Two Decades of Triazine Dendrimers
Abstract
1. Introduction
2. Results
2.1. Synthesis
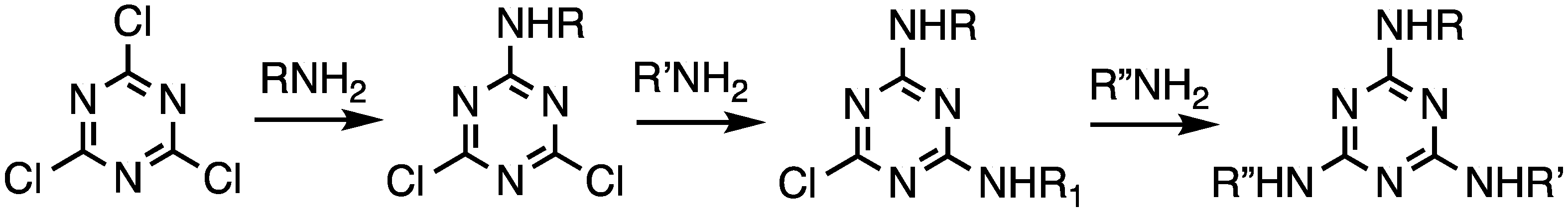
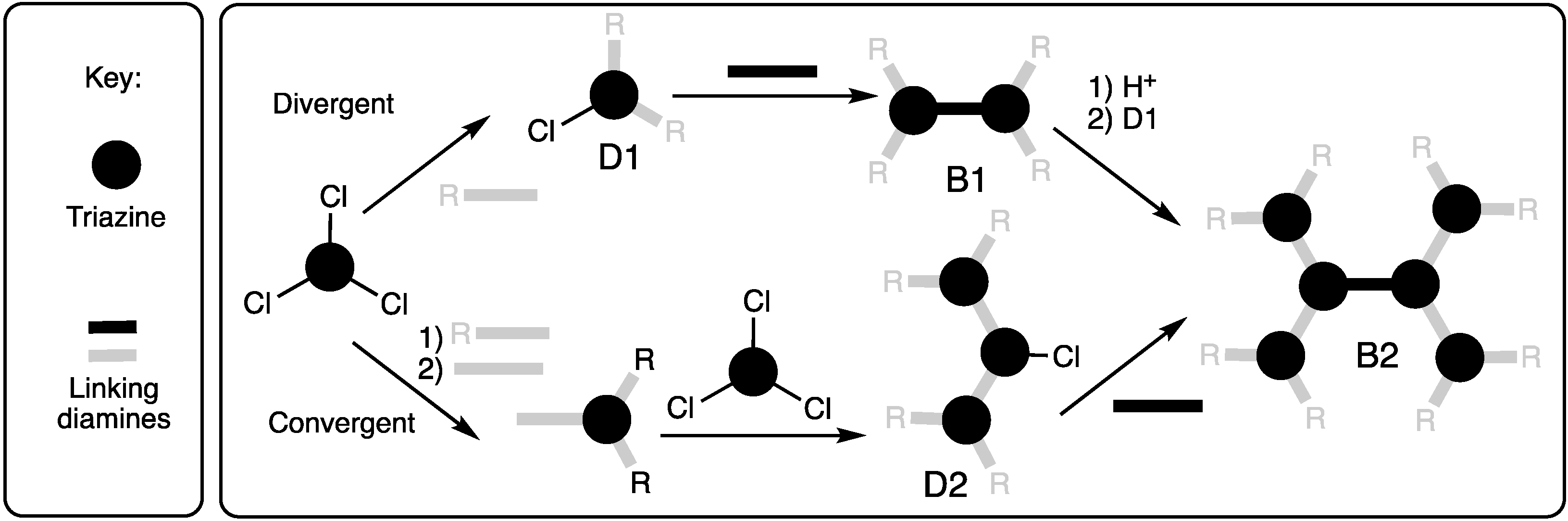
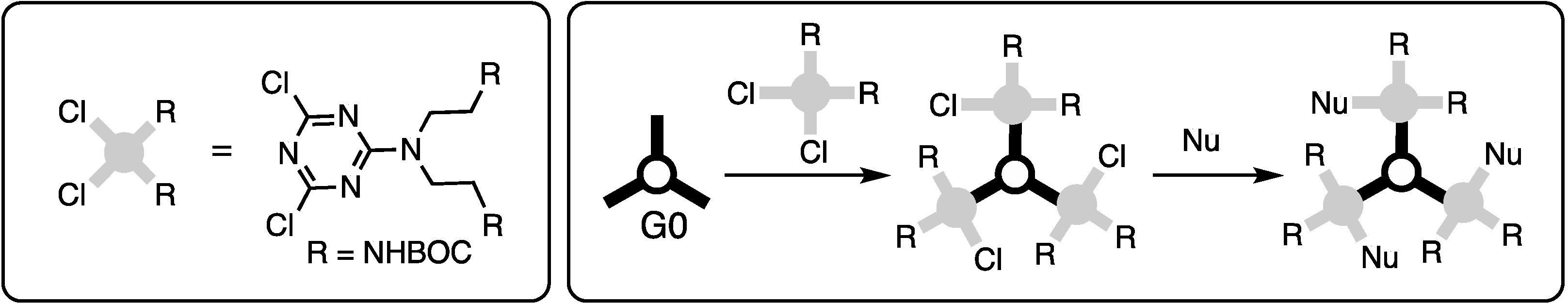
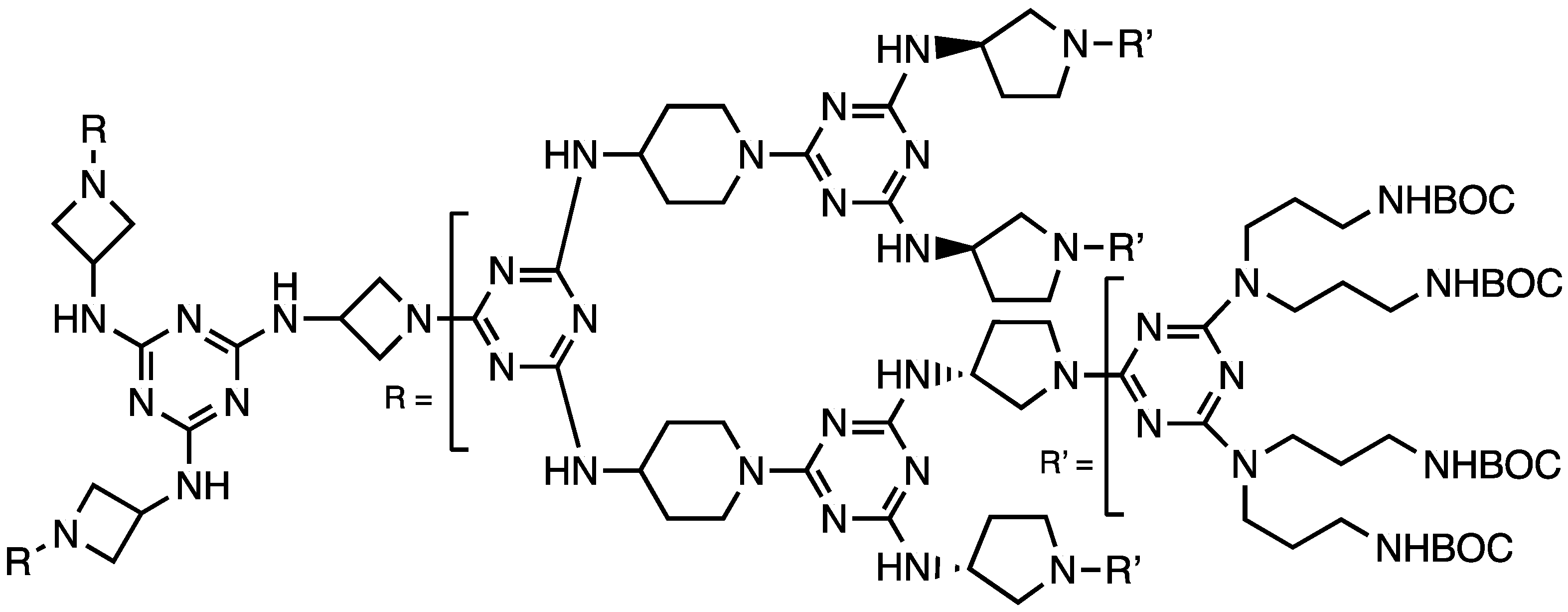
2.1.1. Convergent Methods
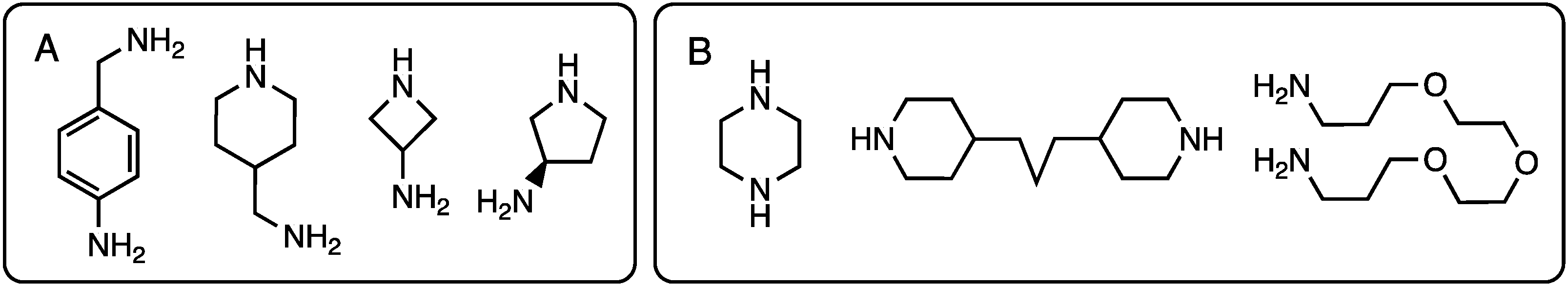
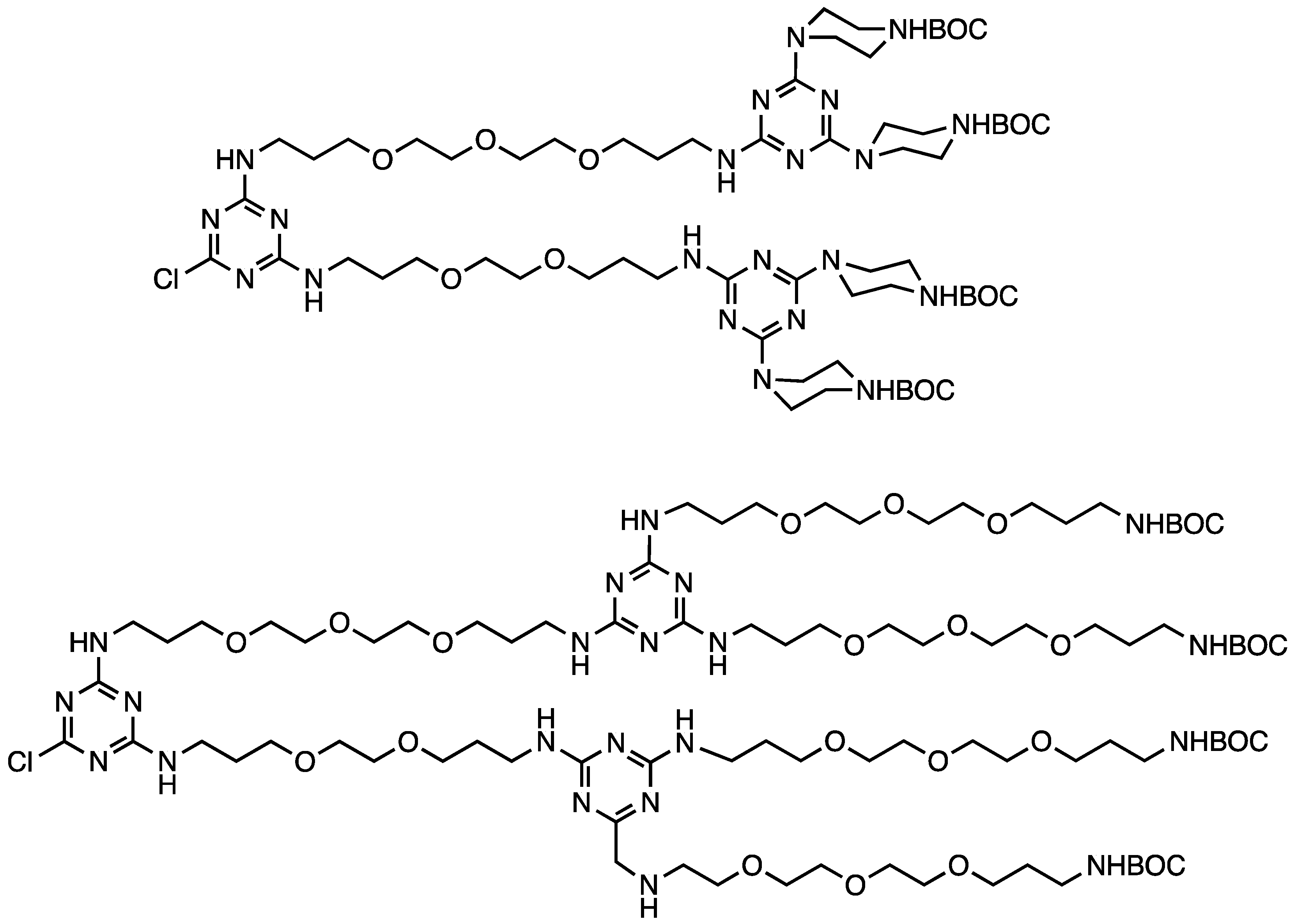
2.1.2. Divergent Methods
2.2. Drivers for Synthesis
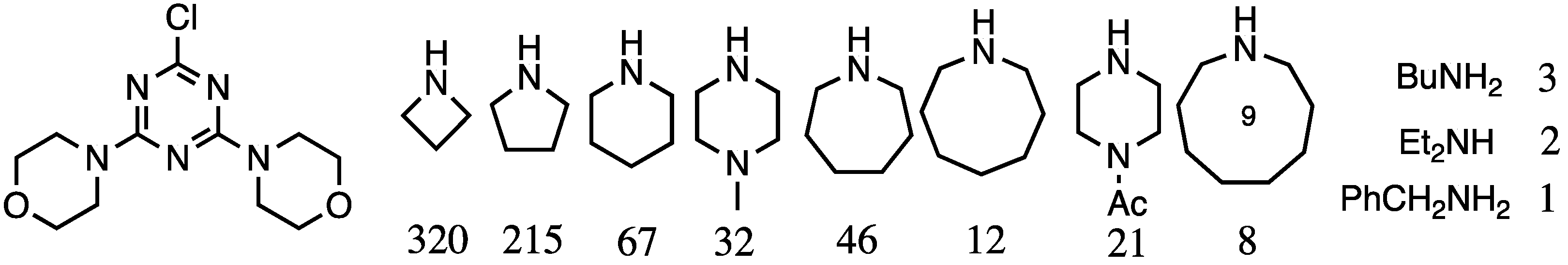
2.2.1. Solubility as a Function of Surface Group and Linking Diamine
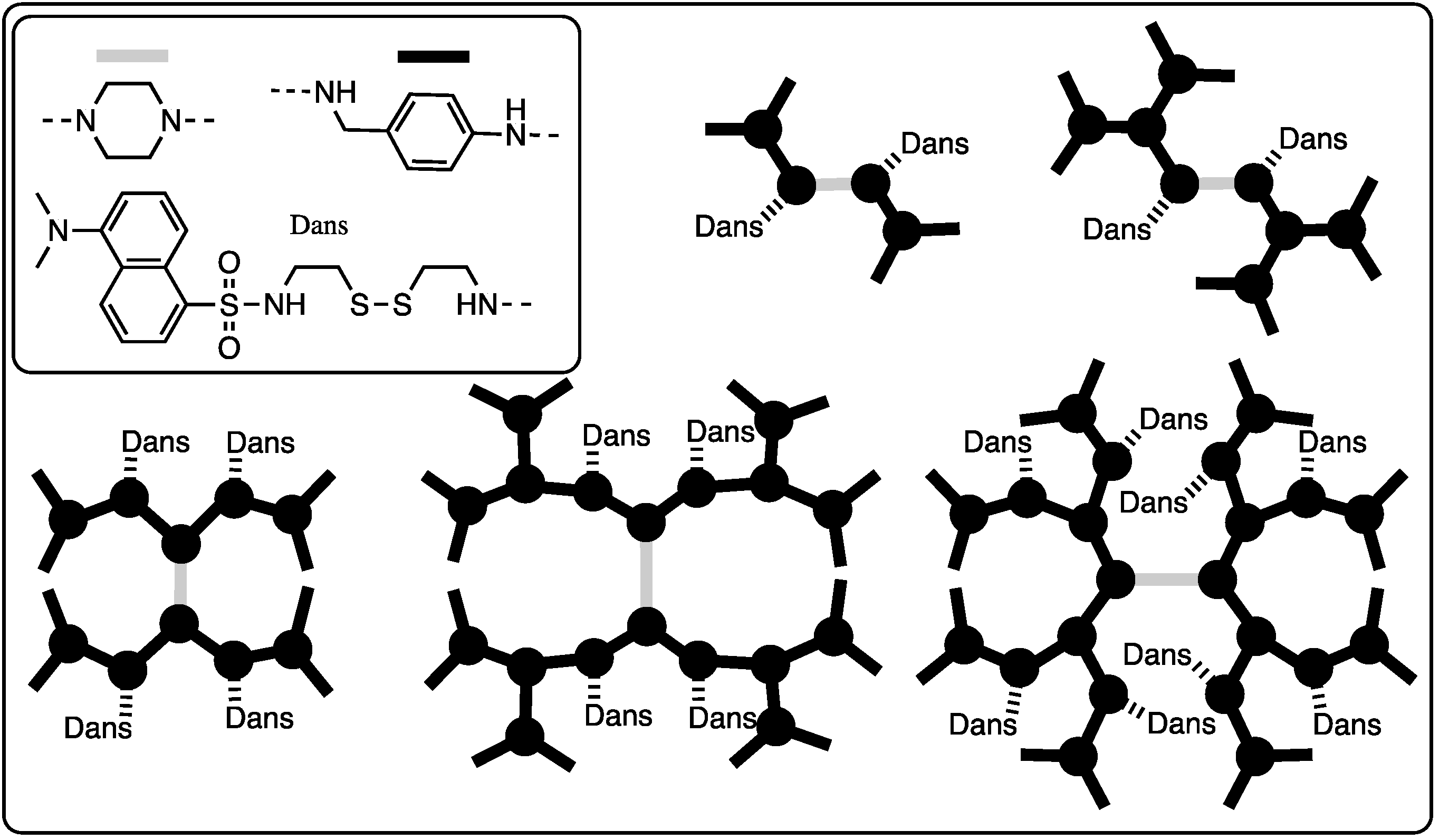
2.2.2. Platforms for Physical Organic Chemistry
2.3. Guests and Biological Activity
2.4. Applications in Materials Science
Membranes
3. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, W.; Simanek, E.E. Dendrimers based on melamine. Divergent and orthogonal, convergent syntheses of a G3 dendrimer. Org. Lett. 2000, 2, 843–845. [Google Scholar] [CrossRef]
- Zhang, W.; Simanek, E.E. Synthesis and characterization of higher generation dendrons based on melamine using p-aminobenzylamine. Evidence for molecular recognition of Cu(II). Tetrahedron Lett. 2001, 42, 5355–5357. [Google Scholar] [CrossRef]
- Zhang, W.; Nowlan, D.T.; Thomson, L.M.; Lackowski, W.M.; Simanek, E.E. Orthogonal, convergent syntheses of dendrimers based on melamine with one or two unique surface sites for manipulation. J. Am. Chem. Soc. 2001, 123, 8914–8922. [Google Scholar] [CrossRef] [PubMed]
- Steffensen, M.B.; Simanek, E.E. Synthesis and manipulation of orthogonally protected dendrimers: Building blocks for library synthesis. Angew. Chem. Int. Ed. 2004, 43, 5178–5180. [Google Scholar] [CrossRef]
- Lim, J.; Simanek, E.E. Toward the next-generation drug delivery vehicle: Synthesis of a dendrimer with four orthogonally reactive groups. Mol. Pharm. 2005, 2, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Umali, A.P.; Crampton, H.L.; Simanek, E.E. Triazine dendrimers with orthogonally protected amines on the periphery. Masking amines with Dde and BOC groups provides an alternative to carrying protected alcohols and disulfides through an iterative synthesis. J. Org. Chem. 2007, 72, 9866–9874. [Google Scholar] [CrossRef]
- Steffensen, M.B.; Simanek, E.E. Chemoselective building blocks for dendrimers from relative reactivity data. Org. Lett. 2003, 5, 2359–2361. [Google Scholar] [CrossRef] [PubMed]
- Moreno, K.X.; Simanek, E.E. Identification of diamine linkers with differing reactivity and their application in the synthesis of melamine dendrimers. Tetrahedron Lett. 2008, 49, 1152–1154. [Google Scholar] [CrossRef]
- Hollink, E.; Simanek, E.E. A divergent route to diversity in macromolecules. Org. Lett. 2006, 8, 2293–2295. [Google Scholar] [CrossRef] [PubMed]
- Crampton, H.; Hollink, E.; Perez, L.M.; Simanek, E.E. A divergent route towards single-chemical entity triazine dendrimers with opportunities for structural diversity. New J. Chem. 2007, 31, 1283–1290. [Google Scholar] [CrossRef]
- Chouai, A.; Venditto, V.J.; Simanek, E.E. Synthesis of 2-[3,3′]-Di-(tert-butoxycarbonyl)-aminoproplamine]-4,6-dichloro-1,3,5-triazine as a monomer and 1,3,5-[tris-piperazine0-triazine as a core for the large scale synthesis of melamine (triazine) dendrimers. Org. Syn. 2009, 86, 141–150. [Google Scholar]
- Chouai, A.; Venditto, V.J.; Simanek, E.E. Large scale synthesis of a generation-1 melamine (triazine) dendrimer. Org. Syn. 2009, 86, 151–158. [Google Scholar]
- Chouai, A.; Simanek, E.E. Kilogram-scale synthesis of a second-generation dendrimer based on 1,3,5-triazine using green and industrially compatible methods with a single chromatographic step. J. Org. Chem. 2008, 73, 2357–2366. [Google Scholar] [CrossRef] [PubMed]
- Venditto, V.J.; Allred, K.; Allred, C.D.; Simanek, E.E. Intercepting the synthesis of triazine dendrimers with nucleophilic pharmacophores: A general strategy toward drug delivery vehicles. Chem. Comm. 2009, 37, 5541–5542. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mintzer, M.A.; Perez, L.M.; Simanek, E.E. Divergent synthesis of triazine dendrimers using a trimethylene-dipiperidine linker that increases efficiency, simplifies analysis, and improves product solubility. Tetrahedron Lett. 2010, 51, 1631–1634. [Google Scholar] [CrossRef] [PubMed]
- Patra, S.; Kozura, B.; Huang, A.Y.; Enciso, A.E.; Sun, X.; Hsieh, J.T.; Kao, C.L.; Chen, H.T.; Simanek, E.E. Dendrimers Terminated with Dichlorotriazine Groups Provide a Route to Compositional Diversity. Org. Lett. 2013, 15, 3808–3811. [Google Scholar] [CrossRef]
- Lim, J.; Mintzer, M.A.; Perez, L.M.; Simanek, E.E. Synthesis of Odd Generation Triazine Dendrimers Using a Divergent, Macromonomer Approach. Org. Lett. 2010, 12, 1148–1151. [Google Scholar] [CrossRef]
- Lim, J.; Kostiainen, M.; Maly, J.; da Costa, V.C.; Annunziata, O.; Pavan, G.M.; Simanek, E.E. Synthesis of Large Dendrimers with the Dimensions of Small Viruses. J. Am. Chem. Soc. 2013, 135, 4660–4663. [Google Scholar] [CrossRef]
- Enciso, A.E.; Abid, Z.M.; Simanek, E.E. Rapid, semi-automated convergent synthesis of low generation triazine dendrimers using microwave assisted reactions. Polym. Chem. 2014, 5, 4635–4640. [Google Scholar] [CrossRef]
- Enciso, A.E.; Ramirez-Crescencio, F.; Zeiser, M.; Redón, R.; Simanek, E.E. Accelerated synthesis of large generation triazine dendrimers using microwave assisted reactions: A 24 h challenge. Polym. Chem. 2015, 6, 5219–5224. [Google Scholar] [CrossRef]
- Zhang, W.; Gonzalez, S.O.; Simanek, E.E. Structure-activity relationships in dendrimers, based on triazines: Gelation depends on choice of linking and surface groups. Macromolecules 2002, 35, 9015–9021. [Google Scholar] [CrossRef]
- Enciso, A.E.; Garzoni, M.; Pavan, G.M.; Simanek, E.E. Influence of linker groups on the solubility of triazine dendrimers. New J. Chem. 2015, 39, 1247–1252. [Google Scholar] [CrossRef]
- Zhang, W.; Tichy, S.E.; Pérez, L.M.; Maria, G.C.; Lindahl, P.A.; Simanek, E.E. Evaluation of multivalent dendrimers based on melamine: Kinetics of thiol-disulfide exchange depends on the structure of the dendrimer. J. Am. Chem. Soc. 2003, 125, 5086–5094. [Google Scholar] [CrossRef]
- Zhang, W.; Lalwani, S.; Chouai, A.; Simanek, E.E. Synthesis and Characterization of Anionic Triazine Dendrimers with a Labile Disulfide Core. Isr. J. Chem. 2009, 49, 23–30. [Google Scholar] [CrossRef]
- Moreno, K.X.; Simanek, E.E. Conformational analysis of triazine dendrimers: Using NMR spectroscopy to probe the choreography of a dendrimer’s dance. Macromolecules 2008, 41, 4108–4114. [Google Scholar] [CrossRef]
- Lim, J.; Pavan, G.M.; Annunziata, O.; Simanek, E.E. Experimental and Computational Evidence for an Inversion in Guest Capacity in High-Generation Triazine Dendrimer Hosts. J. Am. Chem. Soc. 2012, 134, 1942–1945. [Google Scholar] [CrossRef] [PubMed]
- Raut, S.; Enciso, A.E.; Pavan, G.M.; Lee, C.; Yepremyan, A.; Donald, A.; Tomalia, D.A.; Simanek, E.E.; Gryczynski, Z. Intrinsic Fluorescence of Triazine Dendrimers Provides a New Approach to Study Dendrimer Structure and Conformational Dynamics. J. Phys. Chem. C 2017, 121, 6946–6954. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, J.; Qin, C.; Pérez, L.M.; Parrish, A.R.; Safe, S.H.; Simanek, E.E. Triazine dendrimers for drug delivery: Evaluation of solubilization properties, activity in cell culture, and in vivo toxicity of a candidate vehicle. Supramol. Chem. 2003, 15, 607–616. [Google Scholar] [CrossRef]
- Neerman, M.F.; Chen, H.-T.; Parrish, A.R.; Simanek, E.E. Reduction of drug toxicity using dendrimers based on melamine. Mol. Pharm. 2004, 1, 390–393. [Google Scholar] [CrossRef]
- Merkel, O.M.; Zheng, M.; Mintzer, M.A.; Pavan, G.M.; Librizzi, D.; Maly, M.; Höffken, H.; Danani, A.; Simanek, E.E.; Kissel, T. Molecular modeling and in vivo imaging can identify successful flexible triazine dendrimer-based siRNA delivery systems. J. Control. Release 2011, 153, 23–33. [Google Scholar] [CrossRef]
- Merkel, O.M.; Mintzer, M.A.; Librizzi, D.; Samsonova, O.; Dicke, T.; Sproat, B.; Garn, H.; Barth, P.J.; Simanek, E.E.; Kissel, T. Triazine Dendrimers as Nonviral Vectors for in Vitro and in Vivo RNAi: The Effects of Peripheral Groups and Core Structure on Biological Activity. Mol. Pharm. 2010, 7, 969–983. [Google Scholar] [CrossRef] [PubMed]
- Pavan, G.M.; Mintzer, M.A.; Simanek, E.E.; Merkel, O.M.; Kissel, T.; Danani, A. Computational Insights into the Interactions between DNA and siRNA with “Rigid” and “Flexible” Triazine Dendrimers. Biomacromolecules 2010, 11, 721–730. [Google Scholar] [CrossRef]
- Merkel, O.M.; Mintzer, M.A.; Sitterberg, J.; Bakowsky, U.; Simanek, E.E.; Kissel, T. Triazine Dendrimers as Nonviral Gene Delivery Systems: Effects of Molecular Structure on Biological Activity. Bioconj. Chem. 2009, 20, 1799–1806. [Google Scholar] [CrossRef] [PubMed]
- Mintzer, M.A.; Merkel, O.M.; Kissel, T.; Simanek, E.E. Polycationic triazine-based dendrimers: Effect of peripheral groups on transfection efficiency. New J. Chem. 2009, 33, 1918–1925. [Google Scholar] [CrossRef] [PubMed]
- Bell, S.A.; McLean, M.E.; Oh, S.K.; Tichy, S.E.; Zhang, W.; Corn, R.M.; Crooks, R.M.; Simanek, E.E. Synthesis and characterization of covalently linked single-stranded DNA oligonucleotide-dendron conjugates. Bioconj. Chem. 2003, 14, 488–493. [Google Scholar] [CrossRef]
- Umali, A.P.; Simanek, E.E. Thiol-disulfide exchange yields multivalent dendrimers of melamine. Org. Lett. 2003, 5, 1245–1247. [Google Scholar] [CrossRef]
- Lim, J.; Venditto, V.J.; Simanek, E.E. Synthesis and characterization of a triazine dendrimer that sequesters iron(III) using 12 desferrioxamine B groups. Bioorg. Med. Chem. 2010, 18, 5749–5753. [Google Scholar] [CrossRef][Green Version]
- Sreeperumbuduru, R.S.; Abid, Z.M.; Claunch, K.M.; Chen, H.-H.; McGillivray, S.M.; Simanek, E.E. Synthesis and antimicrobial activity of triazine dendrimers with DABCO groups. RSC Adv. 2016, 6, 8806–8810. [Google Scholar] [CrossRef]
- Lalwani, S.; Chouai, A.; Perez, L.M.; Santiago, V.; Shaunak, S.; Simanek, E.E. Mimicking PAMAM Dendrimers with Amphoteric, Hybrid Triazine Dendrimers: A Comparison of Dispersity and Stability. Macromolecules 2009, 42, 6723–6732. [Google Scholar] [CrossRef] [PubMed]
- Lalwani, S.; Venditto, V.J.; Chouai, A.; Rivera, G.E.; Shaunak, S.; Simanek, E.E. Electrophoretic Behavior of Anionic Triazine and PAMAM Dendrimers: Methods for Improving Resolution and Assessing Purity Using Capillary Electrophoresis. Macromolecules 2009, 42, 3152–3161. [Google Scholar] [CrossRef] [PubMed]
- Barata, T.; Teo, I.; Lalwani, S.; Simanek, E.; Zloh, M.; Shaunak, S. Computational design principles for bioactive dendrimer constructs as antagonists of the TLR-MD-2-LPS complex. Biomaterials 2011, 32, 8702–8711. [Google Scholar] [CrossRef]
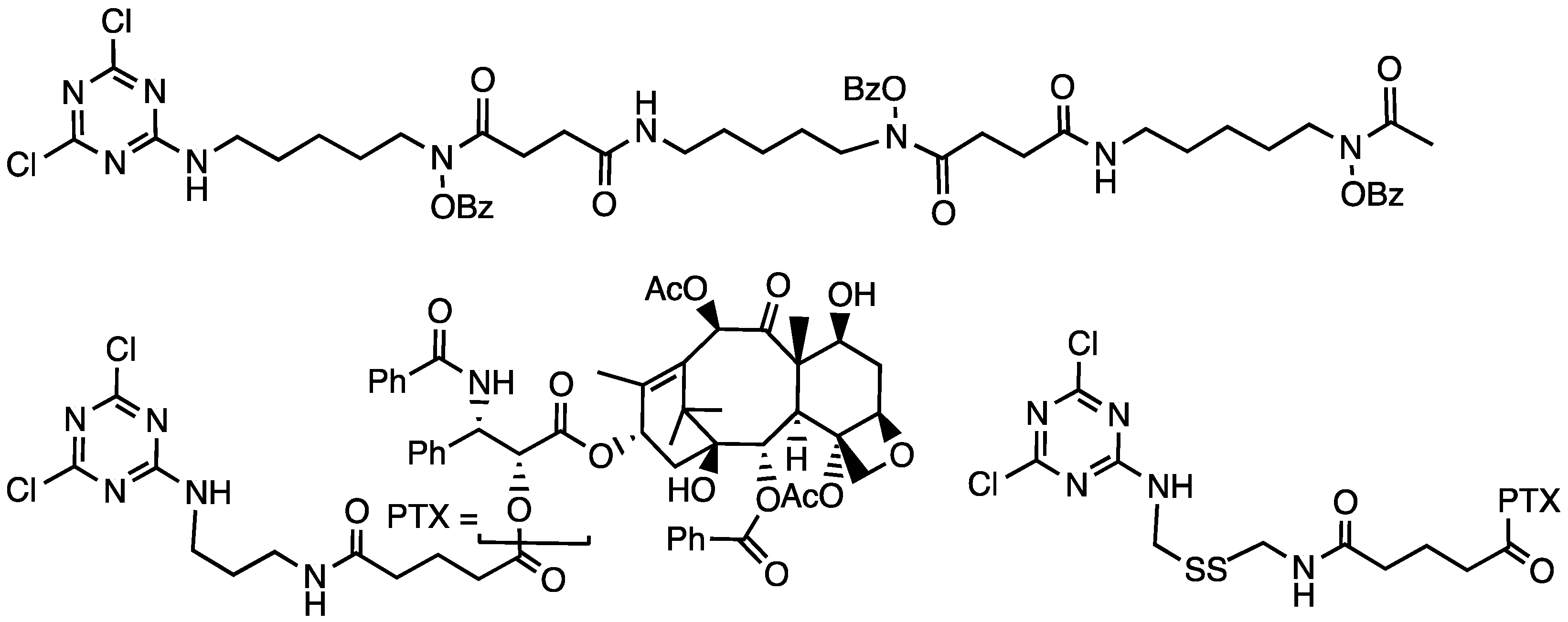
- Lim, J.; Simanek, E.E. Synthesis of water-soluble dendrimers based on melamine bearing 16 paclitaxel groups. Org. Lett. 2008, 10, 201–204. [Google Scholar] [CrossRef]
- Lo, S.-T.; Stern, S.; Clogston, J.D.; Zheng, J.; Adiseshaiah, P.P.; Dobrovolskaia, M.; Lim, J.; Patri, A.K.; Sun, X.; Simanek, E.E. Biological Assessment of Triazine Dendrimer: Toxicological Profiles, Solution Behavior, Biodistribution, Drug Release and Efficacy in a PEGylated, Paclitaxel Construct. Mol. Pharm. 2010, 7, 993–1006. [Google Scholar] [CrossRef]
- Lim, J.; Chouai, A.; Lo, S.T.; Liu, W.; Sun, X.; Simanek, E.E. Design, Synthesis, Characterization, and Biological Evaluation of Triazine Dendrimers Bearing Paclitaxel Using Ester and Ester/Disulfide Linkages. Bioconj. Chem. 2009, 20, 2154–2161. [Google Scholar] [CrossRef]
- Lim, J.; Lo, S.T.; Hill, S.; Pavan, G.M.; Sun, X.; Simanek, E.E. Antitumor Activity and Molecular Dynamics Simulations of Paclitaxel-Laden Triazine Dendrimers. Mol. Pharm. 2012, 9, 404–412. [Google Scholar] [CrossRef]
- Lee, C.; Lo, S.T.; Lim, J.; Da Costa, V.C.; Ramezani, S.; Öz, O.K.; Pavan, G.M.; Annunziata, O.; Sun, X.; Simanek, E.E. Design, Synthesis and Biological Assessment of a Triazine Dendrimer with Approximately 16 Paclitaxel Groups and 8 PEG Groups. Mol. Pharm. 2013, 10, 4452–4461. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Guan, B.; Nham, K.; Hao, G.; Sun, X.; Simanek, E.E. Tumor Uptake of Triazine Dendrimers Decorated with Four, Sixteen, and Sixty-Four PSMA-Targeted Ligands: Passive versus Active Tumor Targeting. Biomolec. 2019, 9, 421. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Guo, Y.; Rostollan, C.L.; Stanfield, J.; Hsieh, J.T.; Sun, X.; Simanek, E.E. The role of the size and number of polyethylene glycol chains in the biodistribution and tumor localization of triazine dendrimers. Mol. Pharm. 2008, 5, 540–547. [Google Scholar] [CrossRef] [PubMed]
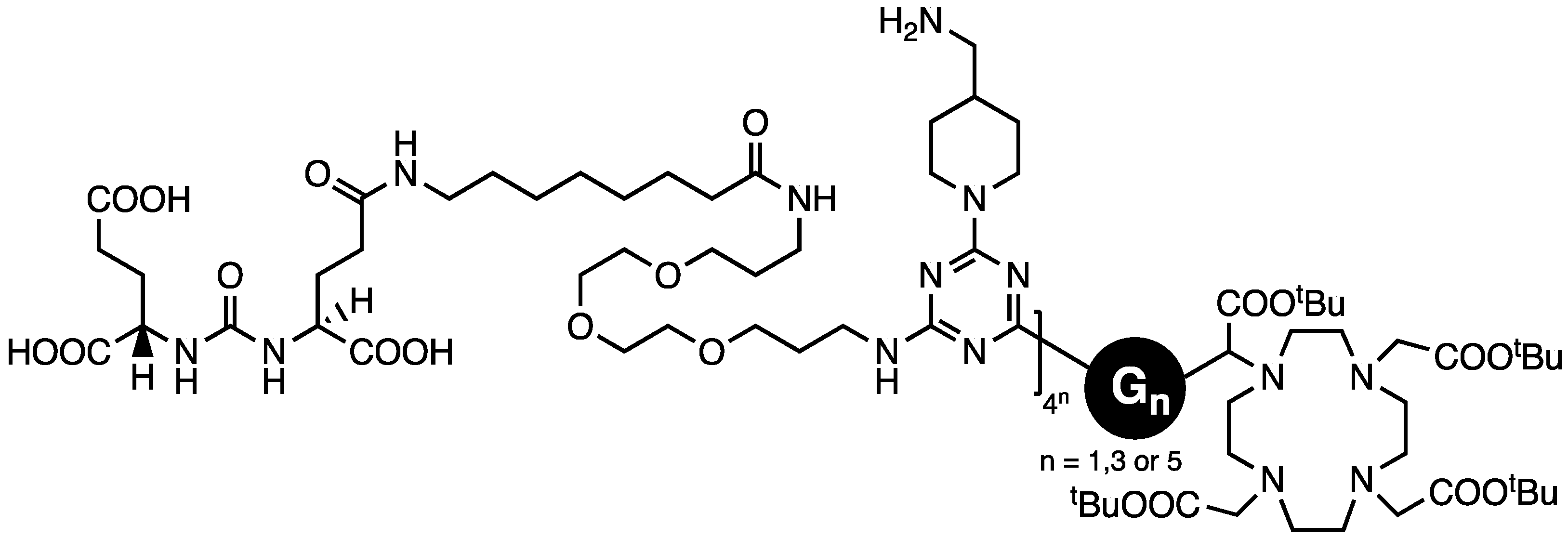
- Lim, J.; Turkbey, B.; Bernardo, M.; Bryant LHJr Garzoni, M.; Pavan, G.M.; Nakajima, T.; Choyke, P.L.; Simanek, E.E.; Kobayashi, H. Gadolinium MRI Contrast Agents Based on Triazine Dendrimers: Relaxivity and In Vivo Pharmacokinetics. Bioconj. Chem. 2012, 23, 2291–2299. [Google Scholar] [CrossRef]
- Lee, C.; Ji, K.; Simanek, E.E. Functionalization of a Triazine Dendrimer Presenting Four Maleimides on the Periphery and a DOTA Group at the Core. Molecules 2016, 21, 335. [Google Scholar] [CrossRef]
- Neerman, M.F.; Zhang, W.; Parrish, A.R.; Simanek, E.E. In vitro and in vivo evaluation of a melamine dendrimer as a vehicle for drug delivery. Int. J. Pharm. 2004, 281, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Enciso, A.E.; Neun, B.; Rodriguez, J.; Ranjan, A.P.; Dobrovolskaia, M.A.; Simanek, E.E. Nanoparticle Effects on Human Platelets in Vitro: A Comparison between PAMAM and Triazine Dendrimers. Molecules 2016, 21, 428. [Google Scholar] [CrossRef] [PubMed]
- Ilinskaya, A.N.; Shah, A.; Enciso, A.E.; Chan, K.C.; Kaczmarczyk, J.A.; Blonder, J.; Simanek, E.E.; Dobrovolskaia, M.A. Nanoparticle physicochemical properties determine the activation of intracellular complement. Nanomed Nanotech. Biol. Med. 2019, 17, 266–275. [Google Scholar] [CrossRef]
- Neerman, M.F.; Umali, A.P.; Chen, H.T.; Waghela, S.D.; Parrish, A.R.; EESimanek, E.E. Biological evaluation of dendrimers based on melamine. J. Drug Deliv. Sci. Tech. 2005, 15, 31–39. [Google Scholar] [CrossRef]
- Acosta, E.J.; Carr, C.S.; Simanek, E.E.; Shantz, D.F. Engineering nanospaces: Iterative synthesis of melamine-based dendrimers on amine-functionalized SBA-15 leading to complex hybrids with controllable chemistry and porosity. Adv. Mater. 2004, 16, 985–989. [Google Scholar] [CrossRef]
- Yoo, S.; Lunn, J.D.; Gonzalez, S.; Ristich, J.A.; Simanek, E.E.; Shantz, D.F. Engineering nanospaces: OMS/dendrimer hybrids possessing controllable chemistry and porosity. Chem. Mater. 2006, 18, 2935–2942. [Google Scholar] [CrossRef]
- Javaid, A.; Gonzalez, S.O.; Simanek, E.E.; Ford, D.M. Nanocomposite membranes of chemisorbed and physisorbed molecules on porous alumina for environmentally important separations. J. Membr. Sci. 2006, 275, 255–260. [Google Scholar] [CrossRef]
- Yoo, S.; Yeu, S.; Sherman, R.L.; Simanek, E.E.; Shantz, D.F.; Ford, D.M. Reverse-selective membranes formed by dendrimers on mesoporous ceramic supports. J. Membr. Sci. 2009, 334, 16–22. [Google Scholar] [CrossRef]
- Acosta, E.J.; Gonzalez, S.O.; Simanek, E.E. Synthesis, characterization, and application of melamine based dendrimers supported on silica gel. J. Polym. Sci. A 2005, 43, 168–177. [Google Scholar] [CrossRef]
- Gonzalez, S.O.; Furyk, S.; Li, C.; Tichy, S.E.; Bergbreiter, D.E.; Simanek, E.E. Latent solid-phase extraction with thermoresponsive soluble polymers. J. Polym. Sci. A 2004, 42, 6309–6317. [Google Scholar] [CrossRef]
- Acosta, E.J.; Steffensen, M.B.; Tichy, S.E.; Simanek, E.E. Removal of atrazine from water using covalent sequestration. J. Agric. Food Chem. 2004, 52, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Hollink, E.; Tichy, S.E.; Simanek, E.E. Piperidine-functionalized supports sequester atrazine from solution. Ind. Eng. Chem. Res. 2005, 44, 1634–1639. [Google Scholar] [CrossRef]
- Huang, A.Y.T.; Patra, S.; Chen, H.T.; Kao, C.L.; Simanek, E.E. Solid-Phase Synthesis of Libraries of Triazine Dendrimers and Orthogonal Staining Methods for Tracking Reactions on Resin. Asian J. Org. Chem. 2016, 5, 860–864. [Google Scholar] [CrossRef]
- Acosta, E.J.; Deng, Y.J.; White, G.N.; Dixon, J.B.; McInnes, K.J.; Senseman, S.A.; Frantzen, S.A.; Simanek, E.E. Dendritic Surfactants show evidence for frustrated intercalation: A new organoclay morphology. Chem. Mat. 2003, 15, 2903–2909. [Google Scholar] [CrossRef][Green Version]
- Neitsch, S.L.; McInnes, K.J.; Senseman, S.A.; White, G.N.; Simanek, E.E. Melamine-based organoclay to sequester atrazine. Chemosphere 2006, 64, 704–710. [Google Scholar] [CrossRef]
- Enciso, A.E.; Doni, G.; Nifosi, R.; Palazzesi, F.; Gonzalez, R.; Ellsworth, A.A.; Coffer, J.L.; Walker, A.V.; Pavan, G.M.; Mohamed, A.A.; et al. Facile synthesis of stable, water soluble, dendron-coated gold nanoparticles. Nanoscale 2017, 9, 10966. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Crescencio, F.; Enciso, A.E.; Hasan, M.; Da Costa, V.C.P.; Annunziata, O.; Redón, R.; Coffer, J.L.; Simanek, E.E. Thermoregulated Coacervation, Metal-Encapsulation and Nanoparticle Synthesis in Novel Triazine Dendrimers. Molecules 2016, 21, 599. [Google Scholar] [CrossRef]
- Redon, R.; Ramirez-Crescencio, F.; Gonzalez-Rodriguez, R.; Coffer, J.; Simanek, E.E. Ir(0) and Pt(0) nanoparticle-triazine dendrimer composites. J. Coord. Chem. 2020, 73, 544–557. [Google Scholar] [CrossRef]
- Cummins, B.M.; Lim, J.; Simanek, E.E.; Pishko, M.V.; Coté, G.L. Encapsulation of a Concanavalin A/dendrimer glucose sensing assay within microporated poly (ethylene glycol) microspheres. Biomed. Opt. Express 2011, 2, 1243–1257. [Google Scholar] [CrossRef]
- Simanek, E.E.; Enciso, A.E. Cationic Triazine Dendrimers: Synthesis, Characterization, and Biological Applications. Cationic Polym. Regen. Med. 2015, 13, 249–267. [Google Scholar]
- Simanek, E.E.; Enciso, A.E.; Pavan, G.M. Computational design principles for the discovery of bioactive dendrimers: [s]-triazines and other examples. Expert Opin. Drug Disc. 2013, 8, 1057–1069. [Google Scholar] [CrossRef]
- Lim, J.; Simanek, E.E. Triazine dendrimers as drug delivery systems: From synthesis to therapy. Adv. Drug Deliv. Rev. 2012, 64, 826–835. [Google Scholar] [CrossRef]
- Simanek, E.E.; Lim, J. Paclitaxel-Triazine Dendrimer Constructs: Efficacy, Toxicity, and Characterization. In Multifunctional Nanoparticles for Drug Delivery Applications: Imaging, Targeting, and Delivery; Springer: Berlin/Heidelberg, Germany, 2012; pp. 85–100. [Google Scholar]
- Simanek, E.E.; Abdou, H.; Lalwani, S.; Lim, J.; Mintzer, M.; Venditto, V.J.; Vittur, B. The 8 year thicket of triazine dendrimers: Strategies, targets and applications. Proc. R. Soc. A 2010, 466, 1445–1468. [Google Scholar] [CrossRef]
- Crampton, H.L.; Simanek, E.E. Dendrimers as drug delivery vehicles: Non-covalent interactions of bioactive compounds with dendrimers. Polym. Int. 2007, 56, 489–496. [Google Scholar] [CrossRef]
- Steffensen, M.B.; Hollink, E.; Kuschel, F.; Bauer, M.; Simanek, E.E. Dendrimers based on [1,3,5]-triazines. J. Polym. Sci. A 2006, 44, 3411–3433. [Google Scholar] [CrossRef]
- Simanek, E.E. Dendrimers based on melamine: Vehicles for drug delivery? Polym. Drug Deliv. I Part. Drug Carr. ACS Symp. Ser. 2003, 923, 121–136. [Google Scholar]
- Hollink, E.; Simanek, E.E.; Bergbreiter, D.E. Strategies for protecting and manipulating triazine derivatives. Tetrahedron Lett. 2005, 46, 2005–2008. [Google Scholar] [CrossRef]
- Bergbreiter, D.E.; Simanek, E.E.; Owsik, I. New synthetic methods for the formation of basic, polyvalent, hyperbranched grafts. J. Poly. Sci. A 2005, 43, 4654–4665. [Google Scholar] [CrossRef]
- Koskela, J.E.; Liljeström, V.; Lim, J.; Simanek, E.E.; Ras, R.H.; Priimagi, A.; Kostiainen, M.A. Light-Fuelled Transport of Large Dendrimers and Proteins. J. Am. Chem. Soc. 2014, 136, 6850–6853. [Google Scholar] [CrossRef]
- Simanek, E.E.; Gonzalez, S.O. Dendrimers: Branching out of polymer chemistry. J. Chem. Ed. 2002, 79, 1222–1231. [Google Scholar] [CrossRef]

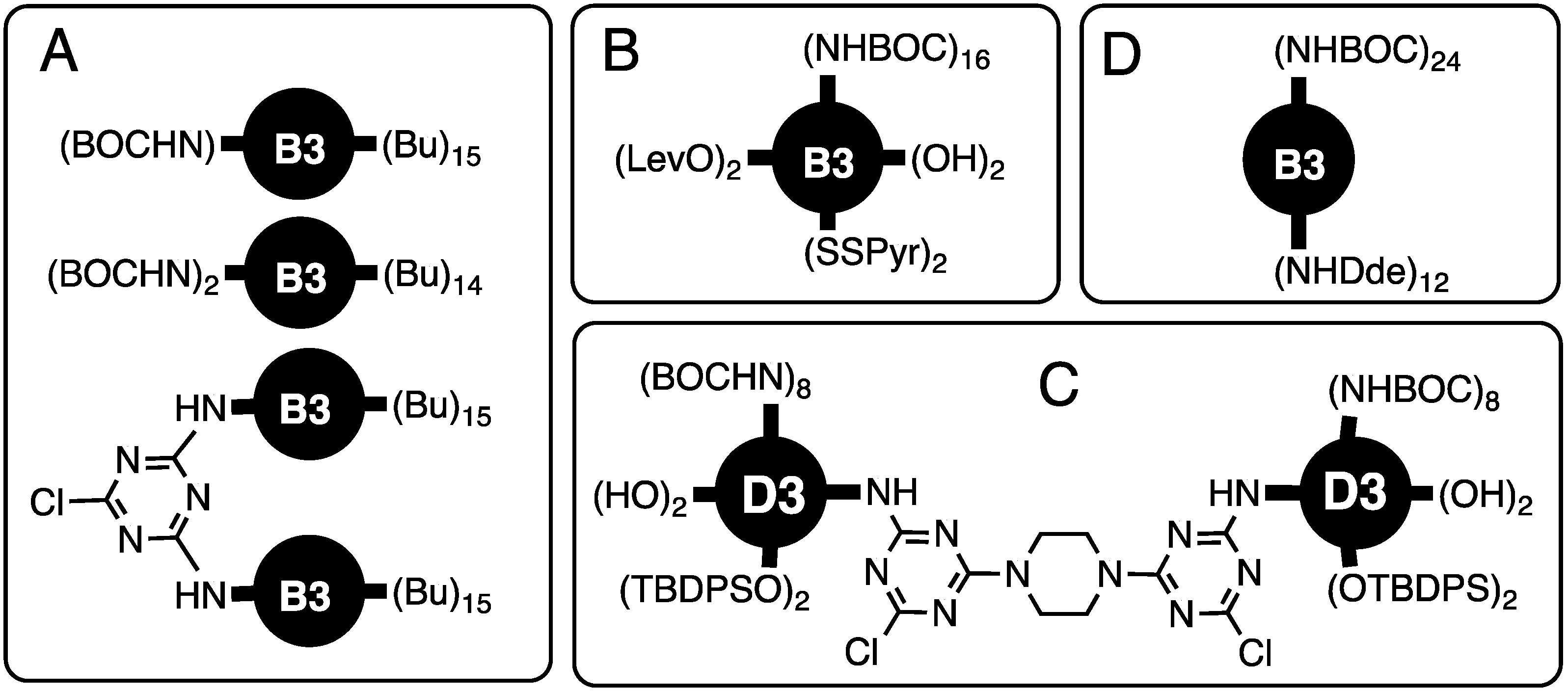
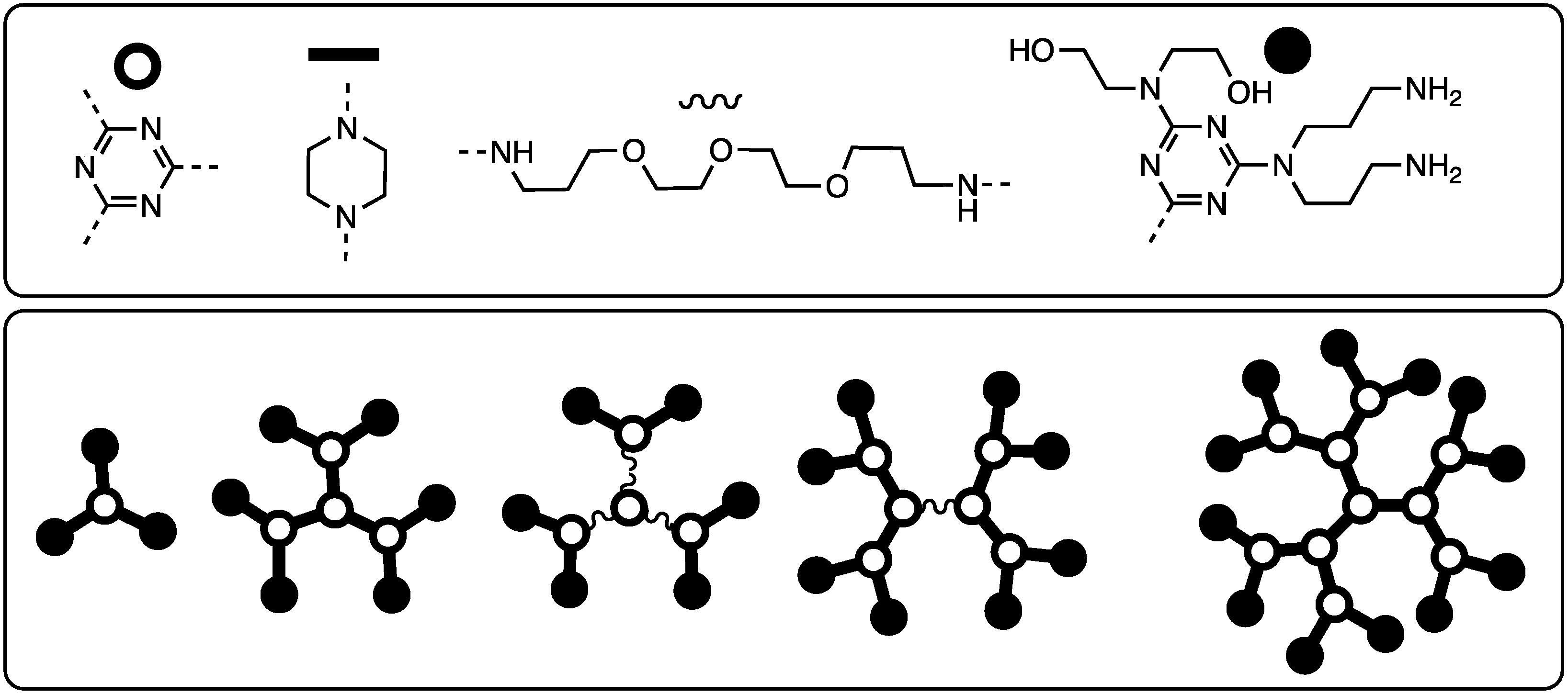
| Focus | Style | Notes | Ref # |
|---|---|---|---|
| Convergent v. divergent methods | B3 | First report, orthogonal linker (p-ABA) | [1] |
| Convergent, GPC analysis | D5 | p-ABA, Cu2+ sequestration | [2] |
| One or two reactive sites of 16 total | B3 | Unique sites, dimers made, computation | [3] |
| Multiple protected peripheral groups | B3 | 16 BOC, 2 OH, 2 TBDPS, 2 levul, 2PyrSS | [4] |
| Multiple protected peripheral groups | B3 | 16 BOC, 4 OH, 4 TBPDS, 2-MCT | [5] |
| Two protected amines on surface | G3 | 24 BOC, 12 DDE | [6] |
| Varied internal diamines | D3 | Relative reactivity data | [7] |
| Larger survey of amine reactivity | G3 | Three different diamines | [8] |
| Focus | Style | Notes | Ref # |
|---|---|---|---|
| Convergent v. divergent methods | B3 | Poor yields | [1] |
| Efficient divergent synthesis | G2,3 | Opportunity for layer-by-layer diversity | [9] |
| BisBOC-dichlorotriazine | G5 | Choice of bisBOC triamine affords big | [10] |
| Kilogram scale monomer | - | Checked by Org. Syn. | [11] |
| Kilogram scale dendrimer | G2 | Checked by Org. Syn. | [12] |
| Full report kg scale | G2 | Complete synthesis | [13] |
| Interception G2 w/bioactive | G2 | Installed 6 camptothecin and 12 PEGs | [14] |
| Trimethylenebispiperidine | G3 | Computation predicts 3 nm dia. | [15] |
| Reaction with trichlorotriazine | D4 | Solution synthesis, different capping amines | [16] |
| Macromonomer routes | G5 | Two generations/iteration | [17] |
| Synthesis of a ‘virus-sized’ dendrimer | G13 | Macromonomer w/flexible hydrophilic linker | [18] |
| Rapid, microwave synthesis | D4 | Iterative, convergent w/flexible diamine | [19] |
| Focus | Gn | Notes | Ref # |
|---|---|---|---|
| Intramolecular (interbranch) reactivity | G1-G3 | Disulfide-linked cleavage not cooperative | [23] |
| Steric protection of the core | B1-B4 | Disulfide core cleavage rates | [24] |
| Conformational dynamics | G5 | NMR studies reveal dynamics | [25] |
| Guest capacity as a function of generation | G1-G11 | Inversion of capacity in large dendrimers | [26] |
| Internal, intermolecular interactions | G1-G9 | Fluorescence studies suggest | [27] |
| Guest/Ligand | Cov/Non | Gn | Notes | Ref # |
|---|---|---|---|---|
| Cu(II) | Non | D1–D5 | Reduction of aggregation by GPC | [2] |
| Pyrene | “” | G1–G3 | Similar to polyethers, 10× than PPI/PAMAM | [28] |
| “” | “” | B1–B4 | Guest concentration correlates with MW | [24] |
| “” | “” | G1–G11 | Inversion of guest capacity at high generation | [26] |
| Methotrexate | “” | G1–G3 | Solubilization study & reduced hepatotoxicity | [28] |
| “” | “” | G3 | 3 molecules/dendrimer solubilized | [29] |
| Indomethacin | “” | G1–G3 | Precipitates with cationic dendrimer | [28] |
| 10-Hydroxycamptothecin | “” | G1–G3 | 4–5 molecules/dendrimer | [28] |
| Bis-indole methane | “” | G1–G3 | 4–5 molecules/dendrimer | [28] |
| Mercaptopurine | “” | G3 | 16 molecules/dendrimer | [29] |
| Camptothecin | Cov | G2 | 6 molecules/dendrimer, | [14] |
| “” | Non | G1–G9 | Inversion of capacity at G7 | [26] |
| DNA | “” | G1–G3, B2 | Flexible G2 outperforms SuperFect, PPI | [30] |
| DNa/siRNA | “” | G1–G3, B2 | Computational models of flexible v. rigid | [31] |
| RNAi | “” | G1–G3, B2 | In vitro and in vivo analysis with SAR | [32] |
| DNA | Cov | D2, D3 | Disulfide linked, 12 base oligos | [35] |
| Captopril | “” | G3, D3 | Disulfide linked tetra- and octavalent | [36] |
| CLKKDDRA peptide | “” | “” | Disulfide linked tetra- and octavalent | [36] |
| Desferrioxamine | “” | G3 | 56 wt% drug | [37] |
| DABCO | “” | D1, B1, G1 | Divalent–hexavalent displays, antibacterial | [38] |
| N-acyl glucosamine | “” | G2 | Anti-sepsis candidate model | [41] |
| Paclitaxel | “” | B3 | Synthesis (con), 16 PTX, 12 PEG, radiolabel | [42] |
| “” | “” | G3 | Ester-linked drug, broad assessment | [43] |
| “” | “” | G3 | Ester/disulfide biodistribution | [44] |
| “” | “” | G3 | “Cures” observed with disulfide in linker | [45] |
| “” | “” | D4 | Synthesis/evaluation of homogeneous target | [46] |
| DUPA | “” | D1, D3, D5 | PSMA-(+) tumors targeted | [47] |
| PEG | “” | Varied | PEGylation strategy influences biodistribution | [48] |
| DOTA/DPTA | “” | G3, G5 | Biodistribution of Gd-MRI agents | [49] |
| DOTA | “” | D2 | DOTA at focus, maleimides on periphery | [50] |
| Support | Gn | Notes | Ref # |
|---|---|---|---|
| Mesoporous Si (SBA-15) | D1-D4 | Synthetic methods; iterative C3N3Cl3 and diamine | [55] |
| “” | D1-D3 | Role of linking diamine, porosity control | [56] |
| Mesoporous alumina | Hyper | Good separation factors, faster than polymer membranes | [57] |
| Ceramic | D1-D3 | Good selectivity for VOCs | [58] |
| Silica | D1-D3 | Convergent and divergent synthesis | [59] |
| LCST polymers | D1 | Solid phase extraction with thermoresponsive polymers | [60] |
| Polystyrene | NA | Piperidine functionalized supports sequester atrazine | [61] |
| Janda gel | NA | Atrazine sequestration with different mesh resins | [62] |
| Solid phase synthesis | D1-D3 | SPPS methods applied to dendrimer synthesis | [63] |
| Smectite clay | D1-D3 | Frustrated intercalation for D2, D3, not D1 or linear D2 | [64] |
| “” | NA | Reactive surfactant sequesters atrazine | [65] |
| Gold nanoparticles | D1-D3 | Synthesis w/ dendritic surfactant w/diazoninum focus | [66] |
| “” | G5 | Au nanoparticles described, others (Cu, Pd, Ir, Pt) observed | [67] |
| Iridium nanoparticles | D1-D3 | Obtained by mechanical milling | [68] |
| Glucose-sensing device | G3 | Optical measurements using glycosylated dendrimer | [69] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simanek, E.E. Two Decades of Triazine Dendrimers. Molecules 2021, 26, 4774. https://doi.org/10.3390/molecules26164774
Simanek EE. Two Decades of Triazine Dendrimers. Molecules. 2021; 26(16):4774. https://doi.org/10.3390/molecules26164774
Chicago/Turabian StyleSimanek, Eric E. 2021. "Two Decades of Triazine Dendrimers" Molecules 26, no. 16: 4774. https://doi.org/10.3390/molecules26164774
APA StyleSimanek, E. E. (2021). Two Decades of Triazine Dendrimers. Molecules, 26(16), 4774. https://doi.org/10.3390/molecules26164774





