In Vitro Anti-Candida Activity and Action Mode of Benzoxazole Derivatives
Abstract
1. Introduction
2. Results
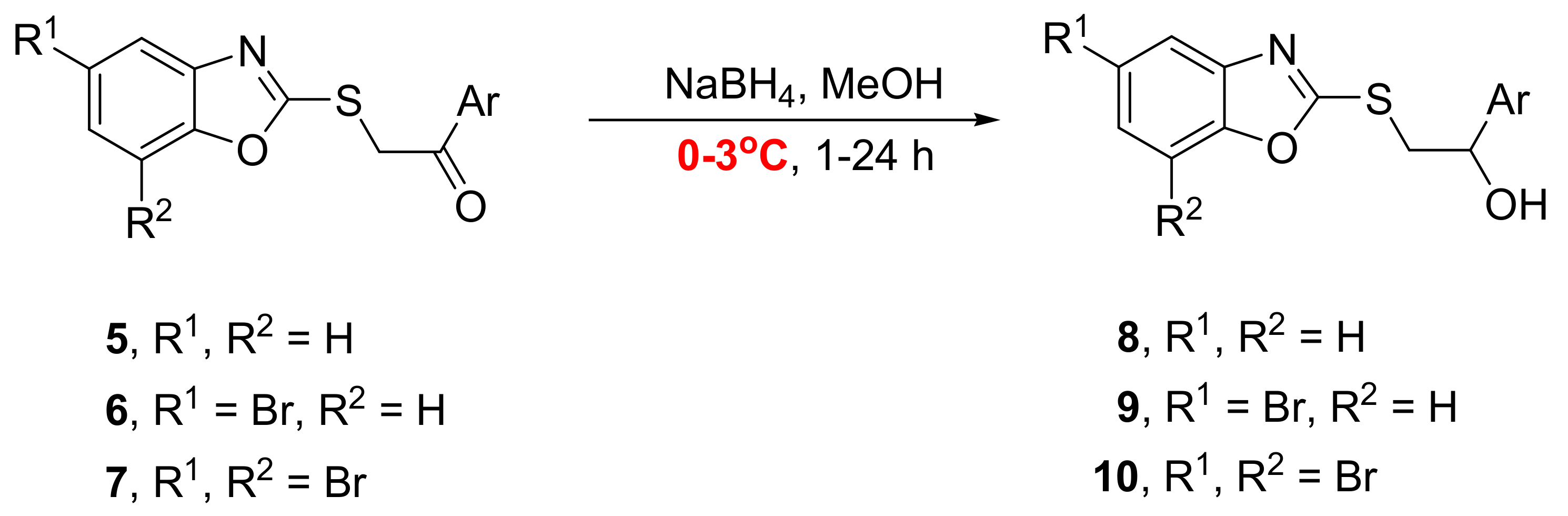
2.1. Synthesis of Benzoxazoles
| Lp. | Benzoxazole | 4, ArCOCH2X a | Ketone (%) | Alcohol (%) | ||
|---|---|---|---|---|---|---|
| 1 | 1 | C6H5 | 5a [24,25,26,27] | 98 | 8a | − b |
| 2 | 1 | 4-FC6H4 | 5b | 91 | 8b | 88 |
| 3 | 1 | 4-ClC6H4 | 5c [24] | 95 | 8c | 91 |
| 4 | 1 | 4-BrC6H4 | 5d [24,27] | 96 | 8d | 95 |
| 5 | 1 | 2,4-F2C6H3 | 5e | 88 | 8e | 57 |
| 6 | 1 | 2,4-Cl2C6H3 | 5f | 87 | 8f | 61 |
| 7 | 1 | 2,5-Cl2C6H3 | 5g | 91 | 8g | 37 c |
| 8 | 1 | 3,4-Cl2C6H3 | 5h | 67 | 8h | 73 |
| 9 | 1 | 2,3,4-Cl3C6H2 | 5i | 92 | 8i | 87 |
| 10 | 1 | 2,4,5-Cl3C6H2 | 5j | 91 | 8j | 89 |
| 11 | 1 | 2,4,6-Cl3C6H2 | 5k | 64 | 8k | − d |
| 12 | 2 | C6H5 | 6a | 71 | 9a | 39 c,e |
| 13 | 2 | 4-FC6H4 | 6b | 55 | 9b | 36 c,e |
| 14 | 3 | C6H5 | 7a | 53 | 10a | 33 c,e |
| 15 | 3 | 4-FC6H4 | 7b | 39 | 10b | − e |
2.2. Anti-Candida Activity of Benzoxazoles
2.3. Assessment of the Cytotoxicity against the Mammalian Cell Lines
2.4. Estimation of the Ergosterol Content in the Benzoxazole-Treated Candida Blastoconidia
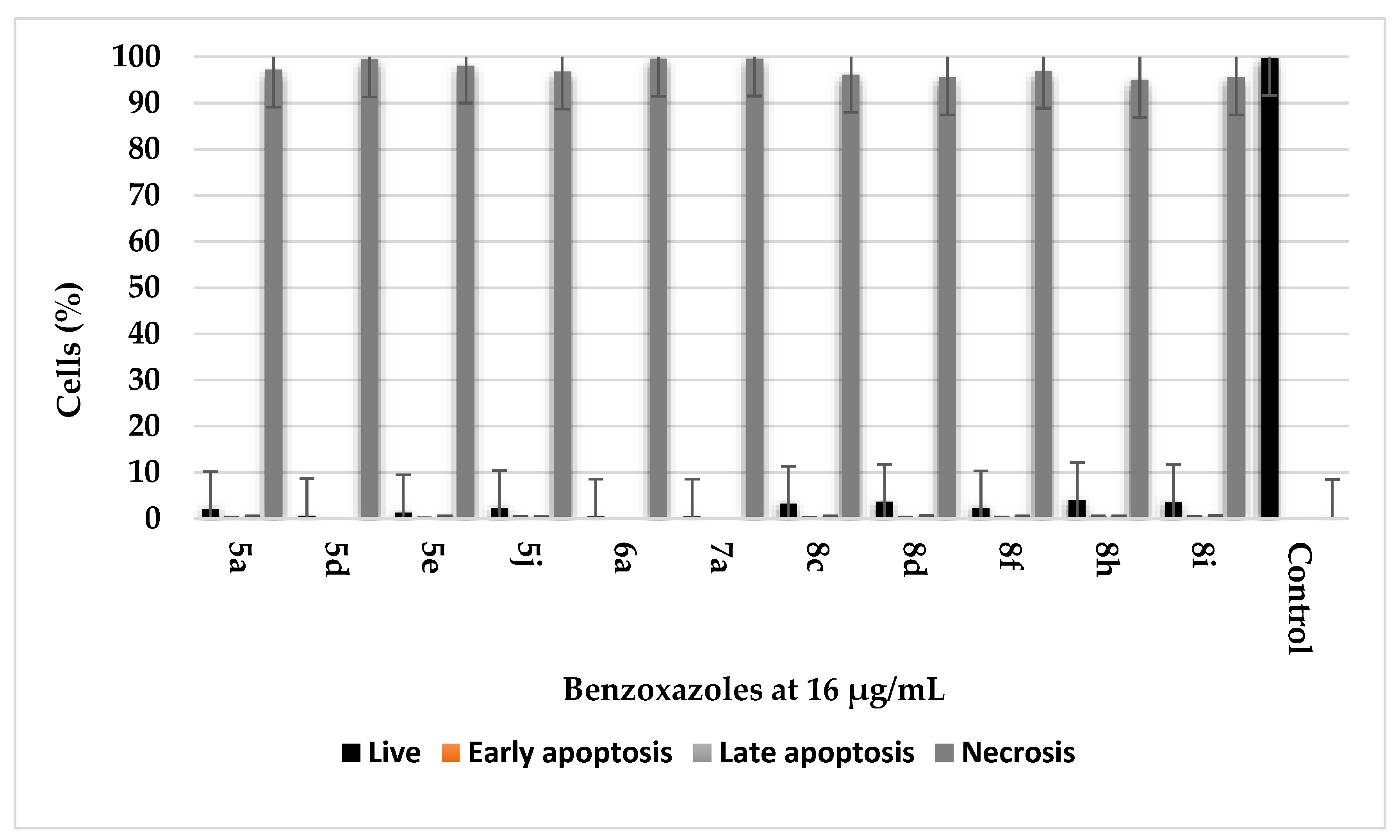
2.5. Cell Death Assessment Using Flow Cytometry
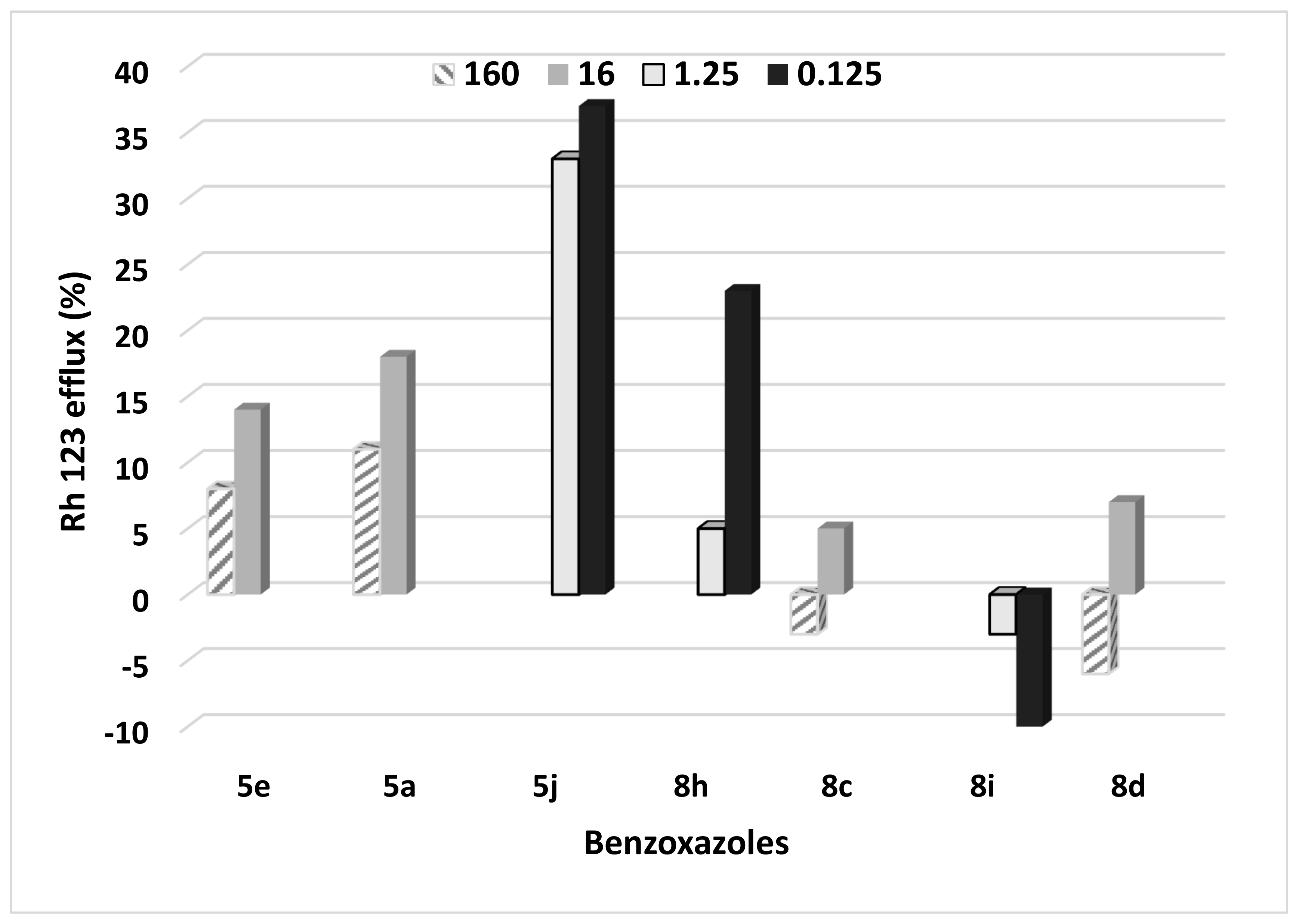
2.6. Efflux Study in the Benzoxazole-Treated C. albicans Cells
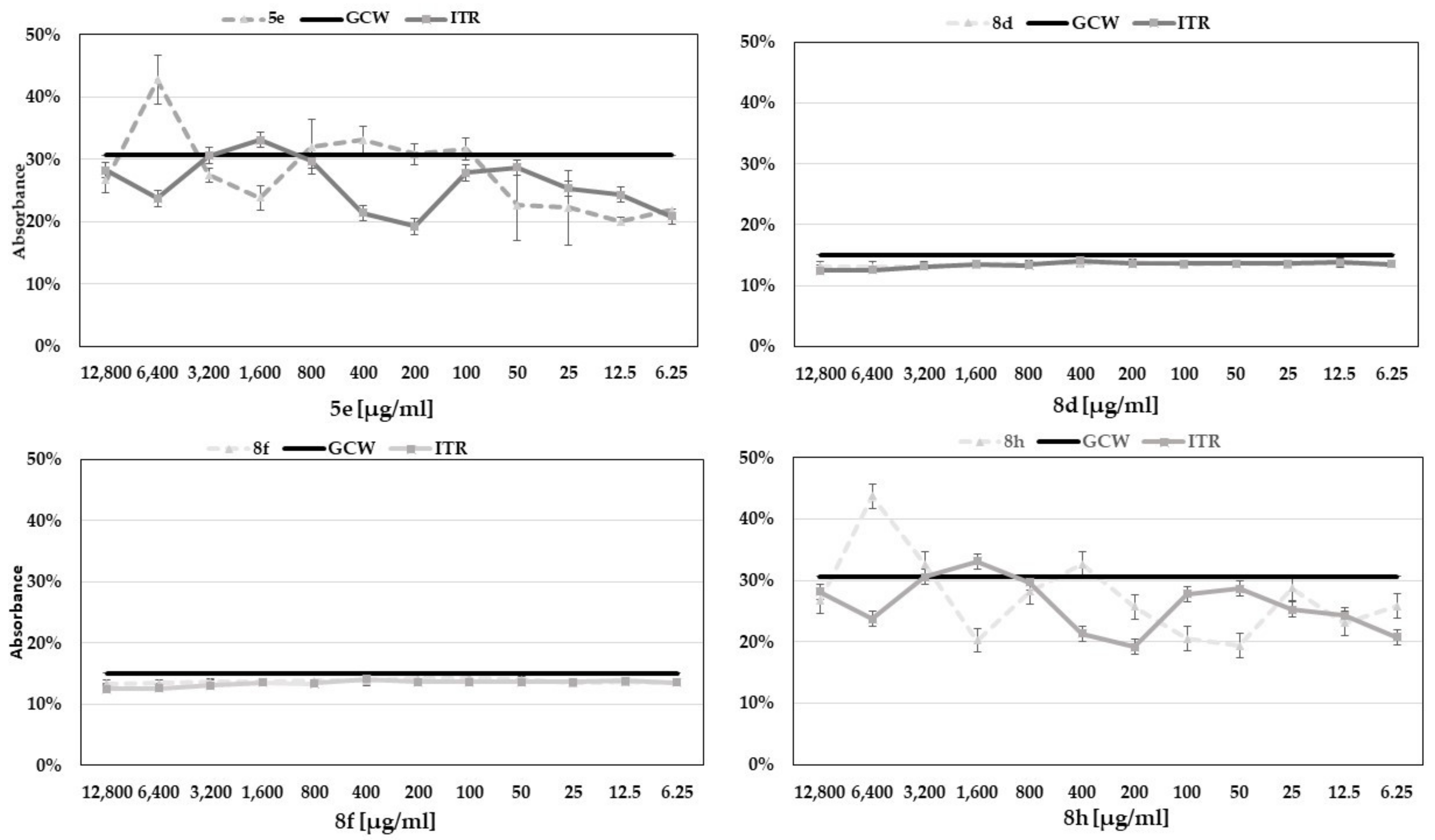
2.7. Determination of Antifungal Activity of Benzoxazole Derivatives in the Presence of Exogenous Ergosterol
2.8. Anti-Candida Effect of Benzoxazole Derivatives Combined with Amphotericin B (AmB). FIC Index Calculation
3. Discussion
4. Materials and Methods
4.1. Synthesis of Benzoxazoles
4.2. Synthesis of 2-amino-4-bromophenol
4.3. Synthesis of 2-amino-4,6-dibromophenol
4.4. General Procedure for Synthesis of Ketones 5–7
4.5. General Procedure for Synthesis of Alcohols 8
4.6. Fungal Strains and Culture Conditions
4.7. In Vitro Antifungal Activity
4.8. Cytotoxicity Assay In Vitro
4.9. Ergosterol Estimation Assay Using Spectrophotometry and HPLC
4.10. Cytometric Analysis of C. albicans Cell Death Type
4.11. Examination of Rhodamine 123 Efflux in the C. albicans SC5314 Treated with Benzoxazole Derivatives
4.12. Antifungal Studies Using Exogenous Ergosterol
4.13. Determination of Antifungal Effect of Benzoxazole Derivatives Combined with Amphotericin B (AmB). FIC Index Calculation
4.14. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- De Oliveira Filho, A.A.; de Oliveira, H.M.B.F.; de Sousa, J.P.; Meireles, D.R.P.; de Azevedo Maia, G.L.; Filho, J.M.B.; de Siqueira Júnior, J.P.; Lima, E.O. In vitro anti-Candida activity and mechanism of action of the flavonoid isolated from Praxelis clematidea against Candida albicans species. J. Appl. Pharm. Sci. 2016, 6, 066–069. [Google Scholar] [CrossRef][Green Version]
- Sattar, R.; Mukhtar, R.; Atif, M.; Hasnain, M.; Irfan, A. Synthetic transformations and biological screening of benzoxazole derivatives: A review. J. Heterocycl. Chem. 2020, 57, 2079–2107. [Google Scholar] [CrossRef]
- Luo, B.; Li, D.; Zhang, A.L.; Gao, J.M. Synthesis, antifungal activities and molecular docking studies of benzoxazole and benzothiazole derivatives. Molecules 2018, 23, 2457. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, S.; Kumar, S.; Narasimhan, B.; Lim, S.M.; Ramasamy, K.; Mani, V.; Shah, S.A.A. Design, synthesis and biological potential of heterocyclic benzoxazole scaffolds as promising antimicrobial and anticancer agents. Chem. Cent. J. 2018, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Veeraswamy, G.; Bhattarai, D.; Goo, J., II; Lee, K.; Choi, Y. Recent Advances in the Development of Pharmacologically Active Compounds that Contain a Benzoxazole Scaffold. Asian J. Org. Chem. 2015, 4, 1338–1361. [Google Scholar] [CrossRef]
- Kakkar, S.; Tahlan, S.; Lim, S.M.; Ramasamy, K.; Mani, V.; Shah, S.A.A.; Narasimhan, B. Benzoxazole derivatives: Design, synthesis and biological evaluation. Chem. Cent. J. 2018, 12, 1–16. [Google Scholar] [CrossRef]
- Erol, M.; Celik, I.; Uzunhisarcikli, E.; Kuyucuklu, G. Synthesis, Molecular Docking, and DFT Studies of Some New 2,5-Disubstituted Benzoxazoles as Potential Antimicrobial and Cytotoxic Agents. Polycycl. Aromat. Compd. 2020, 1–18. [Google Scholar] [CrossRef]
- De Carvalho, L.I.S.; Alvarenga, D.J.; Do Carmo, L.C.F.; De Oliveira, L.G.; Silva, N.C.; Dias, A.L.T.; Coelho, L.F.L.; De Souza, T.B.; Dias, D.F.; Carvalho, D.T. Antifungal Activity of New Eugenol-Benzoxazole Hybrids against Candida spp. J. Chem. 2017, 2017. [Google Scholar] [CrossRef]
- Zomorodian, K.; Khabnadideh, S.; Sakhteman, A.; Mirjalili, B.B.F.; Ranjbar, M.; Zamani, L. Synthesis and antifungal activity of benzoxazole derivatives with their sar analysis by SAS-MAP. Farmacia 2020, 68, 155–163. [Google Scholar] [CrossRef]
- Padalkar, V.S.; Borse, B.N.; Gupta, V.D.; Phatangare, K.R.; Patil, V.S.; Umape, P.G.; Sekar, N. Synthesis and antimicrobial activity of novel 2-substituted benzimidazole, benzoxazole and benzothiazole derivatives. Arab. J. Chem. 2016, 9, S1125–S1130. [Google Scholar] [CrossRef]
- Kuroyanagi, J.I.; Kanai, K.; Sugimoto, Y.; Horiuchi, T.; Achiwa, I.; Takeshita, H.; Kawakami, K. 1,3-Benzoxazole-4-carbonitrile as a novel antifungal scaffold of β-1,6-glucan synthesis inhibitors. Bioorg. Med. Chem. 2010, 18, 7593–7606. [Google Scholar] [CrossRef]
- Jayanna, N.D.; Vagdevi, H.M.; Dharshan, J.C.; Raghavendra, R.; Telkar, S.B. Synthesis, antimicrobial, analgesic activity, and molecular docking studies of novel 1-(5,7-dichloro-1,3-benzoxazol-2-yl)-3-phenyl-1H-pyrazole-4-carbaldehyde derivatives. Med. Chem. Res. 2013, 22, 5814–5822. [Google Scholar] [CrossRef]
- Łukowska-Chojnacka, E.; Mierzejewska, J.; Milner-Krawczyk, M.; Bondaryk, M.; Staniszewska, M. Synthesis of novel tetrazole derivatives and evaluation of their antifungal activity. Bioorg. Med. Chem. 2016, 24, 6058–6065. [Google Scholar] [CrossRef]
- Özdemir, A.; Turan-Zitouni, G.; Asim Kaplancikli, Z.; Revial, G.; Demirci, F.; Işcan, G. Preparation of some pyrazoline derivatives and evaluation of their antifungal activities. J. Enzym. Inhib. Med. Chem. 2010, 25, 565–571. [Google Scholar] [CrossRef]
- Kaplancikli, Z.A.; Yurttaş, L.; Özdemir, A.; Turan-Zitouni, G.; Işcan, G.; Akalin, G.; Abu Mohsen, U. Synthesis, anticandidal activity and cytotoxicity of some tetrazole derivatives. J. Enzym. Inhib. Med. Chem. 2014, 29, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Karaburun, A.Ç.; Çevik, U.A.; Osmaniye, D.; Saglık, B.N.; Çavuşoglu, B.K.; Levent, S.; Özkay, Y.; Koparal, A.S.; Behçet, M.; Asım Kaplancıklı, Z. Synthesis and evaluation of new 1,3,4-thiadiazole derivatives as potent antifungal agents. Molecules 2018, 23, 3129. [Google Scholar] [CrossRef] [PubMed]
- Kaplancikli, Z.A. Synthesis of some oxadiazole derivatives as new anticandidal agents. Molecules 2011, 16, 7662–7671. [Google Scholar] [CrossRef]
- El-Gohary, N.S.; Shaaban, M.I. Synthesis, antimicrobial, antiquorum-sensing and antitumor activities of new benzimidazole analogs. Eur. J. Med. Chem. 2017, 137, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Karaca Gençer, H.; Acar Çevik, U.; Levent, S.; Sağlık, B.N.; Korkut, B.; Özkay, Y.; Ilgın, S.; Öztürk, Y. New Benzimidazole-1,2,4-Triazole Hybrid Compounds: Synthesis, Anticandidal Activity and Cytotoxicity Evaluation. Molecules 2017, 22, 507. [Google Scholar] [CrossRef] [PubMed]
- Yurttaş, L.; Duran, M.; Demirayak, Ş.; Gençer, H.K.; Tunali, Y. Synthesis and initial biological evaluation of substituted 1-phenylamino-2-thio-4,5-dimethyl-1H-imidazole derivatives. Bioorg. Med. Chem. Lett. 2013, 23, 6764–6768. [Google Scholar] [CrossRef] [PubMed]
- Łukowska-Chojnacka, E.; Kowalkowska, A.; Napiórkowska, A. Lipase-catalyzed kinetic resolution of novel antitubercular benzoxazole derivatives. Chirality 2018, 30, 457–468. [Google Scholar] [CrossRef]
- Zeynizadeh, B.; Setamdideh, D. NaBH4/charcoal: A new synthetic method for mild and convenient reduction of nitroarenes. Synth. Commun. 2006, 36, 2699–2704. [Google Scholar] [CrossRef]
- Yamada, N.; Mizuochi, M.; Takeda, M.; Kawaguchi, H.; Morita, H. A facile and efficient one-pot synthesis of thiirans by the reaction of benzoxazolyl β-ketosulfides with NaBH4/NaOH. Tetrahedron Lett. 2008, 49, 1166–1168. [Google Scholar] [CrossRef]
- Loghmani-Khouzani, H.; Hajiheidari, D. Synthesis of difluorinated β-ketosulfones and novel gem-difluoromethylsulfone-containing heterocycles as fluorinated building blocks. J. Fluor. Chem. 2010, 131, 561–569. [Google Scholar] [CrossRef]
- Varun, B.V.; Gadde, K.; Prabhu, K.R. Synthesis of α-sulfenyl monoketones: Via a metal-free oxidative cross dehydrogenative coupling (CDC) reaction. Org. Biomol. Chem. 2016, 14, 7665–7670. [Google Scholar] [CrossRef]
- Yang, Z.; Li, J.; Hua, J.; Yang, T.; Yi, J.; Zhou, C. KI/K2S2O8-Mediated α-C-H Sulfenylation of Carbonyl Compounds with (Hetero)Aryl Thiols. Synlett 2017, 28, 2325–2329. [Google Scholar] [CrossRef]
- Md. Khaja Mohinuddin, P.; Gangi Reddy, N.C. Zinc oxide catalyzed solvent-free mechanochemical route for C-S bond construction: A sustainable process. Eur. J. Org. Chem. 2017, 2017, 1207–1214. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, 3rd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008. [Google Scholar]
- Menezes, C.; Valerio, E.; Dias, E. The Kidney Vero-E6 Cell Line: A Suitable Model to Study the Toxicity of Microcystins. N. Insights Toxic. Drug Test. 2013. [Google Scholar] [CrossRef]
- Abdi Goushbolagh, N.; Farhood, B.; Astani, A.; Nikfarjam, A.; Kalantari, M.; Zare, M.H. Quantitative Cytotoxicity, Cellular Uptake and Radioprotection Effect of Cerium Oxide Nanoparticles in MRC-5 Normal Cells and MCF-7 Cancerous Cells. BioNanoScience 2018, 8, 769–777. [Google Scholar] [CrossRef]
- Staniszewska, M.; Sobiepanek, A.; Małgorzata, G.; Peña-Cabrera, E.; Arroyo-Córdoba, I.J.; Michalina, K.; Kuryk, Ł.; Wieczorek, M.; Koronkiewicz, M.; Kobiela, T.; et al. Sulfone derivatives enter the cytoplasm of Candida albicans sessile cells. Eur. J. Med. Chem. 2020, 191. [Google Scholar] [CrossRef]
- Te Dorsthorst, D.T.A.; Verweij, P.E.; Meis, J.F.G.M.; Punt, N.C.; Mouton, J.W. Comparison of fractional inhibitory concentration index with response surface modeling for characterization of in vitro interaction of antifungals against itraconazole-susceptible and -resistant Aspergillus fumigatus isolates. Antimicrob. Agents Chemother. 2002, 46, 702–707. [Google Scholar] [CrossRef]
- Whaley, S.G.; Berkow, E.L.; Rybak, J.M.; Nishimoto, A.T.; Barker, K.S.; Rogers, P.D. Azole antifungal resistance in Candida albicans and emerging non-albicans Candida Species. Front. Microbiol. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Al-Harthy, T.; Zoghaib, W.; Abdel-Jalil, R. Importance of fluorine in benzazole compounds. Molecules 2020, 25, 4677. [Google Scholar] [CrossRef] [PubMed]
- Arthington-Skaggs, B.A.; Lee-Yang, W.; Ciblak, M.A.; Frade, J.P.; Brandt, M.E.; Hajjeh, R.A.; Harrison, L.H.; Sofair, A.N.; Warnock, D.W. Comparison of visual and spectrophotometric methods of broth microdilution MIC end point determination and evaluation of a sterol quantitation method for in vitro susceptibility testing of fluconazole and itraconazole against trailing and nontrailing Cand. Antimicrob. Agents Chemother. 2002, 46, 2477–2481. [Google Scholar] [CrossRef]
- Suchodolski, J.; Muraszko, J.; Bernat, P.; Krasowska, A. A crucial role for ergosterol in plasma membrane composition, localisation, and activity of Cdr1p and H+-ATPase in Candida albicans. Microorganisms 2019, 7, 378. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Gong, H.; Qi, Y.; Li, J.; Ji, X.; Sun, J.; Tian, R.; Bao, H.; Song, X.; Chen, Q.; et al. In vitro and in vivo antifungal activities and mechanism of heteropolytungstates against Candida species. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef]
- Yumoto, R.; Murakami, T.; Sanemasa, M.; Nasu, R.; Nagai, J.; Takano, M. Pharmacokinetic interaction of cytochrome P450 3A-related compounds with rhodamine 123, a P-glycoprotein substrate, in rats pretreated with dexamethasone. Drug Metab. Dispos. 2001, 29, 145–151. [Google Scholar] [PubMed]
- Forster, S.; Thumser, A.E.; Hood, S.R.; Plant, N. Characterization of rhodamine-123 as a tracer dye for use in in vitro drug transport assays. PLoS ONE 2012, 7. [Google Scholar] [CrossRef]
- Ramsdale, M. Programmed cell death in pathogenic fungi. Biochim. Biophys. Acta Mol. Cell Res. 2008, 1783, 1369–1380. [Google Scholar] [CrossRef] [PubMed]
- François, I.E.J.A.; Cammue, B.P.A.; Borgers, M.; Ausma, J.; Dispersyn, G.D.; Thevissen, K. Azoles: Mode of antifungal action and resistance development. Effect of miconazole on endogenous reactive oxygen species production in Candida albicans. Anti-Infect. Agents Med. Chem. 2006, 5, 3–13. [Google Scholar] [CrossRef]
- Maurya, I.K.; Thota, C.K.; Sharma, J.; Tupe, S.G.; Chaudhary, P.; Singh, M.K.; Thakur, I.S.; Deshpande, M.; Prasad, R.; Chauhan, V.S. Mechanism of action of novel synthetic dodecapeptides against Candida albicans. Biochim. Biophys. Acta-Gen. Subj. 2013, 1830, 5193–5203. [Google Scholar] [CrossRef]
- Te Welscher, Y.M.; Ten Napel, H.H.; Balagué, M.M.; Souza, C.M.; Riezman, H.; De Kruijff, B.; Breukink, E. Natamycin blocks fungal growth by binding specifically to ergosterol without permeabilizing the membrane. J. Biol. Chem. 2008, 283, 6393–6401. [Google Scholar] [CrossRef]
- Escalante, A.; Gattuso, M.; Pérez, P.; Zacchino, S. Evidence for the mechanism of action of the antifungal phytolaccoside B isolated from Phytolacca tetramera Hauman. J. Nat. Prod. 2008, 71, 1720–1725. [Google Scholar] [CrossRef] [PubMed]
- Virág, E.; Pesti, M.; Kunsági-Máté, S. Complex formation between primycin and ergosterol: Entropy–driven initiation of modification of the fungal plasma membrane structure. J. Antibiot. 2012, 65, 193–196. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Staniszewska, M.; Bondaryk, M.; Wieczorek, M.; Estrada-Mata, E.; Mora-Montes, H.M.; Ochal, Z. Antifungal effect of novel 2-bromo-2-chloro-2-(4-chlorophenylsulfonyl)-1-phenylethanone against Candida Strains. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef]
- Promega CellTiter 96 AQueous One Solution Cell Proliferation Assay. Available online: http://www.promega.com (accessed on 14 June 2021).
- Thermofisher Scientific CyQUANTTM MTT Cell Proliferation Assay Kit. Available online: http://www.thermofisher.com (accessed on 14 June 2021).
- Singh, S.; Fatima, Z.; Hameed, S. Octyl gallate reduces ABC multidrug transporter CaCdr1p expression and leads to its mislocalisation in azole-resistant clinical isolates of Candida albicans. J. Glob. Antimicrob. Resist. 2020, 22, 497–503. [Google Scholar] [CrossRef]
- Tian, J.; Ban, X.; Zeng, H.; He, J.; Chen, Y.; Wang, Y. The mechanism of antifungal action of essential oil from dill (Anethum graveolens l.) on Aspergillus flavus. PLoS ONE 2012, 7. [Google Scholar] [CrossRef]
- Borowiecki, P.; Wińska, P.; Bretner, M.; Gizińska, M.; Koronkiewicz, M.; Staniszewska, M. Synthesis of novel proxyphylline derivatives with dual Anti-Candida albicans and anticancer activity. Eur. J. Med. Chem. 2018, 150, 307–333. [Google Scholar] [CrossRef]
- Invitrogen FITC Annexin V/Dead Cell Apoptosis Kit with FITC annexin V and PI, for Flow Cytometry. Available online: http://www.thermofisher.com (accessed on 14 June 2021).
- Gbelska, Y.; Toth Hervay, N.; Dzugasova, V.; Konecna, A. Measurement of Energy-dependent Rhodamine 6G Efflux in Yeast Species. Bio Protoc. 2017, 7. [Google Scholar] [CrossRef]
- Zielinska, P.; Staniszewska, M.; Bondaryk, M.; Koronkiewicz, M.; Urbanczyk-Lipkowska, Z. Design and studies of multiple mechanism of anti-Candida activity of a new potent Trp-rich peptide dendrimers. Eur. J. Med. Chem. 2015, 105, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Leite, M.C.A.; De Brito Bezerra, A.P.; De Sousa, J.P.; Guerra, F.Q.S.; De Oliveira Lima, E. Evaluation of antifungal activity and mechanism of action of citral against Candida albicans. Evid. Based Complement. Altern. Med. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Vale-Silva, L.; Ischer, F.; Leibundgut-Landmann, S.; Sanglard, D. Gain-of-function mutations in PDR1, a regulator of antifungal drug resistance in candida glabrata, control adherence to host cells. Infect. Immun. 2013, 81, 1709–1720. [Google Scholar] [CrossRef] [PubMed]

| Comp. | Conc. (μg/mL) | |||||
|---|---|---|---|---|---|---|
| 16 | 8 | 4 | 2 | 1 | 0.5 | |
| 5a | 11.7 ± 3.8 | 10.0 ± 2.3 | 10.4 ± 4.7 | 8.8 ± 2.4 | 8.1 ± 0.6 | 7.8 ± 1.1 |
| 5b | 99.3 ± 4.3 | 86.9 ± 10.6 | 80.3 ± 13.4 | 82.9 ± 1.5 | 72.0 ± 21.9 | 68.7 ± 16.8 |
| 5c | 96.4 ± 0.6 | 85.3 ± 16.6 | 85.7 ± 16.6 | 79.9 ± 7.3 | 79.4 ± 4.3 | 57.6 ± 16.4 |
| 5d | 100.3 ± 3.2 ** | 96.4 ± 2.6 | 96.1 ± 6.6 | 76.5 ± 10.0 | 74.9 ± 9.5 | 71.0 ± 15.0 |
| 5e | 27.7 ± 3.3 | 10.9 ± 2.9 | 10.9 ± 3.5 | 10.0 ± 2.5 | 9.3 ± 3.1 | 7.2 ± 0.1 |
| 5f | 93.1 ± 8.8 * | 87.6 ± 2.2 | 87.9 ± 11.3 | 82.1 ± 2.6 | 80.0 ± 1.3 | 68.2 ± 20.3 |
| 5g | 73.8 ± 0.4 | 72.9 ± 0.6 | 70.9 ± 2.6 | 71.8 ± 5.1 | 69.4 ± 0.6 | 68.8 ± 0.6 |
| 5h | 98.55 ± 0.26 | 98.52 ± 0.20 | 99.27 ± 0.07 | 98.99 ± 0.15 | 98.98 ± 0.14 | 98.33 ± 0.26 |
| 5i | 78.7 ± 2.1 | 75.6 ± 1.6 | 73.3 ± 2.7 | 73.6 ± 3.5 | 73.3 ± 20.2 | 66.3 ± 10.9 |
| 5j | 17.6 ± 0.8 | 12.5 ± 3.6 | 9.1 ± 0.6 | 8.0 ± 0.2 | 8.1 ± 0.5 | 8.2 ± 0.5 |
| 5k | 76.6 ± 1.2 * | 74.5 ± 1.7 | 73.5 ± 0.8 | 72.5 ± 27.0 | 70.0 ± 14.3 | 65.8 ± 10.2 |
| 6a | 79.7 ± 3.1 * | 74.9 ± 1.2 | 74.9 ± 2.4 | 72.8 ± 15.2 | 70.6 ± 8.0 | 70.7 ± 0.2 |
| 6b | 60.9 ± 12.9 | 45.0 ± 8.3 | 41.3 ± 9.7 | 30.8 ± 7.2 | 5.0 ± 1.1 | 2.0 ± 1.1 |
| 7a | 79.5 ± 12.5 * | 45.0 ± 16.1 | 44.2 ± 8.2 | 42.0 ± 20.1 | 25.2 ± 4.0 | 17.7 ± 3.1 |
| 7b | 65.3 ± 21.8 | 39.7 ± 9.3 | 22.3 ± 4.3 | 31.6 ± 17.6 | 18.6 ± 0.2 | 5.0 ± 1.1 |
| 8b | 98.32 ± 0.23 | 98.47 ± 0.21 | 98.18 ± 0.27 | 98.84 ± 0.18 | 98.55 ± 0.23 | 98.59 ± 0.16 |
| 8c | 8.7 ± 1.9 | 8.8 ± 1.9 | 8.4 ± 1.1 | 7.5 ± 0.5 | 7.4 ± 0.5 | 6.9 ± 0.0 |
| 8d | 7.0 ± 1.5 | 6.5 ± 0.6 | 6.3 ± 0.1 | 6.4 ± 0.1 | 6.5 ± 0.0 | 6.4 ± 0.0 |
| 8e | 98.53 ± 0.12 | 99.43 ± 0.07 | 99.32 ± 0.06 | 98.78 ± 0.10 | 99.17 ± 0.08 | 99.24 ± 0.10 |
| 8f | 7.6 ± 1.3 | 6.8 ± 0.3 | 6.6 ± 0.0 | 6.9 ± 0.2 | 6.8 ± 0.2 | 6.6 ± 0.0 |
| 8h | 14.1 ± 3.9 | 8.1 ± 0.2 | 8.8 ± 0.9 | 8.7 ± 0.5 | 8.1 ± 0.1 | 7.8 ± 0.1 |
| 8i | 10.9 ± 4.7 | 8.9 ± 1.9 | 7.1 ± 0.1 | 7.1 ± 0.1 | 6.8 ± 0.0 | 6.9 ± 0.1 |
| 8j | 98.29 ± 0.02 | 99.28 ± 0.16 | 98.79 ± 0.12 | 98.38 ± 0.22 | 98.40 ± 0.04 | 98.41 ± 0.20 |
| Candida Isolate | Comp. (μg/mL) | % Cell Growth Inhibition (Mean ± SD) | |||||
|---|---|---|---|---|---|---|---|
| 16 | 8 | 4 | 2 | 1 | 0.5 | ||
| C. albicans | 5d | 52.0 ± 15.7 | 44.1 ± 9.1 | 40.6 ± 14.9 | 21.4 ± 4.4 | 20.4 ± 10.3 | 16.0 ± 3.3 |
| 5i | 55.7 ± 13.1 | 42.0 ± 12.2 | 22.9 ± 12.2 | 10.5 ± 2.9 | 6.5 ± 2.9 | 4.6 ± 1.2 | |
| 5k | 64.2 ± 10.6 * | 56.5 ± 16.2 | 44.4 ± 14.3 | 40.0 ± 14.6 | 1.7 ± 0.4 | 1.0 ± 0.4 | |
| 6a | 88.0 ± 9.7 * | 69.4 ± 6.5 | 48.0 ± 6.6 | 27.9 ± 4.3 | 26.5 ± 9.3 | 6.9 ± 3.0 | |
| C. glabrata | 5d | 38.3 ± 3.8 | 26.2 ± 4.6 | 16.6 ± 4.2 | 10.1 ± 2.1 | 14.5 ± 1.0 | 9.2 ± 0.8 |
| 5i | 53.0 ± 3.5 | 34.4 ± 3.0 | 14.4 ± 2.8 | 13.2 ± 1.4 | 11.6 ± 2.1 | 7.5 ± 1.4 | |
| 5k | 35.0 ± 5.1 | 19.1 ± 1.7 | 25.5 ± 2.5 | 26.5 ± 3.4 | 3.5 ± 0.5 | 12.3 ± 1.4 | |
| 6a | 27.0 ± 6.1 | 23.6 ± 3.3 | 10.4 ± 1.3 | 7.0 ± 0.5 | 3.0 ± 0.5 | 1.0 ± 0.5 | |
| Cell Line ATCC | Comp. [µg/mL] | % Cytotoxicity (Mean ± SD) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 512 | 256 | 128 | 64 | 32 | 16 | 8 | 4 | 2 | 1 | ||
| Kidney Vero E6 | 5d | - | 61.3 ± 1.0 | 18.4 ± 0.4 | 17.0 ± 1.0 | 8.7 ± 0.2 | 6.1 ± 0.3 | 4.4 ± 0.2 | 3.6 ± 0.2 | 3.4 ± 0.1 | 2.1 ± 0.1 |
| 6a | - | 60.2 ± 3.3 | 17.2 ± 0.4 | 10.1 ± 0.5 | 5.4 ± 0.3 | 5.0 ± 0.2 | 2.9 ± 0.1 | 0.1 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | |
| 7a | - | 63.8 ± 8.7 | 14.4 ± 0.5 | 9.1 ± 0.7 | 5.4 ± 0.4 | 4.2 ± 0.1 | 1.6 ± 0.1 | 1.4 ± 0.1 | 0.9 ± 0.0 | 0.0 ± 0.0 | |
| Pulmonary Fibroblasts MRC-5 | 5a | 40.64 ± 3.0 | 39.03 ± 3.2 | 38.92 ± 5.5 | 38.69 ± 2.3 | 38.25 ± 3.2 | 36.67 ± 3.0 | 36.71 ± 4.1 | 36.15 ± 2.7 | 33.19 ± 1.6 | 32.59 ± 0.8 |
| 5e | 13.98 ± 0.7 | 12.33 ± 0.4 | 9.97 ± 0.6 | 9.56 ± 0.4 | 9.22 ± 0.5 | 8.44 ± 0.4 | 8.10 ± 0.5 | 3.38 ± 0.2 | 2.59 ± 0.2 | 2.59 ± 0.2 | |
| 5j | 50.75 ± 0.0 | 46.22 ± 0.0 | 45.02 ± 0.0 | 44.01 ± 0.0 | 40.19 ± 0.1 | 39.56 ± 0.0 | 38.92 ± 0.1 | 37.72 ± 0.0 | 35.81 ± 0.0 | 34.46 ± 0.0 | |
| 8c | 77.98 ± 0.0 | 81.91 ± 0.1 | 84.35 ± 0.0 | 8.92 ± 0.0 | 1.69 ± 0.0 | 7.42 ± 0.0 | 4.99 ± 0.0 | 0.65 ± 0.0 | 14.32 ± 0.0 | 20.04 ± 0.0 | |
| 8d | 63.26 ± 0.3 | 83.54 ± 0.0 | 83.99 ± 0.0 | 22.09 ± 0.0 | 15.46 ± 0.0 | 13.21 ± 0.0 | 0.0 ± 0.0 | 12.37 ± 0.0 | 3.60 ± 0.0 | 6.75 ± 0.0 | |
| 8h | 81.13 ± 0.0 | 81.41 ± 0.1 | 81.97 ± 0.0 | 6.13 ± 0.0 | 0.0 ± 0.0 | 1.98 ± 0.0 | 1.86 ± 0.0 | 13.21 ± 0.0 | 7.82 ± 0.0 | 13.32 ± 0.0 | |
| 8i | 71.24 ± 0.2 | 83.86 ± 0.2 | 83.00 ± 0.0 | 61.65 ± 0.6 | 17.65 ± 0.0 | 9.71 ± 0.0 | 11.51 ± 0.0 | 15.25 ± 0.0 | 11.39 ± 0.0 | 14.61 ± 0.0 | |
| Candida albicans | Ergosterol Content (% ± SD) * | |||
|---|---|---|---|---|
| Comp. (µg/mL) | 5d | 6a | 7a | |
| ATCC SC5314 | 16 | 73.8 ± 3.2 | 93.9 ± 4.3 | 95.5 ± 3.7 |
| 4 | 79.8 ± 3.2 | 57.3 ± 1.6 | 84.2 ± 3.8 | |
| Clinical isolate | 16 | 74.3 ± 3.5 | 83.4 ± 4.1 | 99.9 ± 5.0 |
| 4 | 57.8 ± 2.7 | 66.1 ± 4.3 | 68.0 ± 2.0 | |
| Candida albicans | Comp. (µg/mL) | Ergosterol Conc. (% ± SD) | ||
|---|---|---|---|---|
| 5d | 6a | 7a | ||
| ATCC SC5314 | 16 | 114.4 ± 9.2 | 65.9 ± 5.3 | 72.7 ± 5.8 |
| 4 | 78.0 ± 6.2 | 67.9 ± 5.4 | 63.2 ± 3.8 | |
| Clinical isolate | 16 | 51.7 ± 4.1 | 33.1 ± 2.6 | 112.4 ± 9.0 |
| 4 | 97.3 ± 7.8 | 52.6 ± 4.2 | 71.6 ± 5.7 | |
| Comp. | MIC (µg/mL) | Outcomes | |
|---|---|---|---|
| Alone | Combination | ||
| 5d AmB | 4 * 1.25 ** | 16 2.5 | No rules |
| 2 1.25 | Indifference | ||
| 2 2.5 | |||
| 1 2.5 | |||
| 0.5 2.25 | |||
| 0.25 2.5 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Staniszewska, M.; Kuryk, Ł.; Gryciuk, A.; Kawalec, J.; Rogalska, M.; Baran, J.; Łukowska-Chojnacka, E.; Kowalkowska, A. In Vitro Anti-Candida Activity and Action Mode of Benzoxazole Derivatives. Molecules 2021, 26, 5008. https://doi.org/10.3390/molecules26165008
Staniszewska M, Kuryk Ł, Gryciuk A, Kawalec J, Rogalska M, Baran J, Łukowska-Chojnacka E, Kowalkowska A. In Vitro Anti-Candida Activity and Action Mode of Benzoxazole Derivatives. Molecules. 2021; 26(16):5008. https://doi.org/10.3390/molecules26165008
Chicago/Turabian StyleStaniszewska, Monika, Łukasz Kuryk, Aleksander Gryciuk, Joanna Kawalec, Marta Rogalska, Joanna Baran, Edyta Łukowska-Chojnacka, and Anna Kowalkowska. 2021. "In Vitro Anti-Candida Activity and Action Mode of Benzoxazole Derivatives" Molecules 26, no. 16: 5008. https://doi.org/10.3390/molecules26165008
APA StyleStaniszewska, M., Kuryk, Ł., Gryciuk, A., Kawalec, J., Rogalska, M., Baran, J., Łukowska-Chojnacka, E., & Kowalkowska, A. (2021). In Vitro Anti-Candida Activity and Action Mode of Benzoxazole Derivatives. Molecules, 26(16), 5008. https://doi.org/10.3390/molecules26165008







