Proteomics Analysis Reveals Altered Nutrients in the Whey Proteins of Dairy Cow Milk with Different Thermal Treatments
Abstract
1. Introduction
2. Results and Discussion
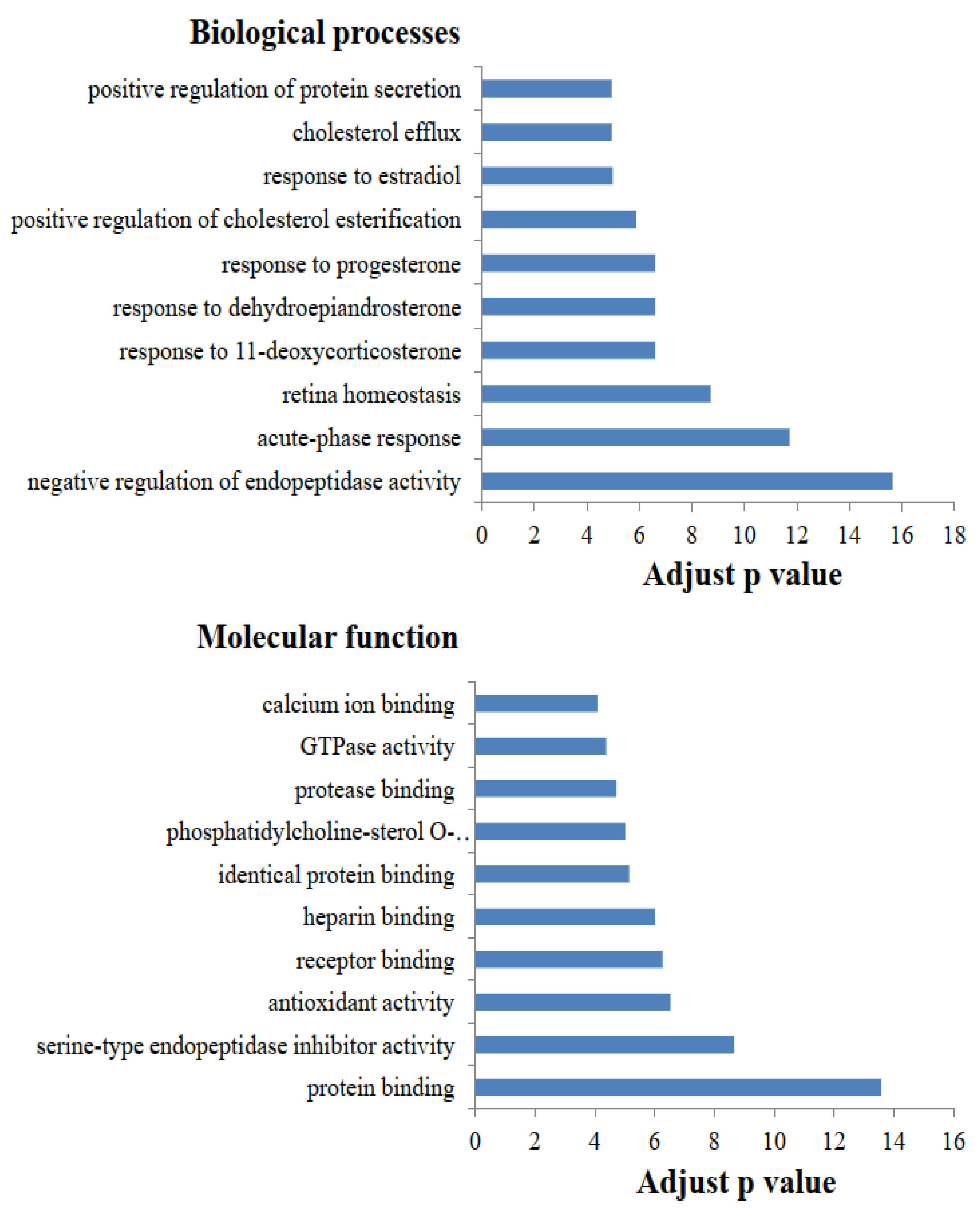
2.1. Functional Category Analysis of Identified Proteins
2.2. Heat Sensitivity in Whey Proteins after Different Thermal Treatments
3. Materials and Methods
3.1. Milk Sampling and Pre-Treatment
3.2. Proteomics Treatment and Analysis
3.3. Data Processing, Protein Identification, and Bioinformatic Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Milkovska-Stamenova, S.; Hoffmann, R. Identification and quantification of bovine protein lactosylation sites in different milk products. J. Proteom. 2016, 134, 112–126. [Google Scholar] [CrossRef] [PubMed]
- Claeys, W.L.; Cardoen, S.; Daube, G.; De Block, J.; Dewettinck, K.; Dierick, K.; de Zutter, L.; Huyghebaert, A.; Imberechts, H.; Thiange, P.; et al. Raw or heated cow milk consumption: Review of risks and benefits. Food Control 2013, 31, 251–262. [Google Scholar] [CrossRef]
- Pizzano, R.; Manzo, C.; Nicolai, M.A.; Addeo, F. Occurrence of major whey proteins in the pH 4.6 insoluble protein fraction from UHT-treated milk. J. Agric. Food Chem. 2012, 60, 8044–8050. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Van Boekel, M.A.J.S. Effect of heating on Maillard reactions in milk. Food Chem. 1998, 62, 403–414. [Google Scholar] [CrossRef]
- Cappozzo, J.C.; Koutchma, T.; Barnes, G. Chemical characterization of milk after treatment with thermal (HTST and UHT) and nonthermal (turbulent flow ultraviolet) processing technologies. J. Dairy Sci. 2015, 98, 5068–5079. [Google Scholar] [CrossRef]
- Halabi, A.; Croguennec, T.; Bouhallab, S.; Dupont, D.; Deglaire, A. Modification of protein structures by altering the whey protein profile and heat treatment affects in vitro static digestion of model infant milk formulas. Food Funct. 2020, 11, 6933–6945. [Google Scholar] [CrossRef]
- Krissansen, G.W. Emerging Health Properties of Whey Proteins and Their Clinical Implications. J. Am. Coll. Nutr. 2007, 26, 713S–723S. [Google Scholar] [CrossRef] [PubMed]
- Akkerman, M.; Rauh, V.M.; Christensen, M.; Johansen, L.B.; Hammershøj, M.; Larsen, L.B. Effect of heating strategies on whey protein denaturation—Revisited by liquid chromatography quadrupole time-of-flight mass spectrometry. J. Dairy Sci. 2016, 99, 152–166. [Google Scholar] [CrossRef]
- Adjonu, R.; Doran, G.; Torley, P.; Agboola, S. Screening of whey protein isolate hydrolysates for their dual functionality: Influence of heat pre-treatment and enzyme specificity. Food Chem. 2013, 136, 1435–1443. [Google Scholar] [CrossRef]
- O’Loughlin, I.; Murray, B.; FitzGerald, R.; Brodkorb, A.; Kelly, P. Pilot-scale production of hydrolysates with altered bio-functionalities based on thermally-denatured whey protein isolate. Int. Dairy J. 2014, 34, 146–152. [Google Scholar] [CrossRef]
- Laleye, L.C.; Jobe, B.; Wasesa, A.A. Comparative study on heat stability and functionality of camel and bovine milk whey proteins. J. Dairy Sci. 2008, 91, 4527–4534. [Google Scholar] [CrossRef]
- Laiho, S.; Ercili-Cura, D.; Forssell, P.; Myllärinen, P.; Partanen, R. The effect of dynamic heat treatments of native whey protein concentrate on its dispersion characteristics. Int. Dairy J. 2015, 49, 139–147. [Google Scholar] [CrossRef]
- Brick, T.; Ege, M.; Boeren, S.; Böck, A.; Von Mutius, E.; Vervoort, J.; Hettinga, K. Effect of Processing Intensity on Immunologically Active Bovine Milk Serum Proteins. Nutrients 2017, 9, 963. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Bu, D.; Zhao, X.; Sun, P.; Wang, J.; Zhou, L. Proteomic analysis of cow, yak, buffalo, goat and camel milk whey proteins: Quantitative differential expression patterns. J. Proteome Res. 2013, 12, 1660–1667. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Boeren, S.; Smits, M.; van Hooijdonk, T.; Vervoort, J.; Hettinga, K. Proteomic study on the stability of proteins in bovine, camel, and caprine milk sera after processing. Food Res. Int. 2016, 82, 104–111. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, C.; Sun, X.; Guo, M. Comparative Proteomics of Whey and Milk Fat Globule Membrane Proteins of Guanzhong Goat and Holstein Cow Mature Milk. J. Food Sci. 2019, 84, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Yang, M.; Yang, N.; Liang, X.; Tao, D.; Liu, B.; Wu, J.; Yue, X. Characterization and comparison of whey N-glycoproteomes from human and bovine colostrum and mature milk. Food Chem. 2019, 276, 266–273. [Google Scholar] [CrossRef]
- Yang, M.; Cao, X.; Wu, R.; Liu, B.; Ye, W.; Yue, X.; Wu, J. Comparative proteomic exploration of whey proteins in human and bovine colostrum and mature milk using iTRAQ-coupled LC-MS/MS. Int. J. Food Sci. Nutr. 2017, 68, 671–681. [Google Scholar] [CrossRef]
- Saint-Sauveur, D.; Gauthier, S.F.; Boutin, Y.; Montoni, A. Immunomodulating properties of a whey protein isolate, its enzymatic digest and peptide fractions. Int. Dairy J. 2008, 18, 260–270. [Google Scholar] [CrossRef]
- Iozzo, R.V.; Sanderson, R.D. Proteoglycans in cancer biology, tumour microenvironment and angiogenesis. J. Cell. Mol. Med. 2011, 15, 1013–1031. [Google Scholar] [CrossRef]
- De La Torre Gomez, C.; Goreham, R.V.; Bech Serra, J.J.; Nann, T.; Kussmann, M. “Exosomics”-A Review of Biophysics, Biology and Biochemistry of Exosomes with a Focus on Human Breast Milk. Front. Genet. 2018, 9, 92. [Google Scholar] [CrossRef]
- Grewal, M.K.; Huppertz, T.; Vasiljevic, T. FTIR fingerprinting of structural changes of milk proteins induced by heat treatment, deamidation and dephosphorylation. Food Hydrocoll. 2018, 80, 160–167. [Google Scholar] [CrossRef]
- Hernández-Castellano, L.E.; Almeida, A.M.; Renaut, J.; Argüello, A.; Castro, N. A proteomics study of colostrum and milk from the two major small ruminant dairy breeds from the Canary Islands: A bovine milk comparison perspective. J. Dairy Res. 2016, 83, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Varghese, P.M.; Murugaiah, V.; Beirag, N.; Temperton, N.; Khan, H.A.; Alrokayan, S.H.; Al-Ahdal, M.N.; Nal, B.; Al-Mohanna, F.A.; Sim, R.B.; et al. C4b Binding Protein Acts as an Innate Immune Effector Against Influenza A Virus. Front. Immunol. 2021, 11, 585361. [Google Scholar] [CrossRef]
- Chen, J.-W.; Scaria, J.; Mao, C.; Sobral, B.; Zhang, S.; Lawley, T.; Chang, Y.-F. Proteomic Comparison of Historic and Recently Emerged Hypervirulent Clostridium difficile Strains. J. Proteome Res. 2013, 12, 1151–1161. [Google Scholar] [CrossRef]
- Yang, Y.; Qiang, X.; Owsiany, K.; Zhang, S.; Thannhauser, T.W.; Li, L. Evaluation of Different Multidimensional LC–MS/MS Pipelines for Isobaric Tags for Relative and Absolute Quantitation (iTRAQ)-Based Proteomic Analysis of Potato Tubers in Response to Cold Storage. J. Proteome Res. 2011, 10, 4647–4660. [Google Scholar] [CrossRef]
- Qin, L.; Walk, T.C.; Han, P.; Chen, L.; Zhang, S.; Li, Y.; Hu, X.; Xie, L.; Yang, Y.; Liu, J.; et al. Adaption of Roots to Nitrogen Deficiency Revealed by 3D Quantification and Proteomic Analysis. Plant Physiol. 2019, 179, 329. [Google Scholar] [CrossRef]
- Kahl, A.; Blanco, I.; Jackman, K.; Baskar, J.; Milaganur Mohan, H.; Rodney-Sandy, R.; Zhang, S.; Iadecola, C.; Hochrainer, K. Cerebral ischemia induces the aggregation of proteins linked to neurodegenerative diseases. Sci. Rep. 2018, 8, 2701. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Yin, Z.; Wu, Y.; Xu, L.; Du, H.; Wang, N.; Huang, L. LaeA Controls Virulence and Secondary Metabolism in Apple Canker Pathogen Valsa mali. Front. Microbiol. 2020, 11, 2693. [Google Scholar] [CrossRef]
- Zhang, H.; Jiang, H.; Fan, Y.; Chen, Z.; Li, M.; Mao, Y.; Karrow, N.A.; Loor, J.J.; Moore, S.; Yang, Z. Transcriptomics and iTRAQ-Proteomics Analyses of Bovine Mammary Tissue with Streptococcus agalactiae-Induced Mastitis. J. Agric. Food Chem. 2018, 66, 11188–11196. [Google Scholar] [CrossRef]
| KEGG Pathway Name | Protein Name | UHT/Pasteurization Fold Change |
|---|---|---|
| Antigen processing and presentation | Protein disulfide-isomerase A3 precursor | 1.29 |
| Cathepsin L1 precursor | 1.31 | |
| Beta-2-microglobulin | 1.23 | |
| Lysosome | Cathepsin L1 precursor | 1.31 |
| Prosaposin precursor | 0.82 | |
| Cathepsin D precursor | 0.81 | |
| Phagosome | Cathepsin L1 precursor | 1.31 |
| Ras-related C3 botulinum toxin substrate 1 precursor | 1.21 | |
| Monocyte differentiation antigen CD14 precursor | 0.78 | |
| Proteoglycans in cancer | Cathepsin L1 precursor | 1.31 |
| Ras-related C3 botulinum toxin substrate 1 precursor | 1.21 | |
| Metalloproteinase inhibitor 3 precursor | 0.83 |
| Protein Name | UHT/Pasteurization Fold Change |
|---|---|
| Alpha-S2-casein precursor | 0.70 |
| Renin receptor precursor | 0.74 |
| Solute carrier family 28 member 3 isoform X1 | 0.74 |
| Ig heavy chain Mem5-like, partial | 0.78 |
| Monocyte differentiation antigen CD14 precursor | 0.78 |
| Ras-related protein Rab-18 | 0.79 |
| Protein kinase C-binding protein NELL2 precursor | 0.81 |
| Cathepsin D precursor | 0.81 |
| Prosaposin precursor | 0.82 |
| Ras-related protein Rab-11A | 0.82 |
| C4b-binding protein alpha chain isoform X6 | 0.82 |
| Alpha-S1-casein isoform X5 | 0.82 |
| Metalloproteinase inhibitor 3 precursor | 0.83 |
| Cysteine-rich secretory protein 3 precursor | 0.83 |
| Alpha-enolase isoform X1 | 0.83 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Min, L.; Zhang, S.; Zheng, N.; Li, D.; Sun, Z.; Wang, J. Proteomics Analysis Reveals Altered Nutrients in the Whey Proteins of Dairy Cow Milk with Different Thermal Treatments. Molecules 2021, 26, 4628. https://doi.org/10.3390/molecules26154628
Zhang Y, Min L, Zhang S, Zheng N, Li D, Sun Z, Wang J. Proteomics Analysis Reveals Altered Nutrients in the Whey Proteins of Dairy Cow Milk with Different Thermal Treatments. Molecules. 2021; 26(15):4628. https://doi.org/10.3390/molecules26154628
Chicago/Turabian StyleZhang, Yangdong, Li Min, Sheng Zhang, Nan Zheng, Dagang Li, Zhihua Sun, and Jiaqi Wang. 2021. "Proteomics Analysis Reveals Altered Nutrients in the Whey Proteins of Dairy Cow Milk with Different Thermal Treatments" Molecules 26, no. 15: 4628. https://doi.org/10.3390/molecules26154628
APA StyleZhang, Y., Min, L., Zhang, S., Zheng, N., Li, D., Sun, Z., & Wang, J. (2021). Proteomics Analysis Reveals Altered Nutrients in the Whey Proteins of Dairy Cow Milk with Different Thermal Treatments. Molecules, 26(15), 4628. https://doi.org/10.3390/molecules26154628










