Phenolic Constituents from Platycodon grandiflorum Root and Their Anti-Inflammatory Activity
Abstract
:1. Introduction
2. Results and Discussion
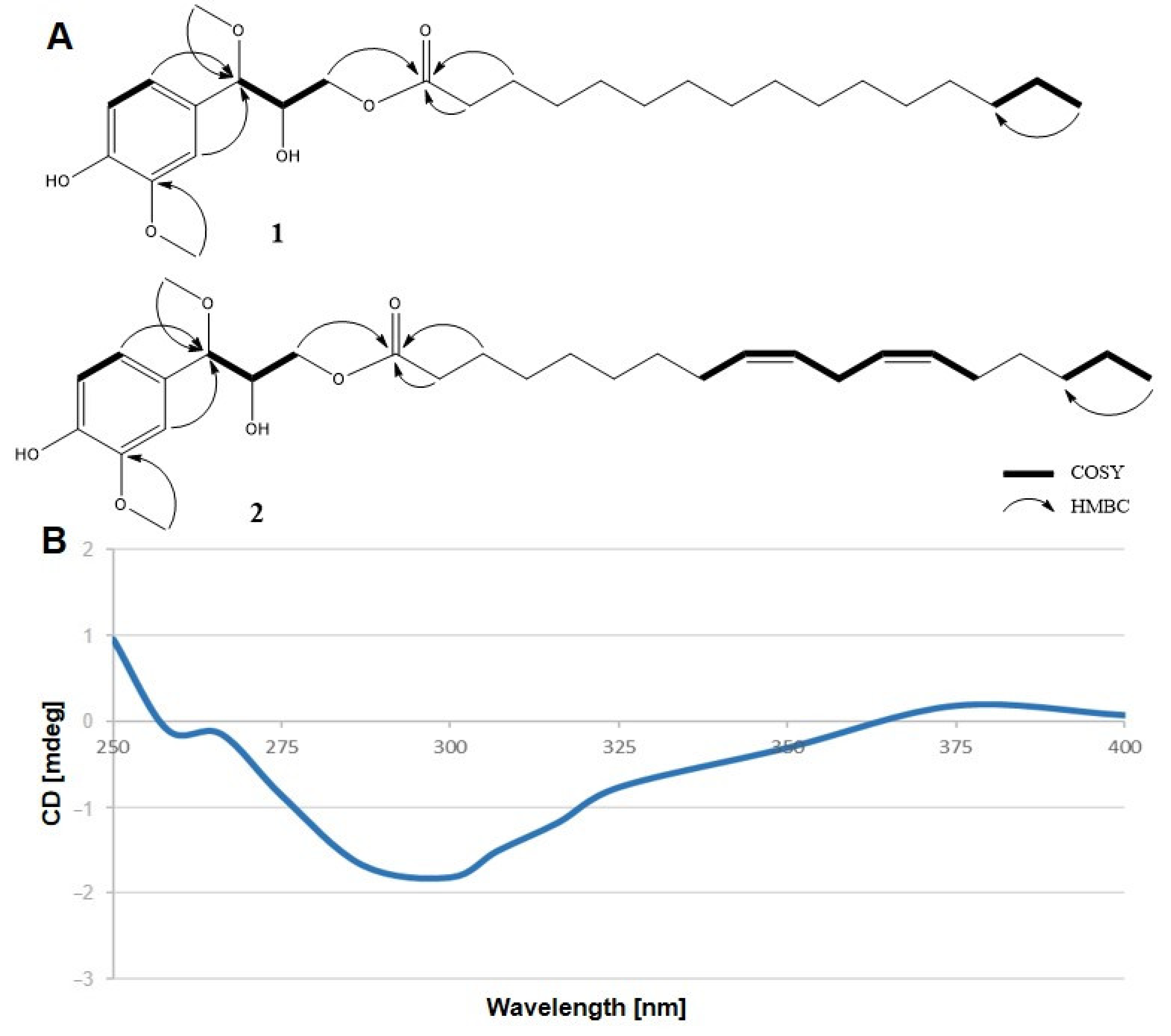
2.1. Isolation and Structural Elucidation of Compounds 1–21
2.2. Bioassay
3. Materials and Methods
3.1. General Information
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Alkaline Hydrolysis
3.5. Absolute Configuration
3.6. Cell Culture
3.7. Cell Viability
3.8. Measurement of Proinflammatory Cytokine Production
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Kim, K.S.; Ezaki, O.; Ikemoto, S.; Itakura, H. Effects of Platycodon grandiflorum feeding on serum and liver lipid concentrations in rats with diet-induced hyperlipidemia. J. Nutr. Sci. Vitaminol. 1995, 41, 485–491. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Wang, Y.L.; Yang, D.W.; Zhang, C.H.; Zhang, N.; Li, M.H.; Liu, Y.Z. Platycodon grandifloras—An ethnopharmacological, phytochemical and pharmacological review. J. Ethnopharmacol. 2015, 164, 147–161. [Google Scholar] [CrossRef]
- Ji, M.Y.; Bo, A.; Yang, M.; Xu, J.F.; Jiang, L.L.; Zhou, B.C.; Li, M.H. The pharmacological effects and health benefits of Platycodon grandifloras—A medicine food homology species. Foods 2020, 9, 142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, J.H.; Hwang, Y.P.; Lee, H.S.; Jeong, H.G. Inhibitory effect of Platycodi Radix on ovalbumin-induced airway inflammation in a murine model of asthma. Food. Chem. Toxicol. 2009, 47, 1272–1279. [Google Scholar] [CrossRef]
- Lee, K.J.; Kim, J.Y.; Choi, J.H.; Kim, H.G.; Chung, Y.C.; Roh, S.H.; Jeong, H.G. Inhibition of tumor invasion and metastasis by aqueous extract of the radix of Platycodon grandiflorum. Food. Chem. Toxicol. 2006, 44, 1890–1896. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Choi, Y.H.; Kang, H.S.; Choi, B.T. An aqueous extract of Platycodi radix inhibits LPS-induced NF-kappaB nuclear translocation in human cultured airway epithelial cells. Int. J. Mol. Med. 2004, 13, 843–847. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; He, J.; Ji, B.; Li, Y.; Zhang, X. Antihyperglycemic effects of Platycodon grandiflorum (Jacq.) A. DC. extract on streptozotocin-induced diabetic mice. Plant Foods Hum. Nutr. 2007, 62, 7–11. [Google Scholar] [CrossRef]
- Khanal, T.; Choi, J.H.; Hwang, Y.P.; Chung, Y.C.; Jeong, H.G. Saponins isolated from the root of Platycodon grandiflorum protect against acute ethanol-induced hepatotoxicity in mice. Food Chem. Toxicol. 2009, 47, 530–535. [Google Scholar] [CrossRef]
- Yoo, D.S.; Choi, Y.H.; Cha, M.R.; Lee, B.H.; Kim, S.J.; Yon, G.H.; Hong, K.S.; Jang, Y.S.; Lee, H.S.; Kim, Y.S.; et al. HPLC-ELSD analysis of 18 platycosides from balloon flower roots (Platycodi Radix) sourced from various regions in Korea and geographical clustering of the cultivation areas. Food Chem. 2011, 129, 645–651. [Google Scholar] [CrossRef]
- Zhang, L.L.; Huang, M.Y.; Yang, Y.; Huang, M.Q.; Shi, J.J.; Zou, L.; Lu, J.J. Bioactive platycodins from Platycodonis Radix: Phytochemistry, pharmacological activities, toxicology and pharmacokinetics. Food Chem. 2020, 327, 127029. [Google Scholar] [CrossRef]
- Han, L.K.; Xu, B.J.; Kimura, Y.; Zheng, Y.N.; Okuda, H. Platycodi radix affects lipid metabolism in mice with high fat diet–induced obesity. J. Nutr. 2000, 130, 2760–2764. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.J.; Choi, C.Y.; Chung, Y.C.; Kim, Y.S.; Ryu, S.Y.; Roh, S.H.; Jeong, H.G. Protective effect of saponins derived from roots of Platycodon grandiflorum on tert-butyl hydroperoxide-induced oxidative hepatotoxicity. Toxicol. Lett. 2004, 147, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Kim, J.S.; Choi, S.U.; Kim, J.S.; Lee, H.S.; Roh, S.H.; Jeong, Y.C.; Kim, Y.K.; Ryu, S.Y. Isolation of a new saponin and cytotoxic effect of saponins from the root of Platycodon grandiflorum on human tumor cell lines. Planta Med. 2005, 71, 566–568. [Google Scholar] [CrossRef]
- Ahn, K.S.; Noh, E.J.; Zhao, H.L.; Jung, S.H.; Kang, S.S.; Kim, Y.S. Inhibition of inducible nitric oxide synthase and cyclooxygenase II by Platycodon grandiflorum saponins via suppression of nuclear factor-κB activation in RAW 264.7 cells. Life Sci. 2005, 76, 2315–2328. [Google Scholar] [CrossRef]
- Wang, C.; Schuller Levis, G.B.; Lee, E.B.; Levis, W.R.; Lee, D.W.; Kim, B.S.; Park, S.Y.; Park, E. Platycodin D and D3 isolated from the root of Platycodon grandiflorum modulate the production of nitric oxide and secretion of TNF-α in activated RAW 264.7 cells. Int. Immunopharmacol. 2004, 4, 1039–1049. [Google Scholar] [CrossRef]
- Chin, Y.W.; Jung, Y.H.; Chae, H.S.; Yoon, K.D.; Kim, J. Anti-inflammatory constituents from the roots of Saposhnikovia divaricate. Bull. Korean Chem. Soc. 2011, 32, 2132–2134. [Google Scholar] [CrossRef] [Green Version]
- Shi, X.L.; Yan, J.K.; Li, W.K.; Donkor, P.O.; Gao, X.M.; Ding, L.Q.; Qiu, F. Two pairs of phenylpropanoid enantiomers from the leaves of Eucommia ulmoides. J. Asian Nat. Prod. Res. 2018, 20, 1045–1054. [Google Scholar] [CrossRef]
- Woo, K.W.; Suh, W.S.; Subedi, L.; Kim, S.Y.; Choi, S.U.; Kim, K.H.; Lee, K.R. Phenolic derivatives from the stems of Lagerstroemia indica and their biological activity. Heterocycles 2015, 91, 2355–2366. [Google Scholar] [CrossRef]
- Vichi, S.; Santini, C.; Natali, N.; Riponi, C.; Lopez-Tamames, E.; Buxaderas, S. Volatile and semi-volatile components of oak wood chips analysed by Accelerated Solvent Extraction (ASE) coupled to gas chromatography-mass spectrometry (GC-MS). Food Chem. 2007, 102, 1260–1269. [Google Scholar] [CrossRef]
- Hirose, K.; Akizawa, T.; Asada, K.; Tanaka, Y.; Negoro, Y.; Yoshioka, M. Syntheses of antigens conjugated with 3-methoxy-4-hydroxyphenyl-glycol by Mannich reaction for enzyme immunoassay. Anal. Chim. Acta 1998, 365, 137–145. [Google Scholar] [CrossRef]
- Landucci, L.L.; Ralph, S.A.; Hammel, K.E. 13C-NMR characterization of guaiacyl, guaiacyl/syringyl, and syringyl dehydrogenation polymers. Holzforschung 1998, 52, 160–170. [Google Scholar] [CrossRef]
- Rao, L.; You, Y.X.; Su, Y.; Fan, Y.; Liu, Y.; He, Q.; Chen, Y.; Meng, J.; Hu, L.; Li, Y.; et al. Lignans and neolignans with antioxidant and human cancer cell proliferation inhibitory activities from Cinnamomum bejolghota confirm its functional food property. J. Agric. Food Chem. 2020, 68, 8825–8835. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Wang, J. Phenolic Compounds from Selaginella moellendorfii. Chem. Biodivers. 2011, 8, 1735–1747. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Moon, E.; Choi, S.U.; Kim, S.Y.; Lee, K.R. Biological evaluation of phenolic constituents from the trunk of Berberis koreana. Bioorg. Med. Chem. Lett. 2011, 21, 2270–2273. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yang, H.J. Isolation and identification of lignans and other phenolic constituents from the stem bark of Albizia julibrissin Durazz and evaluation of their nitric oxide inhibitory activity. Molecules 2020, 25, 2065. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.H.; Akao, T.; Hamasaki, K.; Deyama, T.; Hattori, M. Biotransformation of pinoresinol diglucoside to mammalian lignans by human intestinal microflora, and isolation of Enterococcus faecalis strain PDG-1 responsible for the transformation of (+)-pinoresinol to (+)-lariciresinol. Chem. Pharm. Bull. 2003, 51, 508–515. [Google Scholar] [CrossRef] [Green Version]
- Liang, W.; Sun, L.Q.; Qian, F.; Tian, X.H. Chemical constituents from the whole plant of Liparis japonica. Biochem. Syst. Ecol. 2020, 92, 104126. [Google Scholar] [CrossRef]
- Huang, X.X.; Bai, M.; Zhou, L.; Lou, L.L.; Liu, Q.B.; Zhang, Y.; Li, L.Z.; Song, S.J. Food byproducts as a new and cheap source of bioactive compounds: Lignans with antioxidant and anti-inflammatory properties from Crataegus pinnatifida seeds. J. Agric. Food Chem. 2015, 63, 7252–7260. [Google Scholar] [CrossRef] [PubMed]
- Shataer, D.; Li, J.; Duan, X.M.; Liu, L.; Xin, X.L.; Aisa, H.A. Chemical composition of the hazelnut kernel (Corylus avellana L.) and its anti-inflammatory, antimicrobial, and antioxidant activities. J. Agric. Food Chem. 2021, 69, 4111–4119. [Google Scholar] [CrossRef]
- Nishiwaki, H.; Nakayama, K.; Shuto, Y.; Yamauchi, S. Synthesis of all stereoisomers of 3,3′-dimethoxy-7,7′-epoxylignane-4,4′-diol and their plant growth inhibitory activity. J. Agric. Food Chem. 2014, 62, 651–659. [Google Scholar] [CrossRef]
- Wang, L.Q.; Meselhy, M.R.; Li, Y.; Qin, G.W.; Hattori, M. Human intestinal bacteria capable of transforming secoisolariciresinol diglucoside to mammalian lignans, enterodiol and enterolactone. Chem. Pharm. Bull. 2000, 48, 1606–1610. [Google Scholar] [CrossRef] [Green Version]
- Shi, C.; Xu, M.J.; Bayer, M.; Deng, Z.W.; Kubbutat, M.H.G.; Waejen, W.; Proksch, P.; Lin, W.H. Phenolic compounds and their anti-oxidative properties and protein kinase inhibition from Chinese mangrove plant Laguncularia racemose. Phytochemistry 2010, 71, 435–442. [Google Scholar] [CrossRef]
- Kim, K.H.; Ha, S.K.; Choi, S.U.; Kim, S.Y.; Lee, K.R. Phenolic constituents from the twigs of Euonymus alatus and their cytotoxic and anti-inflammatory activity. Planta Med. 2013, 79, 361–364. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.H.; Zhao, P.; Wang, M.; Liang, Q. Naturally occurring furofuran lignans: Structural diversity and biological activities. Nat. Prod. Res. 2018, 1357–1373. [Google Scholar] [CrossRef] [PubMed]
- Ragab, F.A.; Eid, N.M.; Hassan, G.S.; Nissan, Y.M. Synthesis and anti-inflammatory activity of some benzofuran and benzopyran-4-one derivatives. Chem. Pharm. Bull. 2012, 60, 110–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miao, Y.; Hu, Y.; Yang, J.; Liu, T.; Sun, J.; Wang, X. Natural source, bioactivity and synthesis of benzofuran derivatives. RSC Adv. 2019, 9, 27510–27540. [Google Scholar] [CrossRef] [Green Version]

| 1 | 2 | |||
|---|---|---|---|---|
| Position | δH a (J/Hz) | δC b | δH a (J/Hz) | δC b |
| 1 | 129.2 | 129.2 | ||
| 2 | 6.72 d (1.9) | 109.1 | 6.72 d (1.9) | 109.1 |
| 3 | 146.9 | 146.9 | ||
| 4 | 145.9 | 145.9 | ||
| 5 | 6.82 d (8.0) | 114.4 | 6.84 d (8.0) | 114.4 |
| 6 | 6.70 dd (8.0, 1.9) | 120.9 | 6.70 dd (8.0, 1.9) | 120.9 |
| 7 | 3.97 d (6.1) | 84.2 | 3.98 d (6.1) | 84.2 |
| 8 | 3.80 ddd (6.1, 5.8, 3.4) | 73.6 | 3.80 ddd (6.1, 5.8, 3.4) | 73.6 |
| 9 | 3.99 dd (11.0, 3.4) 3.77 dd (11.0, 5.7) | 64.5 | 4.00 dd (11.0, 3.4) 3.77 dd (11.0, 5.7) | 64.5 |
| 1′ | 173.7 | 173.7 | ||
| 2′ | 2.24 t (7.5) | 34.1 | 2.25 t (7.5) | 34.1 |
| 3′ | 1.54 m c | 24.9 | 1.53 m | 24.9 |
| 4′ | 1.17–1.30 m | 29.1–29.7 | 1.18–1.27 m | 29.1–29.8 |
| 5′ | 1.17–1.30 m | 29.1–29.7 | 1.18–1.27 m | 29.1–29.8 |
| 6′ | 1.17–1.30 m | 29.1–29.7 | 1.18–1.27 m | 29.1–29.8 |
| 7′ | 1.17–1.30 m | 29.1–29.7 | 1.18–1.27 m | 29.1–29.8 |
| 8′ | 1.17–1.30 m | 29.1–29.7 | 1.94 m | 27.2 |
| 9′ | 1.17–1.30 m | 29.1–29.7 | 5.27 m | 129.7 |
| 10′ | 1.17–1.30 m | 29.1–29.7 | 5.27 m | 129.7 |
| 11′ | 1.17–1.30 m | 29.1–29.7 | 1.52 m | 24.9 |
| 12′ | 1.17–1.30 m | 29.1–29.7 | 5.27 m | 130.2 |
| 13′ | 1.17–1.30 m | 29.1–29.7 | 5.27 m | 130.2 |
| 14′ | 1.17–1.30 m | 31.9 | 1.95 m | 27.2 |
| 15′ | 1.17–1.30 m | 22.7 | 1.18–1.27 m | 29.6 |
| 16′ | 0.81 t (7.5) | 14.1 | 1.18–1.27 m | 31.9 |
| 17′ | 1.18–1.27 m | 22.6 | ||
| 18′ | 0.81 t (7.5) | 14.1 | ||
| 3-OMe | 3.82 s | 56.0 | 3.84 s | 56.0 |
| 7-OMe | 3.18 s | 56.7 | 3.19 s | 56.7 |
| IC50 (µM) a | IC50 (µM) a | ||||||
|---|---|---|---|---|---|---|---|
| IL-12 p40 | IL-6 | TNF-α | IL-12 p40 | IL-6 | TNF-α | ||
| 1 | >100 | 8.1 ± 0.2 | 19.6 ± 0.5 | 12 | 56.2 ± 1.3 | 62.7 ± 3.1 | >100 |
| 2 | >100 | 6.5 ± 0.8 | 17.8 ± 1.1 | 13 | >100 | >100 | >100 |
| 3 | >100 | 9.2 ± 1.1 | 20.2 ± 0.9 | 14 | >100 | >100 | >100 |
| 4 | 29.7 ± 3.2 | 5.0 ± 0.1 | 16.5 ± 3.0 | 15 | 29.7 ± 1.5 | 42.6 ± 0.7 | 20.6 ± 0.2 |
| 5 | 35.2 ± 0.7 | 5.1 ± 0.2 | 17.1 ± 2.1 | 16 | 21.3 ± 0.2 | 19.8 ± 0.8 | 18.9 ± 1.0 |
| 6 | 40.0 ± 0.1 | 7.7 ± 0.1 | 14.2 ± 0.2 | 17 | 10.1 ± 0.6 | 19.2 ± 1.8 | 18.1 ± 0.4 |
| 7 | >100 | >100 | >100 | 18 | 60.6 ± 2.1 | 58.7 ± 2.9 | 49.3 ± 0.3 |
| 8 | >100 | >100 | >100 | 19 | 22.3 ± 0.9 | 30.7 ± 2.0 | 46.9 ± 1.3 |
| 9 | >100 | >100 | >100 | 20 | >100 | >100 | >100 |
| 10 | >100 | >100 | >100 | 21 | >100 | >100 | >100 |
| 11 | >100 | >100 | >100 | SB203580 b | 5.3 ± 0.1 | 3.2 ± 0.2 | 8.1 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Yang, H.J. Phenolic Constituents from Platycodon grandiflorum Root and Their Anti-Inflammatory Activity. Molecules 2021, 26, 4530. https://doi.org/10.3390/molecules26154530
Li W, Yang HJ. Phenolic Constituents from Platycodon grandiflorum Root and Their Anti-Inflammatory Activity. Molecules. 2021; 26(15):4530. https://doi.org/10.3390/molecules26154530
Chicago/Turabian StyleLi, Wei, and Hye Jin Yang. 2021. "Phenolic Constituents from Platycodon grandiflorum Root and Their Anti-Inflammatory Activity" Molecules 26, no. 15: 4530. https://doi.org/10.3390/molecules26154530
APA StyleLi, W., & Yang, H. J. (2021). Phenolic Constituents from Platycodon grandiflorum Root and Their Anti-Inflammatory Activity. Molecules, 26(15), 4530. https://doi.org/10.3390/molecules26154530







