A Review on the Degradation of Pollutants by Fenton-Like Systems Based on Zero-Valent Iron and Persulfate: Effects of Reduction Potentials, pH, and Anions Occurring in Waste Waters
Abstract
:1. Introduction
2. Reactive Species: •OH vs. SO4•− vs. •OH + SO4•−
3. Degradation of Pollutants by ZVI-Fenton/Persulfate
3.1. Effect of pH
3.2. Effect of Inorganic Anions
3.2.1. Chloride
3.2.2. Nitrate/Nitrite
3.2.3. Carbonate/Bicarbonate
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ghanbari, F.; Moradi, M.; Manshouri, M. Textile wastewater decolorization by zero valent iron activated peroxymonosulfate: Compared with zero valent copper. J. Environ. Chem. Eng. 2014, 2, 1846–1851. [Google Scholar] [CrossRef]
- Anipsitakis, G.P.; Dionysiou, D.D. Degradation of Organic Contaminants in Water with Sulfate Radicals Generated by the Conjunction of Peroxymonosulfate with Cobalt. Environ. Sci. Technol. 2003, 37, 4790–4797. [Google Scholar] [CrossRef]
- Pignatello, J.J.; Oliveros, E.; Mackay, A. Advanced Oxidation Processes for Organic Contaminant Destruction Based on the Fenton Reaction and Related Chemistry. Crit. Rev. Environ. Sci. Technol. 2006, 36, 1–84. [Google Scholar] [CrossRef]
- Bokare, A.D.; Choi, W. Review of iron-free Fenton-like systems for activating H2O2 in advanced oxidation processes. J. Hazard. Mater. 2014, 275, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, F.; Vione, D. Effect of pH on Zero Valent Iron Performance in Heterogeneous Fenton and Fenton-Like Processes: A Review. Molecules 2018, 23, 3127. [Google Scholar] [CrossRef] [Green Version]
- Weng, C.-H.; Lin, Y.-T.; Chang, C.-K.; Liu, N. Decolourization of direct blue 15 by Fenton/ultrasonic process using a zero-valent iron aggregate catalyst. Ultrason. Sonochem. 2013, 20, 970–977. [Google Scholar] [CrossRef] [PubMed]
- Bogacki, J.; Marcinowski, P.; Zapałowska, E.; Maksymiec, J.; Naumczyk, J. Cosmetic wastewater treatment by the ZVI/H2O2 process. Environ. Technol. 2016, 38, 2589–2600. [Google Scholar] [CrossRef]
- Grčić, I.; Papic, S.; Žižek, K.; Koprivanac, N. Zero-valent iron (ZVI) Fenton oxidation of reactive dye wastewater under UV-C and solar irradiation. Chem. Eng. J. 2012, 195–196, 77–90. [Google Scholar] [CrossRef]
- Pouran, S.R.; Raman, A.A.A.; Daud, W.M.A.W. Review on the application of modified iron oxides as heterogeneous catalysts in Fenton reactions. J. Clean. Prod. 2014, 64, 24–35. [Google Scholar] [CrossRef] [Green Version]
- Matavos-Aramyan, S.; Moussavi, M. Advances in Fenton and Fenton based oxidation processes for industrial effluent contaminants control—A review. Int. J. Environ. Sci. Nat. Resour. 2017, 2, 1–18. [Google Scholar]
- He, J.; Yang, X.; Men, B.; Wang, D. Interfacial mechanisms of heterogeneous Fenton reactions catalyzed by iron-based materials: A review. J. Environ. Sci. 2016, 39, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Pignatello, J.J. Chemical treatment of pesticide wastes. Evaluation of iron (III) chelates for catalytic hydrogen peroxide oxidation of 2,4-D at circumneutral pH. J. Agric. Food Chem. 1992, 40, 322–327. [Google Scholar] [CrossRef]
- Pereira, M.; Oliveira, L.; Murad, E. Iron oxide catalysts: Fenton and Fentonlike reactions—A review. Clay Miner. 2012, 47, 285–302. [Google Scholar] [CrossRef]
- Gumy, D.; Fernandez-Ibañez, P.; Malato, S.; Pulgarin, C.; Enea, O.; Kiwi, J. Supported Fe/C and Fe/Nafion/C catalysts for the photo-Fenton degradation of Orange II under solar irradiation. Catal. Today 2005, 101, 375–382. [Google Scholar] [CrossRef]
- Flores, Y.; Flores, R.; Gallegos, A.A. Heterogeneous catalysis in the Fenton-type system reactive black 5/H2O2. J. Mol. Catal. A Chem. 2008, 281, 184–191. [Google Scholar] [CrossRef]
- Kitis, M.; Kaplan, S. Advanced oxidation of natural organic matter using hydrogen peroxide and iron-coated pumice particles. Chemosphere 2007, 68, 1846–1853. [Google Scholar] [CrossRef] [PubMed]
- Muthuvel, I.; Swaminathan, M. Highly solar active Fe(III) immobilised alumina for the degradation of Acid Violet 7. Sol. Energy Mater. Sol. Cells 2008, 92, 857–863. [Google Scholar] [CrossRef]
- Pramod, L.; Gandhimathi, R.; Lavanya, A.; Ramesh, S.T.; Nidheesh, P.V. Heterogeneous Fenton process coupled with microfiltration for the treatment of water with higher arsenic content. Chem. Eng. Commun. 2019, 207, 1646–1657. [Google Scholar] [CrossRef]
- Santos, F.; Lago, F.R.; Yokoyama, L.; Fonseca, F.V. Synthesis and characterization of zero-valent iron nanoparticles supported on SBA-15. J. Mater. Res. Technol. 2017, 6, 178–183. [Google Scholar] [CrossRef]
- Fontecha-Camara, M.A.; Álvarez-Merino, M.; Carrasco-Marín, F.; Ramon, M.V.L.; Moreno-Castilla, C. Heterogeneous and homogeneous Fenton processes using activated carbon for the removal of the herbicide amitrole from water. Appl. Catal. B Environ. 2011, 101, 425–430. [Google Scholar] [CrossRef]
- Barzegar, G.; Jorfi, S.; Zarezade, V.; Khatebasreh, M.; Mehdipour, F.; Ghanbari, F. 4-Chlorophenol degradation using ultrasound/peroxymonosulfate/nanoscale zero valent iron: Reusability, identification of degradation intermediates and potential application for real wastewater. Chemosphere 2018, 201, 370–379. [Google Scholar] [CrossRef]
- Crane, R.; Scott, T. Nanoscale zero-valent iron: Future prospects for an emerging water treatment technology. J. Hazard. Mater. 2012, 211–212, 112–125. [Google Scholar] [CrossRef]
- Lei, C.; Sun, Y.; Tsang, D.C.; Lin, D. Environmental transformations and ecological effects of iron-based nanoparticles. Environ. Pollut. 2018, 232, 10–30. [Google Scholar] [CrossRef] [PubMed]
- Raman, C.D.; Kanmani, S. Textile dye degradation using nano zero valent iron: A review. J. Environ. Manag. 2016, 177, 341–355. [Google Scholar] [CrossRef] [PubMed]
- Colombo, A.; Dragonetti, C.; Magni, M.; Roberto, D. Degradation of toxic halogenated organic compounds by iron-containing mono-, bi- and tri-metallic particles in water. Inorg. Chim. Acta 2015, 431, 48–60. [Google Scholar] [CrossRef]
- Alowitz, M.J.; Scherer, M.M. Kinetics of nitrate, nitrite, and Cr (VI) reduction by iron metal. Environ. Sci. Technol. 2002, 36, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-Q.; Elliott, D.W.; Zhang, W.-X. Zero-Valent Iron Nanoparticles for Abatement of Environmental Pollutants: Materials and Engineering Aspects. Crit. Rev. Solid State Mater. Sci. 2006, 31, 111–122. [Google Scholar] [CrossRef]
- Guo, X.; Yang, Z.; Dong, H.; Guan, X.; Ren, Q.; Lv, X.; Jin, X. Simple combination of oxidants with zero-valent-iron (ZVI) achieved very rapid and highly efficient removal of heavy metals from water. Water Res. 2016, 88, 671–680. [Google Scholar] [CrossRef]
- Volpe, A.; Pagano, M.; Mascolo, G.; Lopez, A.; Ciannarella, R.; Locaputo, V. Simultaneous Cr(VI) reduction and non-ionic surfactant oxidation by peroxymonosulphate and iron powder. Chemosphere 2013, 91, 1250–1256. [Google Scholar] [CrossRef]
- Diao, Z.-H.; Qian, W.; Zhang, Z.-W.; Jin, J.-C.; Chen, Z.-L.; Guo, P.-R.; Dong, F.-X.; Yan, L.; Kong, L.-J.; Chu, W. Removals of Cr(VI) and Cd(II) by a novel nanoscale zero valent iron/peroxydisulfate process and its Fenton-like oxidation of pesticide atrazine: Coexisting effect, products and mechanism. Chem. Eng. J. 2020, 397, 125382. [Google Scholar] [CrossRef]
- Bruzzoniti, M.C.; Fiore, S. Removal of Inorganic Contaminants from Aqueous Solutions: Evaluation of the Remediation Efficiency and of the Environmental Impact of a Zero-Valent Iron Substrate. Water Air Soil Pollut. 2014, 225, 2098. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Ji, Y.; Kong, D.; Lu, J.; Zhou, Q.; Yin, X. Simultaneous removal of bisphenol A and phosphate in zero-valent iron activated persulfate oxidation process. Chem. Eng. J. 2016, 303, 458–466. [Google Scholar] [CrossRef]
- Klas, S.; Kirk, D.W. Advantages of low pH and limited oxygenation in arsenite removal from water by zero-valent iron. J. Hazard. Mater. 2013, 252–253, 77–82. [Google Scholar] [CrossRef]
- Gil-Díaz, M.; Diez-Pascual, S.; González, A.; Alonso, J.; Rodríguez-Valdés, E.; Rodríguez-Gallego, J.L.; Lobo, M. A nanoremediation strategy for the recovery of an As-polluted soil. Chemosphere 2016, 149, 137–145. [Google Scholar] [CrossRef]
- Baragaño, D.; Alonso, J.; Gallego, J.; Lobo, M.; Gil-Díaz, M. Magnetite nanoparticles for the remediation of soils co-contaminated with As and PAHs. Chem. Eng. J. 2020, 399, 125809. [Google Scholar] [CrossRef]
- Hussain, I.; Li, M.; Zhang, Y.; Huang, S.; Hayat, W.; Li, Y.; Du, X.; Liu, G. Efficient oxidation of arsenic in aqueous solution using zero valent iron- activated persulfate process. J. Environ. Chem. Eng. 2017, 5, 3983–3990. [Google Scholar] [CrossRef]
- Brillas, E.; Garcia-Segura, S. Benchmarking recent advances and innovative technology approaches of Fenton, photo-Fenton, electro-Fenton, and related processes: A review on the relevance of phenol as model molecule. Sep. Purif. Technol. 2020, 237, 116337. [Google Scholar] [CrossRef]
- Martins, R.C.; Henriques, L.R.; Quinta-Ferreira, R.M. Catalytic activity of low cost materials for pollutants abatement by Fenton’s process. Chem. Eng. Sci. 2013, 100, 225–233. [Google Scholar] [CrossRef] [Green Version]
- Lai, B.; Zhang, Y.-H.; Li, R.; Zhou, Y.-X.; Wang, J. Influence of operating temperature on the reduction of high concentration p-nitrophenol (PNP) by zero valent iron (ZVI). Chem. Eng. J. 2014, 249, 143–152. [Google Scholar] [CrossRef]
- Segura, Y.; Martínez, F.; Melero, J.; Fierro, J. Zero valent iron (ZVI) mediated Fenton degradation of industrial wastewater: Treatment performance and characterization of final composites. Chem. Eng. J. 2015, 269, 298–305. [Google Scholar] [CrossRef]
- Yamaguchi, R.; Kurosu, S.; Suzuki, M.; Kawase, Y. Hydroxyl radical generation by zero-valent iron/Cu (ZVI/Cu) bimetallic catalyst in wastewater treatment: Heterogeneous Fenton/Fenton-like reactions by Fenton reagents formed in-situ under oxic conditions. Chem. Eng. J. 2018, 334, 1537–1549. [Google Scholar] [CrossRef]
- Donadelli, J.A.; Carlos, L.; Arques, A.; Einschlag, F.S. Kinetic and mechanistic analysis of azo dyes decolorization by ZVI-assisted Fenton systems: pH-dependent shift in the contributions of reductive and oxidative transformation pathways. Appl. Catal. B 2018, 231, 51–61. [Google Scholar] [CrossRef] [Green Version]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical Review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (OH/O− in Aqueous Solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef] [Green Version]
- Neta, P.; Huie, R.E.; Ross, A.B. Rate Constants for Reactions of Inorganic Radicals in Aqueous Solution. J. Phys. Chem. Ref. Data 1988, 17, 1027–1284. [Google Scholar] [CrossRef]
- Avetta, P.; Pensato, A.; Minella, M.; Malandrino, M.; Maurino, V.; Minero, C.; Hanna, K.; Vione, D. Activation of Persulfate by Irradiated Magnetite: Implications for the Degradation of Phenol under Heterogeneous Photo-Fenton-Like Conditions. Environ. Sci. Technol. 2014, 49, 1043–1050. [Google Scholar] [CrossRef]
- Lee, J.; Von Gunten, U.; Kim, J.-H. Persulfate-Based Advanced Oxidation: Critical Assessment of Opportunities and Roadblocks. Environ. Sci. Technol. 2020, 54, 3064–3081. [Google Scholar] [CrossRef] [PubMed]
- Furman, O.; Teel, A.; Ahmad, M.; Merker, M.C.; Watts, R.J. Effect of Basicity on Persulfate Reactivity. J. Environ. Eng. 2011, 137, 241–247. [Google Scholar] [CrossRef]
- Nidheesh, P.V. Heterogeneous Fenton catalysts for the abatement of organic pollutants from aqueous solution: A review. RSC Adv. 2015, 5, 40552–40577. [Google Scholar] [CrossRef]
- Enami, S.; Sakamoto, Y.; Colussi, A.J. Fenton chemistry at aqueous interfaces. Proc. Natl. Acad. Sci. USA 2014, 111, 623–628. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Wang, J. Reduction of nitrate by zero valent iron (ZVI)-based materials: A review. Sci. Total Environ. 2019, 671, 388–403. [Google Scholar] [CrossRef] [PubMed]
- Wols, B.; Hofman-Caris, C. Review of photochemical reaction constants of organic micropollutants required for UV advanced oxidation processes in water. Water Res. 2012, 46, 2815–2827. [Google Scholar] [CrossRef] [PubMed]
- Gligorovski, S.; Strekowski, R.; Barbati, S.; Vione, D. Environmental Implications of Hydroxyl Radicals (•OH). Chem. Rev. 2015, 115, 13051–13092. [Google Scholar] [CrossRef] [PubMed]
- Wardman, P. Reduction Potentials of One-Electron Couples Involving Free Radicals in Aqueous Solution. J. Phys. Chem. Ref. Data 1989, 18, 1637–1755. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Chen, T.; Chang, D.; Chen, D.; Liu, Y.; He, H.; Yuan, P.; Frost, R. Nitrate reduction over nanoscale zero-valent iron prepared by hydrogen reduction of goethite. Mater. Chem. Phys. 2012, 133, 205–211. [Google Scholar] [CrossRef]
- Ullah, S.; Guo, X.; Luo, X.; Zhang, X.; Leng, S.; Ma, N.; Faiz, P. Rapid and long-effective removal of broad-spectrum pollutants from aqueous system by ZVI/oxidants. Front. Environ. Sci. Eng. 2020, 14, 89. [Google Scholar] [CrossRef]
- Fu, F.; Dionysiou, D.D.; Liu, H. The use of zero-valent iron for groundwater remediation and wastewater treatment: A review. J. Hazard. Mater. 2014, 267, 194–205. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, S.; Chen, Z.; Megharaj, M.; Naidu, R. Kaolinite-supported nanoscale zero-valent iron for removal of Pb2+ from aqueous solution: Reactivity, characterization and mechanism. Water Res. 2011, 45, 3481–3488. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Qin, P.; Shao, J.; Peng, L.; Zeng, Q.; Gu, J.-D. Synthesis of reactive nanoscale zero valent iron using rectorite supports and its application for Orange II removal. Chem. Eng. J. 2013, 223, 1–7. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, L.; Yang, Y.; Sun, P. Degradation of Norfloxacin in an Aqueous Solution by the Nanoscale Zero-Valent Iron-Activated Persulfate Process. J. Nanomater. 2020, 2020, 3286383. [Google Scholar] [CrossRef]
- Dong, H.; Ning, Q.; Li, L.; Wang, Y.; Wang, B.; Zhang, L.; Tian, R.; Li, R.; Chen, J.; Xie, Q. A comparative study on the activation of persulfate by bare and surface-stabilized nanoscale zero-valent iron for the removal of sulfamethazine. Sep. Purif. Technol. 2020, 230, 115869. [Google Scholar] [CrossRef]
- Litter, M.I.; Slodowicz, M. An overview on heterogeneous Fenton and photoFenton reactions using zerovalent iron materials. J. Adv. Oxid. Technol. 2017, 20. [Google Scholar] [CrossRef]
- Minella, M.; Sappa, E.; Hanna, K.; Barsotti, F.; Maurino, V.; Minero, C.; Vione, D. Considerable Fenton and photo-Fenton reactivity of passivated zero-valent iron. RSC Adv. 2016, 6, 86752–86761. [Google Scholar] [CrossRef] [Green Version]
- Furina, F.; Minella, M.; Gosetti, F.; Turci, F.; Sabatino, R.; Di Cesare, A.; Corno, G.; Vione, D. Elimination from wastewater of antibiotics reserved for hospital settings, with a Fenton process based on zero-valent iron. Chemosphere 2021, 283, 131170. [Google Scholar] [CrossRef]
- Liang, S.X.; Jia, Z.; Liu, Y.J.; Zhang, W.; Wang, W.; Lu, J.; Zhang, L.C. Compelling rejuvenated catalytic performance in metallic glasses. Adv. Mater. 2018, 30, 1802764. [Google Scholar] [CrossRef] [Green Version]
- Liang, S.; Jia, Z.; Zhang, L.; Li, X.; Wang, W.; Lin, H. Ultrafast activation efficiency of three peroxides by Fe78Si9B13 metallic glass under photo-enhanced catalytic oxidation: A comparative study. Appl. Catal. B Environ. 2018, 221, 108–118. [Google Scholar] [CrossRef]
- Zhang, L.-C.; Jia, Z.; Lyu, F.; Liang, S.-X.; Lu, J. A review of catalytic performance of metallic glasses in wastewater treatment: Recent progress and prospects. Prog. Mater. Sci. 2019, 105, 100576. [Google Scholar] [CrossRef]
- Liang, S.-X.; Wang, X.; Zhang, W.; Liu, Y.-J.; Wang, W.; Zhang, L.-C. Selective laser melting manufactured porous Fe-based metallic glass matrix composite with remarkable catalytic activity and reusability. Appl. Mater. Today 2020, 19, 100543. [Google Scholar] [CrossRef]
- Deng, J.; Shao, Y.; Gao, N.; Deng, Y.; Tan, C.; Zhou, S. Zero-valent iron/persulfate(Fe0/PS) oxidation acetaminophen in water. Int. J. Environ. Sci. Technol. 2013, 11, 881–890. [Google Scholar] [CrossRef] [Green Version]
- Minella, M.; Bertinetti, S.; Hanna, K.; Minero, C.; Vione, D. Degradation of ibuprofen and phenol with a Fenton-like process triggered by zero-valent iron (ZVI-Fenton). Environ. Res. 2019, 179, 108750. [Google Scholar] [CrossRef]
- Jiang, X.; Wu, Y.; Wang, P.; Li, H.; Dong, W. Degradation of bisphenol A in aqueous solution by persulfate activated with ferrous ion. Environ. Sci. Pollut. Res. 2013, 20, 4947–4953. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Lin, J.; Chen, Z.; Megharaj, M.; Naidu, R. Green synthesized iron nanoparticles by green tea and eucalyptus leaves extracts used for removal of nitrate in aqueous solution. J. Clean. Prod. 2014, 83, 413–419. [Google Scholar] [CrossRef]
- Wu, J.; Wang, B.; Cagnetta, G.; Huang, J.; Wang, Y.; Deng, S.; Yu, G. Nanoscale zero valent iron-activated persulfate coupled with Fenton oxidation process for typical pharmaceuticals and personal care products degradation. Sep. Purif. Technol. 2020, 239, 116534. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, Y.; Gao, J.; Li, X.; Yu, H.; Wang, N.; Du, P.; Yu, R.; Li, H.; Fan, X.; et al. Synergistic degradation of chloramphenicol by ultrasound-enhanced nanoscale zero-valent iron/persulfate treatment. Sep. Purif. Technol. 2020, 240, 116575. [Google Scholar] [CrossRef]
- Du, Y.; Dai, M.; Cao, J.; Peng, C.; Ali, D.I.; Naz, I.; Li, J. Efficient removal of acid orange 7 using a porous adsorbent-supported zero-valent iron as a synergistic catalyst in advanced oxidation process. Chemosphere 2020, 244, 125522. [Google Scholar] [CrossRef]
- Liu, H.; Yao, J.; Wang, L.; Wang, X.; Qu, R.; Wang, Z. Effective degradation of fenitrothion by zero-valent iron powder (Fe0) activated persulfate in aqueous solution: Kinetic study and product identification. Chem. Eng. J. 2019, 358, 1479–1488. [Google Scholar] [CrossRef]
- Zou, X.; Zhou, T.; Mao, J.; Wu, X. Synergistic degradation of antibiotic sulfadiazine in a heterogeneous ultrasound-enhanced Fe0/persulfate Fenton-like system. Chem. Eng. J. 2014, 257, 36–44. [Google Scholar] [CrossRef]
- Tan, C.; Gao, N.; Chu, W.; Li, C.; Templeton, M.R. Degradation of diuron by persulfate activated with ferrous ion. Sep. Purif. Technol. 2012, 95, 44–48. [Google Scholar] [CrossRef]
- Jiang, Z.; Li, J.; Jiang, D.; Gao, Y.; Chen, Y.; Wang, W.; Cao, B.; Tao, Y.; Wang, L.; Zhang, Y. Removal of atrazine by biochar-supported zero-valent iron catalyzed persulfate oxidation: Reactivity, radical production and transformation pathway. Environ. Res. 2020, 184, 109260. [Google Scholar] [CrossRef]
- Nie, M.; Yan, C.; Li, M.; Wang, X.; Bi, W.; Dong, W. Degradation of chloramphenicol by persulfate activated by Fe2+ and zerovalent iron. Chem. Eng. J. 2015, 279, 507–515. [Google Scholar] [CrossRef]
- Dong, S.; Zhai, X.; Pi, R.; Wei, J.; Wang, Y.; Sun, X. Efficient degradation of naproxen by persulfate activated with zero-valent iron: Performance, kinetic and degradation pathways. Water Sci. Technol. 2020, 81, 2078–2091. [Google Scholar] [CrossRef]
- Qiao, J.; Jiao, W.; Liu, Y. Degradation of nitrobenzene-containing wastewater by sequential nanoscale zero valent iron-persulfate process. Green Energy Environ. 2020. [Google Scholar] [CrossRef]
- Gao, C.; Yu, W.; Zhu, Y.; Wang, M.; Tang, Z.; Du, L.; Hu, M.; Fang, L.; Xiao, X. Preparation of porous silicate supported micro-nano zero-valent iron from copper slag and used as persulfate activator for removing organic contaminants. Sci. Total Environ. 2021, 754, 142131. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.-Q.; Gao, N.-Y.; Wang, W.; Kang, S.; Xu, J.-H.; Xiang, H.-M.; Yin, D.-Q. Ultrasound-assisted heterogeneous activation of persulfate by nano zero-valent iron (nZVI) for the propranolol degradation in water. Ultrason. Sonochemistry 2018, 49, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Hussain, I.; Zhang, Y.; Huang, S.; Du, X. Degradation of p-chloroaniline by persulfate activated with zero-valent iron. Chem. Eng. J. 2012, 203, 269–276. [Google Scholar] [CrossRef]
- Hayat, W.; Zhang, Y.; Hussain, I.; Du, X.; Du, M.; Yao, C.; Huang, S.; Si, F. Efficient degradation of imidacloprid in water through iron activated sodium persulfate. Chem. Eng. J. 2019, 370, 1169–1180. [Google Scholar] [CrossRef]
- Le, C.; Wu, J.-H.; Li, P.; Wang, X.; Zhu, N.-W.; Wu, P.; Yang, B. Decolorization of anthraquinone dye Reactive Blue 19 by the combination of persulfate and zero-valent iron. Water Sci. Technol. 2011, 64, 754–759. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Gao, N.; Li, C.; Deng, Y.; Zhou, S.; Li, L. Zero-valent iron (ZVI) activation of persulfate (PS) for oxidation of bentazon in water. Chem. Eng. J. 2016, 285, 660–670. [Google Scholar] [CrossRef]
- Lutze, H.V.; Kerlin, N.; Schmidt, T.C. Sulfate radical-based water treatment in presence of chloride: Formation of chlorate, inter-conversion of sulfate radicals into hydroxyl radicals and influence of bicarbonate. Water Res. 2015, 72, 349–360. [Google Scholar] [CrossRef]
- Kim, C.; Thao, T.T.; Kim, J.-H.; Hwang, I. Effects of the formation of reactive chlorine species on oxidation process using persulfate and nano zero-valent iron. Chemosphere 2020, 250, 126266. [Google Scholar] [CrossRef]
- Rayaroth, M.P.; Lee, C.-S.; Aravind, U.K.; Aravindakumar, C.T.; Chang, Y.-S. Oxidative degradation of benzoic acid using Fe0- and sulfidized Fe 0 -activated persulfate: A comparative study. Chem. Eng. J. 2017, 315, 426–436. [Google Scholar] [CrossRef]
- Vione, D.; Maurino, V.; Minero, C.; Calza, P.; Pelizzetti, E. Phenol Chlorination and Photochlorination in the Presence of Chloride Ions in Homogeneous Aqueous Solution. Environ. Sci. Technol. 2005, 39, 5066–5075. [Google Scholar] [CrossRef]
- Yuan, R.; Wang, Z.; Hu, Y.; Wang, B.; Gao, S. Probing the radical chemistry in UV/persulfate-based saline wastewater treatment: Kinetics modeling and byproducts identification. Chemosphere 2014, 109, 106–112. [Google Scholar] [CrossRef]
- Bu, L.; Sun, J.; Wu, Y.; Zhang, W.; Duan, X.; Zhou, S.; Dionysiou, D.; Crittenden, J.C. Non-negligible risk of chloropicrin formation during chlorination with the UV/persulfate pretreatment process in the presence of low concentrations of nitrite. Water Res. 2020, 168, 115194. [Google Scholar] [CrossRef]
- Bu, L.; Zhou, S.; Zhu, S.; Wu, Y.; Duan, X.; Shi, Z.; Dionysiou, D.D. Insight into carbamazepine degradation by UV/monochloramine: Reaction mechanism, oxidation products, and DBPs formation. Water Res. 2018, 146, 288–297. [Google Scholar] [CrossRef]
- Chu, W.; Ding, S.; Bond, T.; Gao, N.; Yin, D.; Xu, B.; Cao, Z. Zero valent iron produces dichloroacetamide from chloramphenicol antibiotics in the absence of chlorine and chloramines. Water Res. 2016, 104, 254–261. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, T.; Lu, J.; Zhou, L.; Chovelon, J.-M.; Ji, Y. Formation of chloronitrophenols upon sulfate radical-based oxidation of 2-chlorophenol in the presence of nitrite. Environ. Pollut. 2020, 261, 114242. [Google Scholar] [CrossRef]
- Wang, Q.; Shao, Y.; Gao, N.; Liu, S.; Dong, L.; Rao, P.; Chu, W.; Xu, B.; An, N.; Deng, J. Impact of zero valent iron/persulfate preoxidation on disinfection byproducts through chlorination of alachlor. Chem. Eng. J. 2020, 380, 122435. [Google Scholar] [CrossRef]
- Wang, Q.; Shao, Y.; Gao, N.; Chu, W.; Chen, J.; Lu, X.; Zhu, Y.; An, N. Impact of preoxidation of UV/persulfate on disinfection byproducts by chlorination of 2,4-Di-tert-butylphenol. J. Hazard. Mater. 2018, 358, 450–458. [Google Scholar] [CrossRef]
- Bennedsen, L.R.; Muff, J.; Søgaard, E.G. Influence of chloride and carbonates on the reactivity of activated persulfate. Chemosphere 2012, 86, 1092–1097. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Li, H.; Yang, Y.; Li, X. Influence of water matrix species on persulfate oxidation of phenol: Reaction kinetics and formation of undesired degradation byproducts. Water Sci. Technol. 2018, 2017, 340–350. [Google Scholar] [CrossRef]
- Lei, Y.; Chen, C.-S.; Ai, J.; Lin, H.; Huang, Y.-H.; Zhang, H. Selective decolorization of cationic dyes by peroxymonosulfate: Non-radical mechanism and effect of chloride. RSC Adv. 2015, 6, 866–871. [Google Scholar] [CrossRef]
- Guo, W.; Zhao, Q.; Du, J.; Wang, H.; Li, X.; Ren, N. Enhanced removal of sulfadiazine by sulfidated ZVI activated persulfate process: Performance, mechanisms and degradation pathways. Chem. Eng. J. 2020, 388, 124303. [Google Scholar] [CrossRef]
- Ao, X.; Liu, W. Degradation of sulfamethoxazole by medium pressure UV and oxidants: Peroxymonosulfate, persulfate, and hydrogen peroxide. Chem. Eng. J. 2017, 313, 629–637. [Google Scholar] [CrossRef]
- Ji, Y.; Kong, D.; Lu, J.; Jin, H.; Kang, F.; Yin, X.; Zhou, Q. Cobalt catalyzed peroxymonosulfate oxidation of tetrabromobisphenol A: Kinetics, reaction pathways, and formation of brominated by-products. J. Hazard. Mater. 2016, 313, 229–237. [Google Scholar] [CrossRef]

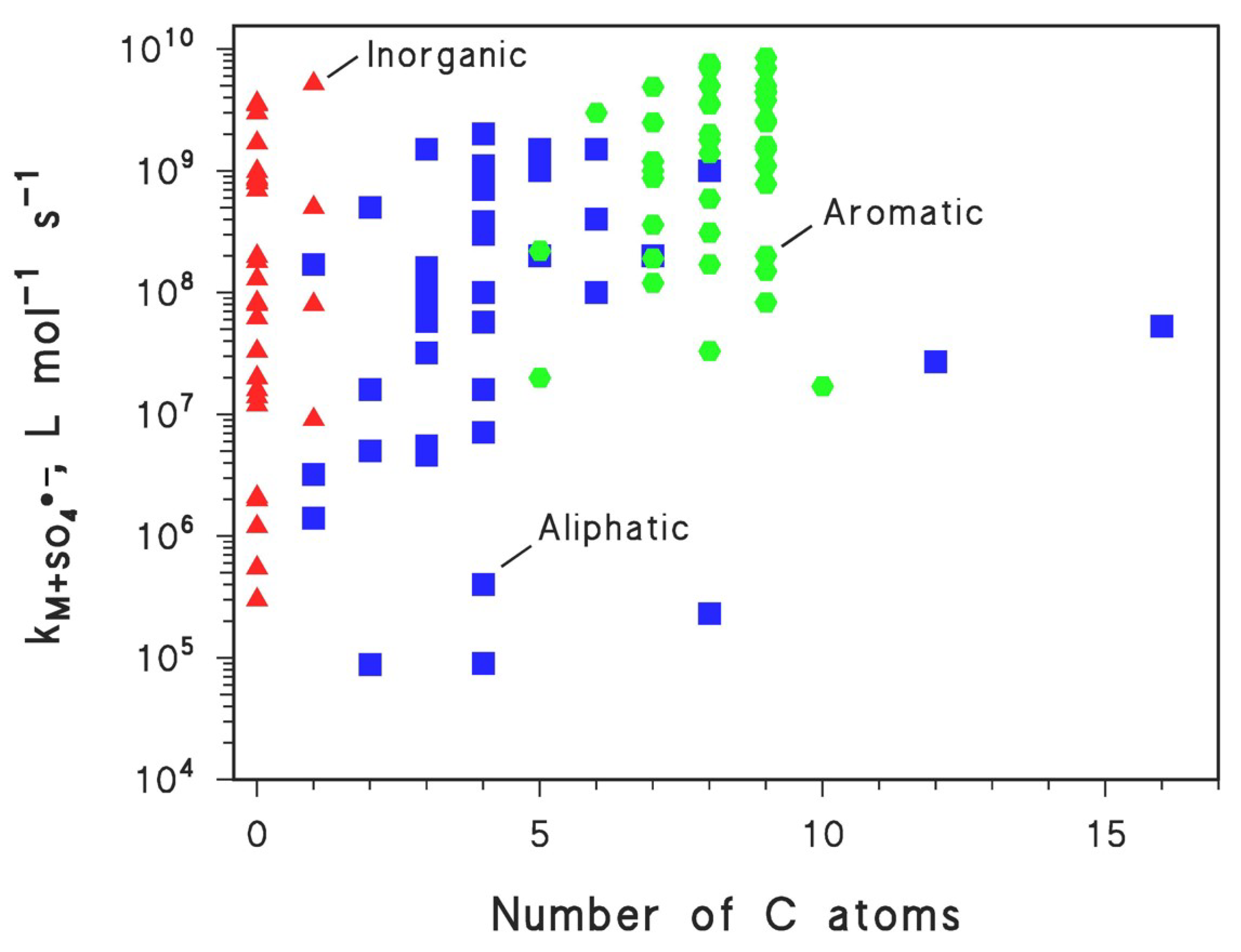
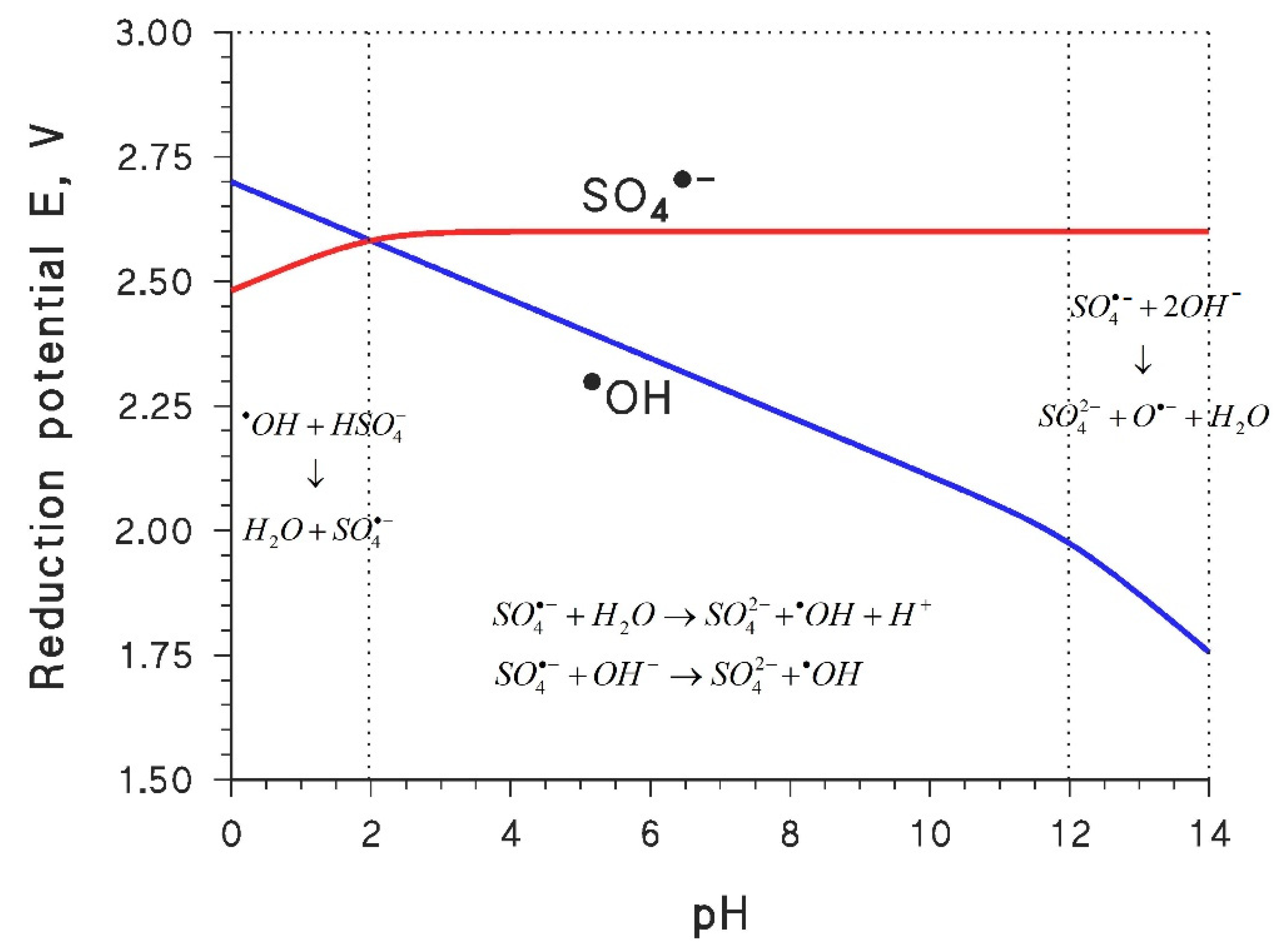
| Pollutants | Experimental Conditions | pH (% Degradation) | Main Reactive Species | Reference |
|---|---|---|---|---|
| Acetaminophen | Pollutant concentration: 0.066 mM ZVI: 0.1–1 g/L PS: 0.4 mM | 3–8.5 (>90%) 1.5 (49.51%) 10.0 (16.57%) | SO4•−, •OH | [68] |
| Acid Orange 7 | Pollutant concentration: 200 mg−1 g Iron source: 0.1–1.4 g/L PS: 5–500 mM | 1.0 (93.95%) 3.0 (93.22%) 5.0 (91.34%) 7.0 (66.25%) 9.0 (52.52%) 11.0 (21.62%) 13.0 (18.26%) | SO4•− | [74] |
| Anisole | Pollutant concentration: 1 mM PS: 0.25–0.5 M | 11.0 (40%) 12.0 (>99%) | SO4•−, •OH | [47] |
| Arsenic | Pollutant concentration: 50 µM ZVI:0.1–0.4 g/L PS: 0.5–10 mM | 3.0(>99%) 5.0 (98%) 7.0 (96%) 9.0 (60%) 11.0(51%) | SO4•−, •OH | [36] |
| Atrazine | Pollutant concentration: 2.5–15 mg/L ZVI: 0.25–1 g/L PS: 0.25–0.50 mM | 4.0 (84%) | SO4•− (84%), •OH | [30] |
| Atrazine | Pollutant concentration: 25 mg/L ZVI/BC: 175 mg/L PS: 2 mM | 3.0 (83.77%) | SO4•−, •OH | [78] |
| Chloramphenicol | Pollutant concentration: 0.05 M ZVI: 0.12–4 mM PS: 0.25–3 mM | 3.0 (95.1) 5.0 (94.3) 7.0 (93.4) 8.5 (93.2) 10.0 (92.5) | SO4•−, •OH (Dominant) | [79] |
| Diuron (3-(3,4-Dichlorophenyl)-1,1-Dimethylurea) | Pollutant concentration: 0.05 M ZVI: 0.05–1 mM PS: 0.5 M | 3.0(82%) 7.0 (65%) 9.0 (No Degradation) 11.0 (No Degradation) | SO4•− (Dominant), •OH | [77] |
| Fenitrothion | Pollutant concentration: 10 mg/L PS:Fe0: 1:1.5 molar ratio PS: 0.1M | 3.0 5.0 7.0 9.0 | SO4•− | [75] |
| Naproxen | Pollutant concentration: 25 µM ZVI:0.25–1.50 mM PS: 0.1–0.5 mM | 3.0 5.0 7.0 9.0 | SO4•−, •OH | [80] |
| Nitrobenzene | Pollutant concentration: 1 mM PS: 0.25–0.5 M | 11.0 (40%) 12.0 (>60%) | •OH | [47] |
| Nitrobenzene | Pollutant concentration: 200 mg/L ZVI: 0.75 g/L Na2S2O8: 26.8mM | 5.0 (100%) | SO4•−, •OH | [81] |
| Norfloxacin | Pollutant concentration: 100 mg/L ZVI: 0.075–0.3 g/L PS: 3 mM | 3.0 (>90%) 4.5(>90%) 7.0 (93.8%) 9.5 (89.9%) 11.0 (80.8%) | SO4•−, •OH | [59] |
| Orange G | Pollutant concentration: 100 mg/L Psi@ZVI: 0.2 g/L PS: 16 mM | 3.0 (99.66%) 5.0 (98.34%) 6.9 (98.93%) 8.0 (98.89%) 10.0 (96.76%) | SO4•−, •OH | [82] |
| Propranolol | Pollutant concentration: 40 µM ZVI: 0.15 g/L PS: 1 mM | 3.0 (97%) 4.5 (94.2%) 7.0 (89.4%) 11.0 (35.4%) | SO4•−, •OH | [83] |
| p-Chloroaniline | Pollutant concentration: 0.05 mM ZVI: 0.70 g/L PS: 2.5 mM | 4.0 (100%) 9.0 (43.59%) 11.0 (41.52%) | SO4•−, •OH | [84] |
| 1-(6-Chloro-3-Pyridylmethyl) -N-Nitro-Imidazolidin-2-Ylideneamine | Pollutant concentration: 30 ppm ZVI: 0.5–3 g/L PS: 2.5–15 mM | 7.0 (88%) | SO4•−, •OH | [85] |
| Sulfadiazine | Pollutant concentration: 20 mg/L ZVI: 0.92 mM PS: 1.84 mM | 3.0–7.0 (95.7–98.4%) 10.0 (35.7%) | SO4•− | [76] |
| Reactive Blue 19 | Pollutant concentration: 0.3 mM ZVI: 0.8 g/L PS: 10 mM | 3.0 (99%) 5.0 7.0 9.0 | SO4•− | [86] |
| Pollutant | pH | Effect of Chloride (+ve/−ve/=) | Chlorinated by-Products | Reference |
|---|---|---|---|---|
| Acid Orange 7 | 3–11 | +ve (1–100 mM) −ve (>100 mM) | 5-Chloroisobenzofuran-1,3-Dione, 1-Chloro- 2-(Dimethoxymethyl)Benzene, 1-(3-Chlorophenyl) Propan-1-One 2-Chlorobenzaldehyde | [92] |
| Bisphenol A | 6–8 | +ve | Trichloronitromethane | [93] |
| Carbamazepine | 6–8 | +ve | Trichloromethane, Trichloroacetonitrile, Trichloronitromethane | [94] |
| Chloramphenicol | 5–8 | Absence of Chloride | Dichloroacetamide | [95] |
| Chloramphenicol | 3–9 | +ve (<1 mM) | [79] | |
| 4-Chlorophenol | 2–7 | +ve (1 mM) −ve (>5 mM) | [21] | |
| 2-Chlorophenol | 7.9 | Absence of Chloride | 2-Chloro-4- Nitrophenol (2C4NP), 2-Chloro-6-Nitrophenol (2C6NP) | [96] |
| (2-Chloro-N-2,6-Diethylphenyl- N-(Methoxymethyl)Acetamide | 7.61–8.76 | +ve | Trichloromethane, 1,1,3-Trichloro-2-Propanone 1,3-Dichloro-2-Propanone | [97] |
| 2,4-Di-Tert-Butylphenol | 7–8 | +ve | Trichloromethane | [98] |
| Perchloroethylene | 7 | No Effect up to 28 mM | [99] | |
| Propanolol | −ve (≥5 mM) | [83] | ||
| Phenol | 2.5 | +ve (25–200 mM) −ve (>400 mM) | [89] | |
| Phenol | 3.9–4.4 | No Effect | [100] | |
| Rhodamine B | 2–12 | +ve up to 50 mM Cl− | [101] | |
| Sulfadiazine | 4.0 | +ve up to ≤10 mM | [102] | |
| Sulfamethoxazole | +ve | [103] |
| Pollutant | pH | Effect of Nitrate/Nitrite | Nitro-Derivatives as Byproducts | Reference |
|---|---|---|---|---|
| Bisphenol A | 7.0 | NO2− +ve | Trichloronitromethane | [93] |
| Chloramphenicol | 3.12–5.4 | NO3−/NO2− −ve/−ve | [79] | |
| 2-Chlorophenol | 7.0 | NO3− +ve (50–100 µM) −ve (>100 µM) | 2-chloro-4- nitrophenol (2C4NP), 2-chloro-6-nitrophenol (2C6NP) | [96] |
| (2-Chloro-N-2,6-Diethylphenyl- N-(Methoxymethyl)Acetamide | No effect | [97] | ||
| Propanolol | NO3− −ve (≥5 mM) | [83] | ||
| Phenol | 3.9–4.4 | No effect | [100] | |
| Sulfadiazine | 4.0 | NO3− (+ve < 10 mM) −ve (10–50 mM) | [102] | |
| Sulfamethoxazole | NO3− +ve | [103] |
| Pollutant | pH | Effect of Bi/Carbonate | Reference |
|---|---|---|---|
| 2-Chlorophenol | 7.9 | +ve | [96] |
| Imidacloprid | 7.0 | +ve | [85] |
| Phenol | 7.4–11.3 | +ve | [100] |
| Propranolol | −ve | [83] | |
| p-Nitrosodimethylaniline | 12.4 | +ve (10–100 mM) in alkaline media −ve in acidic media | [99] |
| Sulfamethoxazole | −ve | [103] | |
| Tetrabromobisphenol A | 7.0–8.5 | −ve | [104] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, N.; Vione, D.; Rivoira, L.; Carena, L.; Castiglioni, M.; Bruzzoniti, M.C. A Review on the Degradation of Pollutants by Fenton-Like Systems Based on Zero-Valent Iron and Persulfate: Effects of Reduction Potentials, pH, and Anions Occurring in Waste Waters. Molecules 2021, 26, 4584. https://doi.org/10.3390/molecules26154584
Ahmed N, Vione D, Rivoira L, Carena L, Castiglioni M, Bruzzoniti MC. A Review on the Degradation of Pollutants by Fenton-Like Systems Based on Zero-Valent Iron and Persulfate: Effects of Reduction Potentials, pH, and Anions Occurring in Waste Waters. Molecules. 2021; 26(15):4584. https://doi.org/10.3390/molecules26154584
Chicago/Turabian StyleAhmed, Naveed, Davide Vione, Luca Rivoira, Luca Carena, Michele Castiglioni, and Maria Concetta Bruzzoniti. 2021. "A Review on the Degradation of Pollutants by Fenton-Like Systems Based on Zero-Valent Iron and Persulfate: Effects of Reduction Potentials, pH, and Anions Occurring in Waste Waters" Molecules 26, no. 15: 4584. https://doi.org/10.3390/molecules26154584
APA StyleAhmed, N., Vione, D., Rivoira, L., Carena, L., Castiglioni, M., & Bruzzoniti, M. C. (2021). A Review on the Degradation of Pollutants by Fenton-Like Systems Based on Zero-Valent Iron and Persulfate: Effects of Reduction Potentials, pH, and Anions Occurring in Waste Waters. Molecules, 26(15), 4584. https://doi.org/10.3390/molecules26154584








