Functional Nanocomposite Films of Poly(Lactic Acid) with Well-Dispersed Chitin Nanocrystals Achieved Using a Dispersing Agent and Liquid-Assisted Extrusion Process
Abstract
:1. Introduction
2. Results
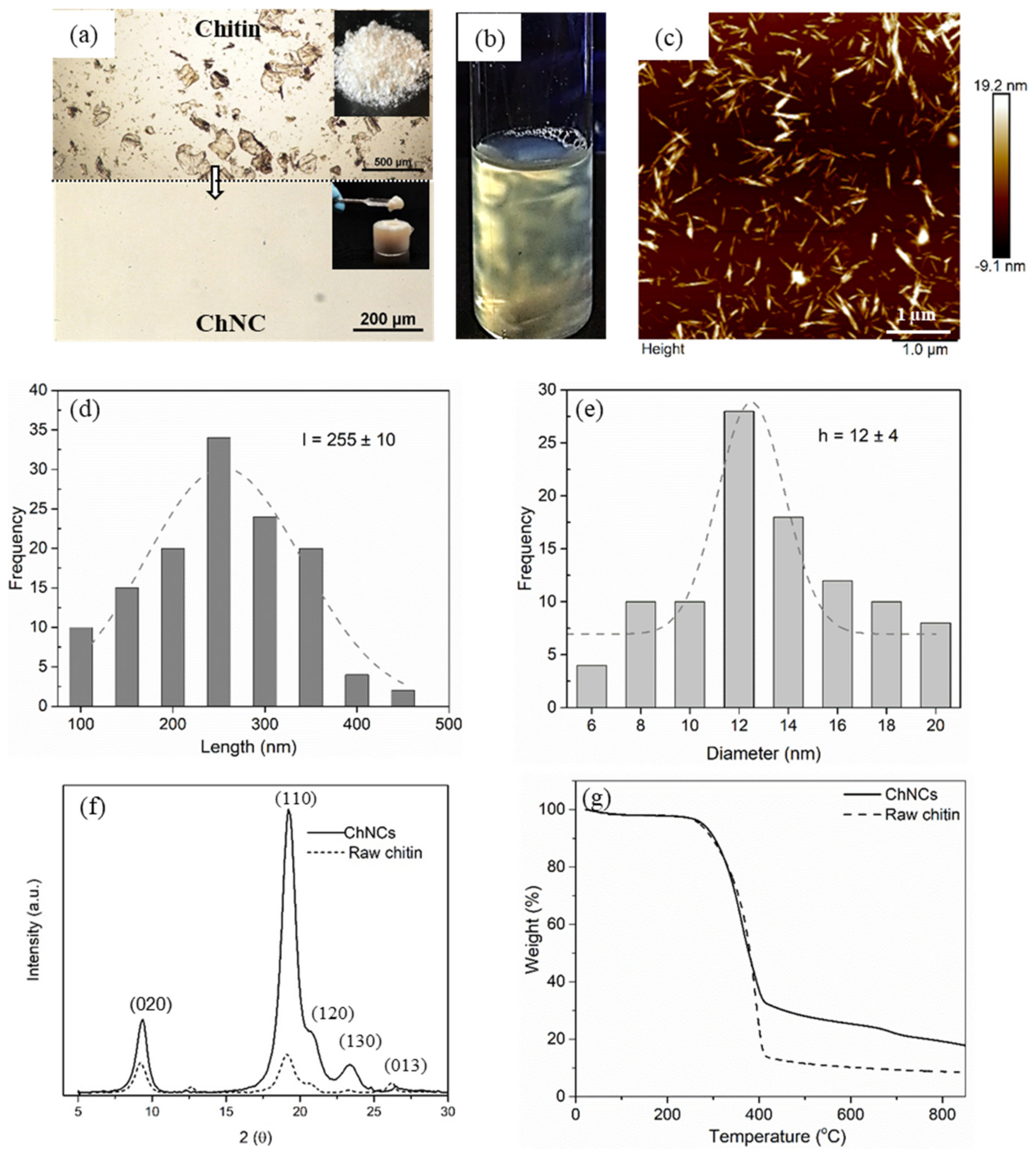
2.1. ChNC Characteristics
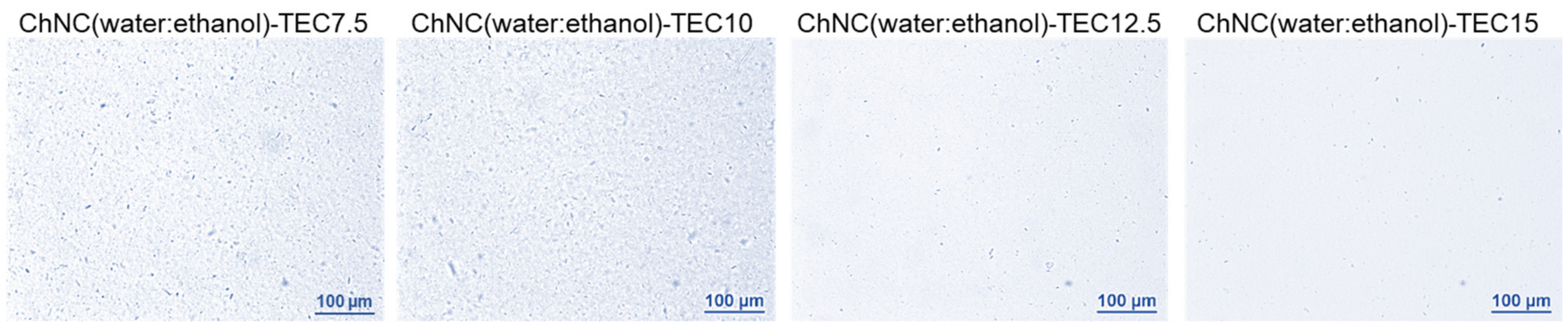
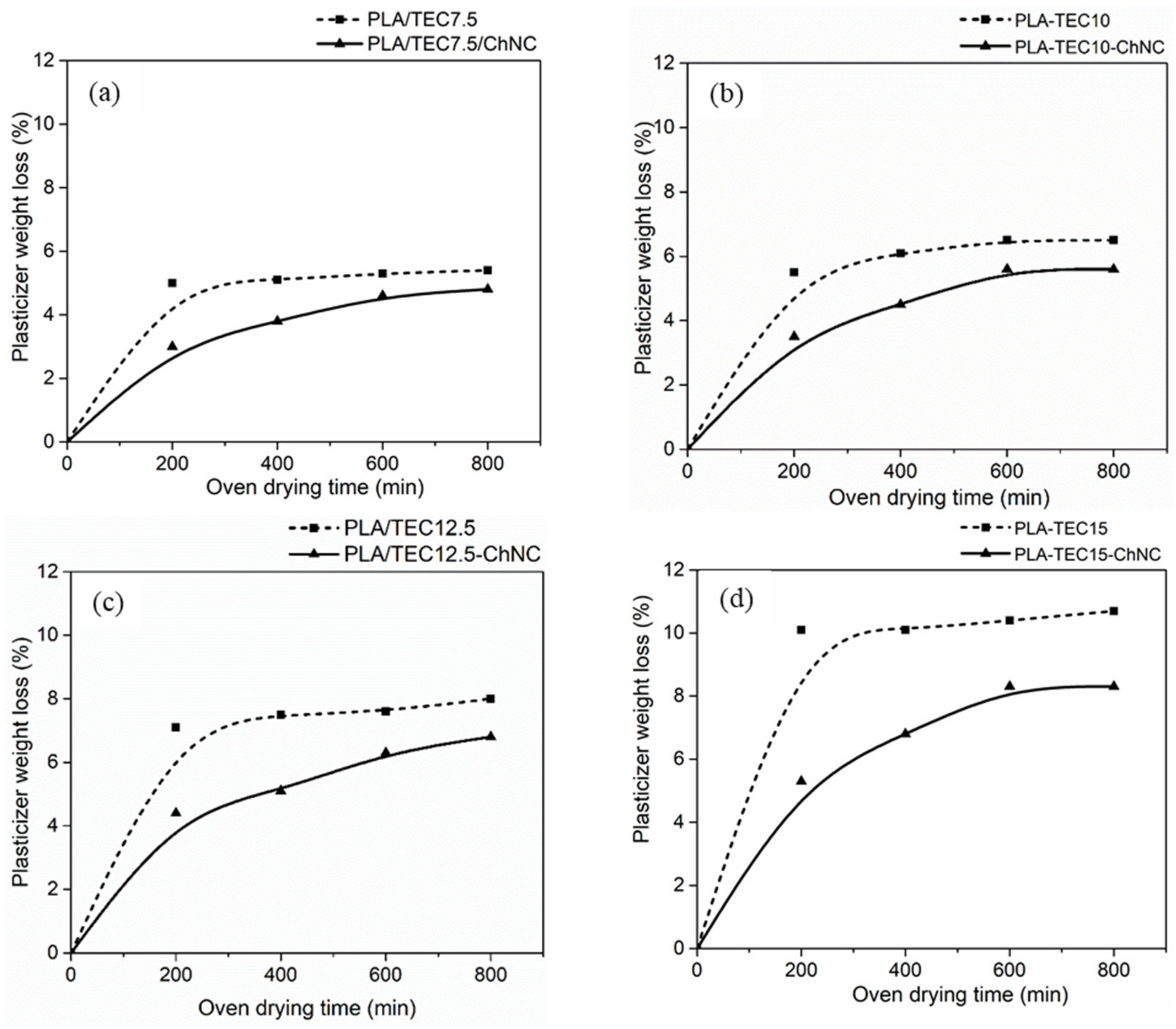
2.2. Determination of Dispersing Agent Amount
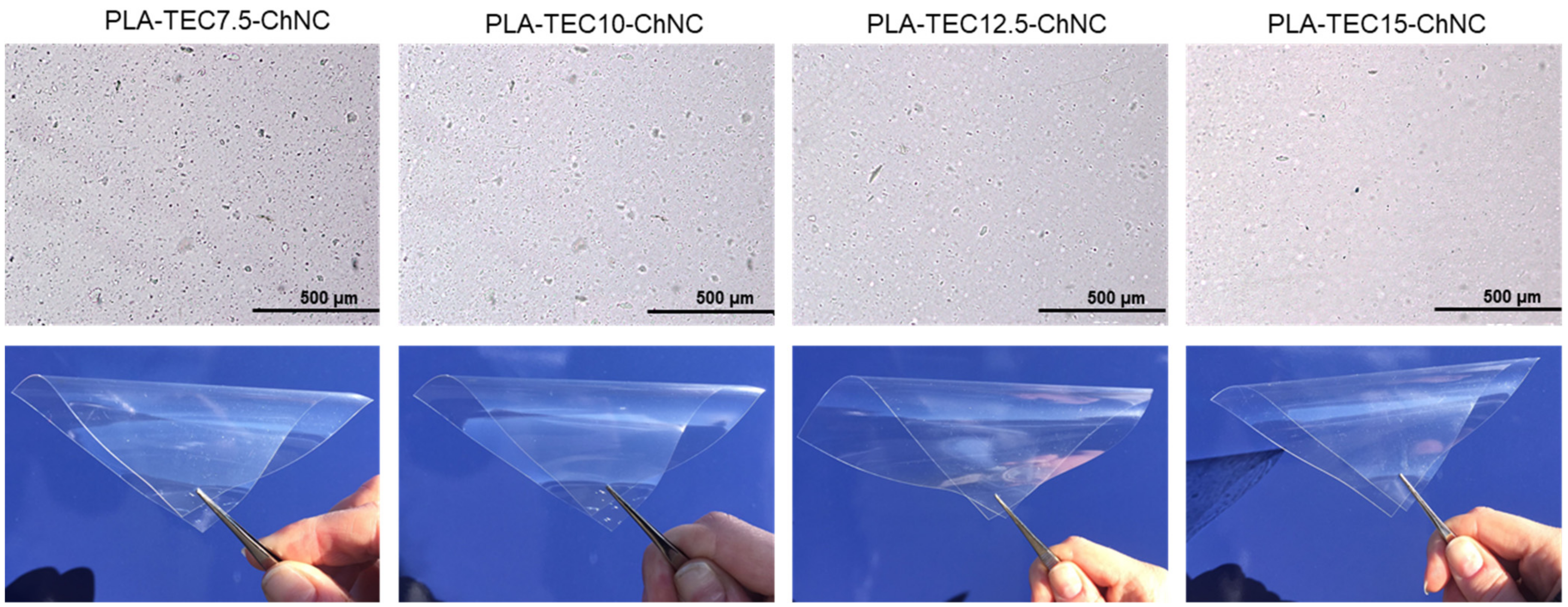
2.3. Dispersion of ChNCs
3. Materials and Methods
3.1. Materials
3.2. Preparation of ChNCs and Suspensions for Liquid Feeding Process
3.3. Liquid-Assisted Extrusion
3.4. Characterizations
3.4.1. Characterizations of ChNCs
3.4.2. Characterization of Feeding Suspension and Nanocomposites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Soulestin, J.; Prashantha, K.; Lacrampe, M.F.; Krawczak, P. Bioplastics Based Nanocomposites for Packaging Applications. In Handbook of Bioplastics and Biocomposites Engineering Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 76–119. ISBN 9780470626078. [Google Scholar]
- Kulinski, Z.; Piorkowska, E. Crystallization, structure and properties of plasticized poly(L-lactide). Polymer 2005, 46, 10290–10300. [Google Scholar] [CrossRef]
- Lim, L.T.; Auras, R.; Rubino, M. Processing technologies for poly(lactic acid). Prog. Polym. Sci. 2008, 33, 820–852. [Google Scholar] [CrossRef]
- Salaberria, A.M.; Diaz, R.H.; Labidi, J.; Fernandes, S.C.M. Preparing valuable renewable nanocomposite films based exclusively on oceanic biomass-Chitin nanofillers and chitosan. React. Funct. Polym. 2015, 89, 31–39. [Google Scholar] [CrossRef]
- Gopalan Nair, K.; Dufresne, A. Crab shell chitin whisker reinforced natural rubber nanocomposites. 1. Processing and swelling behavior. Biomacromolecules 2003, 4, 657–665. [Google Scholar] [CrossRef]
- Tran, T.H.; Nguyen, H.L.; Hwang, D.S.; Lee, J.Y.; Cha, H.G.; Koo, J.M.; Hwang, S.Y.; Park, J.; Oh, D.X. Five different chitin nanomaterials from identical source with different advantageous functions and performances. Carbohydr. Polym. 2019, 205, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Herrera, N.; Singh, A.A.; Salaberria, A.M.; Labidi, J.; Mathew, A.P.; Oksman, K. Triethyl citrate (TEC) as a dispersing aid in polylactic acid/chitin nanocomposites prepared via liquid-assisted extrusion. Polymers 2017, 9, 406. [Google Scholar] [CrossRef] [Green Version]
- Herrera, N.; Salaberria, A.M.; Mathew, A.P.; Oksman, K. Plasticized polylactic acid nanocomposite films with cellulose and chitin nanocrystals prepared using extrusion and compression molding with two cooling rates: Effects on mechanical, thermal and optical properties. Compos. Part A Appl. Sci. Manuf. 2016, 83, 89–97. [Google Scholar] [CrossRef] [Green Version]
- Arias, A.; Heuzey, M.C.; Huneault, M.A.; Ausias, G.; Bendahou, A. Enhanced dispersion of cellulose nanocrystals in melt-processed polylactide-based nanocomposites. Cellulose 2015, 22, 483–498. [Google Scholar] [CrossRef]
- Salaberria, A.M.; Diaz, R.H.; Andrés, M.A.; Fernandes, S.C.M.; Labidi, J. The antifungal activity of functionalized chitin nanocrystals in poly (Lactid Acid) films. Materials 2017, 10, 546. [Google Scholar] [CrossRef] [Green Version]
- Raquez, J.M.; Murena, Y.; Goffin, A.L.; Habibi, Y.; Ruelle, B.; DeBuyl, F.; Dubois, P. Surface-modification of cellulose nanowhiskers and their use as nanoreinforcers into polylactide: A sustainably-integrated approach. Compos. Sci. Technol. 2012, 72, 544–549. [Google Scholar] [CrossRef]
- Bondeson, D.; Oksman, K. Polylactic acid/cellulose whisker nanocomposites modified by polyvinyl alcohol. Compos. Part A Appl. Sci. Manuf. 2007, 38, 2486–2492. [Google Scholar] [CrossRef]
- Oksman, K.; Mathew, A.P.; Bondeson, D.; Kvien, I. Manufacturing process of cellulose whiskers/polylactic acid nanocomposites. Compos. Sci. Technol. 2006, 66, 2776–2784. [Google Scholar] [CrossRef]
- Herrera, N.; Mathew, A.P.; Oksman, K. Plasticized polylactic acid/cellulose nanocomposites prepared using melt-extrusion and liquid feeding: Mechanical, thermal and optical properties. Compos. Sci. Technol. 2015, 106, 149–155. [Google Scholar] [CrossRef]
- Labrecque, L.V.; Kumar, R.A.; Davé, V.; Gross, R.A.; Mccarthy, S.P. Citrate esters as plasticizers for poly(lactic acid). J. Appl. Polym. Sci. 1997, 66, 1507–1513. [Google Scholar] [CrossRef]
- Ljungberg, N.; Wesslén, B. Tributyl citrate oligomers as plasticizers for poly (lactic acid): Thermo-mechanical film properties and aging. Polymer 2003, 44, 7679–7688. [Google Scholar] [CrossRef]
- Ren, Z.; Dong, L.; Yang, Y. Dynamic mechanical and thermal properties of plasticized poly(lactic acid). J. Appl. Polym. Sci. 2006, 101, 1583–1590. [Google Scholar] [CrossRef]
- Kurita, K. Chitin and chitosan: Functional biopolymers from marine crustaceans. Mar. Biotechnol. 2006, 8, 203–226. [Google Scholar] [CrossRef]
- Salaberria, A.M.; Labidi, J.; Fernandes, S.C.M. Different routes to turn chitin into stunning nano-objects. Eur. Polym. J. 2015, 68, 503–515. [Google Scholar] [CrossRef]
- Zeng, J.B.; He, Y.S.; Li, S.L.; Wang, Y.Z. Chitin whiskers: An overview. Biomacromolecules 2012, 13, 1–11. [Google Scholar] [CrossRef]
- Herrera, N.; Roch, H.; Salaberria, A.M.; Pino-Orellana, M.A.; Labidi, J.; Fernandes, S.C.M.; Radic, D.; Leiva, A.; Oksman, K. Functionalized blown films of plasticized polylactic acid/chitin nanocomposite: Preparation and characterization. Mater. Des. 2016, 92, 846–852. [Google Scholar] [CrossRef]
- Li, J.; Gao, Y.; Zhao, J.; Sun, J.; Li, D. Homogeneous dispersion of chitin nanofibers in polylactic acid with different pretreatment methods. Cellulose 2017, 24, 1705–1715. [Google Scholar] [CrossRef]
- Nishino, T.; Matsui, R.; Nakamae, K. Elastic modulus of the crystalline regions of chitin and chitosan. J. Polym. Sci. Part B Polym. Phys. 1999, 37, 1191–1196. [Google Scholar] [CrossRef]
- Paillet, M.; Dufresne, A. Chitin whisker reinforced thermoplastic nanocomposites. Macromolecules 2001, 34, 6527–6530. [Google Scholar] [CrossRef]
- Zhang, Q.; Wei, S.; Huang, J.; Feng, J.; Chang, P.R. Effect of surface acetylated-chitin nanocrystals on structure and mechanical properties of poly(lactic acid). J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Li, C.; Liu, H.; Luo, B.; Wen, W.; He, L.; Liu, M.; Zhou, C. Nanocomposites of poly(l-lactide) and surface-modified chitin whiskers with improved mechanical properties and cytocompatibility. Eur. Polym. J. 2016, 81, 266–283. [Google Scholar] [CrossRef]
- Fleming, K.; Gray, D.G.; Matthews, S. Cellulose crystallites. Chem. Eur. J. 2001, 7, 1831–1836. [Google Scholar] [CrossRef]
- Naseri, N.; Algan, C.; Jacobs, V.; John, M.; Oksman, K.; Mathew, A.P. Electrospun chitosan-based nanocomposite mats reinforced with chitin nanocrystals for wound dressing. Carbohydr. Polym. 2014, 109, 7–15. [Google Scholar] [CrossRef]
- Larbi, F.; García, A.; del Valle, L.J.; Hamou, A.; Puiggalí, J.; Belgacem, N.; Bras, J. Comparison of nanocrystals and nanofibers produced from shrimp shell α-chitin: From energy production to material cytotoxicity and Pickering emulsion properties. Carbohydr. Polym. 2018, 196, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Oksman, K.; Mathew, A.P. Melt Compounding Process of Cellulose Nanocomposites. In Handbook of Green Materials: 2 Bionanocomposites: Processing, Characterization and Properties; Oksman, K., Mathew, A.P., Bismarck, A., Rojas, O., Sain, M., Eds.; World Scientific: Singapore, 2014; pp. 53–68. [Google Scholar]
- Li, X.; Xiao, Y.; Wang, B.; Tang, Y.; Lu, Y.; Wang, C. Effects of poly(1,2-propylene glycol adipate) and nano-CaCO3 on DOP migration and mechanical properties of flexible PVC. J. Appl. Polym. Sci. 2012, 124, 1737–1743. [Google Scholar] [CrossRef]
- Ma, Y.; Liao, S.; Li, Q.; Guan, Q.; Jia, P.; Zhou, Y. Physical and chemical modifications of poly(vinyl chloride) materials to prevent plasticizer migration-Still on the run. React. Funct. Polym. 2020, 147, 104458. [Google Scholar] [CrossRef]
- Callister, W.D., Jr. Materials Science and Engineering: An Introduction; John Wiley & Sons Asia Pte Ltd.: New York, NY, USA, 2015; pp. 580–633. [Google Scholar]
- Liu, X.; Yu, L.; Dean, K.; Toikka, G.; Bateman, S.; Nguyen, T.; Yuan, Q.; Filippou, C. Improving Melt Strength of Polylactic Acid. Int. Polym. Process 2013, 28, 64–71. [Google Scholar] [CrossRef]
- Shojaeiarani, J.; Bajwa, D.S.; Stark, N.M.; Bajwa, S.G. Rheological properties of cellulose nanocrystals engineered polylactic acid nanocomposites. Compos. Part B Eng. 2019, 161, 483–489. [Google Scholar] [CrossRef]
- Azeredo, H.M.C. Nanocomposites for Food Packaging Applications. Food Res. Int. 2009, 42, 1240–1253. [Google Scholar] [CrossRef] [Green Version]
- Cottaz, A.; Bouarab, L.; De Clercq, J.; Oulahal, N.; Degraeve, P.; Joly, C. Potential of incorporation of antimicrobial plant phenolics into polyolefin-based food contact materials to produce active packaging by melt-blending: Proof of concept with isobutyl-4-hydroxybenzoate. Front. Chem. 2019, 7, 148. [Google Scholar] [CrossRef]
- Salaberria, A.M.; Labidi, J.; Fernandes, S.C.M. Chitin nanocrystals and nanofibers as nano-sized fillers into thermoplastic starch-based biocomposites processed by melt-mixing. Chem. Eng. J. 2014, 256, 356–364. [Google Scholar] [CrossRef]
- Nečas, D.; Klapetek, P. Gwyddion: An open-source software for SPM data analysis. Cent. Eur. J. Phys. 2012, 10, 181–188. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Maiza, M.; Benaniba, M.T.; Quintard, G.; Massardier-Nageotte, V. Biobased additive plasticizing Polylactic acid (PLA). Polimeros 2015, 25, 581–590. [Google Scholar] [CrossRef]
- Vasanthakumari, R.; Pennings, A.J. Crystallization kinetics of poly(l-lactic acid). Polymer 1983, 24, 175–178. [Google Scholar] [CrossRef]




| Materials | TEC Content (wt%) | |
|---|---|---|
| Added | Mass Loss TGA | |
| PLA-TEC7.5 | 7.5 | 7.1 |
| PLA-TEC7.5-ChNC | 7.5 | 6.8 |
| PLA-TEC10 | 10 | 9.4 |
| PLA-TEC10-ChNC | 10 | 9.3 |
| PLA-TEC12.5 | 12.5 | 11.0 |
| PLA-TEC12.5-ChNC | 12.5 | 9.9 |
| PLA-TEC15 | 15 | 12.9 |
| PLA-TEC15-ChNC | 15 | 11.8 |
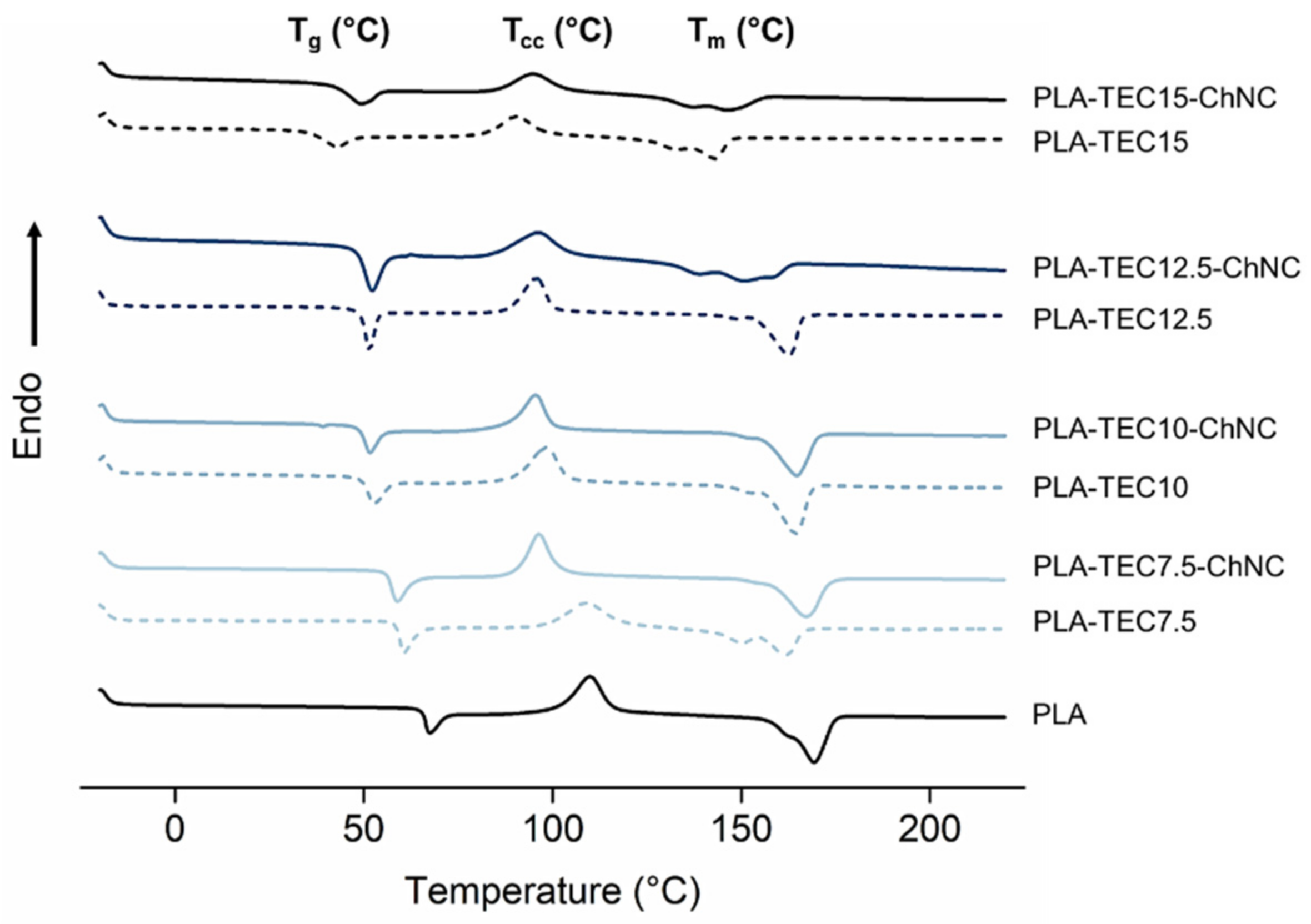
| Materials | Tg (°C) | Tcc Onset (°C) | Tm (°C) | Crystallinity (%) |
|---|---|---|---|---|
| PLA | 65.3 | 101.7 | 169.4 | 1.8 |
| PLA-TEC7.5 | 58.0 | 98.9 | 161.7 | 2.4 |
| PLA-TEC7.5-ChNC | 55.9 | 90.6 | 167.4 | 2.6 |
| PLA-TEC10 | 48.7 | 89.8 | 164.4 | 3.9 |
| PLA-TEC10-ChNC | 48.0 | 88.0 | 164.0 | 4.3 |
| PLA-TEC12.5 | 47.1 | 88.9 | 153.0 | 6.0 |
| PLA-TEC12.5-ChNC | 47.1 | 84.0 | 154.0 | 6.4 |
| PLA-TEC15 | 36.0 | 82.0 | 143.0 | 5.8 |
| PLA-TEC15-ChNC | 42.0 | 85.0 | 146.4 | 7.1 |
| Materials | Young’s Modulus (GPa) | Tensile Strength (MPa) | Elongation at Break (%) | Work of Fracture (MJ/m3) |
|---|---|---|---|---|
| PLA | 1.85 ± 0.03 a | 59.9 ± 0.5 a | 6.3 ± 0.7 a | 2.0 ± 0.4 a |
| PLA-TEC7.5 | 1.76 ± 0.05 a,b | 46.0 ± 2.7 b | 10.2 ± 2.1 a,c | 3.8 ± 0.2 a,c |
| PLA-TEC7.5-ChNC | 1.82 ± 0.03 a,b | 49.0 ± 1.8 b | 8.7 ± 3.35 a | 2.3 ± 0.2 a |
| PLA-TEC10 | 1.69 ± 0.02 c | 41.3 ± 1.5 c | 21.4 ± 2.8 a,c | 6.4 ± 0.9 b |
| PLA-TEC10-ChNC | 1.73 ± 0.02 b | 41.8 ± 1.2 c | 14.9 ± 2.3 a,c | 5.3 ± 1.4 b,c |
| PLA-TEC12.5 | 1.61 ± 0.11 c | 39.4 ± 2.7 c | 65.7 ± 18.7 b | 16.1 ± 2.0 d |
| PLA-TEC12.5-ChNC | 1.62 ± 0.04 c | 40.9 ± 0.9 c | 24.1 ± 5.5 c | 6.6 ± 1.7 b |
| PLA-TEC15 | 1.01 ± 0.08 d | 27.6 ± 3.8 d | 310.8 ± 56.4 α | 49.4 ± 6.3 α |
| PLA-TEC15-ChNC | 1.41 ± 0.10 e | 31.4 ± 0.9 d | 256.2 ± 15.5 α | 46.2 ± 2.4 α |
| Materials | Young’s Modulus (GPa) | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|---|
| PLA-TEC15 | 1.01 | 27.6 | 310.8 |
| PLA-TEC15-ChNC | 1.41 | 31.4 | 256.2 |
| LDPE [33] | 0.17–0.28 | 8.3–31.4 | 100–650 |
| PP [33] | 1.14–1.55 | 31–41.4 | 100–600 |
| Material | Staphylococcus aureus | Escherichia coli | ||
|---|---|---|---|---|
| % Microbial Death | R | % Microbial Death | R | |
| PLA-TEC15 | - | - | 17 | 0.08 |
| PLA-TEC15-ChNC | 22 | 0.11 | 26 | 0.13 |
| Coding | Composition of Materials (wt%) | Feeding Rate (kg/hr) | |||
|---|---|---|---|---|---|
| PLA | TEC | ChNC | PLA | Suspension | |
| PLA | 100 | - | - | 2.00 | - |
| PLA-TEC7.5 | 92.5 | 7.5 | - | 1.85 | 0.70 1 |
| PLA-TEC7.5-ChNC | 91.5 | 7.5 | 1 | 1.83 | 0.72 2 |
| PLA-TEC10 | 90.0 | 10 | - | 1.80 | 0.75 1 |
| PLA-TEC10-ChNC | 89.0 | 10 | 1 | 1.78 | 0.77 2 |
| PLA-TEC12.5 | 87.5 | 12.5 | - | 1.75 | 0.80 1 |
| PLA-TEC12.5-ChNC | 86.5 | 12.5 | 1 | 1.73 | 0.82 2 |
| PLA-TEC15 | 85.0 | 15 | - | 1.70 | 0.85 1 |
| PLA-TEC15-ChNC | 84.5 | 15 | 1 | 1.68 | 0.87 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, M.; Schwendemann, D.; Spigno, G.; Geng, S.; Berglund, L.; Oksman, K. Functional Nanocomposite Films of Poly(Lactic Acid) with Well-Dispersed Chitin Nanocrystals Achieved Using a Dispersing Agent and Liquid-Assisted Extrusion Process. Molecules 2021, 26, 4557. https://doi.org/10.3390/molecules26154557
Patel M, Schwendemann D, Spigno G, Geng S, Berglund L, Oksman K. Functional Nanocomposite Films of Poly(Lactic Acid) with Well-Dispersed Chitin Nanocrystals Achieved Using a Dispersing Agent and Liquid-Assisted Extrusion Process. Molecules. 2021; 26(15):4557. https://doi.org/10.3390/molecules26154557
Chicago/Turabian StylePatel, Mitul, Daniel Schwendemann, Giorgia Spigno, Shiyu Geng, Linn Berglund, and Kristiina Oksman. 2021. "Functional Nanocomposite Films of Poly(Lactic Acid) with Well-Dispersed Chitin Nanocrystals Achieved Using a Dispersing Agent and Liquid-Assisted Extrusion Process" Molecules 26, no. 15: 4557. https://doi.org/10.3390/molecules26154557
APA StylePatel, M., Schwendemann, D., Spigno, G., Geng, S., Berglund, L., & Oksman, K. (2021). Functional Nanocomposite Films of Poly(Lactic Acid) with Well-Dispersed Chitin Nanocrystals Achieved Using a Dispersing Agent and Liquid-Assisted Extrusion Process. Molecules, 26(15), 4557. https://doi.org/10.3390/molecules26154557











