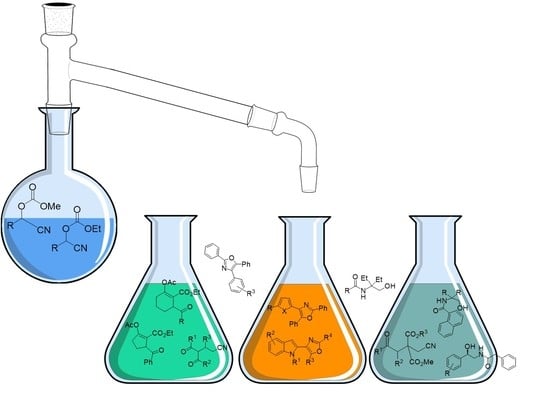
Recent Advances on O-Ethoxycarbonyl and O-Acyl Protected Cyanohydrins
Abstract
:- 1.
- Introduction
- 2.
- Synthesis of O-Protected Cyanohydrins
- 2.1.
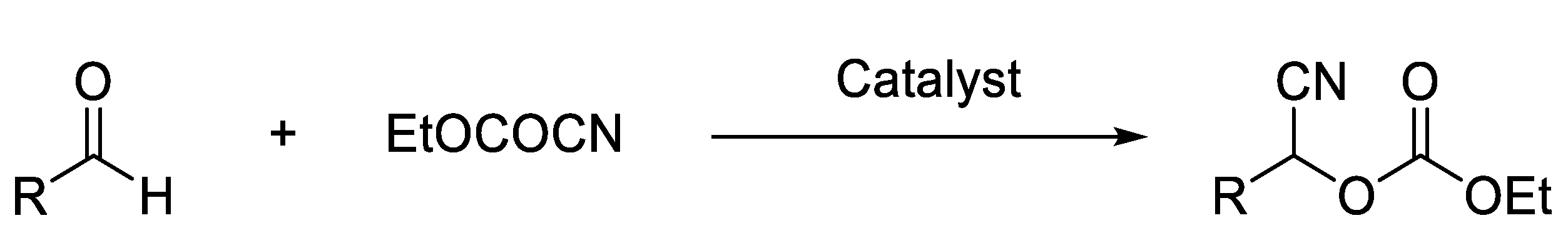
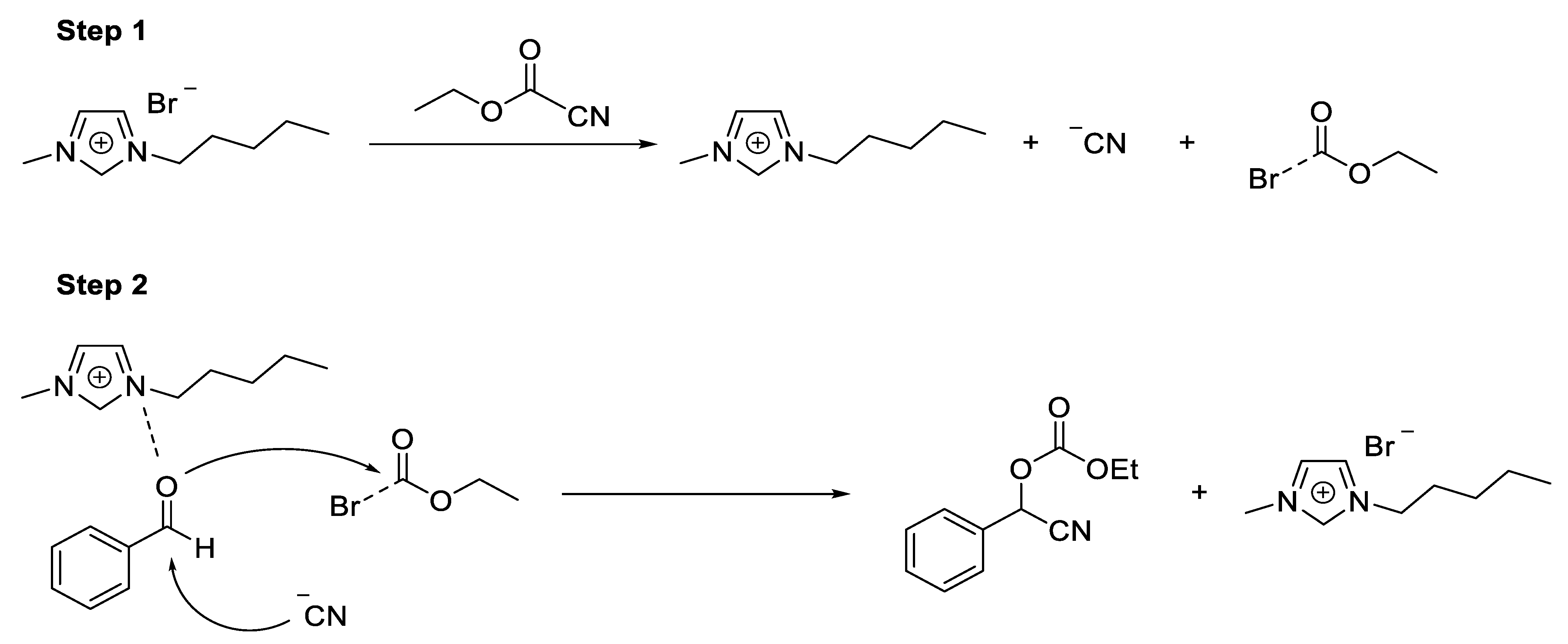
- Synthesis of Ethoxycarbonyl Cyanohydrins
- 2.2.
- Synthesis of O-Acyl Cyanohydrins
- 2.3.
- Synthesis of O-Aroyl Cyanohydrins
- 2.4.
- Asymmetric Cyanation
- 2.4.1.
- Synthesis of O-Acyl Cyanohydrins
- 2.4.2.
- Synthesis of O-Methoxycarbonyl Cyanohydrins
- 2.4.3.
- Synthesis of O-Ethoxycarbonyl Cyanohydrins
- 3.
- Synthetic Applications
- 3.1.
- Synthesis of Substituted Cyclohexenes and Cyclopentenes
- 3.2.
- Synthesis of 4-Heteroaryloxazoles
- 3.3.
- Synthesis of 2-Aminocyclopentanones and 2-Amino-4-azacyclopentanones
- 3.4.
- Synthesis of Cinnamic Esters
- 3.5.
- Synthesis of 4-Amino-2(5H)-furanones
- 3.6.
- Synthesis of Substituted 2-Vinyl-2-cyclopentenones
- 3.7.
- Synthesis of O-Acylcyanohydrins from O-(α-Bromoacyl)cyanohydrins
- 3.8.
- Synthesis of Substituted Cyclopropylamines and 1,4-Diketones
- 3.9.
- Synthesis of α,α-Disubstituted α-Amino-Acids
- 3.10.
- Synthesis of 2-Hydroxy-2-Cyclopentenones
- 3.11.
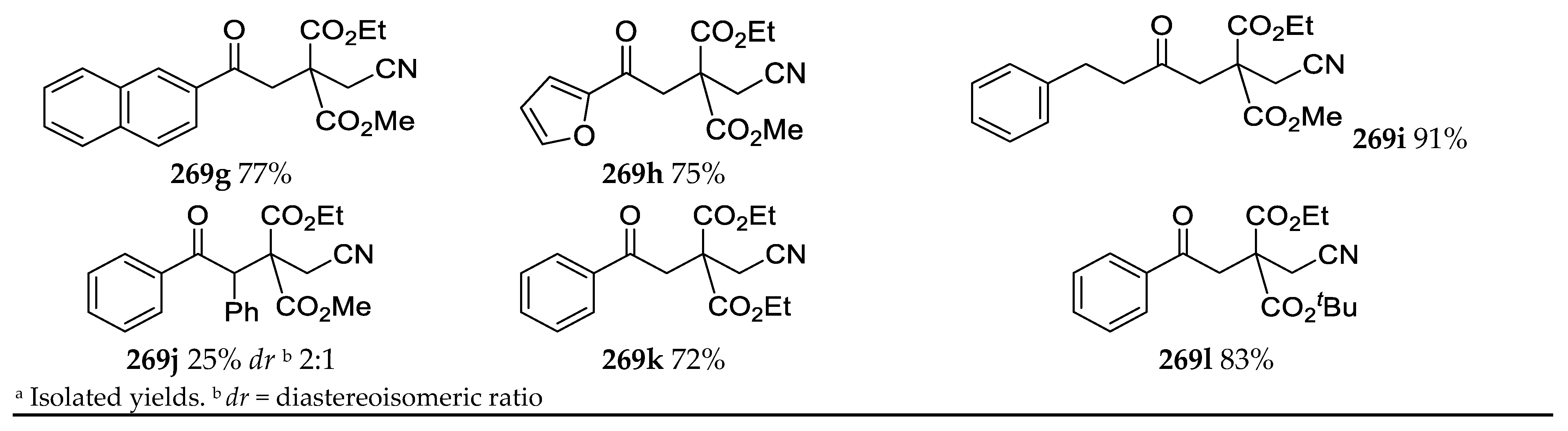
- Synthesis of Highly Functionalized Acyclic Ketones
- 3.l2.
- Synthesis of Substituted 1,3-Diketones
- 3.13.
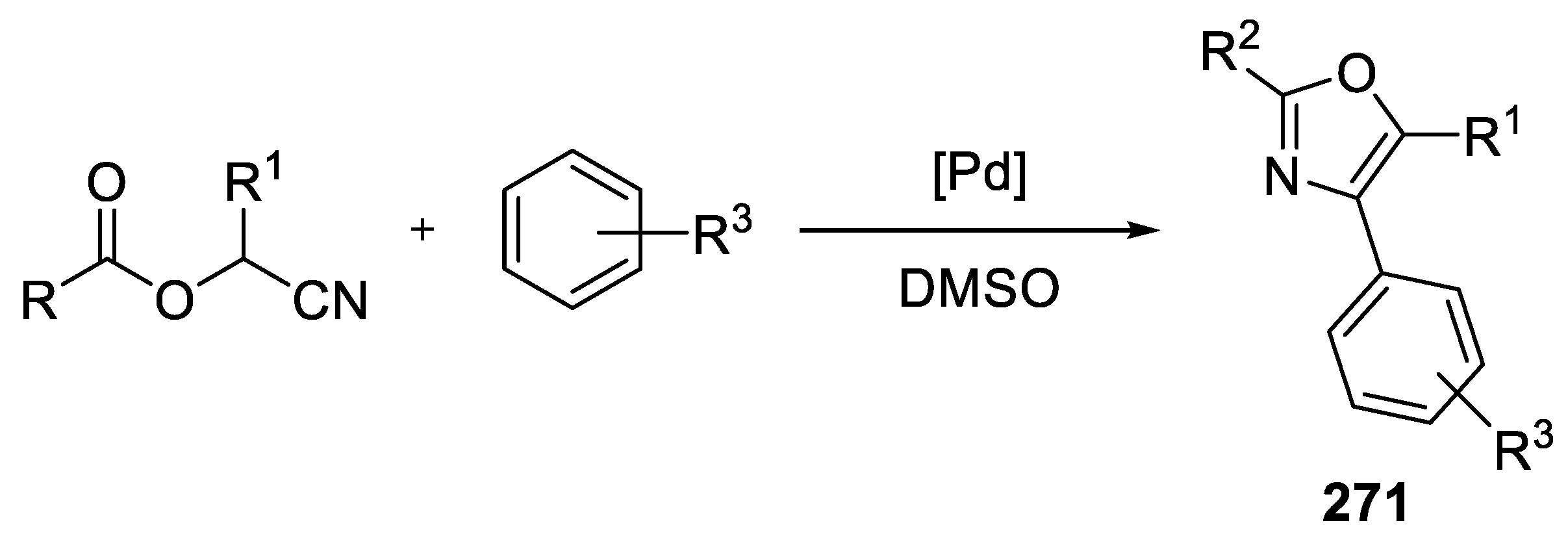
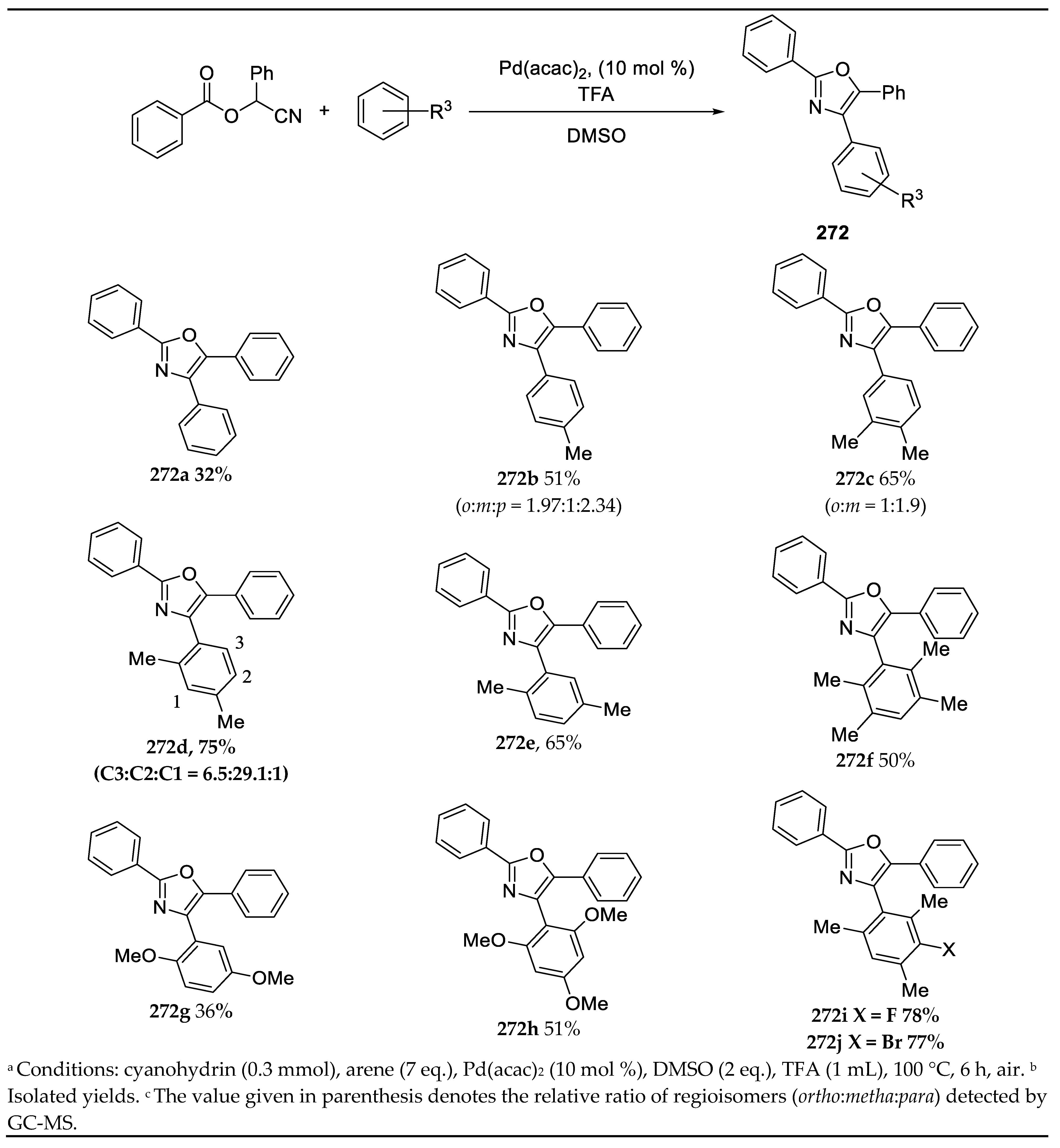
- Synthesis of 2,4,5-Trisubstituted Oxazoles by Palladium Catalyzed C-H Activation
- 4.
- Conclusions
- 5.
- Abbreviations
- 6.
- References
- Table 1.
- Cyanocarbonation of aldehydes
- Table 2.
- Cyanoethoxycarbonilation of aldehydes in ionic liquids
- Table 3.
- Cyanoethoxycarbonilation of aldehydes catalyzed by DMAP under solvent free conditions
- Table 4.
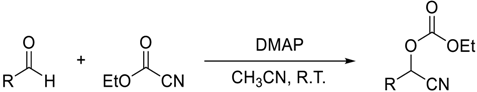
- Cyanation of aldehydes with ethyl cyanoformate catalyzed by DMAP
- Table 5.
- Cyanation of ketones with ethyl cyanoformate catalyzed by DMAP
- Table 6.
- One-pot synthesis of O-acetyl cyanohydrins from aldehydes via O-silylcyanohydrins in [bmim]BF4.
- Table 7.
- Synthesis of O-acyl cyanohydrins with TMSCN, acetic anhydride and aldehydes catalyzed by B(C6F5)3
- Table 8.
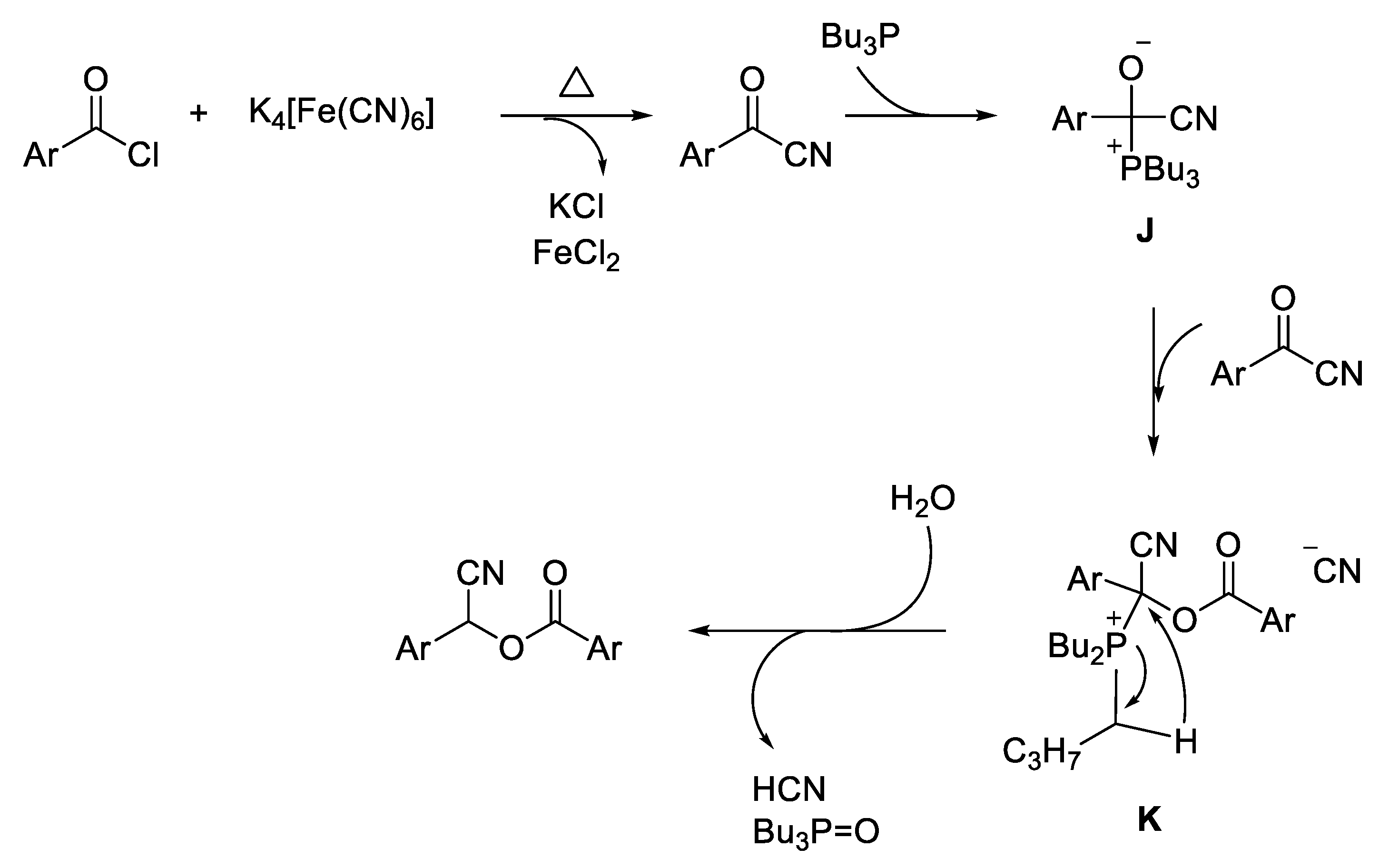
- Synthesis of cyanohydrin esters from aroyl chlorides
- Table 9.
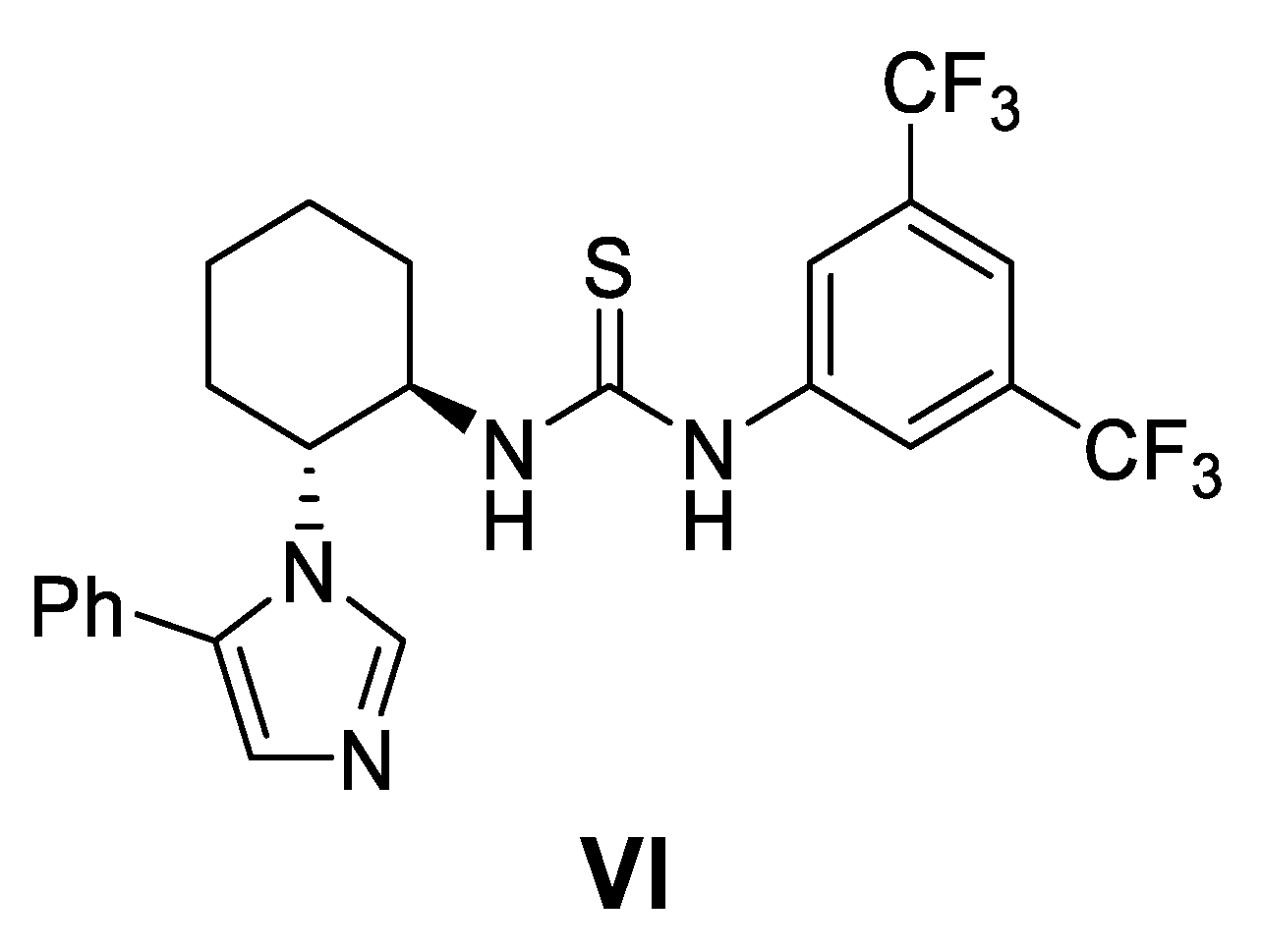
- Asymmetric cyanosilylation of aldehydes catalyzed by a thiourea derivative and conversion to O-acetylcyanohydrins
- Table 10.
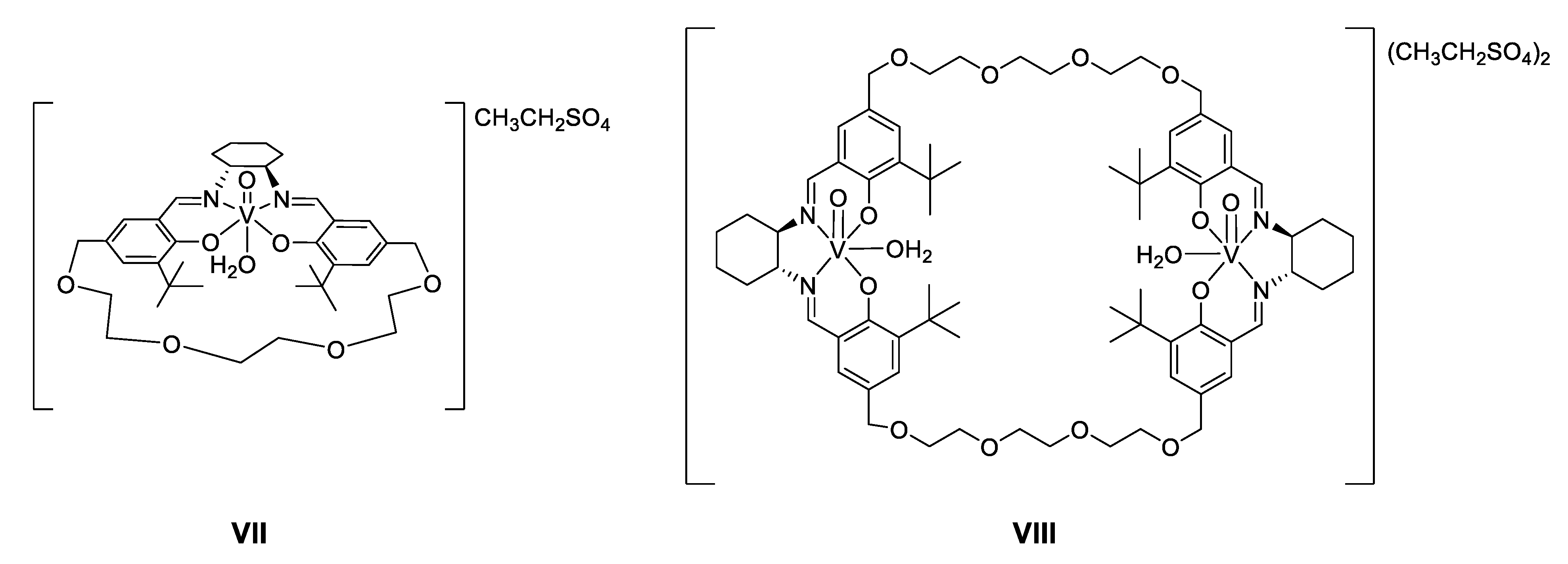
- Asymmetric acetylcyanation of aldehydes catalyzed by vanadium(V) complexes
- Table 11.
- Substrate scope of the asymmetric catalytic formation of cyanohydrin carbonates with complex VII in the presence of lutidine
- Table 12.
- Enantioselective cyanoformylation of aldehydes catalyzed by the Ti(OiPr)4/IX system
- Table 13.
- Enantioselective cyanation of aldehydes catalyzed by alumminium complex
- Table 14.
- Investigation of the substrate scope of the carboxycyanation with pyrocarbonate and KCN
- Table 15.
- Synthesis of compounds 123–130 by addition of anions of ethyl carbonates of cyanohydrins to 2-cycloalkenones
- Table 16.
- Synthesis of aminofuranones via intramolecular Blaise reaction
- Table 17.
- Substrate scope for the cross-coupling of the O-(α-bromoacyl)cyanohydrin with boronic acid
- Table 18.
- Titanium-mediated addition of EtMgBr to nitriles
- Table 19.
- Addition of EtMgBr to acyl cyanohydrins
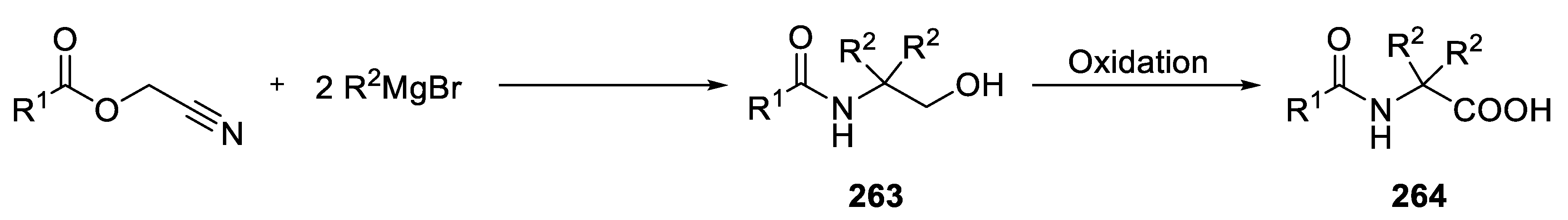
- Table 20.
- Addition of Grignard reagents to acylcyanohydrin
- Table 21.
- Two steps versus one step reaction to prepare 3-substituted-2-hydroxy-2-cyclopentenones
- Table 22.
- Scope of the rearrangement of O-aromatic acylated cyanohydrins
- Table 23.
- Rearrangements of O-aliphatic acylated cyanohydrins
- Table 24.
- Three components coupling reaction to form cyanohydrin derivatives
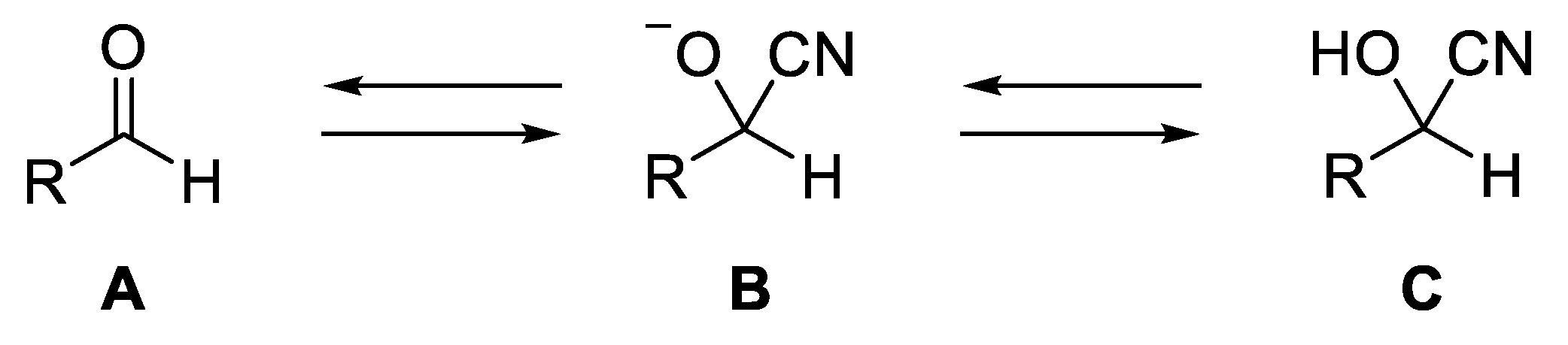
1. Introduction
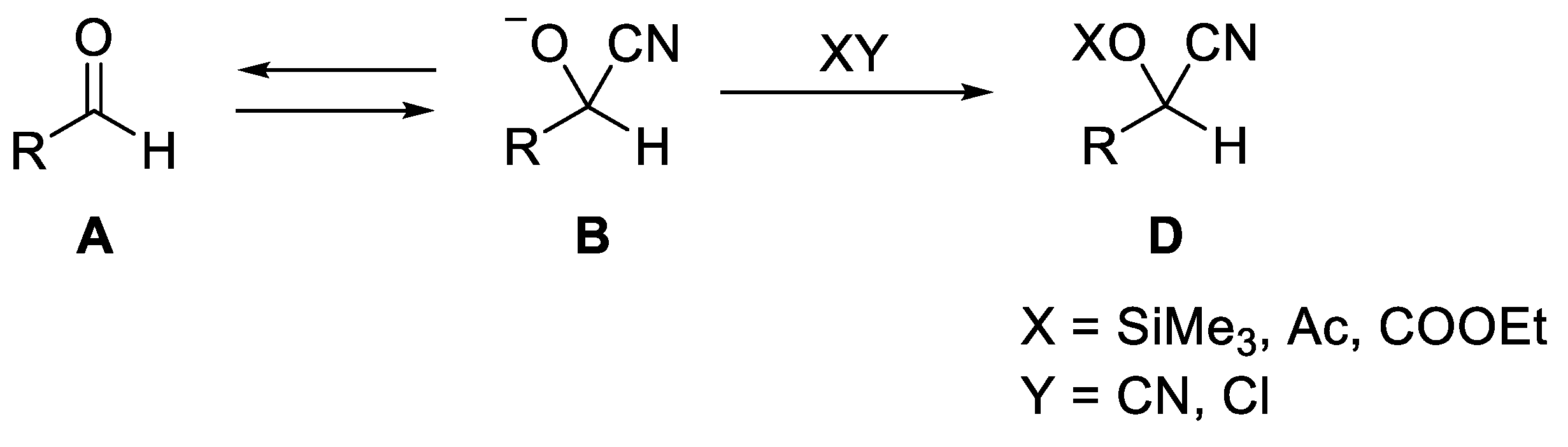
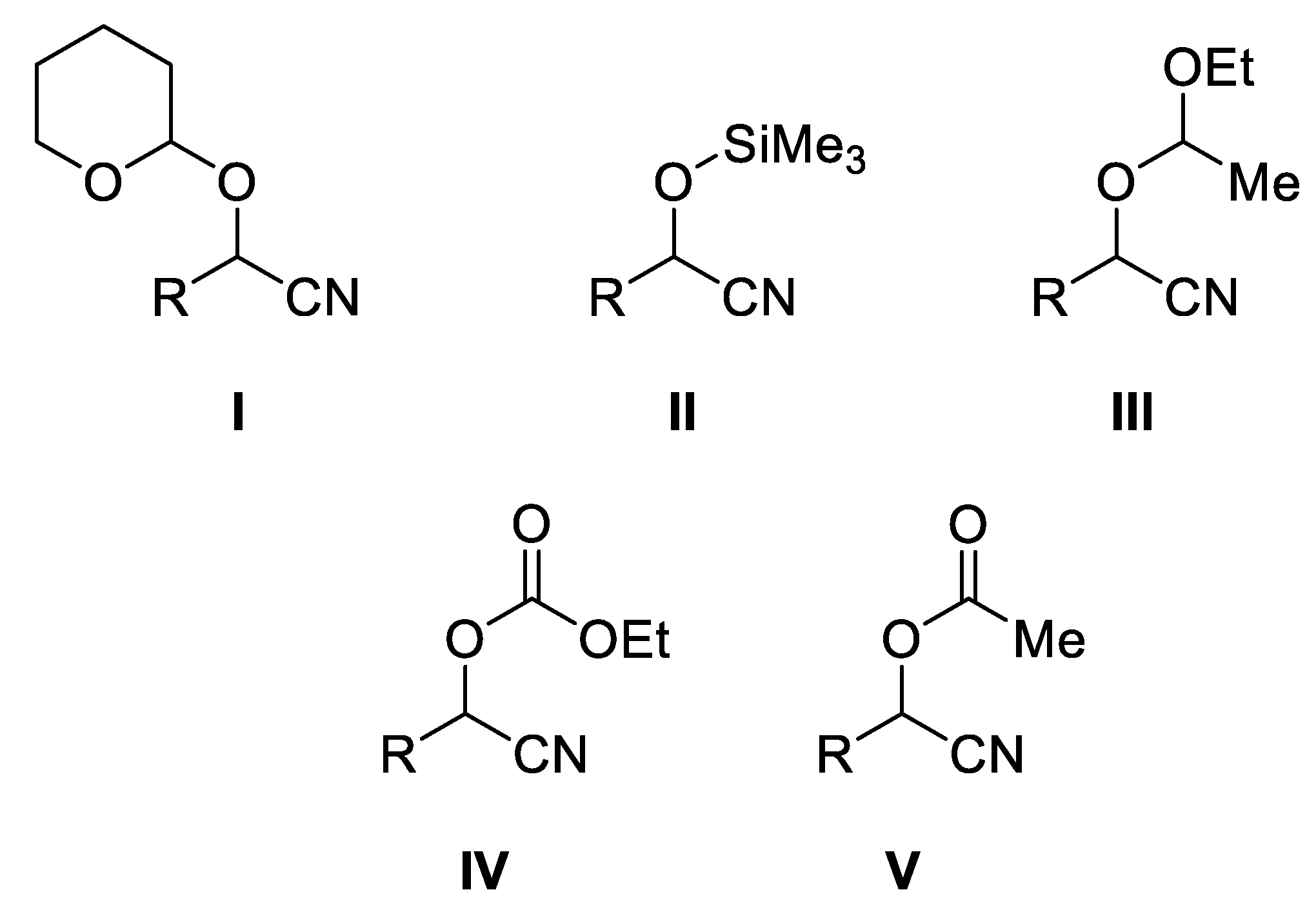
2. Synthesis of O-Protected Cyanohydrins
2.1. Synthesis of Ethoxycarbonyl Cyanohydrins
2.2. Synthesis of O-Acyl Cyanohydrins
2.3. Synthesis of O-Aroyl Cyanohydrins
2.4. Asymmetric Cyanation
2.4.1. Synthesis of O-Acyl Cyanohydrins
2.4.2. Synthesis of O-Methoxycarbonyl Cyanohydrins
2.4.3. Synthesis of O-Ethoxycarbonyl Cyanohydrins
3. Synthetic Applications
3.1. Synthesis of Substituted Cyclohexenes and Cyclopentenes
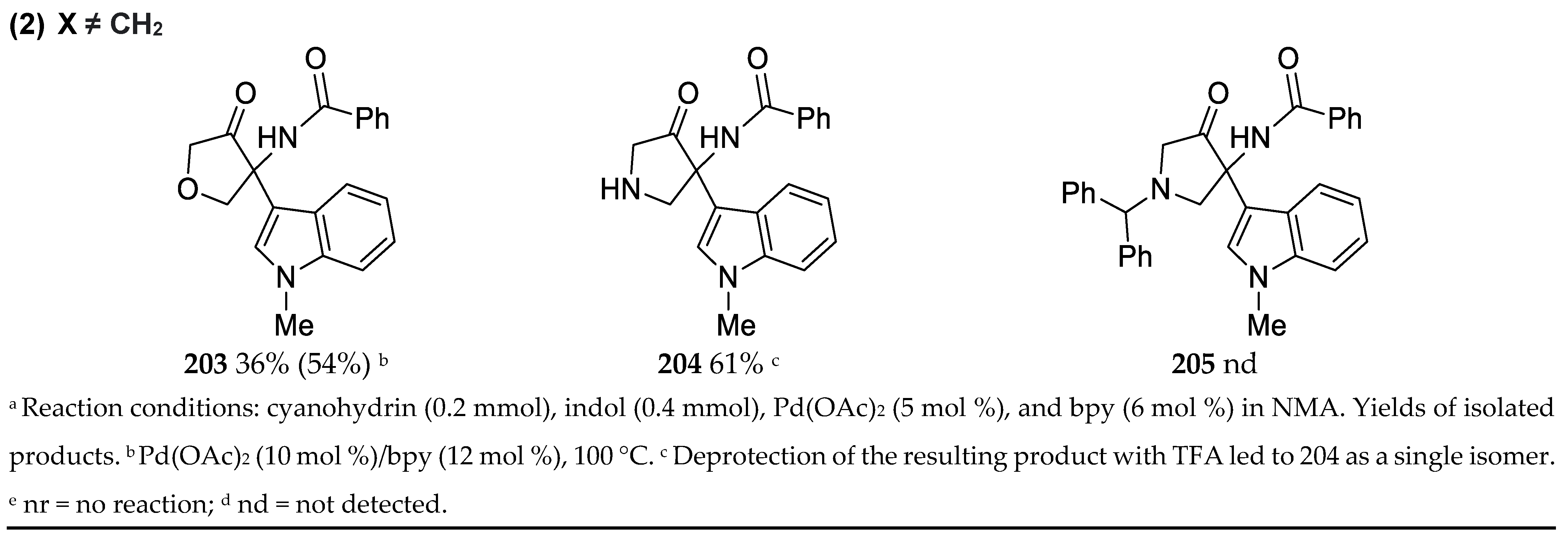
3.2. Synthesis of 4-Heteroaryloxazoles
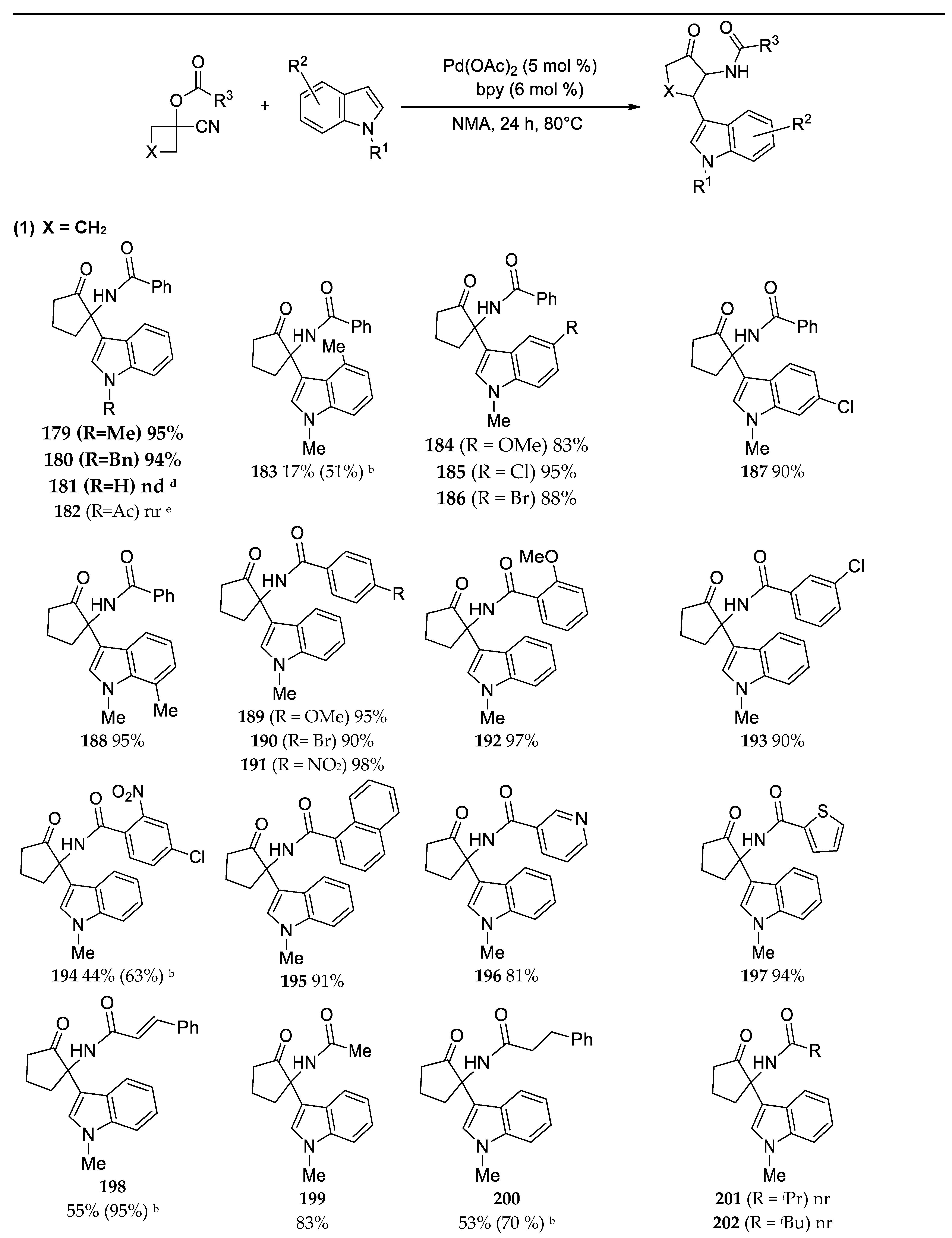
3.3. Synthesis of 2-Aminocyclopentanones and 2-Amino-4-Azacyclopentanones
3.4. Synthesis of Cinnamic Esters
3.5. Synthesis of 4-Amino-2(5H)-Furanones
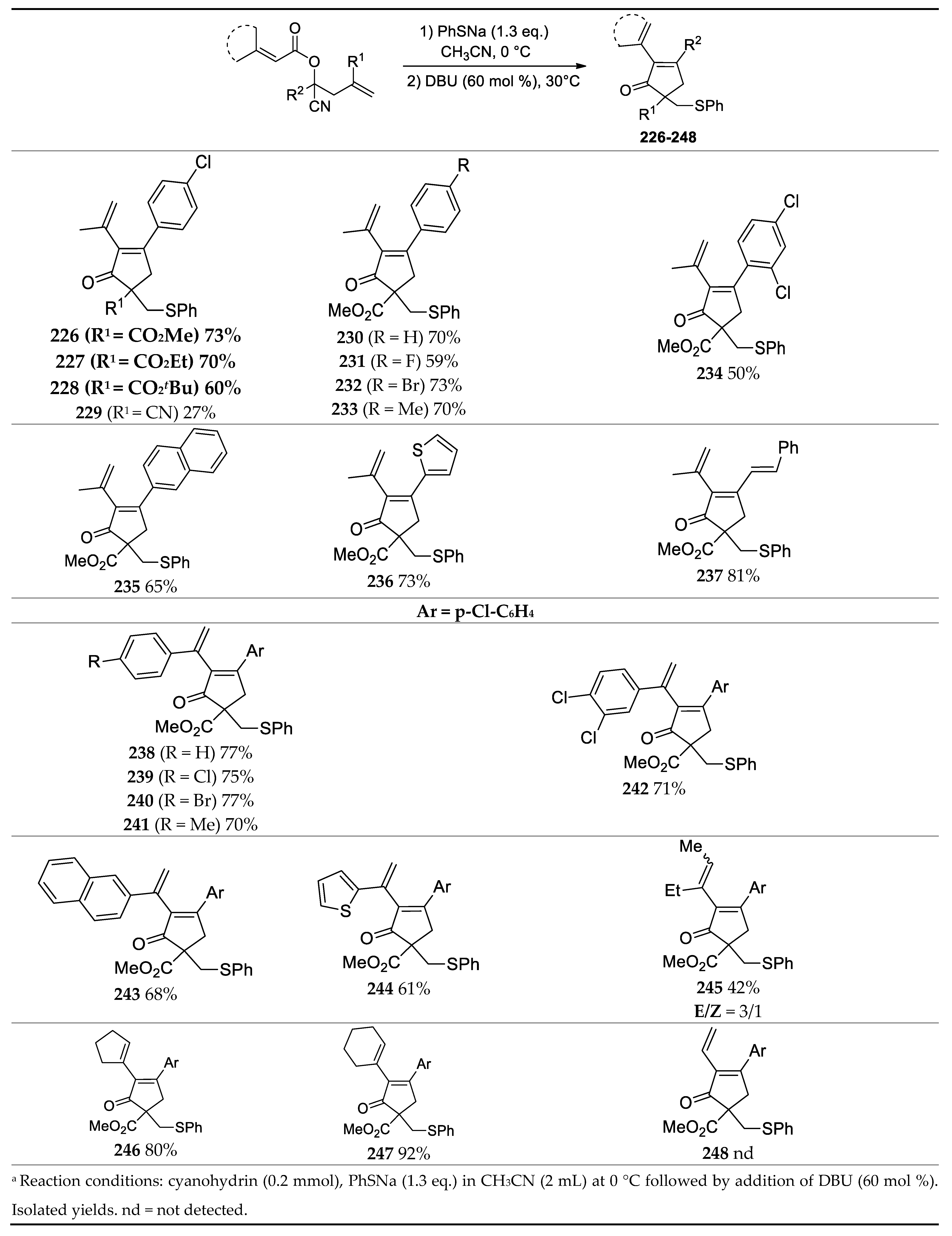
3.6. Synthesis of Substituted 2-Vinyl-2-Cyclopentenones
3.7. Synthesis of O-Acylcyanohydrins from O-(α-Bromoacyl)Cyanohydrins
3.8. Synthesis of Substituted Cyclopropylamines and 1,4-Diketones
3.9. Synthesis of α,α-Disubstituted α-Amino-Acids
3.10. Synthesis of 2-Hydroxy-2-Cyclopentenones
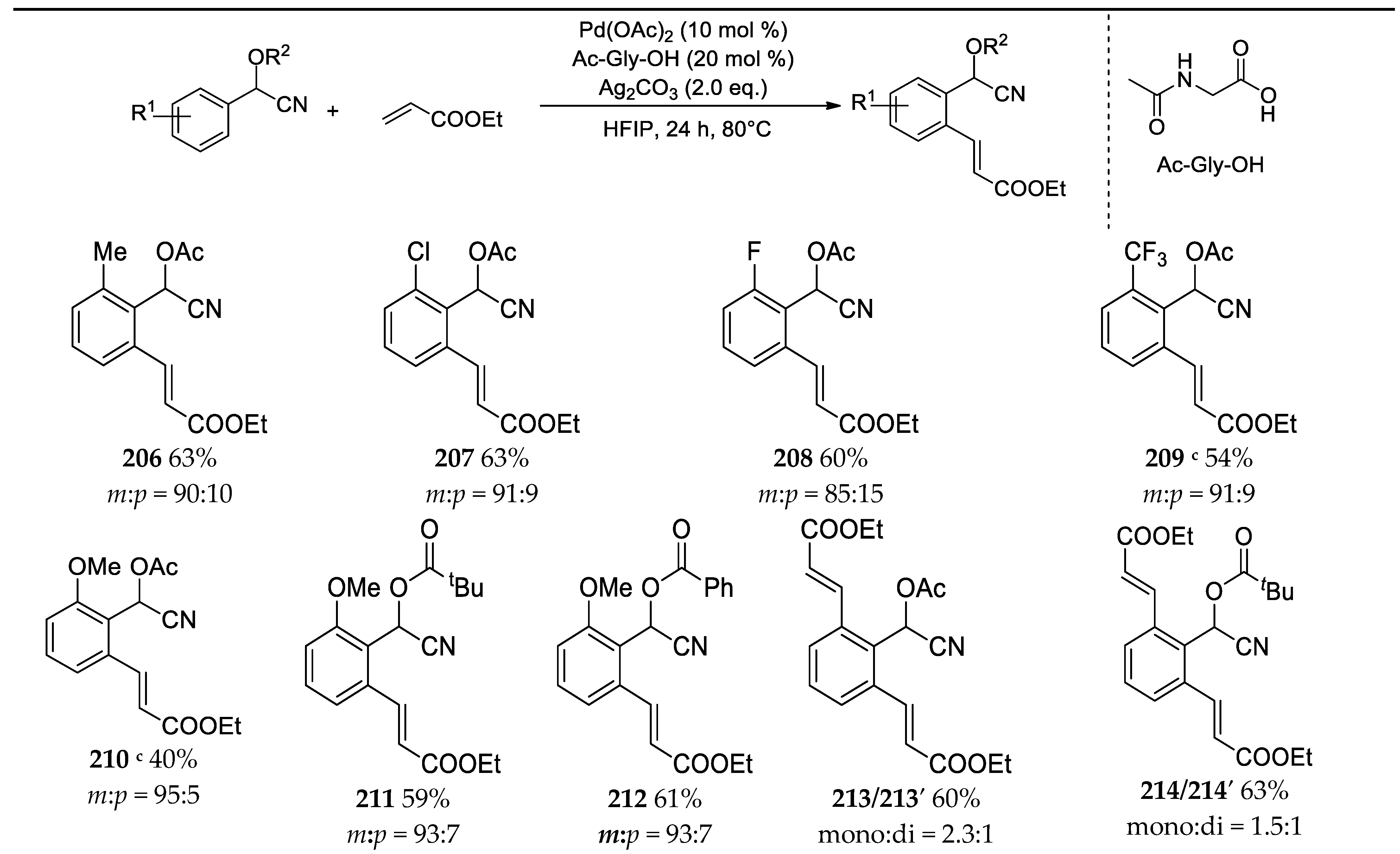
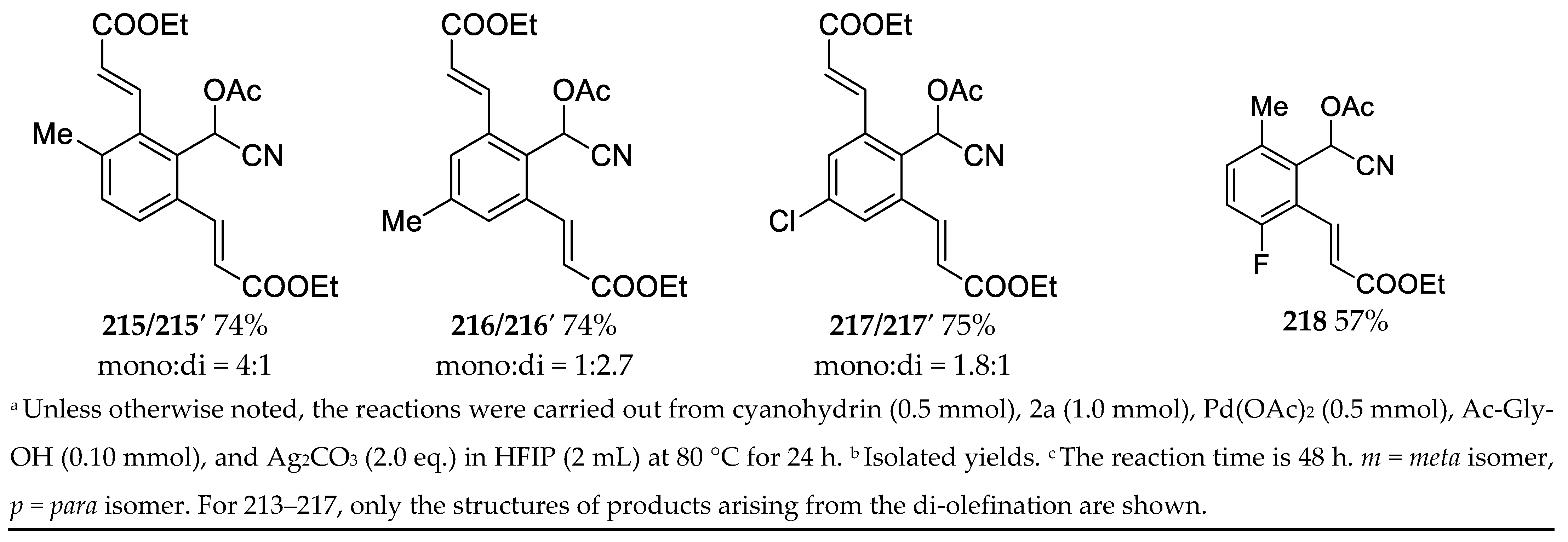
3.11. Synthesis of Highly Functionalized Acyclic Ketones
3.12. Synthesis of Substituted 1,3-Diketones
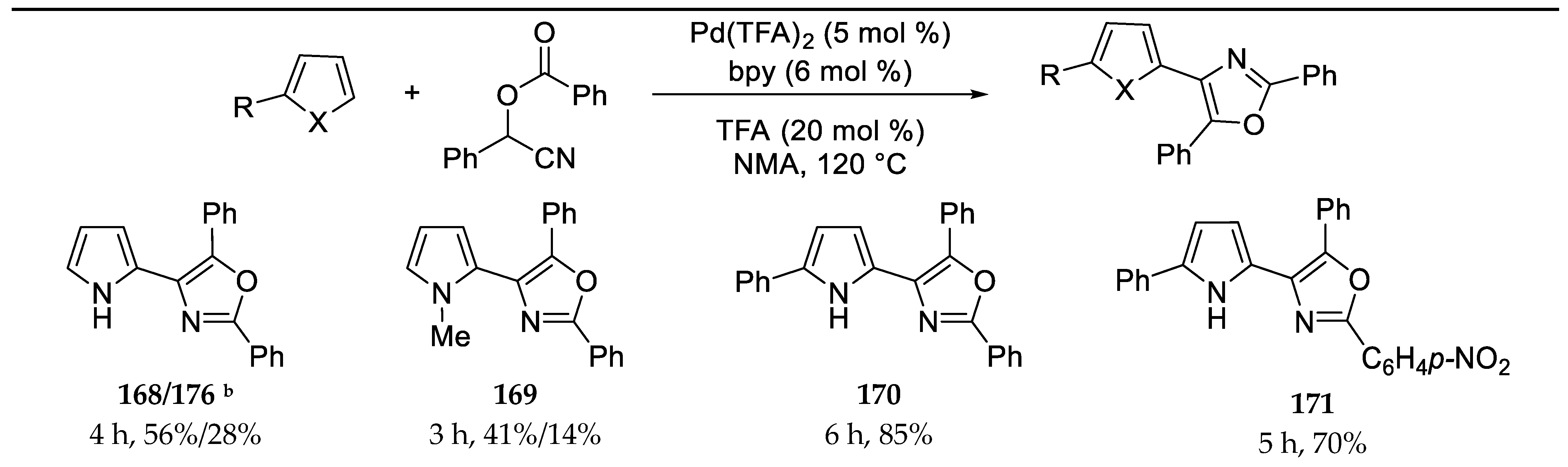
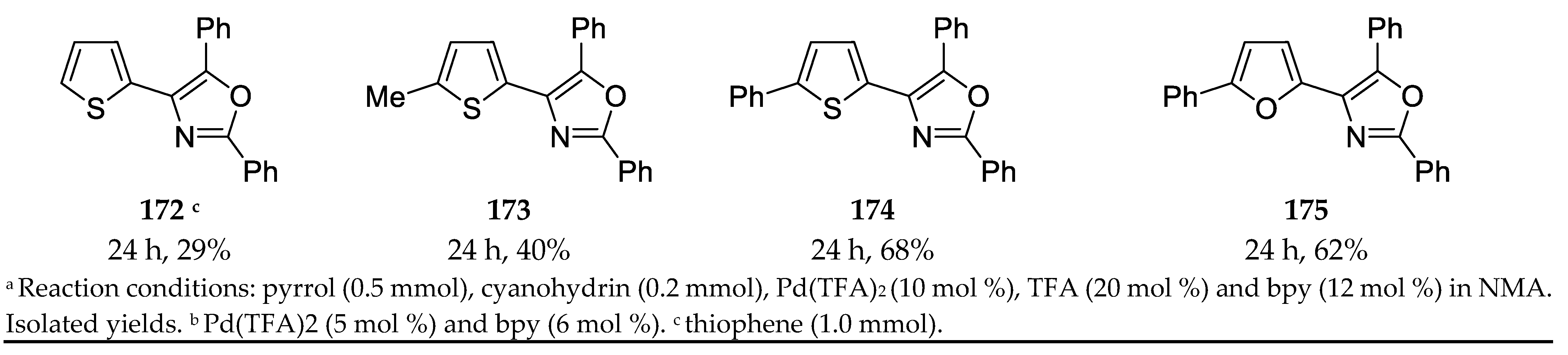
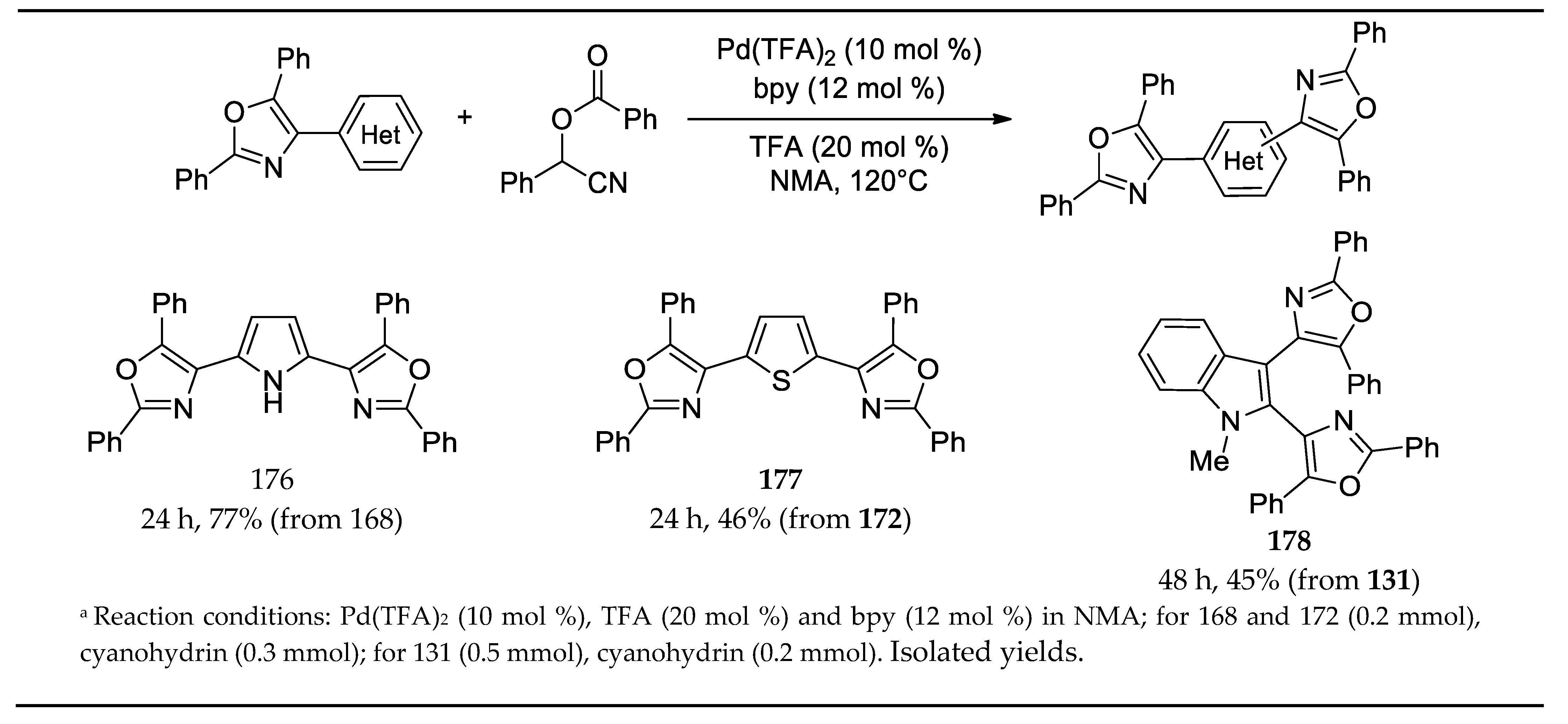
3.13. Synthesis of 2,4,5-Trisubstituted Oxazoles by Palladium Catalyzed C-H Activation
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Ac | Acetyl group |
| Acac | Acetylacetonate |
| AIBN | Azobisisobutyronitrile |
| Ar | Aryl group |
| BMIN | 1-Butyl-3-methylimidazolium |
| bpy | 2,2-Bipyridine |
| Bz | Benzyl group |
| DBU | 1,8-Diazabicyclo[5.4.0]undec-7-ene |
| DCM | Dichloromethane |
| DMAP | 4-Dimethylaminopyridine |
| DMF | Dimethylformamide |
| DMSO | Dimethyl sulfoxide |
| dr | diastereomeric ratio |
| DTAC | Dodecyltrimethylammonium chloride |
| DTMAC | 4-[(n-dodecylthio)methyl]-7-(N,N-dimethylamino)-coumarin |
| EE | Ethoxyethyl acetal |
| ee | Enantiomeric excess |
| er | Enantiomeric ratio |
| GC | Gas chromatography |
| Gly | Glycine |
| HFIP | Hexafluoroisopropanol |
| HPLC | High-performance liquid chromatography |
| Me | Methyl |
| nd | not detected |
| NMA | N-Methylaniline |
| OEt | Ethoxy group |
| OMe | Methoxy group |
| SMA | Sulfa Michael Addition |
| Tf | Triflate |
| TFA | Trifluoroacetic acid |
| THF | Tetrahydrofuran |
| THP | Tetrahydropyran |
| TMS | Trimethylsilyl |
| TMSCN | Trimethylsilyl cyanide |
| TON | Turnover number |
References
- Zeng, X.-P.; Sun, J.-C.; Liu, C.; Ji, C.-B.; Peng, Y. Catalytic Asymmetric Cyanation Reactions of Aldehydes and Ketones in Total Synthesis. Adv. Synth. Catal. 2019, 361, 3281–3305. [Google Scholar] [CrossRef]
- North, M. Synthesis and Applications of Non-Racemic Cyanohydrins. Tetrahedron Asymmetry 2003, 14, 147–176. [Google Scholar] [CrossRef]
- Gregory, J.H.R. Cyanohydrins in Nature and the Laboratory: Biology, Preparations, and Synthetic Applications. Chem. Rev. 1999, 99, 3649–3682. [Google Scholar] [CrossRef]
- Iwanami, K.; Aoyagi, M.; Oriyama, T. Iron(III) triflate-catalyzed one-pot synthesis of acetal-type protected cyanohydrins from carbonyl compounds. Tetrahedron Lett. 2006, 47, 4741–4744. [Google Scholar] [CrossRef]
- Kotke, M.; Schreiner, P.R. Generally Applicable Organocatalytic Tetrahydropyranylation of Hydroxy Functionalities with Very Low Catalyst Loading. Synthesis 2007, 12, 779–790. [Google Scholar] [CrossRef]
- Peris, E.; Porcar, R.; Burguete, M.I.; Garcia, R.P.; Luis, S.V. Supported Ionic Liquid-Like Phases (SILLPs) as Immobilised Catalysts for the Multistep and Multicatalytic Continuous Flow Synthesis of Chiral Cyanohydrins. ChemCatChem 2019, 11, 1955–1962. [Google Scholar] [CrossRef]
- Vinoth, G.; Indira, S.; Bharathi, M.; Alves, L.G.; Martins, A.M.; Bharathi, K.S. Cyanosilylation of carbonyl compounds catalyzed by half-sandwich (η6-p-cymene) Ruthenium(II) complexes bearing heterocyclic hydrazone derivatives. Inorganica Chim. Acta 2021, 514, 120006. [Google Scholar] [CrossRef]
- Rad, N.; Mąkosza, M. Simple Synthesis of Aryl P-Nitroarylacetonitriles by Vicarious Nucleophilic Substitution with Carbanions of Protected Cyanohydrins. Eur. J. Org. Chem. 2018, 2018, 376–380. [Google Scholar] [CrossRef]
- Stork, G.; Maldonado, L. Anions of Protected Cyanohydrins as Acyl Carbanion Equivalents and Their Use in a New Synthesis of Ketones. J. Am. Chem. Soc. 1971, 93, 5286–5287. [Google Scholar] [CrossRef]
- Stork, G.; Maldonado, L. Conjugate Addition of Acyl Carbanion Equivalents via the Protected Cyanohydrin Method. J. Am. Chem. Soc. 1974, 96, 5272–5274. [Google Scholar] [CrossRef]
- North, M.; Usanov, D.L.; Young, C. Lewis Acid Catalyzed Asymmetric Cyanohydrin Synthesis. Chem. Rev. 2008, 108, 5146–5226. [Google Scholar] [CrossRef]
- Kurono, N.; Ohkuma, T. Catalytic Asymmetric Cyanation Reactions. ACS Catal. 2016, 6, 989–1023. [Google Scholar] [CrossRef]
- Wang, W.; Liu, X.; Lin, L.; Feng, X. Recent Progress in the Chemically Catalyzed Enantioselective Synthesis of Cyanohydrins. Eur. J. Org. Chem. 2010, 2010, 4751–4769. [Google Scholar] [CrossRef]
- Bracco, P.; Busch, H.; Von Langermann, J.; Hanefeld, U. Enantioselective Synthesis of Cyanohydrins Catalysed by Hydroxynitrile Lyases-a Review. Org. Biomol. Chem. 2016, 14, 6375–6389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, B.S.; Ribeiro de Souza, F.Z. Enzymatic Synthesis of Enantiopure Alcohols: Current State and Perspectives. RSC Adv. 2019, 9, 2102–2115. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.L.; Lin, X.T. Recent Advances in Catalytic Asymmetric Synthesis of Tertiary Alcohols via Nucleophilic Addition to Ketones. Adv. Synth. Catal. 2019, 361, 876–918. [Google Scholar] [CrossRef]
- Pellissier, H. Enantioselective Titanium-Catalyzed Cyanation Reactions of Carbonyl Compounds. Adv. Synth. Catal. 2015, 357, 857–882. [Google Scholar] [CrossRef]
- Khan, N.H.; Kureshy, R.I.; Abdi, S.H.R.; Agrawal, S.; Jasra, R.V. Metal Catalyzed Asymmetric Cyanation Reactions. Coord. Chem. Rev. 2008, 252, 593–623. [Google Scholar] [CrossRef]
- Torres, H.M.; Maldonado, L.A.; Le Lagadec, R. Efficient Synthesis in Water of Mixed Carbonates of Cyanohydrins from Aromatic Aldehydes. Tetrahedron Lett. 2020, 61, 151414. [Google Scholar] [CrossRef]
- Khan, N.H.; Agrawal, S.; Kureshy, R.; Abdi, S.H.R.; Sadhukhan, A.; Pillar, R.S.; Bajaj, H.C. Ionic Liquid as Catalytic and Reusable Media for Cyanoethoxycarbonylation of Aldehydes. Cat. Comm. 2010, 11, 907–912. [Google Scholar] [CrossRef]
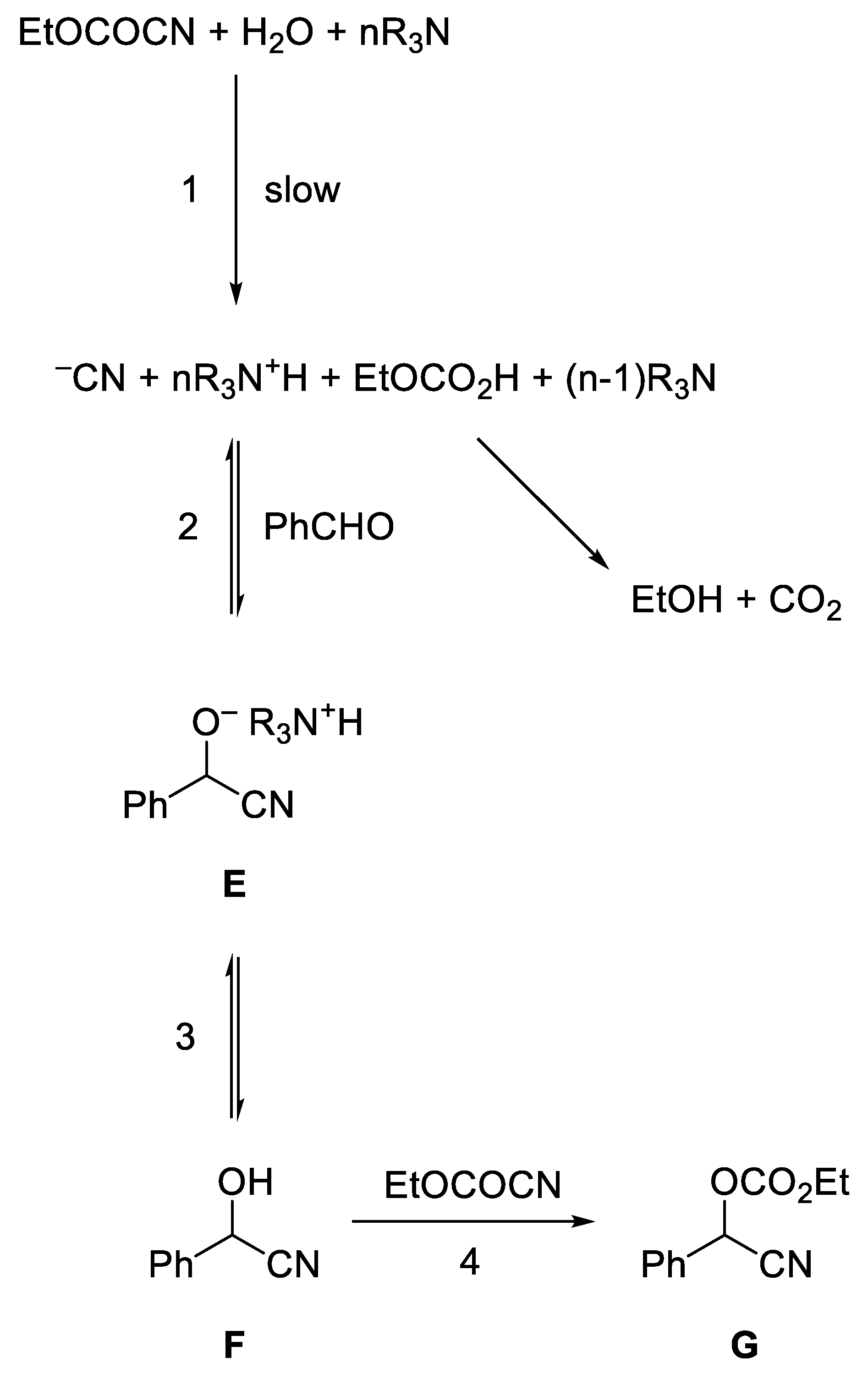
- North, M.; Urwin, S. Kinetic and Mechanism of Base Catalysed Ethyl Cyanoformate Addition to Aldehydes. Tetrahedon 2014, 70, 7100–7105. [Google Scholar] [CrossRef]
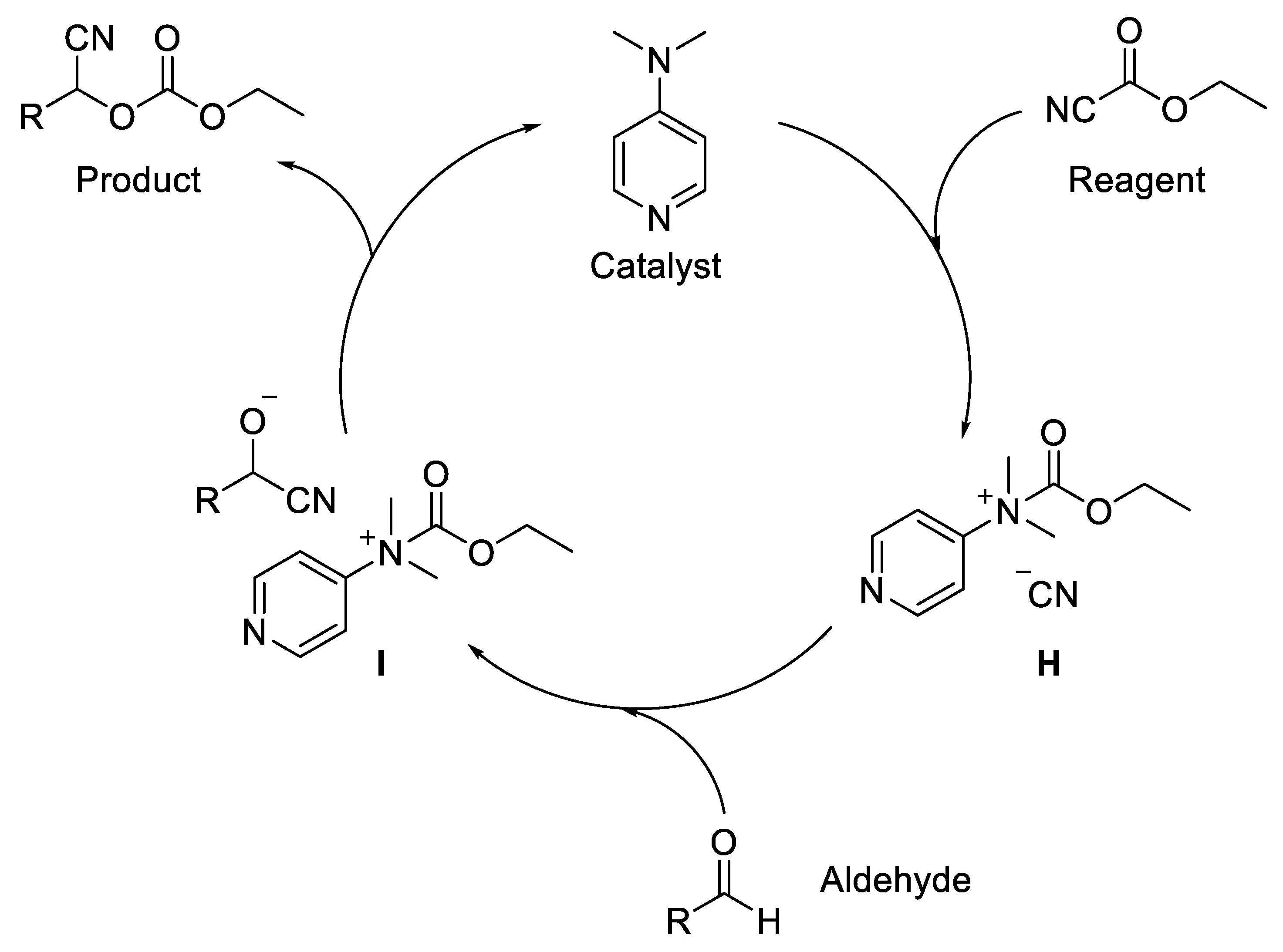
- Khan, N.-U.H.; Agrawal, S.; Kureshy, R.I.; Bera, P.K.; Abdi, S.H.R.; Bajaj, H.C. N,N-Dimethylpyridin-4-Amine Mediated Protocol for Cyanoethoxycarbonylation of Aldehydes Under Solvent-Free Conditions. Catal. Lett. 2010, 137, 255–260. [Google Scholar] [CrossRef]
- Aoki, S.; Kotani, S.; Suguira, M.; Nakajima, M. DMAP-catalyzed Cyanation of Aldehydes and Ketones with Ethyl Cyanoformate. Tetrahedron Lett. 2010, 51, 3547–3549. [Google Scholar] [CrossRef]
- Shen, Z.L.; Ji, S.J. Ionic liquid [bmim] BF4 as an Efficient and Recyclable Reaction Medium for the Synthesis of O-acetyl Cyanohydrins via One-Pot Condensation of Aldehydes, TMSCN, and Ac2O. Synth. Comm. 2009, 39, 808–818. [Google Scholar] [CrossRef]
- Kadam, S.T.; King, S.S. One-pot Three Component Synthesis of O-Acyl Cyanohydrins with TMSCN, Acetic Anhydride and Carbonyl Compounds under Solvent-free Conditions. Tetrahedron 2009, 65, 6330–6334. [Google Scholar] [CrossRef]
- Li, Z.; Zhag, Z. Direct Synthesis of Cyanohydrin Esters from Aroyl Chlorides using Potassium Hexacyanoferrate(II) as an Eco Friendly Cyanide Source. Res. Chem. Intermed. 2015, 41, 3147–3155. [Google Scholar] [CrossRef]
- Zhang, Z.; Lippert, M.; Hausmann, H.; Katku, M.; Schreiner, P.R. Cooperative Thiourea-Bronsted Acid Organocatalysis: Enantioselective Cyanosilylation of Aldehydes with TMSCN. J. Org. Chem. 2011, 76, 9764–9776. [Google Scholar] [CrossRef]
- Khan, N.H.; Sadhukan, A.; Maity, N.C.; Kureshy, R.I.; Abdi, S.H.R.; Saravancen, S.; Bajaj, H.C. Enantioselective O-acetylcyanation/cyanoformylation of Aldehydes using Catalysts with Built-in Crown Ether like Motif in Chiral Macrocyclic V(V) Salen Complexes. Tetrahedron 2011, 67, 7073–7080. [Google Scholar] [CrossRef]
- Ji, N.; Yao, L.; He, W.; Li, Y. Bifunctional Schiff base /Ti(IV) Catalysts for Enantioselective Cyanoformylation of Aldehydes with Ethyl Cyanoformate. Appl. Organomet. Chem. 2013, 27, 209–213. [Google Scholar] [CrossRef]
- Bradbuck, D.; Álvarez-Barcia, S.; Meisner, J.; Broghammer, F.; Klepp, J.; Garnier, D.; Frey, W.; Kastner, J.; Peters, R. Asymmetric Carboxycyanation of Aldehydes by Cooperative AlF/ Onium Salt Catalysts from Cyanoformate to KCN as Cyanide Source. Chem. Eur. J. 2019, 25, 1515–1524. [Google Scholar] [CrossRef] [PubMed]
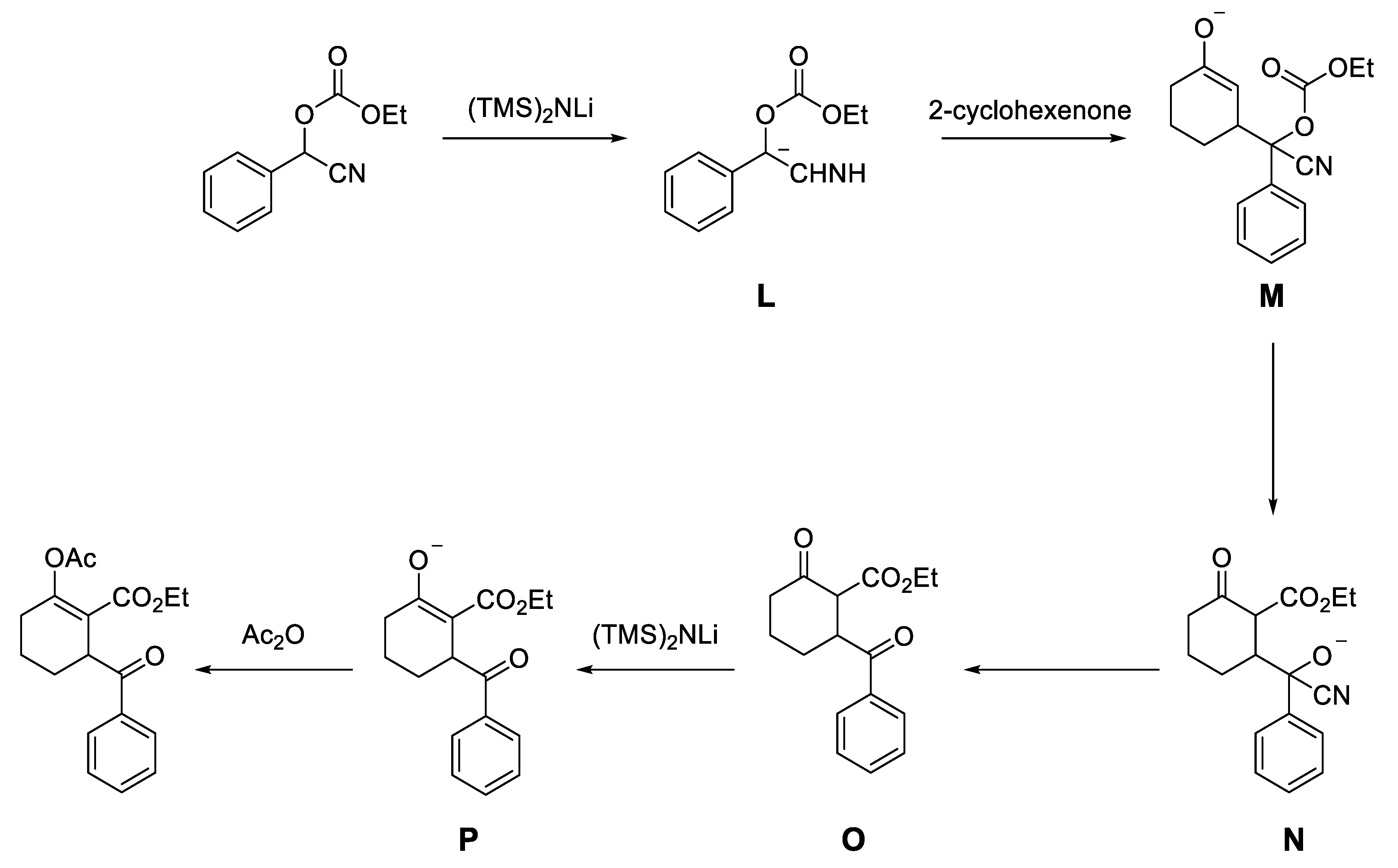
- Torres, H.M.; Maldonado, L.A.; Le Lagadec, R. Tandem Michael Addition-Claisen-type Condensation of Anions of O-ethyl Carbonates of Cyanohydrins to Cyclohex-2-en-1-one. Synth. Comm. 2017, 47, 1250–1255. [Google Scholar] [CrossRef]
- Zhang, D.; Song, H.; Cheng, N.; Liao, W. Synthesis of 2,4,5-Trisubstituted Oxazoles via Pd-Catalyzed C-H Addition to Nitriles/Cyclization Sequences. Org. Lett. 2019, 21, 2745–2749. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.; Cui, S.Q.; Ma, Q.Q.; Wei, Z.L.; Liao, W.W. α-Iminol Rearrangement Triggered by Pd-Catalyzed C-H Addition to Nitriles Sequences: Synthesis of Functionalized α-Amino Cyclopentanones. Org. Lett. 2021, 23, 1021–1025. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.J.; Liang, B.; Xu, Y.H.; Loh, T.P. Palladium Catalyzed Regioselective Olefination of O-Acetyl Cyanohydrins. J. Org. Chem. 2018, 83, 8265–8271. [Google Scholar] [CrossRef]
- Hertzberg, R.; Moberg, C. One-Step Preparation of O-(α-Bromoacyl) Cyanohydrins by Minor Enantiomer Recycling Synthesis of 4-Amino-2(5H)-Furanones. J. Org. Chem. 2013, 78, 9174–9180. [Google Scholar] [CrossRef]
- Jiao, L.C.; He, Z.Y.; Liao, W.W. Sulfa-Michael Addition Initated One-pot Tandem Sequence: Construction of Highly Substituted 2-cyclopentenones from Allylic Cyanohydrins. Tetrahedron 2020, 76, 130922. [Google Scholar] [CrossRef]
- Hertzberg, R.; Dinér, P.; Moberg, C. Palladium-Catalyzed-C(sp3)-C(sp2) Cross-Coupling of O-(α-Bromoacyl) Cyanohydrins with Boronic Acids: An Entry to Enantioenriched N-Acetylated-β-Amino Alcohols. Synthesis 2016, 48, 3175–3182. [Google Scholar] [CrossRef] [Green Version]
- Setzer, P.; Beauseigneur, A.; Pearson-Long, M.S.M.; Bertus, P. Titanium-Mediated Synthesis of 1,4-Diketones from Grignard Reagents and Acyl Cyanohydrins. Angew. Chem. Int. Ed. 2010, 49, 8691–8694. [Google Scholar] [CrossRef]
- Setzer, P.; Forcher, G.; Boeda, F.; Pearson-Long, M.S.M.; Bertus, P. Titanium-Mediated Addition of Grignard Reagents to Acyl Cyanohydrins: Aminocyclopropane versus 1,4-Diketone Formation. Eur. J. Org. Chem. 2014, 2014, 171–180. [Google Scholar] [CrossRef]
- Boukattaya, F.; Caillé, J.; Ammar, H.; Rouzler, F.; Boeda, F.; Pearson-Long, M.S.M.; Bertus, P. A Short Acces to Symmetrically α, α- Disubstituted α-Amino Acids from Acyl Cyanohydrins. Synthesis 2016, 48, 906–916. [Google Scholar] [CrossRef]
- Pantin, M.; Bodinier, F.; Saillour, J.; Youssouf, Y.M.B.; Boeda, F.; Pearson-Long, M.S.M.; Bertus, P. Convenient and Easy Access to 2-hydroxycyclopent-2-enones from Acylcyanohydrins. Tetrahedron 2019, 75, 4657–4662. [Google Scholar] [CrossRef]
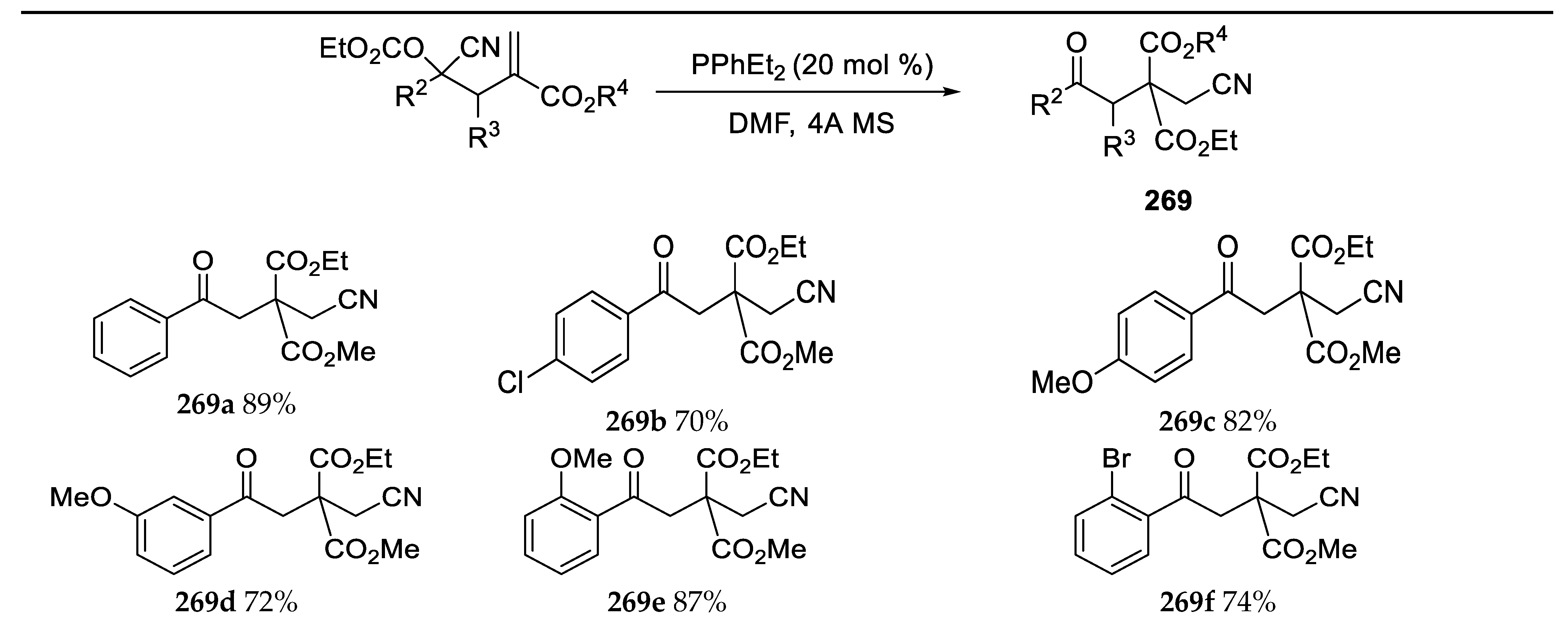
- Zhuang, Z.; Chen, J.M.; Pan, F.; Lia, W.W. Lewis Based Promoted Intramoleculas Acylcyanation of α-Substituted Activated Alkenes: Construction of Ketones Bearing β-Quaternary Carbon Centers. Org. Lett. 2012, 14, 2354–2357. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Hou, Q.L.; Wang, H.J.; Liao, W.W. Lewis-Based Promoted Rearrangement of Allylic Cyanohydrins: Construction of Functionalized Nitriles Bearing 1,3-Diketones Moieties. J. Org. Chem. 2014, 79, 10890–10898. [Google Scholar] [CrossRef]
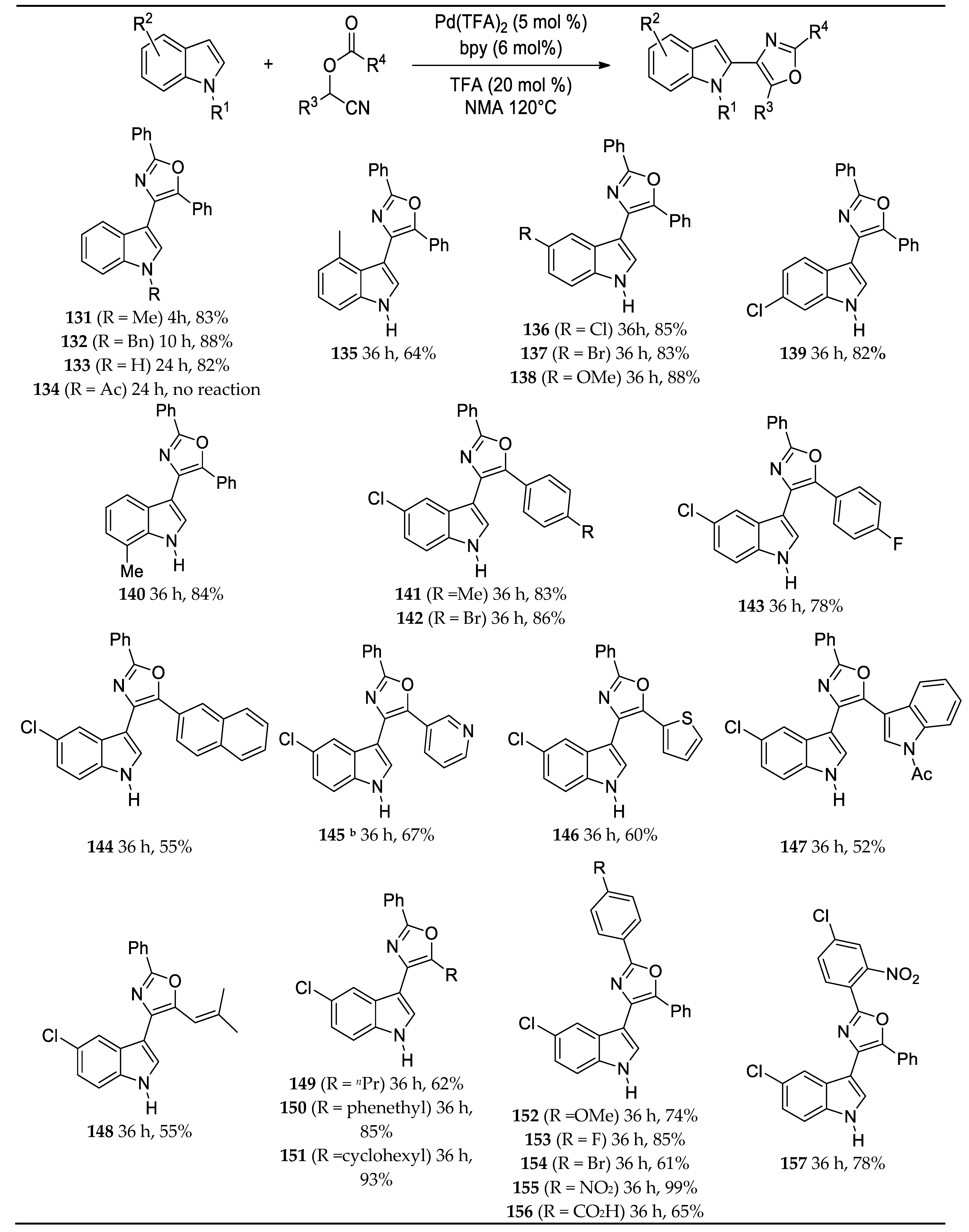
- Dai, L.; Yu, S.; Shao, Y.; Li, R.; Chen, Z.; Lv, N.; Chen, J. Palladium-catalyzed C-H Activation of Simple Arenes and Cascade Reaction with Nitriles: Access to 2,4,5-trisubstituted Oxazoles. Chem. Comm. 2021, 57, 1376–1379. [Google Scholar] [CrossRef]
- Sumino, S.; Fusano, A.; Okai, H.; Fukuyama, T.; Ryu, I. One-pot synthesis of Cyanohydrin Derivatives from Alkyl Bromides via Incorporation of Two One-carbon Components by Consecutive Radical/ionic Reactions. Beil. J. Org. Chem. 2014, 10, 150–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]

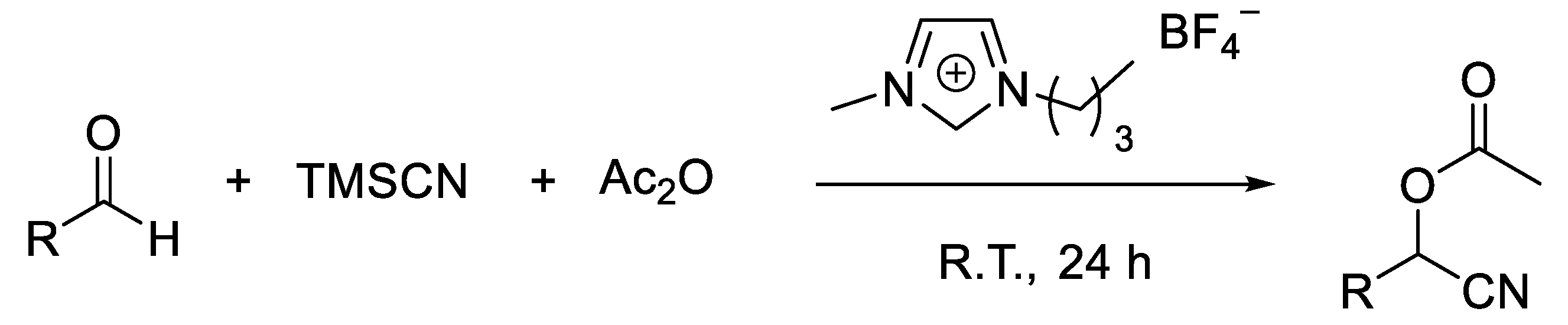
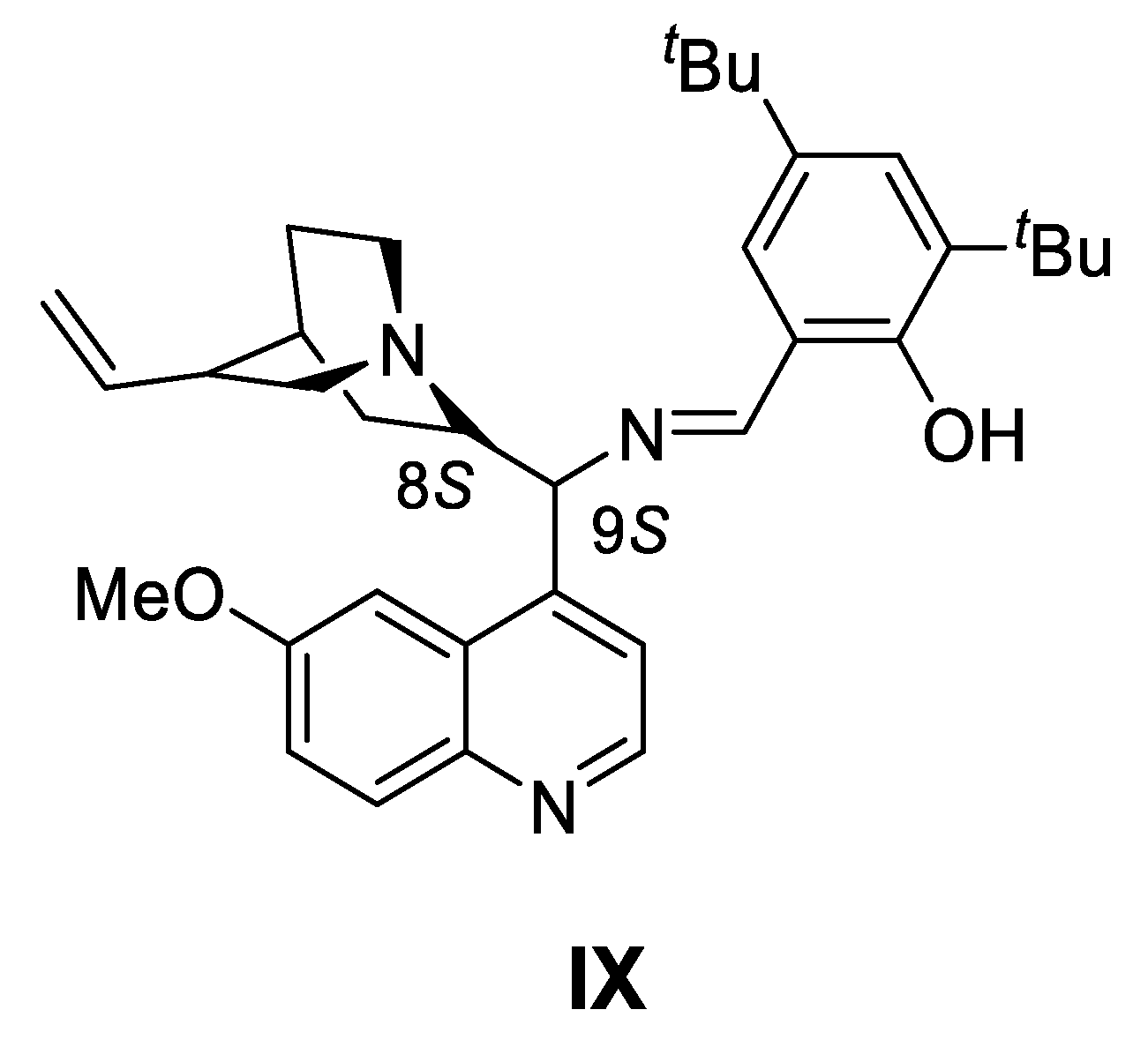
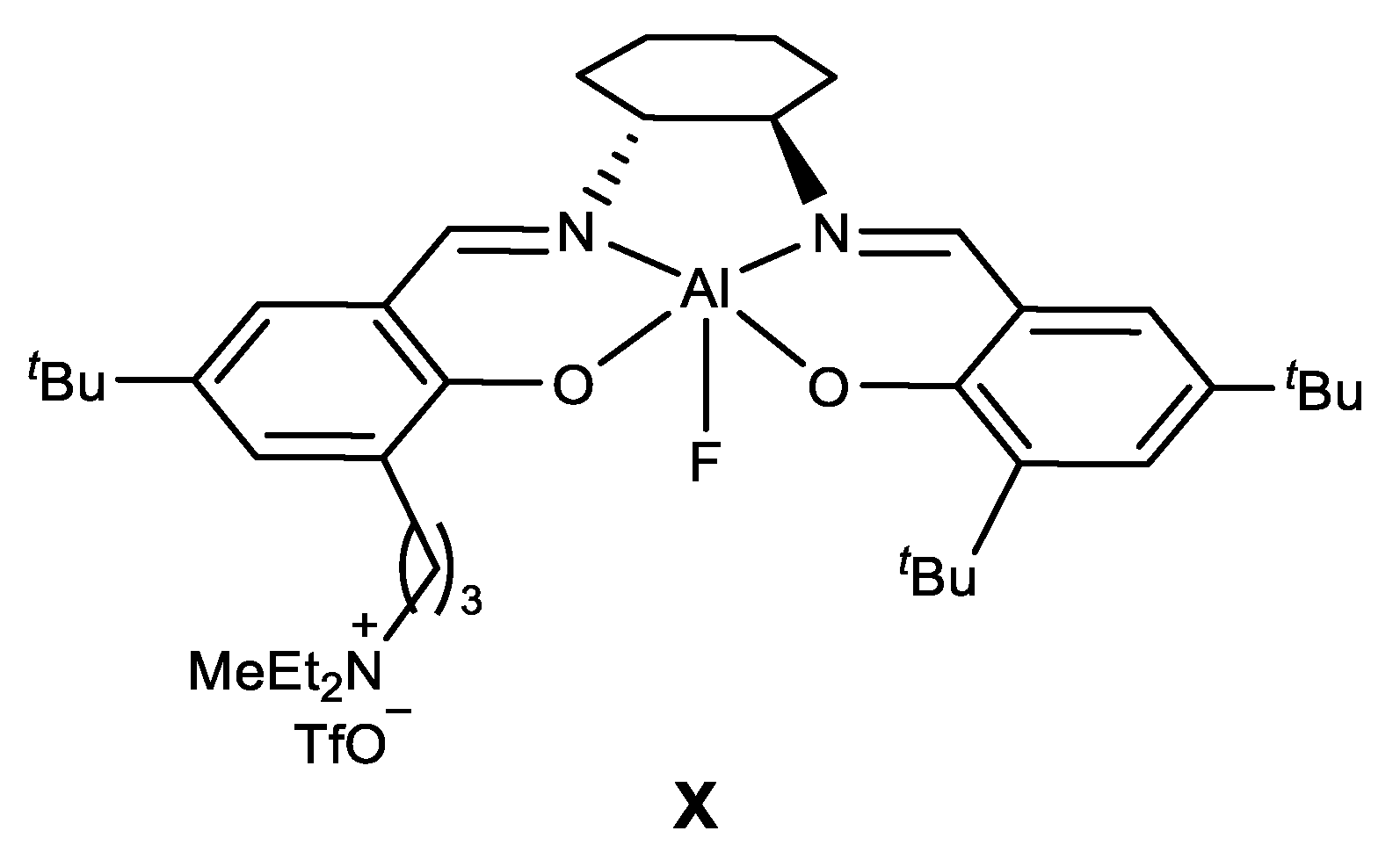



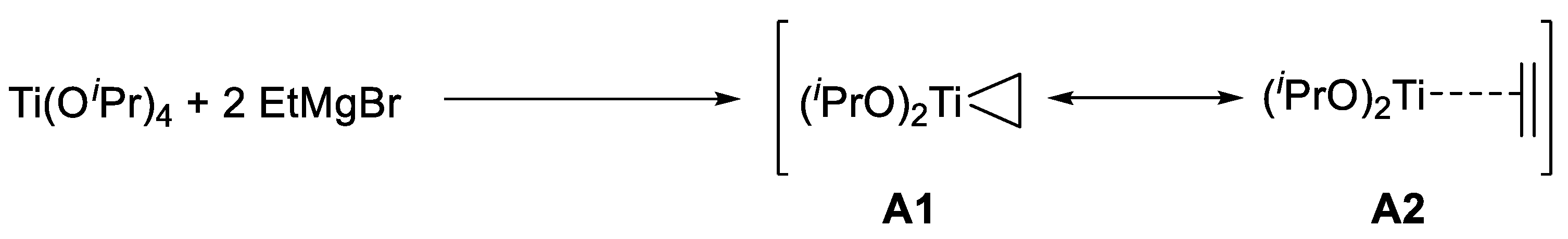

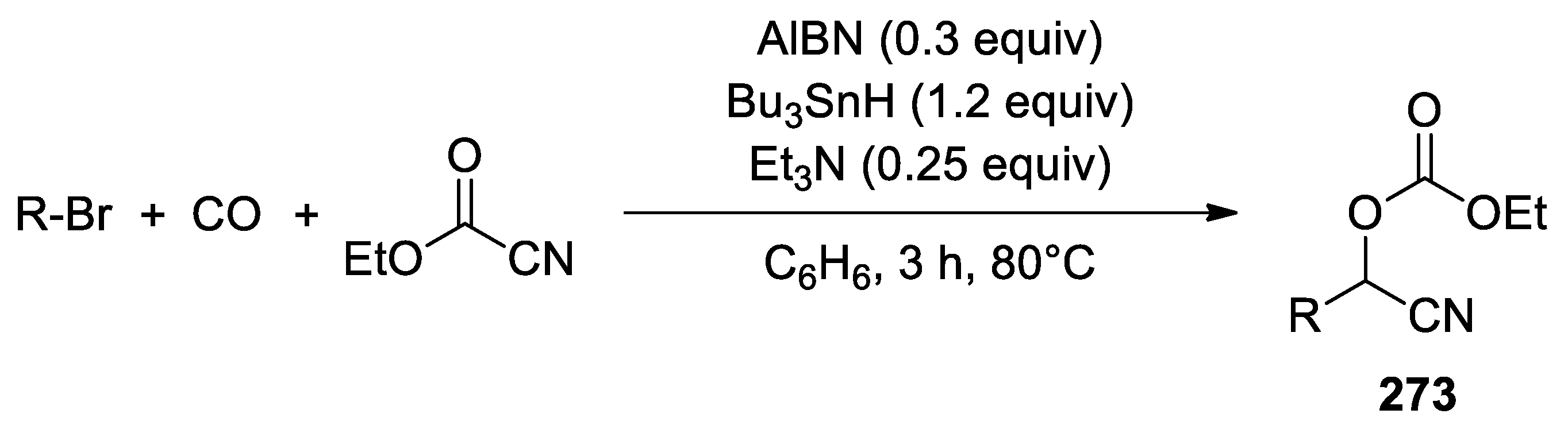
 | |||
|---|---|---|---|
| Entry | Aldehyde | Product | Yield (%) b |
| 1 | 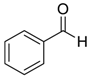 |  1 | 98 |
| 2 |  |  2 | 96 |
| 3 |  | 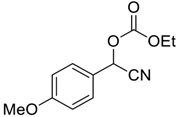 3 | 94 |
| 4 | 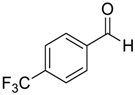 |  4 | 97 |
| 5 |  |  5 | 97 |
| 6 |  | 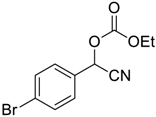 6 | 96 |
| 7 |  |  7 | 97 |
| 8 |  | 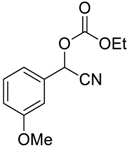 8 | 96 |
| 9 |  | 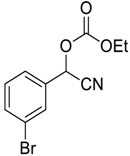 9 | 98 |
| 10 | 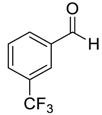 |  10 | 97 |
| 11 | 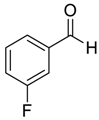 | 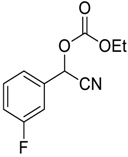 11 | 97 |
| 12 | 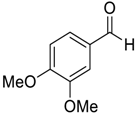 |  12 | 95 c |
| 13 | 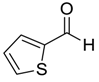 |  13 | 96 |
| 14 | 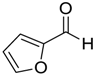 | 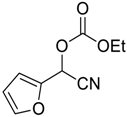 14 | 97 |
 | ||||
|---|---|---|---|---|
| Entry | Substrate | Product | Conversion (%) b | Yield (%) c |
| 1 | 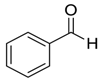 | 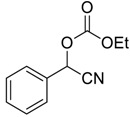 1 | >98 | 94 |
| 2 | 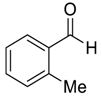 |  15 | >98 | 95 |
| 3 | 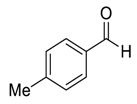 |  2 | >98 | 93 |
| 4 | 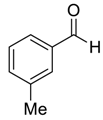 | 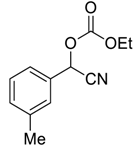 7 | >99 | 95 |
| 5 |  | 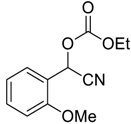 16 | >99 | 96 |
| 6 | 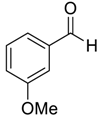 |  8 | >98 | 92 |
| 7 |  | 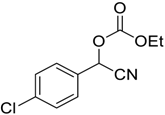 17 | >98 | 93 |
| 8 |  | 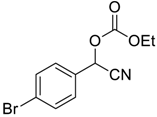 6 | >99 | 95 |
| 9 |  |  5 | >99 | 93 |
| 10 | 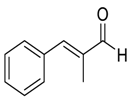 | 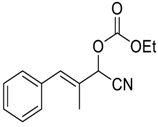 18 | >98 | 94 |
| 11 | 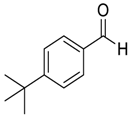 |  19 | >97 | 91 |
| 12 |  | 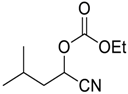 20 | >97 | 90 |
| 13 |  | 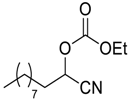 21 | >98 | 92 |
 | ||||
|---|---|---|---|---|
| Entry | Aldehyde | Product | Time (min) | Yield (%) b |
| 1 | 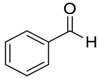 |  1 | 90 | 89 |
| 2 | 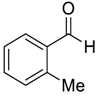 |  15 | 90 | 90 |
| 3 | 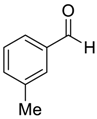 | 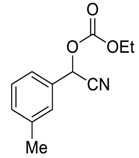 7 | 90 | 91 |
| 4 |  | 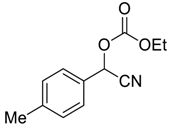 3 | 90 | 87 |
| 5 |  | 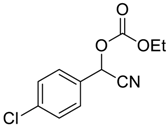 17 | 480 | 62 |
| 6 |  | 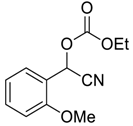 16 | 40 | 94 |
| 7 |  |  8 | 40 | 93 |
| 8 | 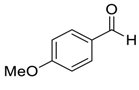 | 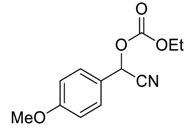 3 | 40 | 92 |
| 9 |  |  22 | 40 | 94 |
| 10 | 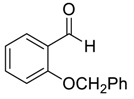 |  23 | 40 | 95 |
| 11 | 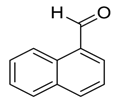 | 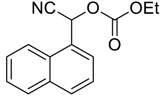 24 | 15 | 93 |
| 12 | 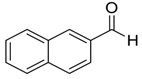 |  25 | 15 | 93 |
| 13 |  |  13 | 35 | 91 |
| 14 | 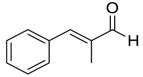 |  18 | 60 | 92 |
| 15 | 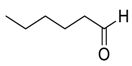 |  26 | 60 | 72 |
| 16 | 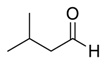 | 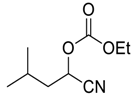 20 | 60 | 86 |
| 17 |  | 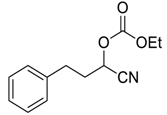 27 | 180 | 80 |
 | |||||
|---|---|---|---|---|---|
| Entry | DMAP mol % | Aldehyde | Product | Time (h) | Yield (%) b |
| 1 | 1 | 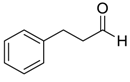 | 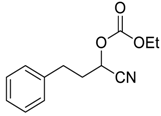 27 | 0.5 | 96 |
| 2 | 1 | 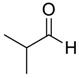 | 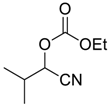 28 | 1 | 83 |
| 3 | 1 | 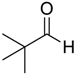 | 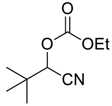 29 | 2 | 55 |
| 4 | 5 | 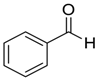 | 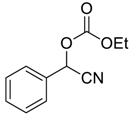 1 | 8 | 99 |
| 5 | 5 | 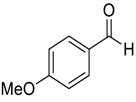 |  3 | 24 | 81 |
| 6 | 5 |  |  6 | 2 | 98 |
| 7 | 5 | 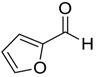 | 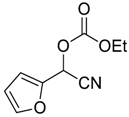 14 | 8 | 97 |
| 8 | 5 |  | 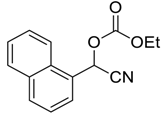 25 | 6 | 97 |
| 9 | 5 | 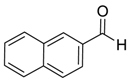 | 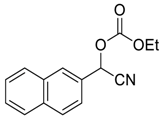 30 | 8 | 99 |
| 10 c,d | 5 |  |  31 | 24 | 78 |
 | ||||
|---|---|---|---|---|
| Entry | Ketone | Product | Time (h) | Yield b (%) |
| 1 c |  | 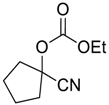 32 | 24 | 20 |
| 2 |  |  32 | 24 | 85 |
| 3 |  | 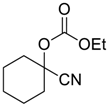 33 | 24 | 99 |
| 4 |  |  34 | 24 | 30 |
| 5 |  |  35 | 24 | 87 |
| 6 |  |  36 | 24 | 73 |
| 7 |  |  37 | 48 | 44 |
| Entry | Aldehyde | Product | Yield (%) b |
|---|---|---|---|
| 1 |  |  38 | 96 |
| 2 | 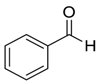 | 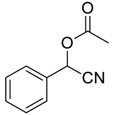 39 | 99 |
| 3 |  |  40 | 91 |
| 4 | 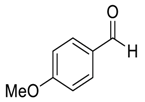 |  41 | 92 |
| 5 |  | 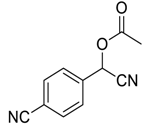 42 | 79 |
| 6 |  |  43 | 91 |
| 7 | 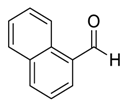 |  44 | 83 |
| 8 |  |  45 | 93 |
| 9 |  | 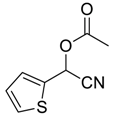 46 | 76 |
| 10 |  |  47 | 87 |
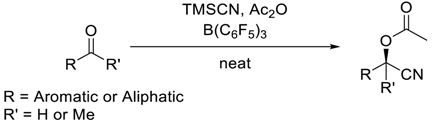 | ||||
|---|---|---|---|---|
| Entry | Aldehyde | Product | Time (h) | Yield (%) b |
| 1 |  | 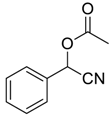 39 | 3 | 92 |
| 2 |  | 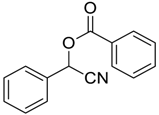 48 | 5 | 88 c |
| 3 | 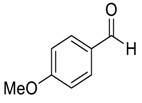 |  41 | 3 | 85 |
| 4 |  |  40 | 3 | 94 |
| 5 |  | 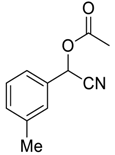 49 | 3 | 90 |
| 6 | 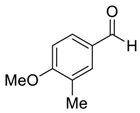 |  50 | 3 | 98 |
| 7 |  | 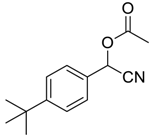 51 | 3 | 89 |
| 8 |  | 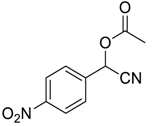 52 | 8 | 71 |
| 9 |  |  38 | 3 | 95 |
| 10 | 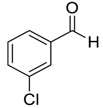 |  53 | 3 | 91 |
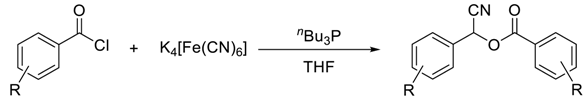 | |||
|---|---|---|---|
| Entry | Aroyl Chloride | Product | Yield (%) b |
| 1 | 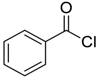 |  48 | 88 |
| 2 | 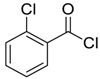 | 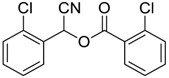 54 | 80 |
| 3 | 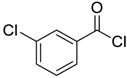 |  55 | 82 |
| 4 |  | 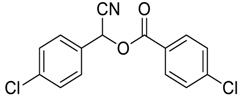 56 | 86 |
| 5 | 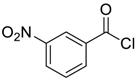 |  57 | 82 |
| 6 |  |  58 | 87 |
| 7 |  | 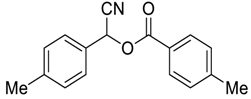 59 | 79 |
| 8 | 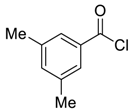 |  60 | 75 |
| 9 |  | 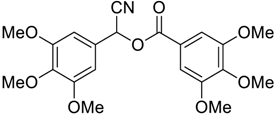 46 | 73 |
| 10 | 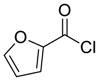 | 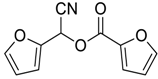 61 | 78 |
 | ||||
|---|---|---|---|---|
| Entry | Aldehyde | Product | Yield (%) b | ee (%) c |
| 1 | 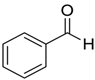 | 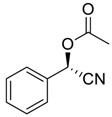 62 | 73 | 73 |
| 2 | 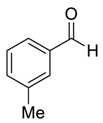 |  63 | 74 | 46 |
| 3 |  |  64 | 80 | 84 |
| 4 | 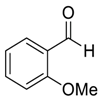 |  65 | 69 | 8 |
| 5 |  | 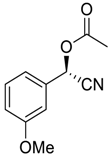 66 | 72 | 58 |
| 6 d |  |  67 | 57 | 68 |
| 7 e | 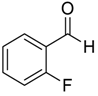 | 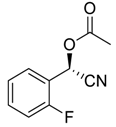 68 | 83 | 42 |
| 8 |  | 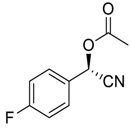 69 | 79 | 82 |
| 9 |  | 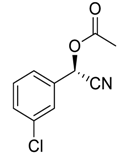 70 | 66 | 65 |
| 10 |  |  71 | 78 | 85 |
| 11 |  |  72 | 87 | 82 |
| 12 | 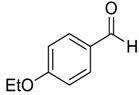 | 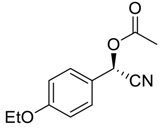 73 | 80 | 77 |
| 13 |  |  74 | 88 | 83 |
| 14 e | 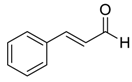 |  75 | 67 | 88 |
| 15 |  |  76 | 90 | 18 |
| 16 |  | 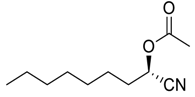 77 | 72 | 51 |
 | ||||||
|---|---|---|---|---|---|---|
| Entry | Substrate | Product | Catalyst VII | Catalyst VIII | ||
| Yield (%) b | ee (%) c | Yield (%) b | ee (%) c | |||
| 1 | 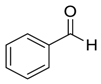 |  1 | 97 | 83 | 99 (98) | 92 (90) |
| 2 |  | 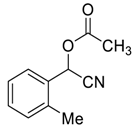 15 | 98 | 89 | 98 (99) | >99 (96) |
| 3 |  |  7 | 97d | 82 | 95 (95) | 91 (88) |
| 4 | 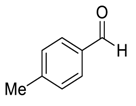 |  2 | 95 | 81 | 95 (93) | 90 (89) |
| 5 | 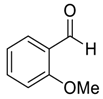 | 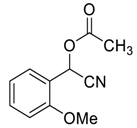 16 | 96 | 86 | 95 (96) | 97 (95) |
| 6 |  |  8 | 95 | 84 | 99 (99) | 96 (95) |
| 7 | 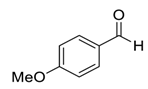 | 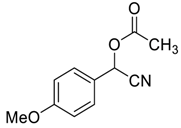 3 | 94 | 82 | 97 (94) | 96 (94) |
| 8 |  | 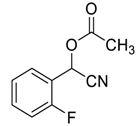 78 | 99 | 87 | 97 (96) | >99 (97) |
| 9 |  |  17 | 98 | 84 | 97 (94) | 92 (90) |
| 10 | 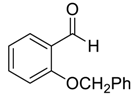 | 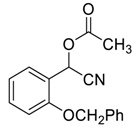 23 | 98 | 78 | 97 (96) | 89 (85) |
| 11 |  |  25 | 99 | 85 | 99 (99) | >99 (97) |
| 12 | 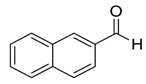 | 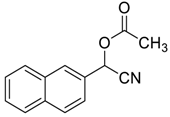 30 | 99 | 89 | 99 (99) | >99 (98) |
| 13 | 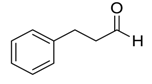 | 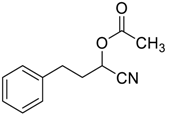 27 | 98 | 65 | 96 (95) | 78 (76) |
| 14 | 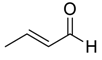 | 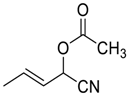 79 | 98 | 82 | 98 (98) | 89 (85) |
| 15 | 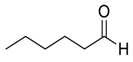 | 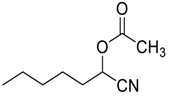 26 | 98 | 53d | 99 (97) | 73 d (72) |
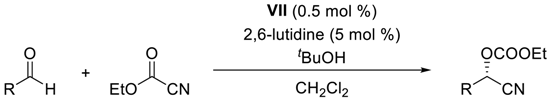 | |||||
|---|---|---|---|---|---|
| Entry | Substrate | Time (h) | Product | Yield b (%) | eec,d (%) |
| 1 |  48 | 12 |  80 | 96 | 95 |
| 2 | 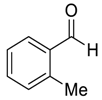 49 | 12 |  81 | 97 | 93 |
| 3 | 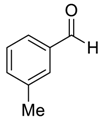 50 | 15 |  82 | 94 | 85 |
| 4 |  51 | 12 | 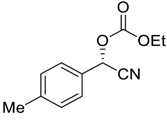 83 | 97 | 96 |
| 5 | 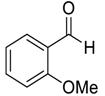 52 | 16 |  84 | 95 | 92 |
| 6 |  53 | 18 |  85 | 90 | 87 |
| 7 |  54 | 12 |  86 | 96 | 97 |
| 8 | 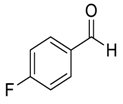 56 | 16 |  87 | 95 | 93 |
| 9 |  58 | 18 |  88 | 93 | 91 |
| 10 |  61 | 12 |  89 | 95 | 95 |
| 11 | 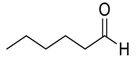 64 | 15 | 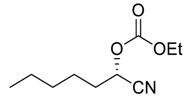 90 | 88 | 81 |
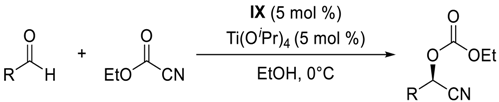 | |||||
|---|---|---|---|---|---|
| Entry | Substrate | Product | Time (h) | Yield (%) b | ee (%) c |
| 1 | 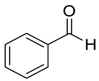 |  91 | 6 | 93 | 83 |
| 2 |  | 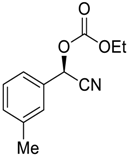 92 | 6 | 91 | 77 |
| 3 | 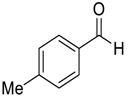 | 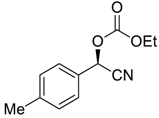 93 | 6 | 92 | 75 |
| 4 |  | 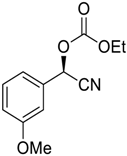 94 | 6 | 94 | 71 |
| 5 |  |  95 | 6 | 93 | 73 |
| 6 |  | 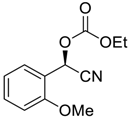 96 | 6 | 90 | 85 |
| 7 |  | 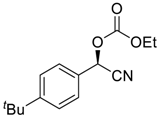 97 | 6 | 91 | 75 |
| 8 | 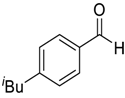 |  98 | 6 | 92 | 71 |
| 9 | 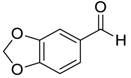 |  99 | 10 | 94 | 65 |
| 10 d | 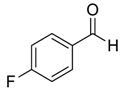 |  100 | 12 | 95 | 71 |
| 11 d | 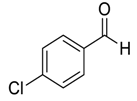 |  101 | 12 | 95 | 63 |
| 12 e |  |  102 | 10 | 96 | 73 |
| 13 e |  |  103 | 10 | 93 | 61 |
| 14 | 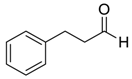 | 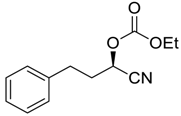 104 | 6 | 91 | 67 |
| 15 e | 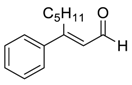 |  105 | 10 | 90 | 71 |
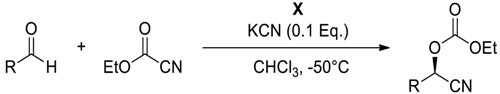 | ||||||
|---|---|---|---|---|---|---|
| Entry | Substrate | Product | Catalyst (mol %) | Time (h) | Yield (%) b | ee (%) c |
| 1 | 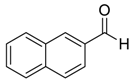 |  106 | 0.1 | 24 | >99 | 93 |
| 2 |  |  107 | 0.1 | 24 | 92 | 93 |
| 3 |  | 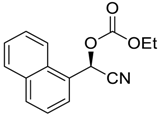 108 | 0.1 | 24 | 80 | 79 |
| 4 |  |  91 | 0.1 | 24 | 99 | 91 |
| 5 |  | 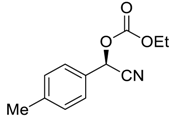 93 | 0.1 | 72 | 85 | 88 |
| 6 |  | 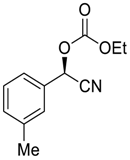 92 | 0.1 | 48 | 98 | 90 |
| 7 | 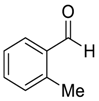 | 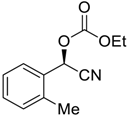 109 | 0.1 | 48 | 99 | 82 |
| 8 | 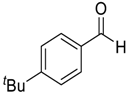 |  97 | 0.5 | 72 | 51 | 85 |
| 9 |  | 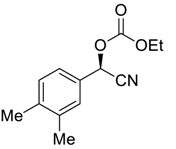 110 | 0.1 | 72 | 83 | 86 |
| 10 | 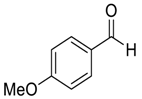 | 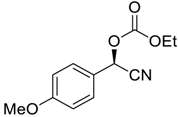 95 | 0.5 | 72 | 78 | 93 |
| 11 |  | 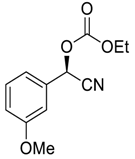 94 | 0.1 | 24 | >99 | 92 |
| 12 |  |  96 | 0.1 | 48 | 61 | 84 |
| 13 |  |  111 | 0.5 | 72 | 97 | 96 |
| 14 |  |  101 | 0.1 | 48 | >99 | 89 |
| 15 |  |  112 | 0.1 | 24 | 93 | 79 |
| 16 |  |  100 | 0.1 | 48 | >99 | 89 |
| 17 | 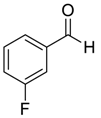 |  113 | 0.1 | 24 | 98 | 78 |
| 18 | 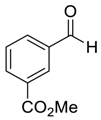 | 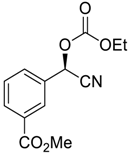 114 | 0.1 | 48 | 99 | 80 |
| 19 |  | 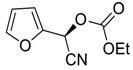 102 | 0.1 | 48 | 98 | 82 |
| 20 |  | 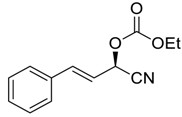 115 | 0.1 | 48 | >99 | 96 |
| 21 | 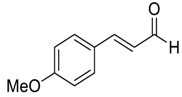 |  116 | 0.1 | 48 | 90 | 97 |
| 22 d | 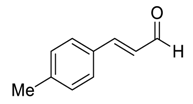 |  117 | 0.1 | 72 | 96 | 94 |
| 23 e | 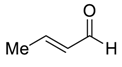 |  118 | 0.01 | 72 | >99 | 93 |
| 24 d |  |  118 | 0.1 | 48 | >99 | 80 |
| 25 d |  |  119 | 0.1 | 72 | 99 | 81 |
| 26 d | 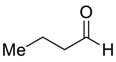 | 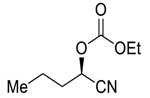 120 | 0.1 | 72 | 89 | 78 |
| 27 d | 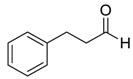 | 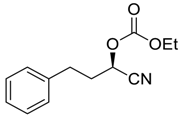 104 | 0.1 | 72 | 99 | 90 |
| 28 d |  | 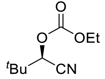 121 | 0.1 | 72 | 99 | 78 |
 | |||||
|---|---|---|---|---|---|
| Entry | Substrate | Product | Temperature (°C) | Yield (%) b | ee (%) c |
| 1 |  |  106 | −60 | 96 | 93 |
| 2 |  | 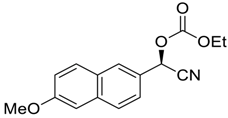 107 | −60 | 93 | 93 |
| 3 c | 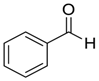 | 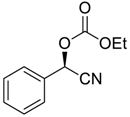 91 | −60 | 92 | 88 |
| 4 d |  |  93 | −80 | 85 | 90 |
| 5 | 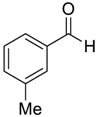 |  92 | −60 | 98 | 91 |
| 6 | 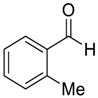 |  109 | −60 | >99 | 82 |
| 7 f |  | 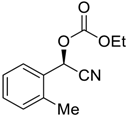 109 | −60 | 98 | 90 |
| 8 | 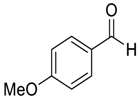 |  95 | −60 | 99 | 93 |
| 9 | 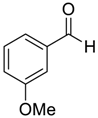 |  94 | −60 | >99 | 85 |
| 10 |  | 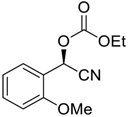 96 | −60 | >99 | 90 |
| 11 |  | 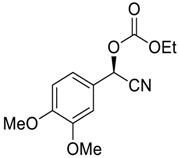 111 | −60 | 90 | 96 |
| 12 |  | 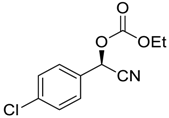 101 | −60 | >99 | 80 |
| 13 | 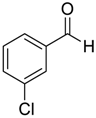 | 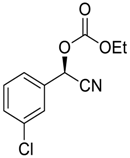 112 | −60 | >99 | 80 |
| 14 |  |  100 | −60 | >99 | 80 |
| 15 | 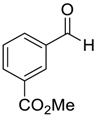 | 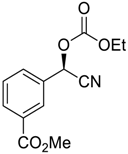 114 | −60 | 92 | 82 |
| 16 d |  | 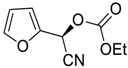 102 | −80 | 93 | 90 |
| 17 | 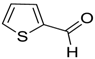 |  122 | −60 | 91 | 80 |
| 18 |  |  115 | −60 | >99 | 92 |
| 19 |  | 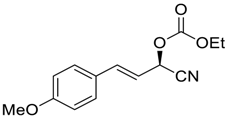 116 | −60 | 99 | 93 |
| 20 e | 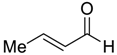 |  118 | −60 | 90 | 95 |
| 21 f |  | 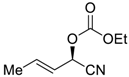 118 | −60 | 81 | 94 |
| 22 |  | 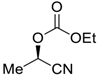 119 | −60 | >99 | 55 |
| 23 d |  | 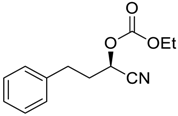 104 | −80 | 95 | 69 |
| Entry | Cyanohydrin | Product | Yield (%) b |
|---|---|---|---|
| 1 |  1 |  123 | 77 |
| 2 | 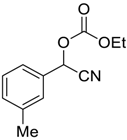 7 |  124 | 75 |
| 3 | 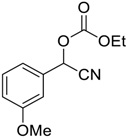 8 |  125 | 69 |
| 4 | 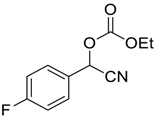 5 |  126 | 70 |
| 5 |  14 | 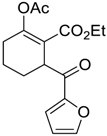 127 | 62 |
| 6 | 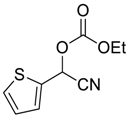 13 | 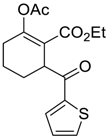 128 | 65 |
| 7 c |  1 | 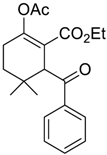 129 | 0 |
| 8 d |  1 |  130 | 70 |
 | ||||
|---|---|---|---|---|
| Entry | Substrate | Product | Yield (%) b | erc |
| 1 | 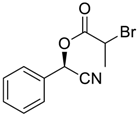 |  219 | 86 | 98.6:1.4 |
| 2 |  | 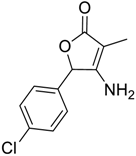 220 | 97 | 98.4:1.6 |
| 3 |  | 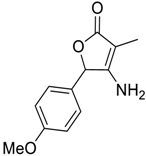 221 | 98 | 97.7:2.1 |
| 4 |  |  222 | 73 | 97.3:2.7 |
| 5 | 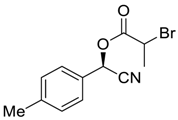 |  223 | 93 | 97.3:2.7 |
| 6 | 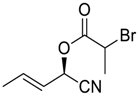 |  224 | 94 | 95.4:4.6 |
| 7 |  | 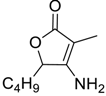 225 | 71 | 98.8:1.2 |
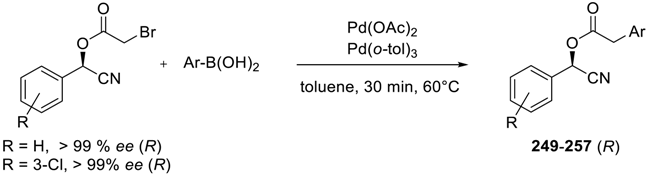 | |||||
|---|---|---|---|---|---|
| Entry | Substrate | Boronic Acid | Product | Yield (%) b | ee (%) |
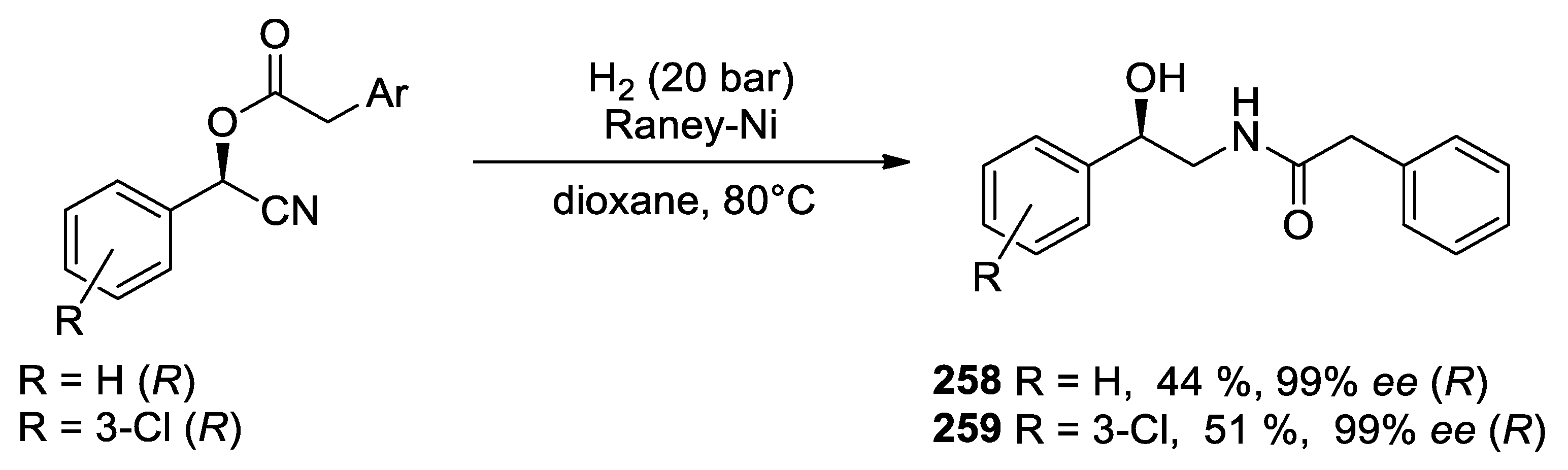
| 1 | 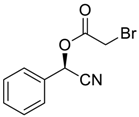 | 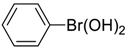 | 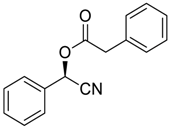 249 | 89 | >99 |
| 2 |  |  |  250 | 90 | >99 |
| 3 |  | 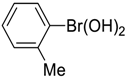 | 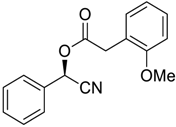 251 | 90 | >99 |
| 4 |  |  |  252 | 83 | 99 |
| 5 | 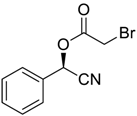 | 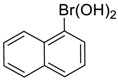 |  253 | 92 | 99 |
| 6 | 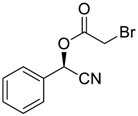 | 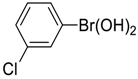 |  254 | 73 | 99 |
| 7 |  | 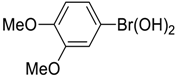 | 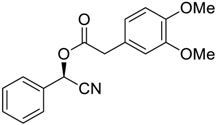 255 | 66 | 95 |
| 8 |  |  |  256 | 91 | 99 |
| 9 |  |  |  257 | 87 | 96 |
 | |||||
|---|---|---|---|---|---|
| Entry | Substract | Solvent | 260/261 Ratio a | 260 | 261 (Yield %) b |
| 1 | 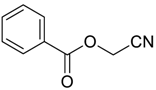 | Et2O | 14:86 | 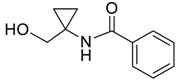 260a | 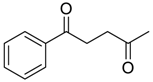 261a (69) |
| 2 | 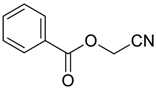 | THF | 37:63 |  260a |  261a (45) |
| 3 | 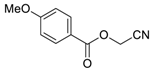 | Et2O | 13:87 | 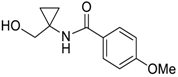 260b |  261b (65) |
| 4 | 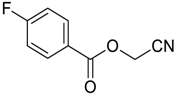 | Et2O | 7:93 | 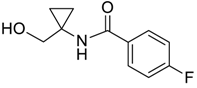 260c | 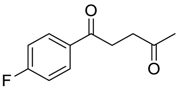 261c (60) |
| 5 |  | Et2O | 20:80 |  260d |  261d (60) |
| 6 |  | Et2O | 60:40 | 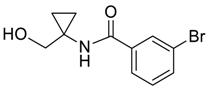 260e | 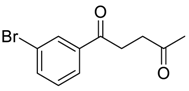 261e (74) |
| 7 | 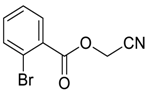 | Et2O | 7:93 | 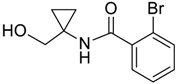 260f |  261f (62) |
| 8 | 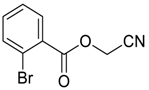 | THF | 22:78 |  260f (40) |  261f (31) |
| 9 |  | Et2O | 7:93 | 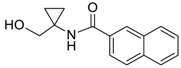 260g |  261g (62) |
| 10 | 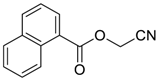 | Et2O | 22:78 |  260h |  261h (66) |
| 11 |  | THF | 59:41 |  260h | 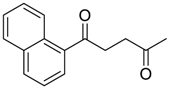 261h (32) |
| 12 | 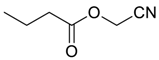 | Et2O | 7:93 | 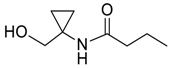 260i |  261i (50) |
| 13 |  | THF | 13:87 |  260i |  261i (48) |
| 14 |  | Et2O | 13:87 |  260j |  261j (50) |
| 15 |  | Et2O | 8:92 |  260k |  261k (55) |
| 16 |  | Et2O | 20:80 | 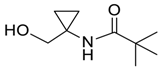 260l |  261l (56) |
| 17 | 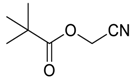 | THF | 90:10 |  260l |  261l (60) |
| 18 | 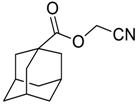 | Et2O | 30:70 |  260m | 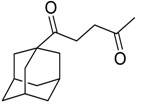 261m (58) |
| 19 |  | THF | 81:19 |  260m |  261m (69) |
| 20 | 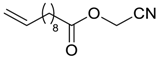 | Et2O | 12:88 |  260n | 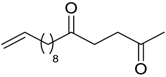 261n (62) |
| 21 | 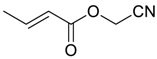 | Et2O | 3:97 | 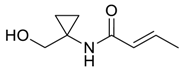 260o |  261o (42) |
| 22 |  | Et2O | 8:92 | 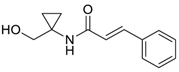 260p | 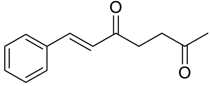 261p (45) |
| 23 |  | Et2O | 22:78 |  260q |  261q (42) |
| 24 | 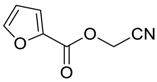 | Et2O | 12:88 |  260r |  216r (50) |
| 25 |  | Et2O | 19:81 | 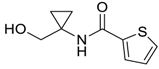 260s | 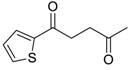 261s (65) |
| 26 | 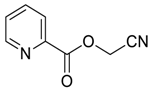 | Et2O | [c] | 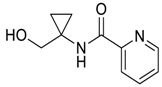 260t | 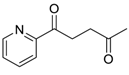 261t (0) |
| 27 | 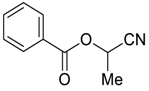 | Et2O | 15:85 |  260u | 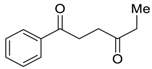 261u (56) |
| 28 | 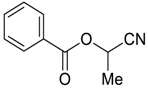 | THF | 28:72 | 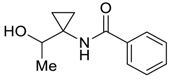 260u |  261u (52) |
| 29 | 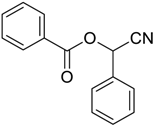 | Et2O | 22:78 | 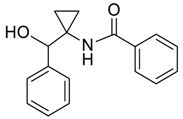 260v | 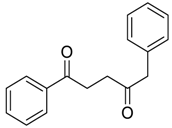 261v (52) |
| 30 |  | Et2O | 12:88 | 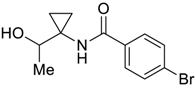 260w |  261w (50) |
| 31 | 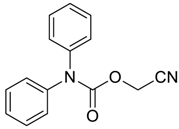 | Et2O | 54:46 | 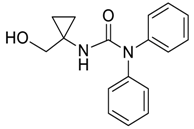 260xd | 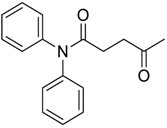 261x (34) |
| 32 |  | THF | 57:43 | 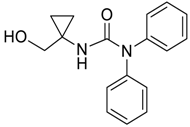 260xd | 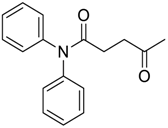 261x (27) |
| 33 | 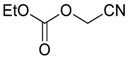 | Et2O | 100:0 | 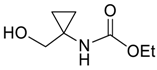 260y (31) + 262 (30) e |  261y |
 | ||||
|---|---|---|---|---|
| Entry | Cyanohydrin | Product | 263 Yield a (%) | Ratio b 263/265 |
| 1 |  | 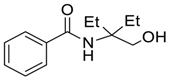 263a | 65 | 83:17 |
| 2 c |  |  263a | 14 | 26:74 |
| 3 |  | 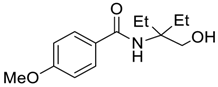 263b | 73 | 84:16 |
| 4 | 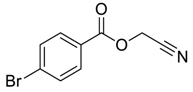 | 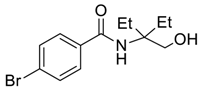 263c | 58 | 71:29 |
| 5 |  | 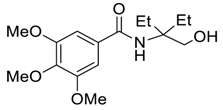 263d | 68 | 84:16 |
| 6 | 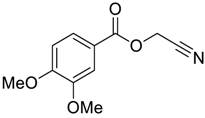 | 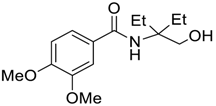 263e | 65 | 87:13 |
| 7 | 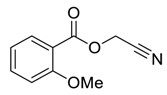 |  263f | d | 0:100 |
| 8 | 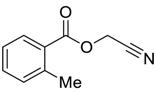 | 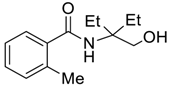 263g | 72 | 96:4 |
| 9 |  | 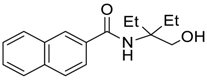 263h | 59 | 71:29 |
| 10 |  | 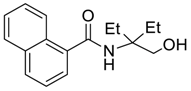 263i | 74 | 96:4 |
| 11 e | 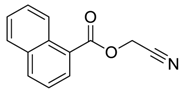 | 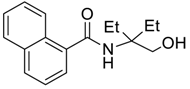 263i | 77 | >98:2 |
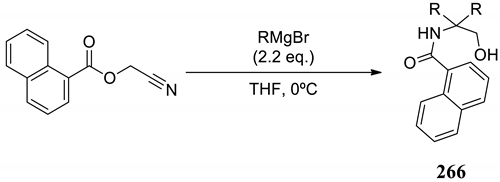 | |||
|---|---|---|---|
| Entry | Grignard Reagent | Product | Yield (%) a |
| 1 | EtMgBr | 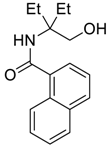 266a | 77 |
| 2 | MeMgBr | 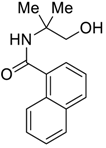 266b | 73 |
| 3 | nC5H11MgBr | 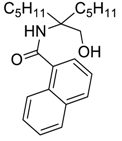 266c | 68 |
| 4 | Ph(CH2)3MgBr |  266d | 79 |
| 5 | PhMgBr | 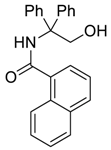 266e | 70 |
| 6 | 4-MeO-C6H4MgBr |  266f | 82 |
| 7 | H2C=CHMgBr |  266g | 71 |
| 8 | H2C=CH(CH2)2MgBr |  266h | 72 |
| 9 | H2C=CH(CH2)3MgBr |  266i | 60 |
| 10 | H2C=CHCH2MgBr |  266j | 26 |
| 11 | H2C=CHCH2MgBr |  266j | 57 b |
 | ||||
|---|---|---|---|---|
| Entry | Substrate | Product | Two-Step Yield | One-Pot a Yield |
| 1 |  |  268a | 62% | 60% b |
| 2 |  |  268b | 49% | 78% |
| 3 | 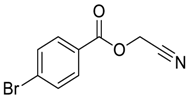 | 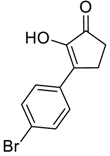 268c | 52% | 80% |
| 4 |  | 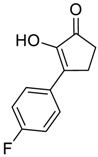 268d | 46% | 73% |
| 5 | 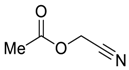 | 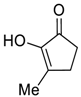 268e | - | 72% |
| 6 |  |  268f | - | 68% |
| 7 | 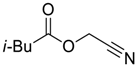 |  268g | - | 63% |
| 8 |  | 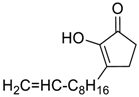 268h | - | 69% |
| 9 |  |  268i | - | 21% |
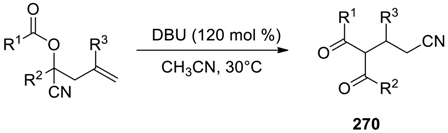 | |||||
|---|---|---|---|---|---|
| Entry | Substrate | Product | Time (h) | Yield (%) b | dr c |
| 1 |  | 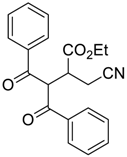 270a | 43 | 67 | - |
| 2 | 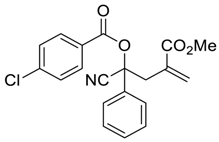 |  270b | 24 | 65 | 1:1 |
| 3 |  |  270c | 1 | 32 | 1.1:1 |
| 4 |  |  270d | 53 | 31 | 1:1 |
| 5 |  |  270e | 6 | 83 | 1:1 |
| 6 |  |  270f | 7 | 77 | 1.2:1 |
| 7 |  |  270g | 48 | 38 | 1.1:1 |
| 8 | 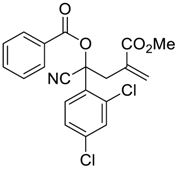 | 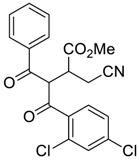 270h | 16 | 60 | 1.1:1 |
| 9 | 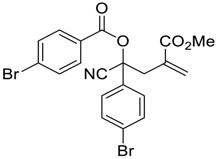 | 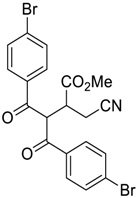 270i | 6 | 56 | - |
| 10 | 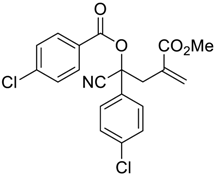 | 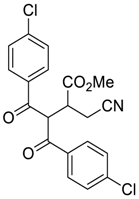 270j | 3 | 53 | - |
| 11 |  |  270k | 1 | 72 | 1.4:1 |
| 12 | 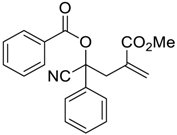 |  270l | 24 | 38 | - |
| Entry | Substrate | Product | Time (h) | Yield (%) b | drc |
|---|---|---|---|---|---|
| 1 | 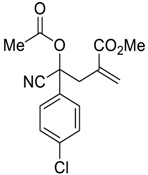 | 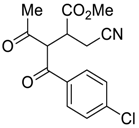 270m | 1.5 | 78 | 1.1:1 |
| 2 |  |  270n | 4 | 54 | 1:1 |
| 3 d |  |  270o | 5 | - | - |
| 4 | 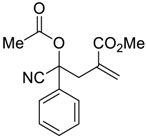 |  270p | 2 | 77 | 1:1 |
| 5 | 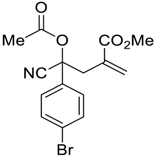 | 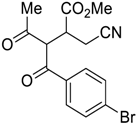 270q | 2 | 63 | 1.2:1 |
| 6 |  |  270r | 6 | 72 | 1.1:1 |
| 7 | 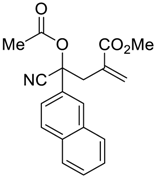 |  270s | 3.5 | 69 | 1:1 |
| 8 | 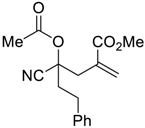 | 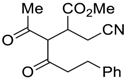 270t | 6 | 20 | 1:1 |
| Entry | Alkyl Bromide | CO (atm) | Product | Yield (%) b |
|---|---|---|---|---|
| 1 c |  | 120 | 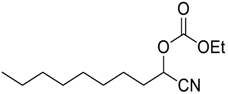 273a | 79 |
| 2 |  | 80 |  273b | 60 |
| 3 | 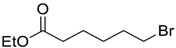 | 80 |  273c | 83 |
| 4 |  | 120 | 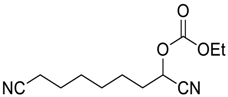 273d | 76 |
| 5 | 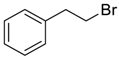 | 120 | 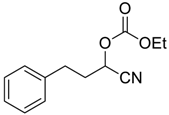 273e | 61 |
| 6 |  | 120 | 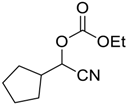 273f | 61 |
| 7 | 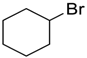 | 120 | 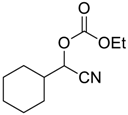 273g | 74 |
| 8 |  | 120 | 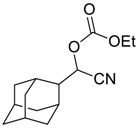 273h | 73 |
| 9 |  | 110 | 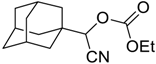 273i | 82 |
| 10 | 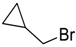 | 110 |  273j | 45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres Domínguez, H.M.; Hernández Villaverde, L.M.; Le Lagadec, R. Recent Advances on O-Ethoxycarbonyl and O-Acyl Protected Cyanohydrins. Molecules 2021, 26, 4691. https://doi.org/10.3390/molecules26154691
Torres Domínguez HM, Hernández Villaverde LM, Le Lagadec R. Recent Advances on O-Ethoxycarbonyl and O-Acyl Protected Cyanohydrins. Molecules. 2021; 26(15):4691. https://doi.org/10.3390/molecules26154691
Chicago/Turabian StyleTorres Domínguez, Héctor Manuel, Luis Mauricio Hernández Villaverde, and Ronan Le Lagadec. 2021. "Recent Advances on O-Ethoxycarbonyl and O-Acyl Protected Cyanohydrins" Molecules 26, no. 15: 4691. https://doi.org/10.3390/molecules26154691
APA StyleTorres Domínguez, H. M., Hernández Villaverde, L. M., & Le Lagadec, R. (2021). Recent Advances on O-Ethoxycarbonyl and O-Acyl Protected Cyanohydrins. Molecules, 26(15), 4691. https://doi.org/10.3390/molecules26154691