp. Tradit. Med. Formulae 2012, 18, 125–128. [Google Scholar]
- Lin, S.D.; Dai, Q.W.; Zhang, H.C.; Hu, J. Advances in chemical constituents and biological activities of radix pseudostellariae. Chin. J. Ethnomed. Ethnopharm. 2010, 19, 33–34. [Google Scholar]
- Shen, X.C.; Tao, L.; Bo, S.; Gan, H.R.; Duan, J.A. Ameliorated effects of radix pseudostellariae on oxidative stress in rat chronic heart failure induced by acute cardiac infarction. West Chin. Med. J. 2008, 4, 413–416. [Google Scholar]
- Wang, Z.; Liao, S.G.; He, Y.; Li, J.; Zhong, R.F.; He, X.; Liu, Y.; Xiao, T.T.; Lan, Y.Y.; Long, D.Q.; et al. Protective effects of fractions from Pseudostellaria heterophylla against cobalt chloride-induced hypoxic injury in H9c2 cell. J. Ethnopharmacol. 2013, 147, 540–545. [Google Scholar] [CrossRef]
- Şöhretoğlu, D.; Baran, M.Y.; Arroo, R.; Kuruüzüm-Uz, A. Recent advances in chemistry, therapeutic properties and sources of polydatin. Phytochem. Rev. 2018, 17, 973–1005. [Google Scholar] [CrossRef]
- Venturo, N.; Suffritti, G. Improving dermatological signs and symptoms with polydatin-based dermocosmetic products. J. Cosmo. Trichol. 2017, 3, 124–136. [Google Scholar] [CrossRef]
- Kammerer, D.; Claus, A.; Carle, R.; Schieber, A. Polyphenol screening of pomace from red and white grape varieties (Vitis vinifera L.) by HPLC-DAD-MS/MS. J. Agric. Food Chem. 2004, 52, 4360–4367. [Google Scholar] [CrossRef] [PubMed]
- Brahmbhatt, K.G.; Ahmed, N.; Sabde, S.; Mitra, D.; Singh, I.P.; Bhutani, K.K. Synthesis and evaluation of β-carboline derivatives as inhibitors of human immunodeficiency virus. Bioorg. Med. Chem. Lett. 2010, 20, 4416–4419. [Google Scholar] [CrossRef]
- Jiang, Y.; Yu, S.-W.; Yang, Y.; Liu, Y.-L.; Xu, X.-Y.; Zhang, X.-M.; Yuan, W.-C. Facile synthesis of fused polycyclic compounds via intramolecular oxidative cyclization/aromatization of β-tetralone or β-tetralone oximes. Org. Biomol. Chem. 2018, 16, 9003–9010. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Jian, R.; Kang, J.; Huang, X.; Li, Y.; Zhuang, C.; Yang, F.; Zhang, L.; Fan, X.; Wu, T.; et al. Anti-inflammatory effects of caper (Capparis spinosa L.) fruit aqueous extract and the isolation of main phytochemicals. J. Agric. Food Chem. 2010, 58, 12717–12721. [Google Scholar] [CrossRef]
- Zhou, C.X.; Zou, L.; Gan, L.S.; Cao, Y.L. Kleinhospitines A-D, new cycloartane triterpenoid alkaloids from Kleinhovia hospital. Org. Lett. 2013, 15, 2734–2737. [Google Scholar] [CrossRef]
- Ren, C.; Bao, Y.; Meng, X.S.; Diao, Y.P.; Kang, T.G. Comparison of the protective effects of ferulic acid and its drug-containing plasma on primary cultured neonatal rat cardiomyocytes with hypoxia/reoxygenation injury. Pharmacogn. Mag. 2013, 9, 202–209. [Google Scholar] [PubMed] [Green Version]
- Du, Q.H.; Peng, C.; Zhang, H. Polydatin: A review of pharmacology and pharmacokinetics. Pharm. Biol. 2013, 51, 1347–1354. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.P.; Ma, H.J.; Bu, H.M.; Wang, M.L.; Li, Q.; Qi, Z.; Zhang, Y. Polydatin attenuates ischemia/reperfusion-induced apoptosis in myocardium of the rat. Acta Physiol. Sin. 2009, 61, 367–372. [Google Scholar]
- Li, X.; Arslan, F.; Ren, Y.; Adav, S.S.; Poh, K.K.; Sorokin, V.; Lee, C.N.; Kleijn, D.; Lim, S.K.; Sze, S.K. Metabolic adaptation to a disruption in oxygen supply during myocardial ischemia and reperfusion is underpinned bytemporal and quantitative changes in the cardiac proteome. J. Proteome Res. 2012, 11, 2331–2346. [Google Scholar] [CrossRef]
- Kokura, S.; Yoshida, N.; Yoshikawa, T. Anoxia/ reoxygenation-induced leukocyte-endothelial cell interactions. Free Radic. Biol. Med. 2002, 33, 427–432. [Google Scholar] [CrossRef]
- Li, C.; Jackson, R.M. Reactive species mechanisms of cellular hypoxia-reoxygenation injury. Am. J. Physiol. Cell. Physiol. 2002, 282, C227–C241. [Google Scholar] [CrossRef] [Green Version]
- Chu, D.; Zhang, Z. Trichosanthis pericarpium aqueous extract protects H9c2 cardiomyocytes from hypoxia/reoxygenation injury by regulating PI3K/Akt/NO pathway. Molecules 2018, 23, 2409. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.P.; Yang, C.Y.; Wang, Y.P.; Cui, F.; Zhang, Y. Protective effect of polydatin against ischemia/reperfusion injury in rat heart. Acta Physiol. Sin. 2008, 60, 161–168. [Google Scholar] [CrossRef]
- Granger, D.N.; Kvietys, P.R. Reperfusion injury and reactive oxygen species: The evolution of a concept. Redox Biol. 2015, 6, 524–551. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.R.; Zweier, J.L. Cardiac mitochondria and reactive oxygen species generation. Circ. Res. 2014, 114, 524–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herraiz, T.; Galisteo, J. Hydroxyl radical reactions and the radical scavenging activity of β-carboline alkaloids. Food Chem. 2015, 172, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.C.; Quang, T.H.; Yoon, C.S.; Ngan, N.T.T.; Lim, S.I.; Lee, S.Y.; Kim, Y.C.; Oh, H. Anti-neuroinflammatory activities of indole alkaloids from kanjang (Korean fermented soy source) in lipopolysaccharide-induced BV2 microglial cells. Food Chem. 2016, 213, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.B.; Yuan, C.M.; Xue, C.M.; Li, D.H.; Jing, Y.K.; He, H.P.; Hao, X.J.; Di, Y.T.; Li, Z.L.; Hua, H.M. Pegaharmalines A and B, two novel β-carboline alkaloids with unprecedented carbon skeletons from Peganum harmala. RSC Adv. 2014, 4, 53725–53729. [Google Scholar] [CrossRef]
- Herraiz, T.; Guillén, H. Monoamine oxidase—A inhibition and associated antioxidant activity in plant extracts with potential antidepressant actions. Biomed Res. Int. 2018, 2018, 4810394. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.L.; Xu, G.B.; Liu, J.; Liao, S.G.; He, X. Chemical constituents of Pseudostellaria heterophylla. Nat. Prod. Res. Dev. 2017, 29, 1132–1135, 1164. [Google Scholar]
- McGinnis, G.D. Preparation of aldononitrile acetates using N-methylimidazole as catalyst and solvent. Carbohyd. Res. 1982, 108, 284–292. [Google Scholar] [CrossRef]
- Zhang, W.; He, H.; Zhang, X. Determination of neutral sugars in soil by capillary gas chromatography after derivatization to aldononitrile acetates. Soil Biol. Biochem. 2007, 39, 2665–2669. [Google Scholar] [CrossRef]
- Fujii, K.; Ikai, Y.; Oka, H.; Suzuki, M.; Harada, K. A nonempirical method using LC/MS for determination of the absolute configuration of constituent amino acids in a peptide: Combination of Marfey’s method with mass spectrometry and its practical application. Anal. Chem. 1997, 69, 5146–5151. [Google Scholar] [CrossRef]
- Goebel, F. Über das harmalin. Eur. J. Org. Chem. 1842, 38, 363–366. [Google Scholar]
- Khan, F.A.; Maalik, A.; Iqbal, Z.; Malik, I. Recent pharmacological developments in β-carboline alkaloid “harmaline”. Eur. J. Pharmacol. 2013, 721, 391–394. [Google Scholar] [CrossRef]
- Khan, H.; Patel, S.; Kamal, M.A. Pharmacological and toxicological profile of harmane-β-carboline alkaloid: Friend or Foe. Curr. Drug Metab. 2017, 18, 853–857. [Google Scholar] [CrossRef]
- Dai, J.; Dan, W.; Schneider, U.; Wang, J. β-Carboline alkaloid monomers and dimers: Occurrence, structural diversity, and biological activities. Eur. J. Med. Chem. 2018, 157, 622–656. [Google Scholar] [CrossRef]
- Wavefunction Inc. Spartan 18; Wavefunction Inc.: Irvine, CA, USA, 2018. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegek, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Stephens, P.J.; Harada, N. ECD cotton effect approximated by Gaussian curve and other methods. Chirality 2010, 22, 229–233. [Google Scholar] [CrossRef]



| No. | δH (J in Hz) | δC | ||||
|---|---|---|---|---|---|---|
| 1 a | 2 b | 3 c | 1 a | 2 b | 3 c | |
| 1 | 145.6 | 145.9 | 143.9 | |||
| 3 | 139.2 | 135.4 | 139.0 | |||
| 4 | 8.74 (1H, s) | 9.34 (1H, s) | 8.75 (1H, s) | 114.4 | 117.0 | 113.2 |
| 4a | 131.4 | 130.6 | 129.4 | |||
| 4b | 122.6 | 124.1 | 120.9 | |||
| 5 | 8.23 (1H, d, 7.8) | 8.31 (1H, br d, 7.4) | 8.34 (1H, br d, 8.0) | 122.5 | 122.4 | 121.9 |
| 6 | 7.30 (1H, dd, 7.8, 7.4) | 7.35 (1H, br dd, 7.4, 7.3) | 7.27 (1H, br dd, 8.0, 8.0) | 121.4 | 120.6 | 120.0 |
| 7 | 7.57 (1H, dd, 8.2, 7.4) | 7.55 (1H, br dd, 7.7, 7.3) | 7.57 (1H, br dd, 8.0, 8.0) | 129.8 | 128.9 | 128.5 |
| 8 | 7.63 (1H, d, 8.2) | 7.77 (1H, br d, 7.7) | 7.66 (1H, br d, 8.0) | 113.3 | 112.9 | 112.4 |
| 8a | 142.8 | 142.4 | 141.0 | |||
| 9 | 12.5 (1H, s) | 11.76 (1H, s) | ||||
| 9a | 136.0 | 136.1 | 134.1 | |||
| 10 | 167.7 | 168.8 | 166.9 | |||
| 11 | 5.63 (1H, q, 6.6) | 6.03 (1H, q-like) | 5.48 (1H, q, 6.5) | 78.4 | 77.4 | 76.9 |
| 12 | 1.81 (3H, d, 6.6) | 1.97 (3H, d, 5.5) | 1.74 (3H, d, 6.5) | 21.7 | 22.3 | 21.4 |
| 1′ | 4.52 (1H, d, 7.7) | 5.19 (1H, d, 7.3) | 4.47 (1H, d, 7.7) | 102.9 | 102.9 | 101.9 |
| 2′ | 3.45 (1H, dd, 8.3, 7.7) | 4.29 (1H, dd, 7.9, 7.3) | 3.25 (1H) o | 75.7 | 75.7 | 74.0 |
| 3′ | 3.34 (1H, m) | 4.21 (1H, dd, 8.6, 7.9) | 3.16 (1H) o | 78.1 | 78.5 | 77.1 |
| 4′ | 3.35 (1H, m) | 4.33 (1H, br d, 8.6) | 3.14 (1H) o | 71.7 | 71.7 | 70.2 |
| 5′ | 3.27 (1H, m) | 3.92 (1H, br s) | 3.18 (1H) o | 78.1 | 78.8 | 76.5 |
| 6′ | 3.93 (1H, dd, 11.8, 2.2) 3.74 (1H, dd, 11.8, 5.8) | 4.53 (1H, br d, 11.4) 4.41 (1H, dd, 11.4, 4.6) | 3.73 (1H, dd, 11.2, 5.1) 3.50 (1H, m) | 62.7 | 62.6 | 61.1 |
| 1″ | 175.8 | |||||
| 2″ | 4.71 (1H, br s) | 53.9 | ||||
| 3″ | 2.38 (1H, m) 2.18 (1H, m) | 28.8 | ||||
| 4″ | 2.48 (2H, m) | 31.5 | ||||
| 5″ | 176.8 | |||||
| -CONH a | 7.98 (1H, d, 2.0) | |||||
| -CONH b | 7.45 (1H, d, 2.0) | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, G.-B.; Zhu, Q.-F.; Wang, Z.; Zhang, C.-L.; Yang, X.; Zhang, J.-J.; Wang, F.-R.; Liu, J.; Zhou, M.; Wang, Y.-L.; et al. Pseudosterins A–C, Three 1-Ethyl-3-formyl-β-carbolines from Pseudostellaria heterophylla and Their Cardioprotective Effects. Molecules 2021, 26, 5045. https://doi.org/10.3390/molecules26165045
Xu G-B, Zhu Q-F, Wang Z, Zhang C-L, Yang X, Zhang J-J, Wang F-R, Liu J, Zhou M, Wang Y-L, et al. Pseudosterins A–C, Three 1-Ethyl-3-formyl-β-carbolines from Pseudostellaria heterophylla and Their Cardioprotective Effects. Molecules. 2021; 26(16):5045. https://doi.org/10.3390/molecules26165045
Chicago/Turabian StyleXu, Guo-Bo, Qin-Feng Zhu, Zhen Wang, Chun-Li Zhang, Xin Yang, Jin-Juan Zhang, Fu-Rui Wang, Jun Liu, Meng Zhou, Yong-Lin Wang, and et al. 2021. "Pseudosterins A–C, Three 1-Ethyl-3-formyl-β-carbolines from Pseudostellaria heterophylla and Their Cardioprotective Effects" Molecules 26, no. 16: 5045. https://doi.org/10.3390/molecules26165045
APA StyleXu, G.-B., Zhu, Q.-F., Wang, Z., Zhang, C.-L., Yang, X., Zhang, J.-J., Wang, F.-R., Liu, J., Zhou, M., Wang, Y.-L., He, X., Gan, L.-S., & Liao, S.-G. (2021). Pseudosterins A–C, Three 1-Ethyl-3-formyl-β-carbolines from Pseudostellaria heterophylla and Their Cardioprotective Effects. Molecules, 26(16), 5045. https://doi.org/10.3390/molecules26165045






