Production of Functional Buttermilk and Soymilk Using Pediococcus acidilactici BD16 (alaD+)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pediococcus acidilactici BD16 (alaD+) Strain and Culture Conditions
2.2. Procurement of Buttermilk and Preparation of Soymilk Samples
2.3. Fermentation of Buttermilk and Soymilk Using P. acidilactici BD16 (alaD+)
2.4. Analysis of Bacterial Growth during Fermentation of Buttermilk and Soymilk
2.5. Variation in pH and Milk Consistency during LAB Fermentation
2.6. Estimation of In-Situ L-Alanine Production
2.7. Determination of Physicochemical and Quality Attributes of Buttermilk and Soymilk during the Course of Fermentation
2.7.1. Estimation of Total Solids and Moisture Content
2.7.2. Estimation of Total Protein Concentration and Titratable Acidity
- f = 0.1 N NaOH factor;
- m = volume of test sample (mL)
2.7.3. Estimation of Carbohydrate Content
2.7.4. Estimation of Antioxidant Activity
2.7.5. Estimation of Total Phenolic Content
2.7.6. Estimation of Total Flavonoid Content
2.8. Sensory Evaluation
2.9. GC-MS Based Metabolomic Fingerprinting for Identification of Bioactive and Health-Promoting Compounds
2.9.1. Sample Preparation for GC-MS
2.9.2. GC-MS Analysis
2.10. Statistical Analysis
3. Results
3.1. Production of Functional Buttermilk and Soymilk Using P. acidilactici BD16 (alaD+)
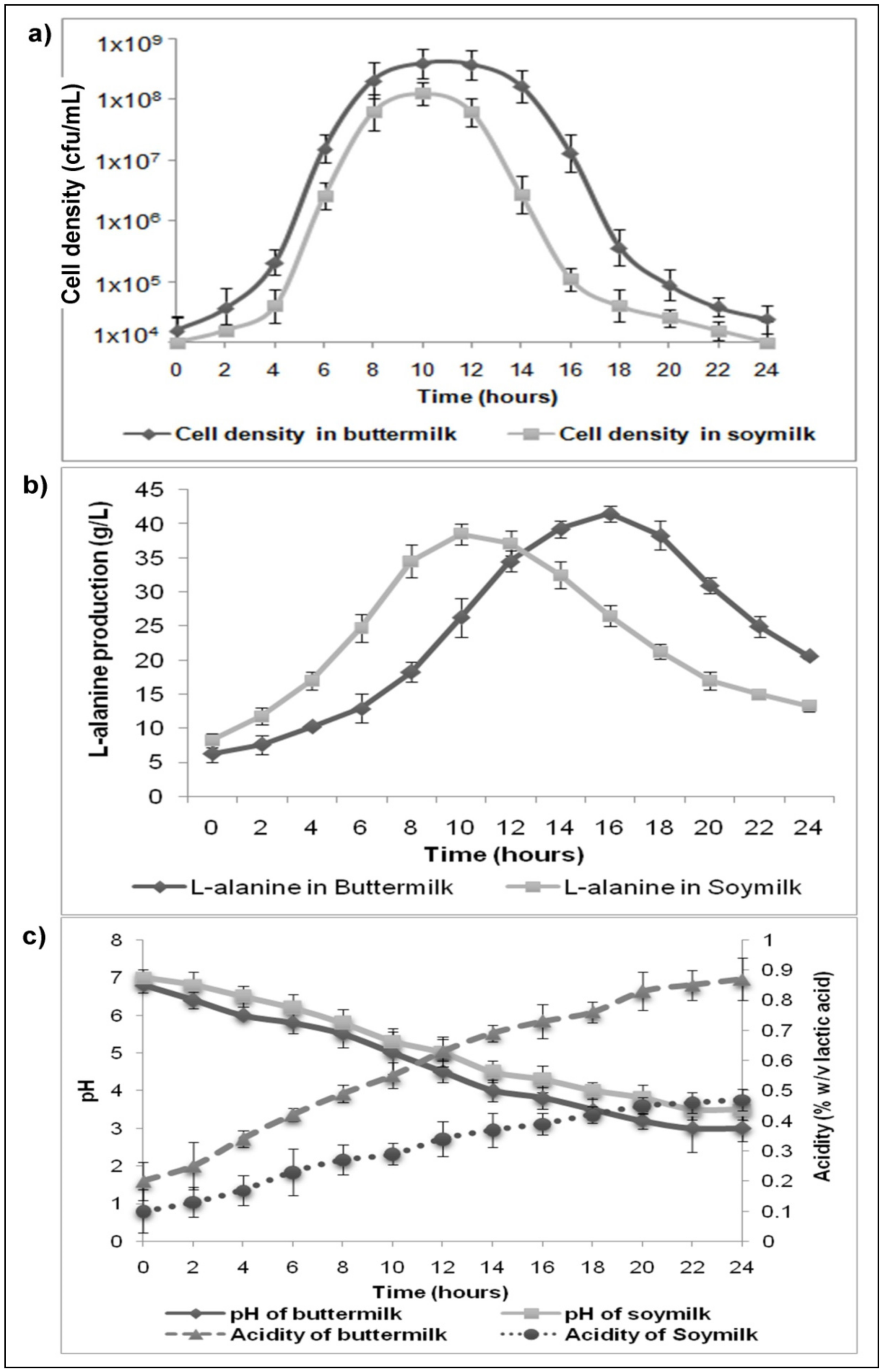
3.2. Analysis of Bacterial Growth during LAB Fermentation
3.3. Variations in pH and Milk Consistency during the Course of Fermentation
3.4. In-Situ L-Alanine Production during Fermentation of Buttermilk and Soymilk
3.5. Physico-Chemical and Quality Attributes of Buttermilk and Soymilk during Fermentation of Buttermilk and Soymilk
3.5.1. Variation in Physicochemical Parameters during the Course of Fermentation
3.5.2. Variation in Protein and Carbohydrate Contents
3.5.3. Variation in Total Phenolic Content
3.5.4. Variation in Antioxidant Activity
3.5.5. Variation in Total Flavonoid Content
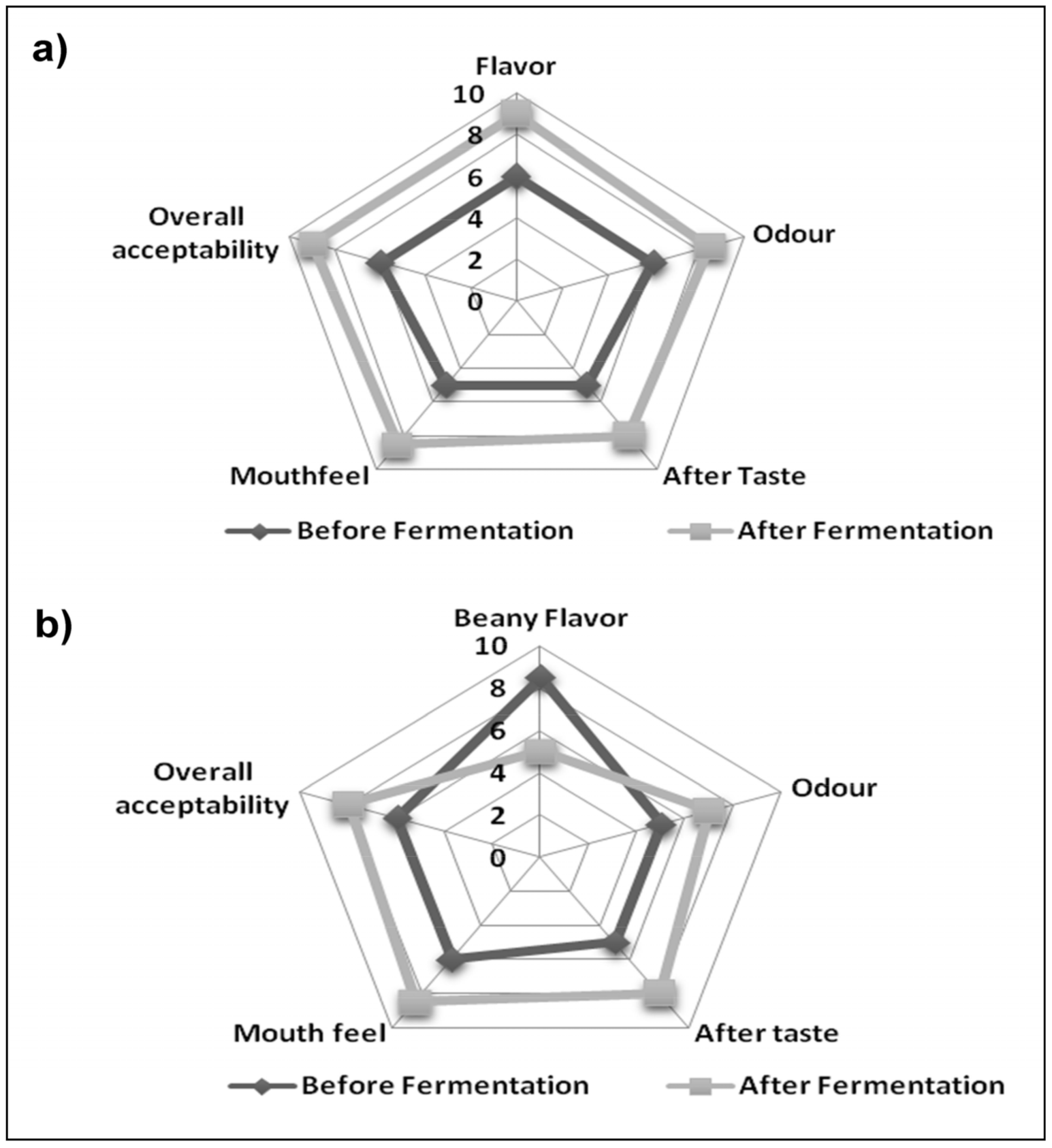
3.6. Comparison of Sensory Characteristics of the Fermented and Unfermented Buttermilk and Soymilk
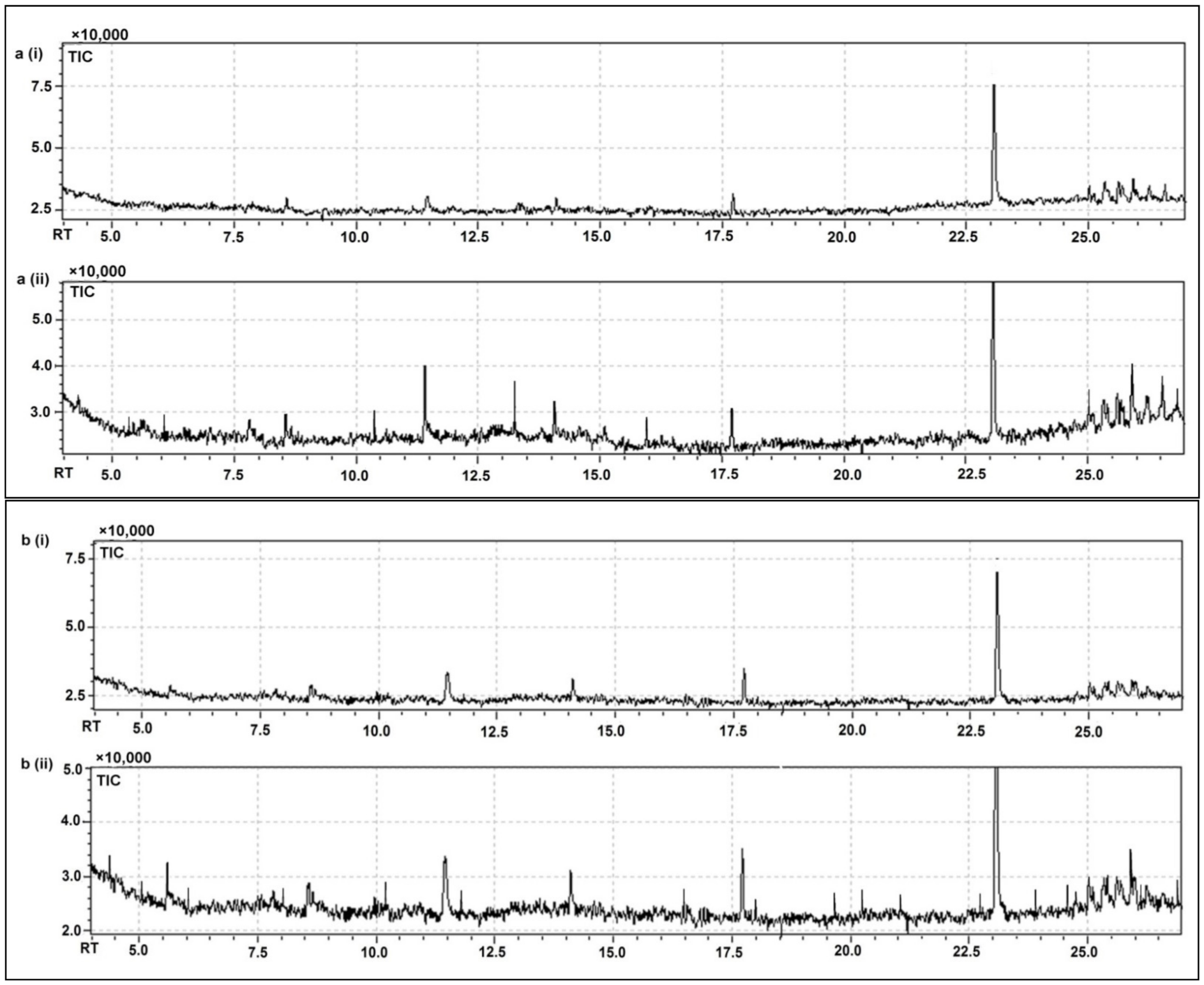
3.7. GC-MS Based Metabolomic Fingerprinting for the Identification of Significant Bioactive and Heath Health-Promoting Compounds
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Bultosa, G. Functional foods: Dietary fibers, prebiotics, probiotics, and synbiotics. Ref. Modul. Food Sci. 2016, 11–16. [Google Scholar] [CrossRef]
- Jiang, S.; Cai, W.; Xu, B. Food quality improvement of soy milk made from short-time germinated soybeans. Foods 2013, 2, 198–212. [Google Scholar] [CrossRef] [Green Version]
- Alghamdi, S.; Migdadi, H.; Khan, M.; El-Harty, E.H.; Ammar, M.; Farooq, M.; Afzal, M. Phytochemical profiling of soybean (Glycine max (L.) Merr.) genotypes using GC-MS Analysis. Phytochem. Source Antioxid. Role Dis. Prev. 2018. [Google Scholar] [CrossRef] [Green Version]
- de Moraes Filho, M.L.; Busanello, M.; Prudencio, S.H.; Garcia, S. Soymilk with okara flour fermented by Lactobacillus acidophilus: Simplex-centroid mixture design applied in the elaboration of probiotic creamy sauce and storage stability. LWT 2018, 93, 339–345. [Google Scholar] [CrossRef]
- Myagmardorj, B.; Purev, M.-E.; Batdorj, B. Functional properties of fermented soymilk by Lactobacillus fermentum BM-325. Mong. J. Chem. 2018, 19, 32–37. [Google Scholar] [CrossRef] [Green Version]
- LeBlanc, J.; Garro, M.S.; Silvestroni, A.; Connes, C.; Piard, J.C.; Sesma, F.; Savoy de Giori, G. Reduction of α-galactooligosaccharides in soyamilk by Lactobacillus fermentum CRL 722: In vitro and in vivo evaluation of fermented soyamilk. J. Appl. Microbiol. 2004, 97, 876–881. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, H.; Pihlanto, A. Food-derived bioactive peptides-opportunities for designing future foods. Curr. Pharm. Des. 2003, 9, 1297–1308. [Google Scholar] [CrossRef] [Green Version]
- Burns, P.; Molinari, F.; Beccaria, A.; Paez, R.; Meinardi, C.; Reinheimer, J.; Vinderola, G. Suitability of buttermilk for fermentation with Lactobacillus helveticus and production of a functional peptide-enriched powder by spray-drying. J. Appl. Microbiol. 2010, 109, 1370–1378. [Google Scholar] [CrossRef]
- Gebreselassie, N.; Abay, F.; Beyene, F. Biochemical and molecular identification and characterization of lactic acid bacteria and yeasts isolated from Ethiopian naturally fermented buttermilk. J. Food Sci. Technol. 2016, 53, 184–196. [Google Scholar] [CrossRef] [Green Version]
- Gascon, M. Masking agents for use in foods. In Modifying Flavour in Food; Woodhead Publishing Limited: Duxford, UK, 2007; pp. 232–242. [Google Scholar]
- Leffingwell, J.C. Flavor-Base 2004-Database; Leffingwell & Associates: Canton, GA, USA, 2004. [Google Scholar]
- Braverman, E.R. The Healing Nutrients Within: Facts, Findings, and New Research on Amino Acids: Easyread Large Bold Edition; Accessible Publishing Systems Pty Ltd.: Sydney, Austraila, 2009. [Google Scholar]
- Brennan, L.; Shine, A.; Hewage, C.; Malthouse, J.P.G.; Brindle, K.M.; McClenaghan, N.; Flatt, P.R.; Newsholme, P. A nuclear magnetic resonance-based demonstration of substantial oxidative L-alanine metabolism and L-alanine-enhanced glucose metabolism in a clonal pancreatic β-cell line: Metabolism of L-alanine is important to the regulation of insulin secretion. Diabetes 2002, 51, 1714–1721. [Google Scholar] [CrossRef] [Green Version]
- Tessem, M.B.; Swanson, M.G.; Keshari, K.R.; Albers, M.J.; Joun, D.; Tabatabai, Z.L.; Simko, J.P.; Shinohara, K.; Nelson, S.J.; Vigneron, D.B. Evaluation of lactate and alanine as metabolic biomarkers of prostate cancer using 1H HR-MAS spectroscopy of biopsy tissues. Magn. Reson. Med. 2008, 60, 510–516. [Google Scholar] [CrossRef] [Green Version]
- Dave, U.C.; Kadeppagari, R.-K. Alanine dehydrogenase and its applications—A review. Crit. Rev. Biotechnol. 2019, 39, 648–664. [Google Scholar] [CrossRef] [PubMed]
- Leuchtenberger, W.; Huthmacher, K.; Drauz, K. Biotechnological production of amino acids and derivatives: Current status and prospects. Appl. Microbiol. Biotechnol. 2005, 69, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Oprea, E.; Ruta, L.L.; Farcasanu, I.C. Pharmacological aspects and health impact of sports and energy drinks. In Sports and Energy Drinks; Woodhead Publishing: Duxford, UK, 2019; pp. 65–129. [Google Scholar]
- Wada, T.; Noda, M.; Kashiwabara, F.; Jeon, H.J.; Shirakawa, A.; Yabu, H.; Matoba, Y.; Kumagai, T.; Sugiyama, M. Characterization of four plasmids harboured in a Lactobacillus brevis strain encoding a novel bacteriocin, brevicin 925A, and construction of a shuttle vector for lactic acid bacteria and Escherichia coli. Microbiology 2009, 155, 1726–1737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noda, M.; Miyauchi, R.; Danshiitsoodol, N.; Matoba, Y.; Kumagai, T.; Sugiyama, M. Expression of Genes Involved in Bacteriocin Production and Self-Resistance in Lactobacillus brevis 174A Is Mediated by Two Regulatory Proteins. Appl. Environ. Microbiol. 2018, 84, e02707-17. [Google Scholar] [CrossRef] [Green Version]
- Kaur, B.; Chakraborty, D.; Kumar, B. Metabolic engineering of Pediococcus acidilactici BD16 for production of vanillin through ferulic acid catabolic pathway and process optimization using response surface methodology. Appl. Microbiol. Biotechnol. 2014, 98, 8539–8551. [Google Scholar] [CrossRef]
- Sebastian, A.; Barus, T.; Mulyono, N.; Yanti. Effects of fermentation and sterilization on quality of soybean milk. Int. Food Res. J. 2018, 25, 2428–2434. [Google Scholar]
- Nurliyani; Harmayani, E.; Sunarti. Microbiological quality, fatty acid and amino acid profiles of kefir produced from combination of goat and soy milk. Pak. J. Nutr. 2014, 13, 107–115. [Google Scholar]
- Shah, S.; Rathod, I.; Kanakia, D. Colorimetry method for estimation of glycine, alanine and isoleucine. Indian J. Pharm. Sci. 2007, 69, 462. [Google Scholar] [CrossRef] [Green Version]
- Shibatani, T.; Kakimoto, T.; Chibata, I. Stimulation of L-asparate beta-decarboxylase formation by L-glutamate in Pseudomonas dacunhae and Improved production of L-alanine. Appl. Environ. Microbiol. 1979, 38, 359–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lowry, O. Protein determination with the phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Kim, D.-M.; Lee, H.; Yoo, S.-H. Compositional changes and physical properties of soymilk prepared with pre-soaked-fermented soybean. J. Korean Soc. Appl. Biol. Chem. 2012, 55, 121–126. [Google Scholar] [CrossRef]
- Layne, E. Spectrophotometric and Turbidimetric Methods for Measuring Proteins; Elsevier: Amsterdam, The Netherlands, 1957. [Google Scholar]
- Mu, P.; Plummer, D.T. Introduction to Practical Biochemistry; Tata McGraw-Hill Education: New York, NY, USA, 2001. [Google Scholar]
- Fakri, E.M.; Lim, S.; Musa, N.; Hasan, M.H.; Adam, A.; Ramasamy, K. Lactobacillus fermentum LAB 9-fermented soymilk with enriched isoflavones and antioxidants improved memory in vivo. Sains Malays. 2016, 45, 1289–1297. [Google Scholar]
- Ma, Y.; Huang, H. Characterisation and comparison of phenols, flavonoids and isoflavones of soymilk and their correlations with antioxidant activity. Int. J. Food Sci. Technol. 2014, 49, 2290–2298. [Google Scholar] [CrossRef]
- Lim, J.; Wood, A.; Green, B.G. Derivation and evaluation of a labeled hedonic scale. Chem. Senses 2009, 34, 739–751. [Google Scholar] [CrossRef] [Green Version]
- Kaur, B.; Kumar, B.; Kaur, G.; Chakraborty, D.; Kaur, K. Application of recombinant Pediococcus acidilactici BD16 (fcs(+)/ech(+)) in malolactic fermentation. Appl. Microbiol. Biotechnol. 2015, 99, 3015–3028. [Google Scholar] [CrossRef]
- Halket, J.M.; Waterman, D.; Przyborowska, A.M.; Patel, R.K.; Fraser, P.D.; Bramley, P.M. Chemical derivatization and mass spectral libraries in metabolic profiling by GC/MS and LC/MS/MS. J. Exp. Bot. 2005, 56, 219–243. [Google Scholar] [CrossRef] [Green Version]
- Mariani, T.J.; Budhraja, V.; Mecham, B.H.; Gu, C.C.; Watson, M.A.; Sadovsky, Y. A variable fold-change threshold determines significance for expression microarrays. FASEB J. 2003, 17, 321–323. [Google Scholar] [CrossRef] [Green Version]
- Obadina, A.O.; Akinola, O.J.; Shittu, T.A.; Bakare, H.A. Effect of natural fermentation on the chemical and nutritional composition of fermented soymilk nono. Niger. Food J. 2013, 31, 91–97. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.R.; Jeong, D.Y.; Cha, Y.S.; Baik, S.H. Exopolysaccharide produced by Pediococcus acidilactici M76 isolated from the Korean traditional rice wine, makgeolli. J. Microbiol. Biotechnol. 2013, 23, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Bansal, P.; Singh, J.; Dhanda, S. Purification, partial structural characterization and health benefits of ex-opolysaccharides from potential probiotic Pediococcus acidilactici NCDC 252. Process. Biochem. 2020, 99, 79–86. [Google Scholar] [CrossRef]
- Korcz, E.; Varga, L. Exopolysaccharides from lactic acid bacteria: Techno-functional application in the food indus-try. Trends Food Sci. Technol. 2021, 110, 375–384. [Google Scholar] [CrossRef]
- Song, Y.-R.; Kim, Y.-E.; Kim, J.-H.; Song, N.-E.; Jeong, D.-Y.; Baik, S.-H. Preparation of fermented sugar-soaked black soybean snacks (FSBSS) and characterization of their quality changes. Food Sci. Biotechnol. 2011, 20, 1547–1553. [Google Scholar] [CrossRef]
- Rekha, C.; Vijayalakshmi, G. Isoflavone phytoestrogens in soymilk fermented with β-glucosidase producing probiotic lactic acid bacteria. Int. J. Food Sci. Nutr. 2011, 62, 111–120. [Google Scholar] [CrossRef]
- Shilpa, V.; Subrota, H.; Deepika, Y. Biofunctionality of probiotic soy yoghurt. Food Nutr. Sci. 2011, 2011, 5819. [Google Scholar]
- Tachakittirungrod, S.; Okonogi, S.; Chowwanapoonpohn, S. Study on antioxidant activity of certain plants in Thailand: Mechanism of antioxidant action of guava leaf extract. Food Chem. 2007, 103, 381–388. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, inflammation and immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Al-Nabulsi, A.; Shaker, R.; Osaili, T.; Al-Taani, M.; Olaimat, A.; Awaisheh, S.; Abushelaibi, A.; Holley, R. Sensory evaluation of flavored soy milk-based yogurt: A comparison between Jordanian and Malaysian consumers. J. Food Sci. Eng. 2014, 4, 27. [Google Scholar]
- Alañón, M.; Pérez-Coello, M.; Marina, M. Wine science in the metabolomics era. TrAC Trends Anal. Chem. 2015, 74, 1–20. [Google Scholar] [CrossRef]
- Ahuja, V.; Macho, M.; Ewe, D.; Singh, M.; Saha, S.; Saurav, K. Biological and Pharmacological Potential of Xylitol: A Molecular Insight of Unique Metabolism. Foods 2020, 9, 1592. [Google Scholar] [CrossRef]
- Belakhdar, G.; Benjouad, A.; Abdennebi, E. Determination of some bioactive chemical constituents from Thesium humile Vahl. J. Mater. Environ. Sci. 2015, 6, 2778–2783. [Google Scholar]
- El-Emary, T.I. Synthesis and Biological Activity of Some New Pyrazolo[3,4-b] Pyrazines. J. Chin. Chem. Soc. 2006, 53, 391–401. [Google Scholar] [CrossRef]
- Elsayed, T.R.; Galil, D.F.; Sedik, M.Z.; Hassan, H.M.; Sadik, M.W. Antimicrobial and Anticancer Activities of Actinomycetes Isolated from Egyptian Soils. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 2020. [Google Scholar] [CrossRef]
- Fajriah, S.; Megawati; Hudiyono, S.; Kosela, S.; Hanafi, M. Chemical constituents and potential cytotoxic activity of n-hexane fraction from Myristica fatua Houtt leaves. In AIP Conference Proceedings; American Institute of Physics: College Park, MD, USA, 2017; p. 030087. [Google Scholar]
- Ganesh, M.; Mohankumar, M. Extraction and identification of bioactive components in Sida cordata (Burm. f.) using gas chromatography–mass spectrometry. J. Food Sci. Technol. 2017, 54, 3082–3091. [Google Scholar] [CrossRef] [PubMed]
- Joseph, D.D.; Veerasamy, K.; Singaram, S.S. Identification of bioactive compounds by gas chromatography-mass spectrometry analysis of Syzygium jambos (L.) collected from Western Ghats region Coimbatore, Tamil Nadu. Asian J. Pharm. Clin. Res. 2016, 10, 364–369. [Google Scholar] [CrossRef] [Green Version]
- Lochner, M.; Thompson, A.J. The muscarinic antagonists scopolamine and atropine are competitive antagonists at 5-HT3 receptors. Neuropharmacology 2016, 108, 220–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madhubala, M.; Santhi, G. Phytochemical and GC-MS analysis on leaves of selected medicinal plants in Boraginaceae family Cordia dichotoma L. Pramana Res. J. 2019, 9, 2249–2276. [Google Scholar]
- PubChem. PubChem Substance and Compound Databases. Available online: https://pubchem.ncbi.nlm.nih.gov (accessed on 17 April 2021).
- Akpuaka, A.; Ekwenchi, M.; Dashak, D.; Dildar, A. Biological activities of characterized isolates of n-hexane extract of Azadirachta indica A. Juss (Neem) leaves. Nat. Sci. 2013, 11, 141–147. [Google Scholar]
- Vijayakumari, J.; Raj, T.L. GC-MS analysis of secondary metabolites from acetone and chloroform extract of Dicranopteris linearis (Burm. F.) Underw. Int. Res. J. Biol. Sci. 2019, 8, 39–43. [Google Scholar]
- Yogeswari, S.; Ramalakshmi, S.; Neelavathy, R.; Muthumary, J. Identification and comparative studies of different volatile fractions from Monochaetia kansensis by GCMS. Glob. J. Pharmacol. 2012, 6, 65–71. [Google Scholar]
- Seebacher, W.; Wolkinger, V.; Faist, J.; Kaiser, M.; Brun, R.; Saf, R.; Bucar, F.; Gröblacher, B.; Brantner, A.; Merino, V. Synthesis of 3-azabicyclo[3.2.2]nonanes and their antiprotozoal activities. Bioorg. Med. Chem. Lett. 2015, 25, 1390–1393. [Google Scholar] [CrossRef]
- Lakshmi, M.; Nair, B.R. GC-MS analysis of the chloroform extract of bark of Terminalia travancorensis Wight & Arn.(Combretaceae). Int. J. Pharm. Sci. Res. 2017, 8, 794. [Google Scholar]
- Premathilaka, U.L.R.R.; Mayumi, G.; Silva, S.W. Bioactive compounds and antioxidant activity of Bunchosia armeniaca. World J. Pharm. Pharm. Sci. 2016, 5, 1237–1247. [Google Scholar]
- Jahan, I.; Tona, M.R.; Sharmin, S.; Sayeed, M.A.; Tania, F.Z.; Paul, A.; Chy, M.; Uddin, N.; Rakib, A.; Emran, T.B. GC-MS phytochemical profiling, pharmacological properties, and in silico studies of Chukrasia velutina leaves: A novel source for bioactive agents. Molecules 2020, 25, 3536. [Google Scholar] [CrossRef]
- Kumari, D.; Bansal, H. Benzohydrazides: As potential bio-active agents. Pharma Innov. J. 2018, 7, 543–550. [Google Scholar]
- Kumari, N.; Menghani, E.; Mithal, R. Bioactive Compounds characterization and Antibacterial Potentials of Actinomycetes isolated from Rhizospheric soil. J. Sci. Ind. Res. 2019, 78, 793–798. [Google Scholar]
- Achi, N.K.; Ohaeri, O. GC-MS determination of bioactive constituents of the methanolic fractions of Cnidoscolus aconitifolius. J. Pharm. Res. Int. 2015, 5, 163–172. [Google Scholar] [CrossRef]
- Wishart, D.S.; Feunang, Y.D.; Marcu, A.; Guo, A.C.; Liang, K.; Vázquez-Fresno, R.; Sajed, T.; Johnson, D.; Li, C.; Karu, N. HMDB 4.0: The human metabolome database for 2018. Nucleic Acids Res. 2018, 46, D608–D617. [Google Scholar] [CrossRef]
- Kavitha, R.; Uduman, M. Identification Of Bioactive Components And Its Biological Activities of Abelmoschas moschatus Flower Extrtact-A Gc-Ms Study. IOSR J. Appl. Chem. 2017, 10, 19–22. [Google Scholar]
- Gunalan, G.; Krishnamurthy, V.; Saraswathy, A. GC-MS and HPTLC fingerprinting of Bauhinia variegata leaves for anticancer activity. World J. Pharm. Res. 2014, 3, 1313–1336. [Google Scholar]
- Kafarski, P. Phosphonates: Their natural occurrence and physiological role. In Contemporary Topics about Phosphorus in Biology and Materials; IntechOpen: London, UK, 2019. [Google Scholar]
- El-Shemy, H.; Shalaby, E.; Lightfoot, D. Potential Use of Secoyohimban Derivatives isolated from Water hyacinth as inhibitors of the COVID-19 Virus. OSF 2020. [Google Scholar] [CrossRef]
- Dickinson, R.; Smith, E.; Franks, N.; Lieb, W. Synthesis and use of the n-bromododecane-1,12-diols as conformational probes for general anesthetic target sites. J. Med. Chem. 1993, 36, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Togashi, N.; Shiraishi, A.; Nishizaka, M.; Matsuoka, K.; Endo, K.; Hamashima, H.; Inoue, Y. Antibacterial activity of long-chain fatty alcohols against Staphylococcus aureus. Molecules 2007, 12, 139–148. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; More, P.; Mohan, S.M. Curry leaves (Murraya koenigii Linn. Sprengal)—A mircale plant. Indian J. Sci. Res. 2014, 4, 46–52. [Google Scholar]
- Raman, B.V.; Samuel, L.; Saradhi, M.P.; Rao, B.N.; Krishna, N.; Sudhakar, M.; Radhakrishnan, T. Antibacterial, antioxidant activity and GC-MS analysis of Eupatorium odoratum. Asian J. Pharm. Clin. Res. 2012, 5, 99–106. [Google Scholar]
- Kaškonienė, V.; Kaškonas, P.; Maruška, A. Volatile compounds composition and antioxidant activity of bee pollen collected in Lithuania. Chem. Pap. 2015, 69, 291–299. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Jain, S.; Sharma, P.C. Thiazolidin-4-one and hydrazone derivatives of capric acid as possible anti-inflammatory, analgesic and hydrogen peroxide-scavenging agents. J. Enzym. Inhib. Med. Chem. 2011, 26, 546–552. [Google Scholar] [CrossRef]
- Božanić, R.; Lovković, S.; Jeličić, I. Optimising fermentation of soymilk with probiotic bacteria. Czech J. Food Sci. 2011, 29, 51–56. [Google Scholar] [CrossRef] [Green Version]
- Ishibashi, N.; Shimamura, S. Bifidobacteria: Research and development in Japan. Food Technol. 1993, 47, 126–136. [Google Scholar]
- Oyedeji, A.B.; Mellem, J.J.; Ijabadeniyi, O.A. Improvement of some quality attributes of soymilk through optimization of selected soybean sprouting parameters using response surface methodology. CyTA-J. Food 2018, 16, 230–237. [Google Scholar] [CrossRef] [Green Version]
- Libudzisz, Z.; Stepaniak, L. Buttermilk. In Encyclopedia of Dairy Sciences; Roginski, H., Fuquay, J.W., Fox, P.F., Eds.; Academic Press: Cambridge, MA, USA, 2003; Volume 2. [Google Scholar]
- Sodini, I.; Morin, P.; Olabi, A.; Jiménez-Flores, R. Compositional and functional properties of buttermilk: A comparison between sweet, sour, and whey buttermilk. J. Dairy Sci. 2006, 89, 525–536. [Google Scholar] [CrossRef] [Green Version]
- Gebreselassie, N.; Abrahamsen, R.K.; Beyene, F.; Abay, F.; Narvhus, J.A. Chemical composition of naturally fermented buttermilk. Int. J. Dairy Technol. 2016, 69, 200–208. [Google Scholar] [CrossRef]
- Ruoff, K.L. Leuconostoc, Pediococcus, Stomatococcus, and miscellaneous gram-positive cocci that grow aerobically. In Manual of Clinical Microbiology, 7th ed.; Murray, P.R., Baron, E.J., Pfaller, M.A., Tenover, F.C., Yolken, R.H., Eds.; American Society for Microbiology: Washington, DC, USA, 1995; pp. 315–323. [Google Scholar]
- Kaur, T.; Balgir, P.P.; Kaur, B. Construction of a shuttle expression vector for lactic acid bacteria. J. Genet. Eng. Biotechnol. 2019, 17, 10. [Google Scholar] [CrossRef]
- Wade, M.E.; Strickland, M.T.; Osborne, J.P.; Edwards, C.G. Role of Pediococcus in winemaking. Aust. J. Grape Wine Res. 2019, 25, 7–24. [Google Scholar] [CrossRef] [Green Version]
- Papagianni, M.; Anastasiadou, S. Pediocins: The bacteriocins of Pediococci. Sources, production, properties and applications. Microb. Cell Fact. 2009, 8, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ricci, A.; Allende, A.; Bolton, D.; Chemaly, M.; Davies, R.; Escámez, P.S.F.; Girones, R.; Koutsoumanis, K.; Lindqvist, R.; Nørrung, B.; et al. Update of the list of QPS-recommended biological agents intentionally added to food or feed as notified to EFSA 8: Suitability of taxonomic units notified to EFSA until March 2018. EFSA J. 2018, 16, 5315. [Google Scholar]
- Kaur, B.; Chakraborty, D. Statistical media and process optimization for biotransformation of rice bran to vanillin using Pediococcus acidilactici. Indian J. Exp. Biol. 2013, 51, 935–943. [Google Scholar] [PubMed]
- Kaur, B.; Chakraborty, D.; Kaur, G.; Kaur, G. Biotransformation of rice bran to ferulic acid by pediococcal isolates. Appl. Biochem. Biotechnol. 2013, 170, 854–867. [Google Scholar] [CrossRef]

| S. No. | Physicochemical Attributes | Buttermilk | Soymilk | ||
|---|---|---|---|---|---|
| Before Fermentation | After Fermentation | Before Fermentation | After Fermentation | ||
| 1. | pH | 6.8 ± 0.15 | 3.0 ± 0.25 | 7.0 ± 0.40 | 3.5 ± 0.20 |
| 2. | Titratable acidity (%) | 0.21 ± 0.02 | 0.87 ± 0.04 | 0.13 ± 0.02 | 0.42 ± 0.03 |
| 3. | Total solids (%) | 2.6 ± 0.1 | 1.8 ± 0.20 | 4.9 ± 0.35 | 2.3 ± 0.25 |
| 4. | Moisture content (%) | 95.6 ± 0.5 | 98.3 ± 0.31 | 93.4 ± 0.26 | 97.6 ± 0.40 |
| 5. | Total carbohydrates (%) | 3.5 ± 0.15 | 2.6 ± 0.35 | 9.18 ± 0.04 | 7.43 ± 0.03 |
| 6. | Total antioxidants (%) | 27.2 ± 0.51 | 67.3 ± 0.41 | 24.3 ± 0.36 | 44.72 ± 0.09 |
| 7. | Total flavonoids (mg/mL) | 3.71 ± 0.29 | 6.19 ± 0.03 | 5.76 ± 0.05 | 7.89 ± 0.10 |
| 8. | Total phenolics (mg/mL) | 5.98 ± 0.16 | 6.25 ± 0.04 | 4.51 ± 0.04 | 7.82 ± 0.05 |
| 9. | Total protein (mg/mL) | 3.06 ± 0.19 | 2.17 ± 0.05 | 6.48 ± 0.06 | 4.21 ± 0.07 |
| 10. | L-alanine (g/L) | 6.75 ± 0.26 | 41.5 ± 0.5 | 8.25 ± 0.18 | 38.5 ± 0.39 |
| S. No. | RT (min) | Compound | Chemical Nature | Occurrence | Biological Activity and Applications | Reference | ||
|---|---|---|---|---|---|---|---|---|
| BF | AF | FC | ||||||
| 1. | 23.06 | 1,2-Benezenedicarboxylic acid | Dicarboxylic acid | + | + | 1.02 | Anti-alzheimer; anti-arthritic; anti-cancer; anti-inflammatory; lipoxygenase inhibitor | [54] |
| 2. | 7.82 | 4-Octen-3-One | Ketone | − | + | − | Flavoring agent | [55] |
| 3. | 17.71 | 1-Acetoxynonadecane | Alkane | + | − | − | Anti-microbial | [56] |
| 4. | 25.90 | 1-Bromoeicosane | Alkane | − | + | − | Anti-cancer; anti-inflammatory | [57] |
| 5. | 25.90 | 1-Bromohexadecane | Alkene | − | + | − | Anti-microbial | [58] |
| 6. | 8.58 | Butafume | Amine | − | + | − | Anti-diabetic; anti-fungal | [55] |
| 7. | 17.70 | 1-Docosene | Alkene | − | + | − | Anti-bacterial; anti-cancer; anti-inflammatory | [49,50] |
| 8. | 8.58 | 1-Dodecene | Alkene | + | + | 1.2 | Imparts pleasant odor | [55] |
| 9. | 8.58 | 8-Azanonane | Amide | − | + | − | Anti-malarial; anti-protozoal; anti-trypanosomal; used for morphine drug synthesis | [59] |
| 10. | 11.45 | 1H-Indole-2-carboxylic acid | Carboxylic acid | − | + | − | Anti-asthmatic; anti-tussive | [52] |
| 11. | 11.40 | 1-Heptadecene | Alkene | + | − | − | Anti-microbial | [60] |
| 12. | 14.07 | 1-Hexadecanol | Fatty alcohol | − | + | − | Anti-microbial | [49] |
| 13. | 11.40 | 1-Hexadecene | Alkene | − | + | − | Anti-microbial | [58] |
| 14. | 8.58 | 1-Nonadecene | Alkene | + | + | 1.04 | Anti-cancer; anti-fungal | [60,61] |
| 15. | 11.40 | 1-Pentadecene | Alkene | − | + | − | - | - |
| 16. | 25.02 | 2-Bromopropionic acid | Carboxylic acid | + | + | − | - | - |
| 17. | 7.82 | 2-Hydroxybenzoic acid | Carboxylic acid | − | + | − | Anti-microbial; food preservative; lipase and lipoxygenase inhibitor | [55] |
| 18. | 8.58 | 2-Aminononadecane | Amino alkane | + | + | 0.9 | Anti-microbial; antioxidant | [55] |
| 19. | 25.02 | 2-Nonyloxirane | - | + | − | − | - | - |
| 20. | 25.02 | 2-Propenyl Decanoate | - | + | + | 0.10 | Anti-microbial; anti-viral | [47] |
| 21. | 25.90 | 2-Undecenoic acid | Fatty acid | − | + | − | Anti-fungal | [55] |
| 22. | 14.10 | 3-Chloropropionic Acid | Chlorocarboxylic acid | + | − | − | Anti-depressant | [62] |
| 23. | 8.58 | 3-Trifluroacetoxytridecane | Alkane | + | − | − | Anti-nephrotoxic; Antioxidant | [51] |
| 24. | 11.40 | 9-Eicosene | - | − | + | − | anti-hyperglycemic; anti-microbial; antioxidant; cytotoxic; Insecticidal | [49] |
| 25. | 11.44 | 9-Octadecene | Alkene | + | + | 1.95 | Analgesic; anti-diabetic; anti-inflammatory; anti-microbial; anti-pyretic; antioxidant; anti-tumor | [49] |
| 26. | 7.82 | Benzaldehyde | Aldehyde | − | + | − | Aromatic and flavoring agent; nitrilase and triacylglycerol lipase inhibitor | [55] |
| 27. | 7.82 | Benzenecarbothioic acid | Organosulfur compound | − | + | − | - | - |
| 28. | 7.82 | Benzo-hydrazide | Heterocyclic compound | − | + | − | Anti-cancer; anti-convulsant; anti-microbial; anti-tubercular | [63] |
| 29. | 7.82 | Benzoic acid | Carboxylic acid | + | + | 1.5 | Anti-microbial; food preservative; lipase and lipoxygenase inhibitor | [55] |
| 30. | 11.44 | Cachalot | Fatty alcohol | − | + | − | Anti-microbial | [49] |
| 31. | 17.70 | Choloroacetic acid | Carboxylic acid | + | + | 1.6 | Herbicide, food preservative | [55] |
| 32. | 25.90 | Colchicine | - | + | + | 1.9 | Anti-gout; anti-inflammatory; inhibits urate crystallization in joints | [55] |
| 33. | 14.07 | Cyclohexadecane | Alkane | + | + | 1.8 | Anti-microbial | [64] |
| 34. | 11.40 | Cyclotetradecane | Cycloalkane | − | + | − | Anti-diuretic; Anti-microbial | [65] |
| 35. | 25.91 | Cysteamine | Amine/Aminothiol | + | − | − | Anti-nephropathic; radioprotective | [55] |
| 36. | 25.90 | Decanoic acid | - | + | + | − | - | - |
| 37. | 14.07 | Dichloroacetic acid | Carboxylic acid, Organochlorine compound | − | + | − | Anti-cancer;pyruvatedehydrogenase kinase inhibitor | [55] |
| 38. | 23.06 | Diisooctyl phthalate | Carboxylic acid ester | − | + | − | Anti-microbial; anti-fouling | [64] |
| 39. | 17.70 | Dioctadecyl Phosphonate | - | − | + | − | - | - |
| 40. | 25.56 | D-Xylitol | Sugar alcohol | + | − | − | Low- calorie sweetener; anti-cancer; anti-diabetic; anti-inflammatory; anti-microbial | [46] |
| 41. | 7.825 | Ethanone | - | − | + | − | - | - |
| 42. | 8.58 | Fluoroacetic Acid | Organofluorine compound/haloacetic acid. | + | − | − | Aconitatehydratase inhibitor | [55] |
| 43. | 25.24 | Glucitol | Sugar alcohol | + | − | − | Low calorie sweetener; diuretic; laxative; cathartic | [55] |
| 44. | 17.71 | Formic acid | Carboxylic acid | + | + | −0.12 | Anti-bacterial; | [66] |
| 45. | 25.90 | Glucopyranoside | - | + | + | − | - | - |
| 46. | 11.40 | Heptadecanenitrile | Organic compound | + | + | 1.58 | Anti-bacterial; anti-cancer; antioxidant | [67] |
| 47. | 14.07 | Heptadecyl acetate | - | − | + | − | Anti-microbial | [49] |
| 48. | 25.02 | Hexadecane | Alkane | − | + | − | Anti-bacterial | [64] |
| 49. | 25.91 | Isochipane B | - | + | − | − | Anti-bacterial | [49] |
| 50. | 25.91 | Lyxitol | Sugar alcohol | + | − | − | Anti-fungal | [66] |
| 51. | 8.58 | Heptacosanol | Alcohol | − | + | − | Anti-inflammatory; Anti-thrombotic; cholesterol lowering drug | [68] |
| 52. | 23.06 | Pyrazinediyl | Heterocyclic compound | − | + | − | Anti-allergic; Anti-cancer; anti-inflammatory; anti-microbial; flavoring agent | [48] |
| 53. | 25.90 | Pentadecanoic acid methyl ester | + | + | − | − | Anti-microbial | [47] |
| 54. | 14.07 | Phosphonic acid | Organophosphorus compound | + | + | 2.2 | Anti-microbial; anti-viral; used in nuclear medicine | [69] |
| 55. | 7.825 | Propionic acid | Carboxylic acid | + | + | −0.01 | Anti-fungal; imparts unpleasant flavor | [55] |
| 56. | 25.90 | Quercetin | Flavonoid | + | + | 1.04 | Anti-allergic; anti-bacterial; anti-inflammatory; antioxidant; anti-neoplastic; anti-rheumatic;anti-viral; cytotoxic; aurora kinase, lipoxygenase, cyclooxygenase and protein kinase inhibitor; cardioprotective; cholesterol lowering drug | [43,55] |
| 57. | 25.90 | Scopolamine- M | - | − | + | − | Anti-cholinergic; anti-inflammatory; gastro-protective; improves CNS functioning | [53] |
| 58. | 25.02 | Secoyohimban | - | + | − | − | Anti-cancer; antioxidant; anti-microbial | [70] |
| 59. | 25.90 | Triacontane | Alkane | + | + | Anti-bacterial; Anti-diabetic; Anti-tumor | [64] | |
| 60. | 17.71 | Tricosyl Acetate | - | − | + | − | - | - |
| S. No. | RT (min) | Compound | Chemical Nature | Occurrence | Biological Activity and Applications | Reference | ||
|---|---|---|---|---|---|---|---|---|
| BF | AF | FC | ||||||
| 1. | 23.06 | 1,2-Benzenedicarboxylic Acid | Dicarboxylic acid | + | + | 0.8 | Anti-alzheimer; anti-arthritic; anti-cancer; anti-inflammatory; lipoxygenase inhibitor | [54] |
| 2. | 25.61 | 1-Bromododecane | Organobromine compound | + | − | − | Anesthetic | [71] |
| 3. | 25.02 | 1-Bromoeicosane | Alkane | − | + | − | Anti-cancer; Anti-inflammatory | [57] |
| 4. | 25.91 | 1-Decanol | Fatty alcohol | − | + | − | Anti-bacterial; drug delivery agent | [72] |
| 5. | 17.71 | 1-Docosene | Alkene | + | − | − | Anti-bacterial; anti-cancer; anti-inflammatory | [49,50] |
| 6. | 11.45 | 1-Hexadecanol | Fatty alcohol | − | + | − | Anti-microbial | [49] |
| 7. | 11.45 | 1-Hexadecene | Alkene | + | + | −0.12 | Anti-microbial | [58] |
| 8. | 14.12 | 1-Nonadecane | Alkane | + | + | −0.10 | Anti-microbial | [56] |
| 9. | 17.78 | 1-Octadecanol | Fatty alcohol | − | + | − | Anti-bacterial; antioxidant | [58] |
| 10. | 25.91 | 1-Octanamine | Amine | + | + | 1.3 | Anti-microbial | [73] |
| 11. | 25.02 | 1-Octanol | Fatty alcohol | + | − | − | Anti-microbial | [72] |
| 12. | 14.12 | 1-Pentadecanol | Fatty alcohol | − | + | − | Anti-microbial | [72] |
| 13. | 11.45 | 1-Pentadecene | Alkene | + | + | −0.5 | - | - |
| 14. | 14.12 | 1-Tetradecyl acetate | Ester | − | + | − | - | - |
| 15. | 17.71 | 1-Tricosanol | Alcohol | + | − | − | Anti-microbial | [74] |
| 16. | 11.45 | 1-Tridecene | Alkene | + | + | 1.4 | Antioxidant | [75] |
| 17. | 11.45 | 2-Aminononadecane | Amino alkane | + | + | 0.7 | Anti-microbial; antioxidant | [55] |
| 18. | 14.12 | 2-Bromopropionic acid | Carboxylic acid | + | − | − | - | - |
| 19. | 25.02 | 2-Propenyl decanoate | - | + | + | 1.2 | Anti-microbial; anti-viral | [47] |
| 20. | 11.45 | 3-Chloropropionic acid | Chlorocarboxylic acid | + | + | 1.5 | Anti-depressant | [62] |
| 21. | 17.71 | 3-Eicosene | - | − | + | − | Anti-microbial; antioxidant; anti-hyperglycemic; cytotoxic; insecticidal | [49] |
| 22. | 11.45 | 3-Octadecene | Alkene | + | + | 2.05 | Analgesic; anti-diabetic; anti-inflammatory; anti-microbial; antioxidant; anti-pyretic; anti-tumor | [49] |
| 23. | 11.45 | 3-Trifluoracetoxypentadecane | Alkane | + | + | 0.78 | Antioxidant | [51] |
| 24. | 11.45 | 8-Heptadecene | Alkene | + | − | − | Anti-microbial | [60] |
| 25. | 14.10 | 9,12,15-Octadecadienoic acid | Fatty acid | − | + | − | Anti-acne; anti-androgenic; anti-arthritic; anti-cancer; anti-histaminic; anti-inflammatory; anti-eczemic; cardio-protective; hepatoprotective; 5-alpha reductase inhibitor | [51] |
| 26. | 11.45 | 9-Octadecene | Alkene | + | + | 1.56 | Anti-bacterial; anti-cancer; antioxidant | [47] |
| 27. | 14.12 | Acetic acid | Carboxylic acid | + | + | 1.10 | Anti-microbial; food acidity regulator; aromatic and flavor compound; food preservative | [55] |
| 28. | 25.02 | Azanonane | Amide | + | + | 0.97 | Anti-malarial; anti-protozoan; anti-trypanosomal; used for morphine drug synthesis | [59] |
| 29. | 23.06 | Benzoic acid | Carboxylic acid | + | − | − | Anti-microbial; food preservative; lipase and lipoxygenase inhibitor | [55] |
| 30. | 17.71 | Cachalot | Fatty alcohol | + | − | − | Anti-microbial | [49] |
| 31. | 11.45 | Chloroacetic acid | Carboxylic acid | + | + | −0.8 | Anti-microbial; herbicide | [55] |
| 32. | 14.12 | Crodacol | Fatty alcohol | + | + | 1.1 | Used as ingredient for ophthalmic, rectal and vaginal preparations | [55] |
| 33. | 17.74 | Cyclohexadecane | Alkane | + | + | 0.75 | Anti-microbial | [64] |
| 34. | 17.71 | Cyclotetracosane | - | + | − | − | - | - |
| 35. | 14.12 | Cyclotetradecane | Cycloalkane | − | + | − | Anti-diuretic; anti-microbial | [65] |
| 36. | 25.91 | Decanohydrazide | - | + | + | 1.3 | Anti-bacterial; Anti-convulsant; Anti-inflammatory; Anti-protozoal; tuberculostatic | [76] |
| 37. | 25.91 | Decanoic acid | - | + | − | − | - | - |
| 38. | 14.12 | Dichloroacetic acid | Carboxylic acid, Organochlorine compound | + | + | 1.4 | Anti-cancer; pyruvatedehydrogenase kinase inhibitor | [55] |
| 39. | 23.06 | Diisooctylphthalate | Carboxylic acid ester | − | + | − | Anti-microbial; anti-fouling | [64] |
| 40. | 25.34 | D-Xylitol | Sugar alcohol | − | + | − | Low calorie sweetener; anti-cancer; anti-diabetic; anti-inflammatory; anti-microbial | [46] |
| 41. | 11.45 | Eicosane | - | − | + | − | Anti-microbial; anti-tumor; cytotoxic | [47] |
| 42. | 25.61 | Eicosenoic acid | Fatty acid | − | + | − | - | - |
| 43. | 25.34 | Glucitol | Sugar alcohol | − | + | − | Low calorie sweetener; diuretic; laxative; cathartic | [55] |
| 44. | 25.02 | Glucopyranoside | - | − | + | − | - | - |
| 45. | 11.45 | Heptadecanenitrile | Organic compound | + | + | 0.8 | Anti-bacterial; anti-cancer; antioxidant | [67] |
| 46. | 17.74 | Heptadecylactetate | - | − | + | − | Anti-microbial | [49] |
| 47. | 25.91 | Hexadecane | Alkane | − | + | − | Anti-bacterial | [64] |
| 48. | 11.45 | Hexadecenal | Fatty aldehyde | + | + | 1.11 | Anti-microbial; Anti-viral; promotes apoptosis | [52] |
| 49. | 11.45 | Hexadecanoic acid, methyl ester | Ester | − | + | − | Antioxidant; hypocholesterolemic; nematicide; flavor compound | [3] |
| 50. | 25.61 | Hexadecylpyridinium bromide | Pyridinium salt | + | + | 0.78 | Antiseptic; surfactant; myosin-light-chain kinase inhibitor | [55] |
| 51. | 25.91 | IdeuteroOctadecanal | Fatty Aldehyde | − | + | − | - | - |
| 52. | 25.34 | Lyxitol | Sugar alcohol | + | + | 1.9 | Anti-fungal | [66] |
| 53. | 25.02 | Octadecatrieonic acid | Fatty acid | + | + | 1.2 | Anti-cancer; anti-inflammatory; hypocholesterolemic; hepatoprotective | [52] |
| 54. | 11.45 | Phosphonic acid | Organophosphorus compound | + | + | 1.3 | Anti-microbial; anti-viral; used in nuclear medicine | [69] |
| 55. | 25.61 | Propenyl decanoate | - | − | + | − | - | - |
| 56. | 25.02 | Quercetin | Flavonoid | − | + | − | Anti-allergic; anti-bacterial; anti-inflammatory; antioxidant; anti-neoplastic; anti-rheumatic; anti-viral; cytotoxic; aurora kinase, lipoxygenase, cyclooxygenase and protein kinase inhibitor; cardioprotective; cholesterol lowering drug | [43,55] |
| 57. | 25.34 | Rhamnitol | Carbohydrate | − | + | − | - | - |
| 58. | 25.61 | Scopolamine-M | - | − | + | − | Anti-cholinergic; anti-inflammatory; gastro protective; improves CNS functioning | [53] |
| 59. | 25.91 | Triacontane | Alkane | + | + | 1.08 | Anti-bacterial; anti-diabetic; anti-tumor | [64] |
| 60. | 25.91 | Vinyl decanoate | Fatty acid ester | + | − | − | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, A.; Noda, M.; Sugiyama, M.; Ahmad, A.; Kaur, B. Production of Functional Buttermilk and Soymilk Using Pediococcus acidilactici BD16 (alaD+). Molecules 2021, 26, 4671. https://doi.org/10.3390/molecules26154671
Sharma A, Noda M, Sugiyama M, Ahmad A, Kaur B. Production of Functional Buttermilk and Soymilk Using Pediococcus acidilactici BD16 (alaD+). Molecules. 2021; 26(15):4671. https://doi.org/10.3390/molecules26154671
Chicago/Turabian StyleSharma, Anshula, Masafumi Noda, Masanori Sugiyama, Ajaz Ahmad, and Baljinder Kaur. 2021. "Production of Functional Buttermilk and Soymilk Using Pediococcus acidilactici BD16 (alaD+)" Molecules 26, no. 15: 4671. https://doi.org/10.3390/molecules26154671
APA StyleSharma, A., Noda, M., Sugiyama, M., Ahmad, A., & Kaur, B. (2021). Production of Functional Buttermilk and Soymilk Using Pediococcus acidilactici BD16 (alaD+). Molecules, 26(15), 4671. https://doi.org/10.3390/molecules26154671





