Comparative and Combinatorial Effects of Resveratrol and Sacubitril/Valsartan alongside Valsartan on Cardiac Remodeling and Dysfunction in MI-Induced Rats
Abstract
1. Introduction
2. Results
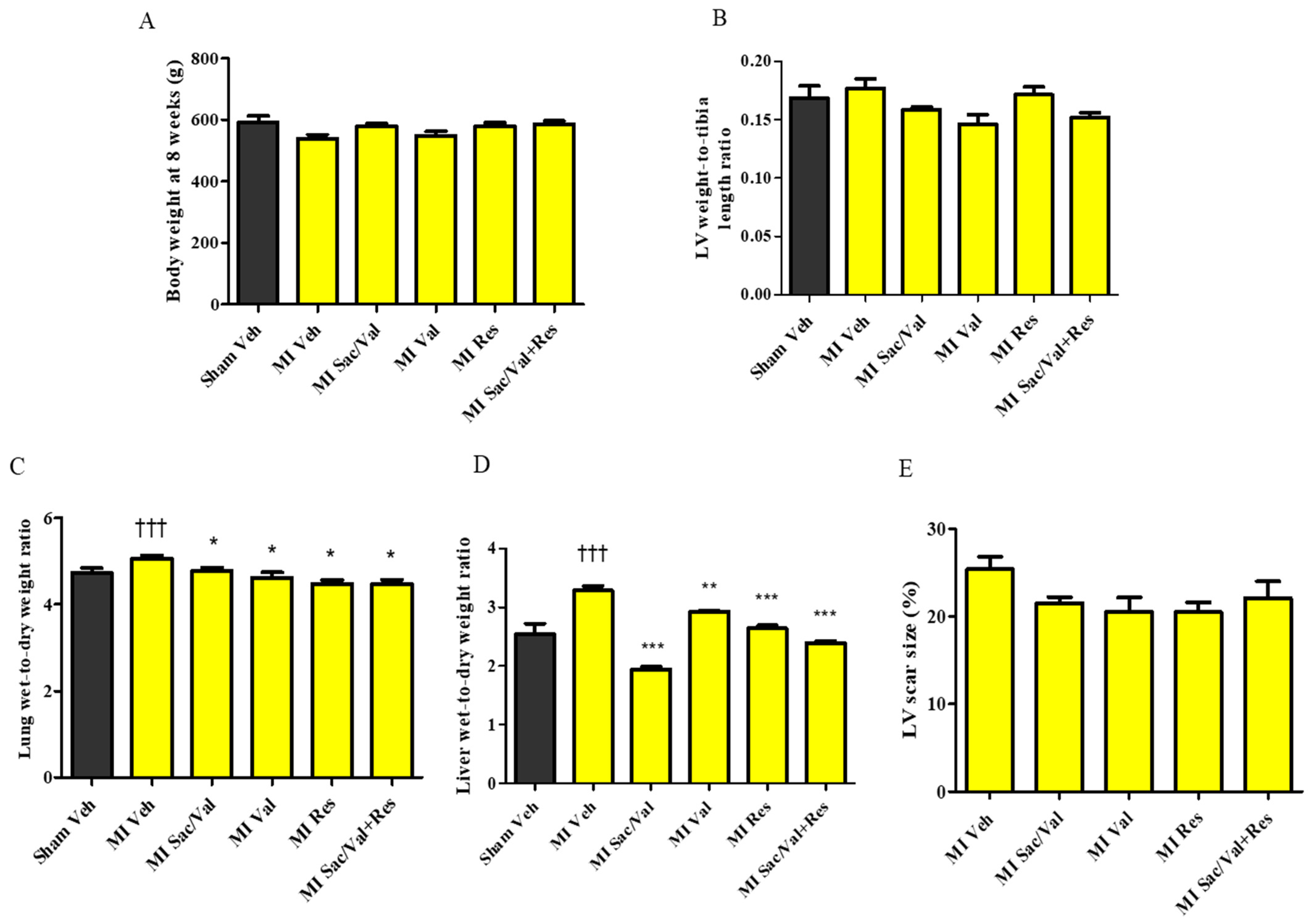
2.1. General Characteristics
2.2. Treatment with Resveratrol, Sacubitril/Valsartan, Valsartan and Sacubitril/Valsartan + Resveratrol Prevents Post-MI LV Dilatation
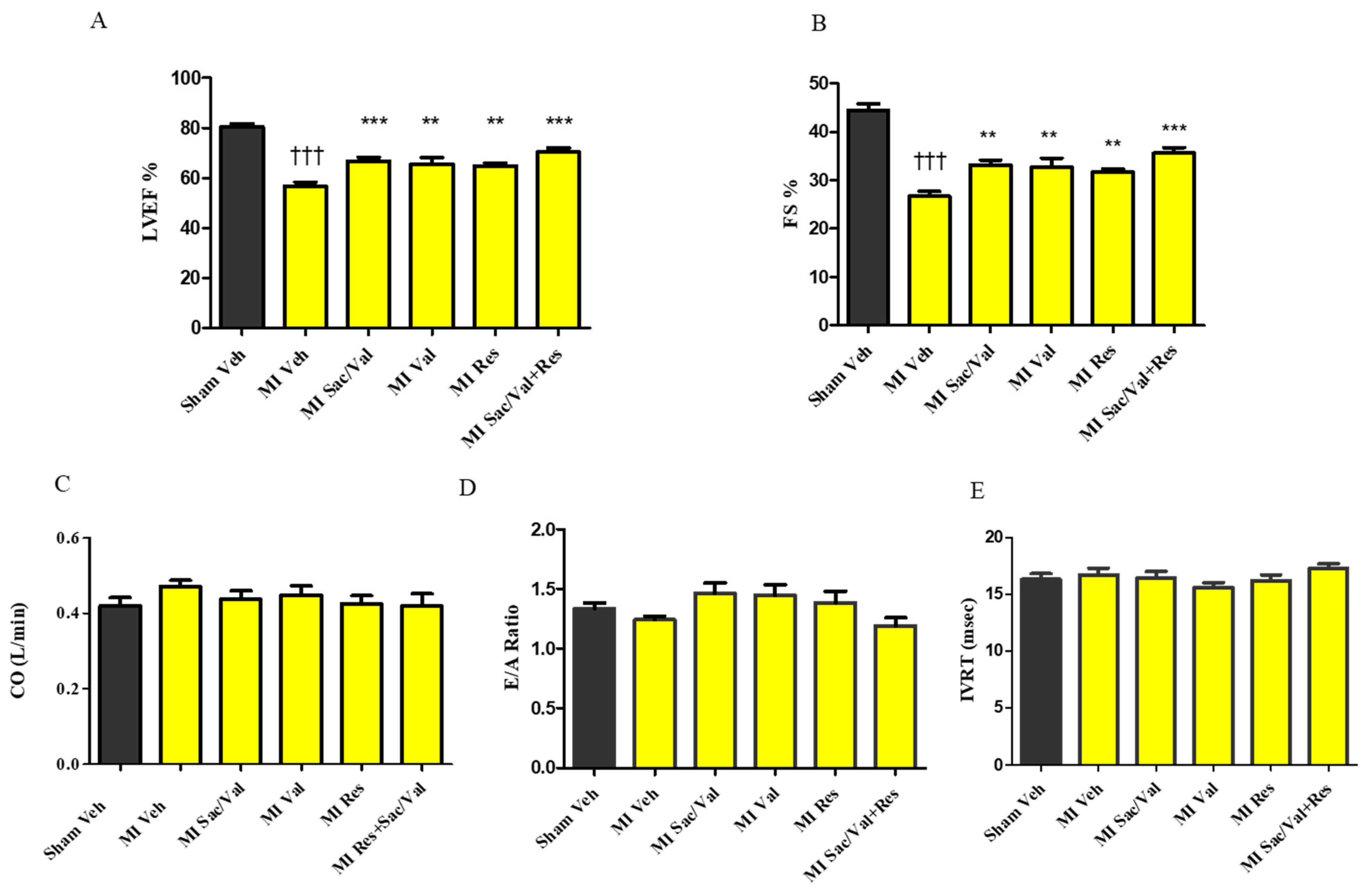
2.3. Treatment with Resveratrol, Sacubitril/Valsartan, Valsartan and Sacubitril/Valsartan+ Resveratrol Prevents Post-MI Cardiac Dysfunction
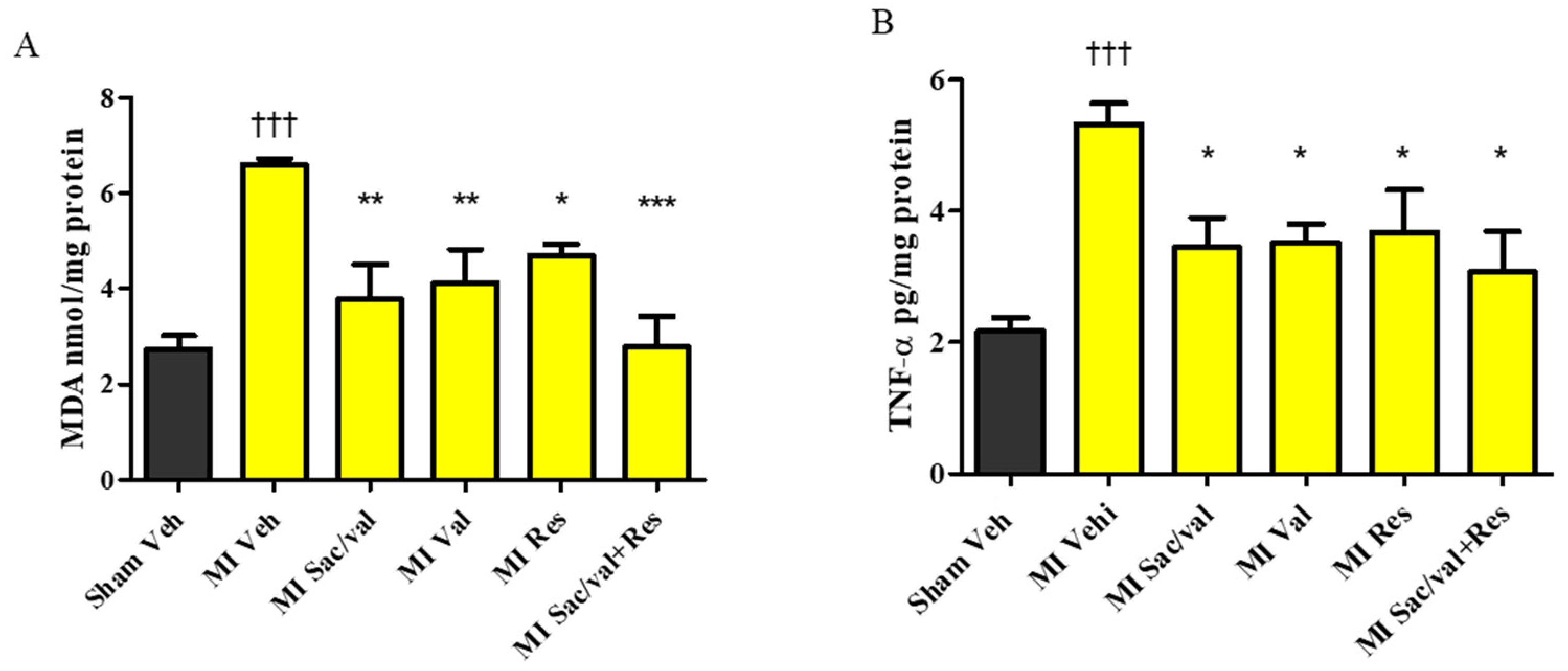
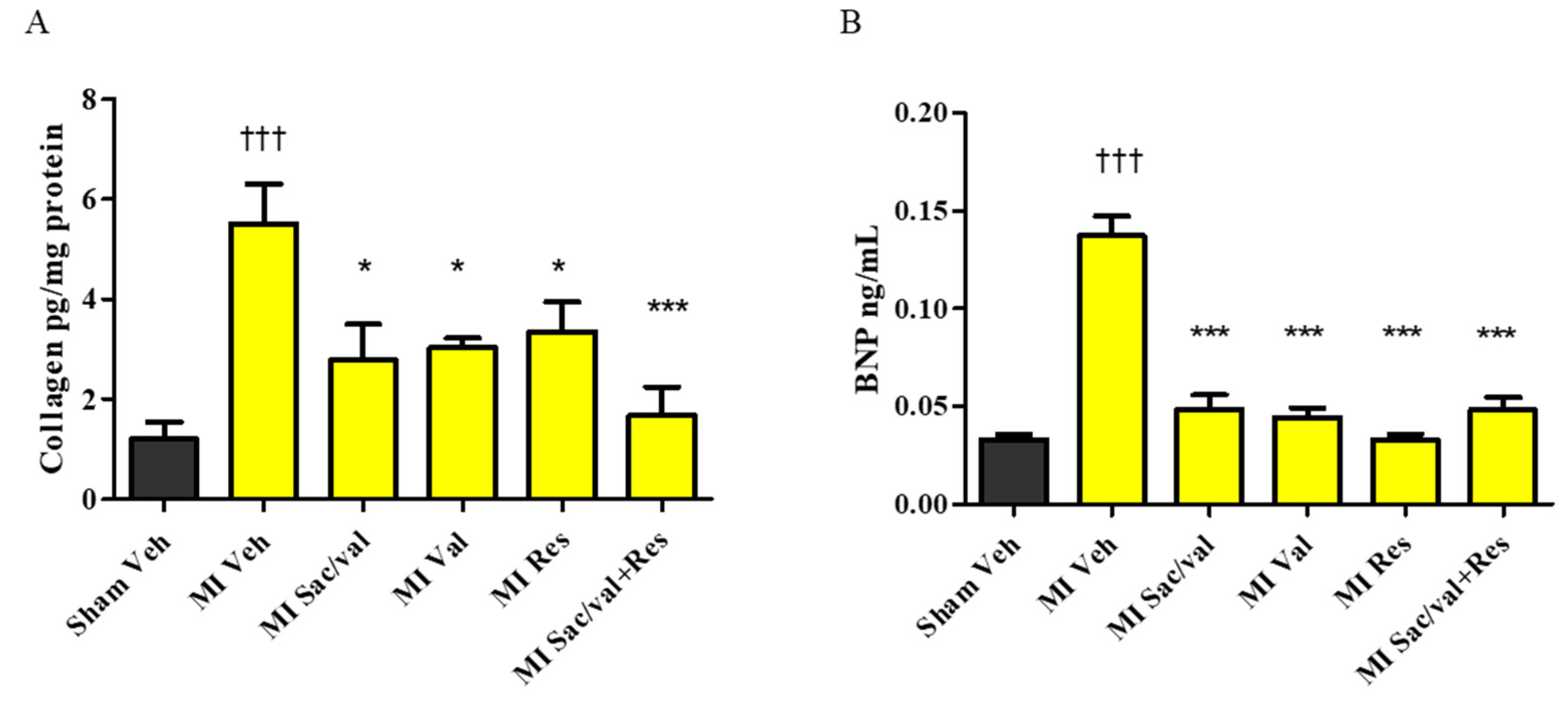
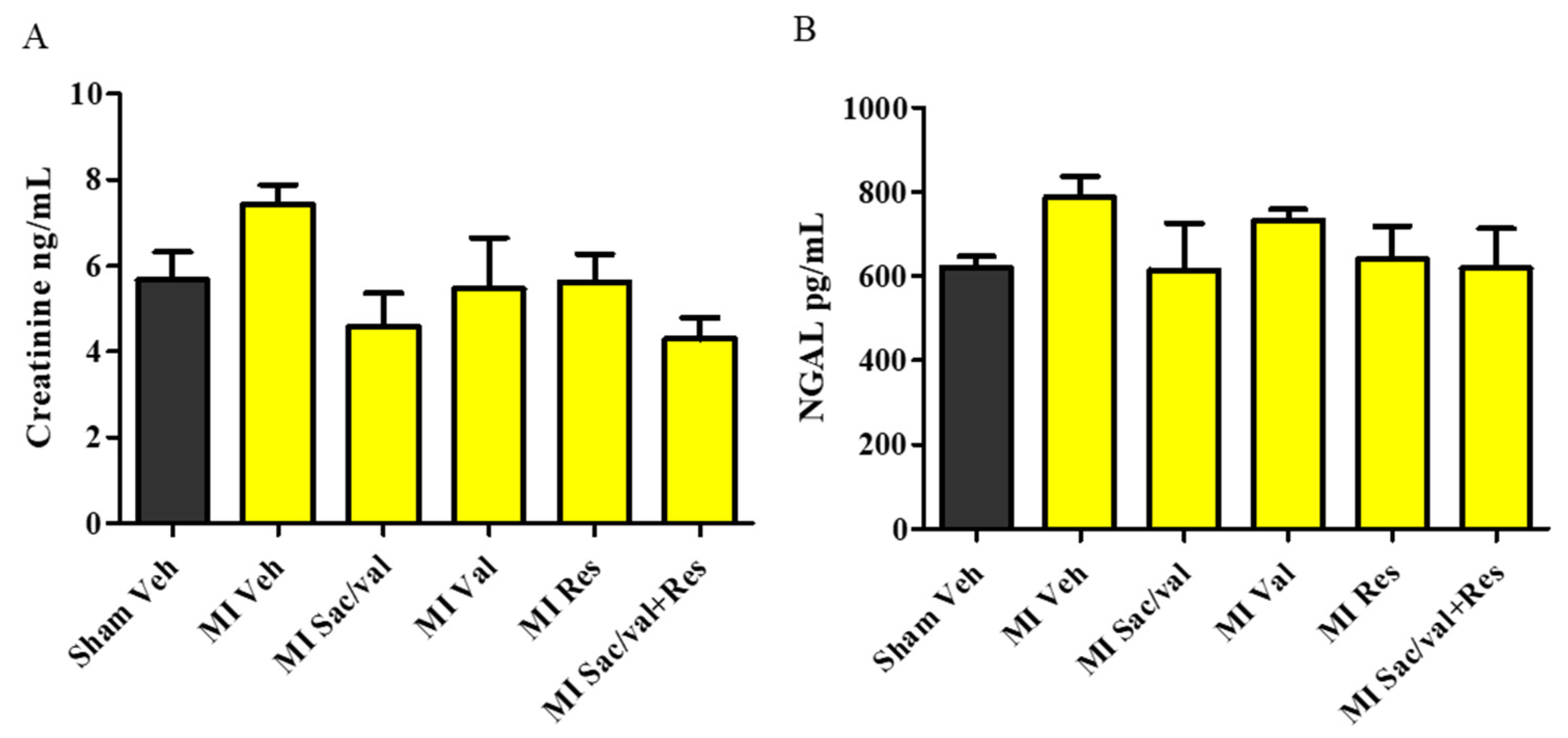
2.4. Treatment with Resveratrol, Sacubitril/Valsartan, Valsartan and Sacubitril/Valsartan + Resveratrol Lowers Post-MI Increase in MDA, TNF-α, Collagen, and BNP
3. Discussion
4. Materials and Methods
4.1. Animal Care and Experimental Design
4.2. Transthoracic Echocardiography (TTE)
4.3. Blood and Tissue Collection
4.4. LV Scar Size and Lung and Liver Wet-to-Dry Weight Ratio Determination
4.5. Oxidative Stress Marker Assay
4.6. Proinflammatory and Cardiac Fibrosis Marker Assays
4.7. NP and Renal Dysfunction and Injury Marker Assays
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E.; Colvin, M.M.; Drazner, M.H.; Filippatos, G.S.; Fonarow, G.C.; Givertz, M.M.; et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure. J. Am. Coll. Cardiol. 2017, 70, 776–803. [Google Scholar] [CrossRef]
- de Lemos, J.A.; McGuire, D.K.; Drazner, M.H. B-type natriuretic peptide in cardiovascular disease. Lancet 2003, 362, 316–322. [Google Scholar] [CrossRef]
- Federico, C. Natriuretic Peptide system and cardiovascular disease. Heart Views Off. J. Gulf Heart Assoc. 2010, 11, 10–15. [Google Scholar]
- Kostis, J.B.; Packer, M.; Black, H.R.; Schmieder, R.; Henry, D.; Levy, E. Omapatrilat and enalapril in patients with hypertension: The Omapatrilat Cardiovascular Treatment vs. Enalapril (OCTAVE) trial. Am. J. Hypertens. 2004, 17, 103–111. [Google Scholar] [CrossRef]
- O’Connor, C.M.; Starling, R.C.; Hernandez, A.F.; Armstrong, P.W.; Dickstein, K.; Hasselblad, V.; Heizer, G.M.; Komajda, M.; Massie, B.M.; McMurray, J.J.V.; et al. Effect of Nesiritide in Patients with Acute Decompensated Heart Failure. N. Engl. J. Med. 2011, 365, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Rademaker, M.T.; Charles, C.J.; Espiner, E.A.; Nicholls, M.G.; Richards, A.M.; Kosoglou, T. Combined neutral endopeptidase and angiotensin-converting enzyme inhibition in heart failure: Role of natriuretic peptides and angiotensin II. J. Cardiovasc. Pharmacol. 1998, 31, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Trippodo, N.C.; Fox, M.; Monticello, T.M.; Panchal, B.C.; Asaad, M.M. Vasopeptidase inhibition with omapatrilat improves cardiac geometry and survival in cardiomyopathic hamsters more than does ACE inhibition with captopril. J. Cardiovasc. Pharmacol. 1999, 34, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Packer, M.; Califf, R.M.; Konstam, M.A.; Krum, H.; McMurray, J.J.; Rouleau, J.L.; Swedberg, K. Comparison of omapatrilat and enalapril in patients with chronic heart failure: The Omapatrilat Versus Enalapril Randomized Trial of Utility in Reducing Events (OVERTURE). Circulation 2002, 106, 920–926. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.J.V.; Packer, M.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.L.; Shi, V.C.; Solomon, S.D.; Swedberg, K.; et al. Angiotensin–Neprilysin Inhibition versus Enalapril in Heart Failure. N. Engl. J. Med. 2014, 371, 993–1004. [Google Scholar] [CrossRef]
- Zordoky, B.N.; Robertson, I.M.; Dyck, J.R. Preclinical and clinical evidence for the role of resveratrol in the treatment of cardiovascular diseases. Biochim. Biophys. Acta 2015, 1852, 1155–1177. [Google Scholar] [CrossRef]
- Raj, P.; Zieroth, S.; Netticadan, T. An overview of the efficacy of resveratrol in the management of ischemic heart disease. Ann. N. Y. Acad. Sci. 2015, 1348, 55–67. [Google Scholar] [CrossRef]
- Raj, P.; Aloud, B.M.; Louis, X.L.; Yu, L.; Zieroth, S.; Netticadan, T. Resveratrol is equipotent to perindopril in attenuating post-infarct cardiac remodeling and contractile dysfunction in rats. J. Nutr. Biochem. 2016, 28, 155–163. [Google Scholar] [CrossRef]
- Magyar, K.; Halmosi, R.; Palfi, A.; Feher, G.; Czopf, L.; Fulop, A.; Battyany, I.; Sumegi, B.; Toth, K.; Szabados, E. Cardioprotection by resveratrol: A human clinical trial in patients with stable coronary artery disease. Clin. Hemorheol. Microcirc. 2012, 50, 179–187. [Google Scholar] [CrossRef] [PubMed]
- von Lueder, T.G.; Wang, B.H.; Kompa, A.R.; Huang, L.; Webb, R.; Jordaan, P.; .Atar, D.; Krum, H. Angiotensin Receptor Neprilysin Inhibitor LCZ696 Attenuates Cardiac Remodeling and Dysfunction After Myocardial Infarction by Reducing Cardiac Fibrosis and Hypertrophy. Circ. Heart Fail. 2015, 8, 71–78. [Google Scholar] [CrossRef]
- Ishii, M.; Kaikita, K.; Sato, K.; Sueta, D.; Fujisue, K.; Arima, Y.; Oimatsu, Y.; Mitsuse, T.; Onoue, Y.; Araki, S.; et al. Cardioprotective Effects of LCZ696 (Sacubitril/Valsartan) After Experimental Acute Myocardial Infarction. JACC Basic Transl. Sci. 2017, 2, 655. [Google Scholar] [CrossRef] [PubMed]
- Torrado, J.; Cain, C.; Mauro, A.G.; Romeo, F.; Ockaili, R.; Chau, V.Q.; Nestler, J.A.; Devarakonda, T.; Ghosh, S.; Das, A.; et al. Sacubitril/Valsartan Averts Adverse Post-Infarction Ventricular Remodeling and Preserves Systolic Function in Rabbits. J. Am. Coll. Cardiol. 2018, 72, 2342–2356. [Google Scholar] [CrossRef]
- Matsumura, N.; Takahara, S.; Maayah, Z.H.; Parajuli, N.; Byrne, N.J.; Shoieb, S.M.; Soltys, C.M.; Beker, D.L.; Masson, G.; El-Kadi, A.O.S.; et al. Resveratrol improves cardiac function and exercise performance in MI-induced heart failure through the inhibition of cardiotoxic HETE metabolites. J. Mol. Cell. Cardiol. 2018, 125, 162–173. [Google Scholar] [CrossRef]
- Kanamori, H.; Takemura, G.; Goto, K.; Tsujimoto, A.; Ogino, A.; Takeyama, T.; Kawaguchi, T.; Watanabe, T.; Morishita, K.; Kawasaki, M.; et al. Resveratrol reverses remodeling in hearts with large, old myocardial infarctions through enhanced autophagy-activating AMP kinase pathway. Am. J. Pathol. 2013, 182, 701–713. [Google Scholar] [CrossRef]
- Vaskova, E.; Ikeda, G.; Tada, Y.; Wahlquist, C.; Mercola, M.; Yang, P.C. Sacubitril/Valsartan Improves Cardiac Function and Decreases Myocardial Fibrosis Via Downregulation of Exosomal miR-181a in a Rodent Chronic Myocardial Infarction Model. J. Am. Heart Assoc. 2020, 9, e015640. [Google Scholar] [CrossRef]
- Chew, D.S.; Wilton, S.B.; Kavanagh, K.; Southern, D.A.; Tan-Mesiatowsky, L.E.; Exner, D.V.; Investigators, A. Left ventricular ejection fraction reassessment post-myocardial infarction: Current clinical practice and determinants of adverse remodeling. Am. Heart J. 2018, 198, 91–96. [Google Scholar] [CrossRef]
- Solomon, S.D.; Glynn, R.J.; Greaves, S.; Ajani, U.; Rouleau, J.L.; Menapace, F.; Arnold, J.M.; Hennekens, C.; Pfeffer, M.A. Recovery of ventricular function after myocardial infarction in the reperfusion era: The healing and early afterload reducing therapy study. Ann. Intern. Med. 2001, 134, 451–458. [Google Scholar] [CrossRef]
- Ottervanger, J.P.; van ‘t Hof, A.W.; Reiffers, S.; Hoorntje, J.C.; Suryapranata, H.; de Boer, M.J.; Zijlstra, F. Long-term recovery of left ventricular function after primary angioplasty for acute myocardial infarction. Eur. Heart J. 2001, 22, 785–790. [Google Scholar] [CrossRef]
- Almufleh, A.; Marbach, J.; Chih, S.; Stadnick, E.; Davies, R.; Liu, P.; Mielniczuk, L. Ejection fraction improvement and reverse remodeling achieved with Sacubitril/Valsartan in heart failure with reduced ejection fraction patients. Am. J. Cardiovasc. Dis. 2017, 7, 108–113. [Google Scholar]
- Moris, D.; Spartalis, M.; Spartalis, E.; Karachaliou, G.S.; Karaolanis, G.I.; Tsourouflis, G.; Tsilimigras, D.I.; Tzatzaki, E.; Theocharis, S. The role of reactive oxygen species in the pathophysiology of cardiovascular diseases and the clinical significance of myocardial redox. Ann. Transl. Med. 2017, 5, 326. [Google Scholar] [CrossRef]
- Zhou, R.; Xu, Q.; Zheng, P.; Yan, L.; Zheng, J.; Dai, G. Cardioprotective effect of fluvastatin on isoproterenol-induced myocardial infarction in rat. Eur. J. Pharmacol. 2008, 586, 244–250. [Google Scholar] [CrossRef]
- Zhou, S.X.; Zhou, Y.; Zhang, Y.L.; Lei, J.; Wang, J.F. Antioxidant probucol attenuates myocardial oxidative stress and collagen expressions in post-myocardial infarction rats. J. Cardiovasc. Pharmacol. 2009, 54, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Dolinsky, V.W.; Chan, A.Y.M.; Frayne, I.R.; Light, P.E.; Rosiers, C.D.; Dyck, J.R.B. Resveratrol Prevents the Prohypertrophic Effects of Oxidative Stress on LKB1. Circulation 2009, 119, 1643–1652. [Google Scholar] [CrossRef]
- Bagul, P.K.; Deepthi, N.; Sultana, R.; Banerjee, S.K. Resveratrol ameliorates cardiac oxidative stress in diabetes through deacetylation of NFkB-p65 and histone 3. J. Nutr. Biochem. 2015, 26, 1298–1307. [Google Scholar] [CrossRef]
- Cheng, L.; Jin, Z.; Zhao, R.; Ren, K.; Deng, C.; Yu, S. Resveratrol attenuates inflammation and oxidative stress induced by myocardial ischemia-reperfusion injury: Role of Nrf2/ARE pathway. Int. J. Clin. Exp. Med. 2015, 8, 10420–10428. [Google Scholar]
- Xia, N.; Daiber, A.; Forstermann, U.; Li, H. Antioxidant effects of resveratrol in the cardiovascular system. Br. J. Pharmacol. 2017, 174, 1633–1646. [Google Scholar] [CrossRef]
- Tanno, M.; Kuno, A.; Yano, T.; Miura, T.; Hisahara, S.; Ishikawa, S.; Shimamoto, K.; Horio, Y. Induction of manganese superoxide dismutase by nuclear translocation and activation of SIRT1 promotes cell survival in chronic heart failure. J. Biol. Chem. 2010, 285, 8375–8382. [Google Scholar] [CrossRef] [PubMed]
- Khan, B.V.; Sola, S.; Lauten, W.B.; Natarajan, R.; Hooper, W.C.; Menon, R.G.; Lerakis, S.; Helmy, T. Quinapril, an ACE Inhibitor, Reduces Markers of Oxidative Stress in the Metabolic Syndrome. Diabetes Care 2004, 27, 1712. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Khaper, N.; Singal, P.K. Modulation of oxidative stress by a selective inhibition of angiotensin II type 1 receptors in MI rats. J. Am. Coll. Cardiol. 2001, 37, 1461–1466. [Google Scholar] [CrossRef]
- Mikrut, K.; Kupsz, J.; Kozlik, J.; Krauss, H.; Pruszynska-Oszmalek, E.; Gibas-Dorna, M. Angiotensin-converting enzyme inhibitors reduce oxidative stress intensity in hyperglicemic conditions in rats independently from bradykinin receptor inhibitors. Croat. Med. J. 2016, 57, 371–380. [Google Scholar] [CrossRef]
- Marti, C.N.; Khan, H.; Mann, D.L.; Georgiopoulou, V.V.; Bibbins-Domingo, K.; Harris, T.; Koster, A.; Newman, A.; Kritchevsky, S.B.; Kalogeropoulos, A.P.; et al. Soluble tumor necrosis factor receptors and heart failure risk in older adults: Health, Aging, and Body Composition (Health ABC) Study. Circ. Heart Fail. 2014, 7, 5–11. [Google Scholar] [CrossRef]
- Satoh, M.; Minami, Y.; Takahashi, Y.; Nakamura, M. Immune modulation: Role of the inflammatory cytokine cascade in the failing human heart. Curr. Heart Fail. Rep. 2008, 5, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Saba, S.; Janczewski, A.M.; Baker, L.C.; Shusterman, V.; Gursoy, E.C.; Feldman, A.M.; Salama, G.; McTiernan, C.F.; London, B. Atrial contractile dysfunction, fibrosis, and arrhythmias in a mouse model of cardiomyopathy secondary to cardiac-specific overexpression of tumor necrosis factor-α. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H1456–H1467. [Google Scholar] [CrossRef] [PubMed]
- Tome-Carneiro, J.; Gonzalvez, M.; Larrosa, M.; Yanez-Gascon, M.J.; Garcia-Almagro, F.J.; Ruiz-Ros, J.A.; Garcia-Conesa, M.T.; Tomas-Barberan, F.A.; Espin, J.C. One-year consumption of a grape nutraceutical containing resveratrol improves the inflammatory and fibrinolytic status of patients in primary prevention of cardiovascular disease. Am. J. Cardiol. 2012, 110, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Ceconi, C.; Fox, K.M.; Remme, W.J.; Simoons, M.L.; Deckers, J.W.; Bertrand, M.; Parrinello, G.; Kluft, C.; Blann, A.; Cokkinos, D.; et al. ACE inhibition with perindopril and biomarkers of atherosclerosis and thrombosis: Results from the PERTINENT study. Atherosclerosis 2009, 204, 273–275. [Google Scholar] [CrossRef]
- Weber, K.T.; Sun, Y.; Bhattacharya, S.K.; Ahokas, R.A.; Gerling, I.C. Myofibroblast-mediated mechanisms of pathological remodelling of the heart. Nat. Rev. Cardiol. 2013, 10, 15–26. [Google Scholar] [CrossRef]
- Zhou, X.; Lu, X.; Xu, W.; Chen, J. Protective effects of hydrogen sulfide against chronic alcohol intake-induced left ventricular remodeling in rats. Cardiovasc. Drugs 2013, 27, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Li, G.N.; Xie, J.; Li, R.; Chen, Q.H.; Chen, J.Z.; Wei, Z.H.; Kang, L.N.; Xu, B. Resveratrol ameliorates myocardial fibrosis by inhibiting ROS/ERK/TGF-β/periostin pathway in STZ-induced diabetic mice. BMC Cardiovasc. Disord. 2016, 16, 5. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Li, J.; Liu, J.; Li, N.; Wang, S.; Liu, H.; Zeng, M.; Zhang, Y.; Bu, P. Activation of SIRT3 by resveratrol ameliorates cardiac fibrosis and improves cardiac function via the TGF-β/Smad3 pathway. Am. J. Physiol. Heart Circ. Physiol. 2014, 308, H424–H434. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, Y.; Ong’achwa, M.J.; Ge, L.; Qian, Y.; Chen, L.; Hu, X.; Li, F.; Wei, H.; Zhang, C.; et al. Resveratrol Inhibits the TGF-β1-Induced Proliferation of Cardiac Fibroblasts and Collagen Secretion by Downregulating miR-17 in Rat. BioMed Res. Int. 2018, 2018, 8730593. [Google Scholar] [CrossRef]
- Burke, R.M.; Lighthouse, J.K.; Mickelsen, D.M.; Small, E.M. Sacubitril/Valsartan Decreases Cardiac Fibrosis in Left Ventricle Pressure Overload by Restoring PKG Signaling in Cardiac Fibroblasts. Circ. Heart Fail. 2019, 12, e005565. [Google Scholar] [CrossRef]
- Fazlinezhad, A.; Rezaeian, M.K.; Yousefzadeh, H.; Ghaffarzadegan, K.; Khajedaluee, M. Plasma Brain Natriuretic Peptide (BNP) as an Indicator of Left Ventricular Function, Early Outcome and Mechanical Complications after Acute Myocardial Infarction. Clin. Med. Insights Cardiol. 2011, 5, 77–83. [Google Scholar] [CrossRef]
- Riba, A.; Deres, L.; Sumegi, B.; Toth, K.; Szabados, E.; Halmosi, R. Cardioprotective Effect of Resveratrol in a Postinfarction Heart Failure Model. Oxidative Med. Cell. Longev. 2017, 2017, 6819281. [Google Scholar] [CrossRef]
- Dudka, J.; Gieroba, R.; Korga, A.; Burdan, F.; Matysiak, W.; Jodlowska-Jedrych, B.; Mandziuk, S.; Korobowicz, E.; Murias, M. Different Effects of Resveratrol on Dose-Related Doxorubicin-Induced Heart and Liver Toxicity. Evid. Based Complementary Altern. Med. 2012, 2012, 10. [Google Scholar] [CrossRef]
- Lin, C.H.; Lin, C.C.; Ting, W.J.; Pai, P.Y.; Kuo, C.H.; Ho, T.J.; Kuo, W.W.; Chang, C.H.; Huang, C.Y.; Lin, W.T. Resveratrol enhanced FOXO3 phosphorylation via synergetic activation of SIRT1 and PI3K/Akt signaling to improve the effects of exercise in elderly rat hearts. Age 2014, 36, 9705. [Google Scholar] [CrossRef]
- Windt, W.A.K.M.; Eijkelkamp, W.B.A.; Henning, R.H.; Kluppel, A.C.A.; de Graeff, P.A.; Hillege, H.L.; Schäfer, S.; de Zeeuw, D.; van Dokkum, R.P.E. Renal Damage after Myocardial Infarction Is Prevented by Renin-Angiotensin-Aldosterone-System Intervention. J. Am. Soc. Nephrol. 2006, 17, 3059. [Google Scholar] [CrossRef]
- Louis, X.L.; Thandapilly, S.J.; MohanKumar, S.K.; Yu, L.; Taylor, C.G.; Zahradka, P.; Netticadan, T. Treatment with low-dose resveratrol reverses cardiac impairment in obese prone but not in obese resistant rats. J. Nutr. Biochem. 2012, 23, 1163–1169. [Google Scholar] [CrossRef]
- Almeida, L.; Vaz-da-Silva, M.; Falcão, A.; Soares, E.; Costa, R.; Loureiro, A.I.; Fernandes-Lopes, C.; Rocha, J.F.; Nunes, T.; Wright, L.; et al. Pharmacokinetic and safety profile of trans-resveratrol in a rising multiple-dose study in healthy volunteers. Mol. Nutr. Food Res. 2009, 53 (Suppl. 1), S7–15. [Google Scholar] [CrossRef]
- Sergides, C.; Chirila, M.; Silvestro, L.; Pitta, D.; Pittas, A. Bioavailability and safety study of resveratrol 500 mg tablets in healthy male and female volunteers. Exp. Med. 2016, 11, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, M.A.; McMurray, J.J.V.; Velazquez, E.J.; Rouleau, J.-L.; Køber, L.; Maggioni, A.P.; Solomon, S.D.; Swedberg, K.; Van de Werf, F.; White, H.; et al. Valsartan, Captopril, or Both in Myocardial Infarction Complicated by Heart Failure, Left Ventricular Dysfunction, or Both. N. Engl. J. Med. 2003, 349, 1893–1906. [Google Scholar] [CrossRef] [PubMed]
- Raj, P.; McCallum, J.L.; Kirby, C.; Grewal, G.; Yu, L.; Wigle, J.T.; Netticadan, T. Effects of cyanidin 3-0-glucoside on cardiac structure and function in an animal model of myocardial infarction. Food Funct. 2017, 8, 4089–4099. [Google Scholar] [CrossRef]
- Jassal, D.S.; Han, S.Y.; Hans, C.; Sharma, A.; Fang, T.; Ahmadie, R.; Lytwyn, M.; Walker, J.R.; Bhalla, R.S.; Czarnecki, A.; et al. Utility of tissue Doppler and strain rate imaging in the early detection of trastuzumab and anthracycline mediated cardiomyopathy. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2009, 22, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Ju, H.; Zhao, S.; Jassal, D.S.; Dixon, I.M. Effect of AT1 receptor blockade on cardiac collagen remodeling after myocardial infarction. Cardiovasc. Res. 1997, 35, 223–232. [Google Scholar] [CrossRef]
- Thandapilly, S.J.; Wojciechowski, P.; Behbahani, J.; Louis, X.L.; Yu, L.; Juric, D.; Kopilas, M.A.; Anderson, H.D.; Netticadan, T. Resveratrol prevents the development of pathological cardiac hypertrophy and contractile dysfunction in the SHR without lowering blood pressure. Am. J. Hypertens. 2010, 23, 192–196. [Google Scholar] [CrossRef]

| Sham Veh | MI Veh | MI Sac/Val | MI Val | MI Res | MI Sac/Val + Res | |
|---|---|---|---|---|---|---|
| LVIDd (mm) | 8.86 ± 0.19 | 10.66 ± 0.18 ††† | 9.78 ± 0.20 * | 9.85 ± 0.23 * | 9.82 ± 0.21 * | 9.01 ± 0.31 *** |
| LVIDs (mm) | 4.93 ± 0.19 | 7.57 ± 0.31 ††† | 6.54 ± 0.19 * | 6.59 ± 0.30 ** | 6.53 ± 0.27 * | 5.81 ± 0.25 *** |
| LVPWTd (mm) | 1.94 ± 0.13 | 2.28 ± 0.09 | 2.02 ± 0.12 | 2.33 ± 0.07 | 2.21 ± 0.12 | 2.31 ± 0.06 |
| LVPWTs (mm) | 3.03 ± 0.16 | 2.99 ± 0.11 | 2.80 ± 0.16 | 3.14 ± 0.09 | 3.01 ± 0.15 | 3.14 ± 0.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raj, P.; Sayfee, K.; Parikh, M.; Yu, L.; Wigle, J.; Netticadan, T.; Zieroth, S. Comparative and Combinatorial Effects of Resveratrol and Sacubitril/Valsartan alongside Valsartan on Cardiac Remodeling and Dysfunction in MI-Induced Rats. Molecules 2021, 26, 5006. https://doi.org/10.3390/molecules26165006
Raj P, Sayfee K, Parikh M, Yu L, Wigle J, Netticadan T, Zieroth S. Comparative and Combinatorial Effects of Resveratrol and Sacubitril/Valsartan alongside Valsartan on Cardiac Remodeling and Dysfunction in MI-Induced Rats. Molecules. 2021; 26(16):5006. https://doi.org/10.3390/molecules26165006
Chicago/Turabian StyleRaj, Pema, Karen Sayfee, Mihir Parikh, Liping Yu, Jeffrey Wigle, Thomas Netticadan, and Shelley Zieroth. 2021. "Comparative and Combinatorial Effects of Resveratrol and Sacubitril/Valsartan alongside Valsartan on Cardiac Remodeling and Dysfunction in MI-Induced Rats" Molecules 26, no. 16: 5006. https://doi.org/10.3390/molecules26165006
APA StyleRaj, P., Sayfee, K., Parikh, M., Yu, L., Wigle, J., Netticadan, T., & Zieroth, S. (2021). Comparative and Combinatorial Effects of Resveratrol and Sacubitril/Valsartan alongside Valsartan on Cardiac Remodeling and Dysfunction in MI-Induced Rats. Molecules, 26(16), 5006. https://doi.org/10.3390/molecules26165006






