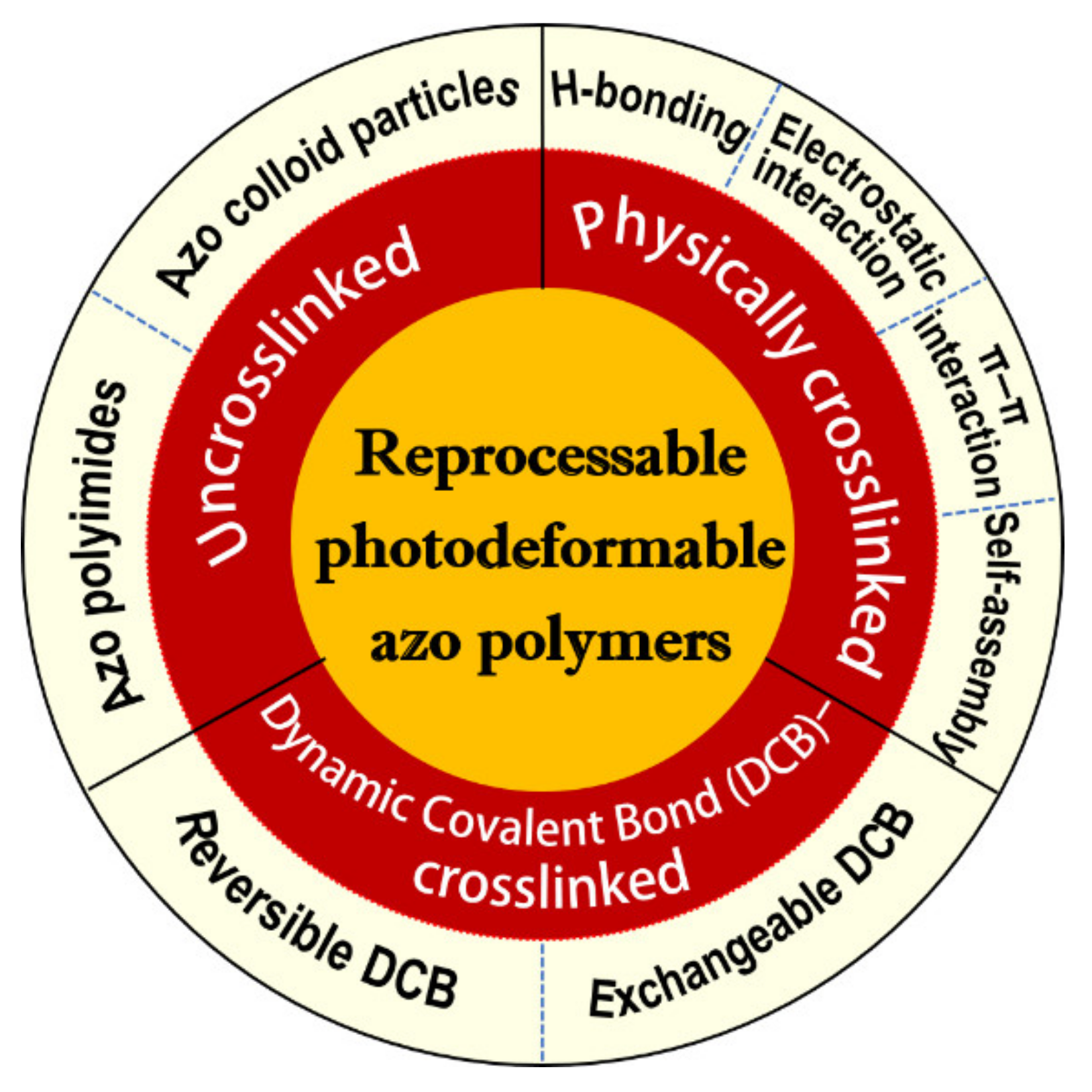
Reprocessable Photodeformable Azobenzene Polymers
Abstract
:1. Introduction
2. Reprocessable Physically Crosslinked Photodeformable Azo Polymers
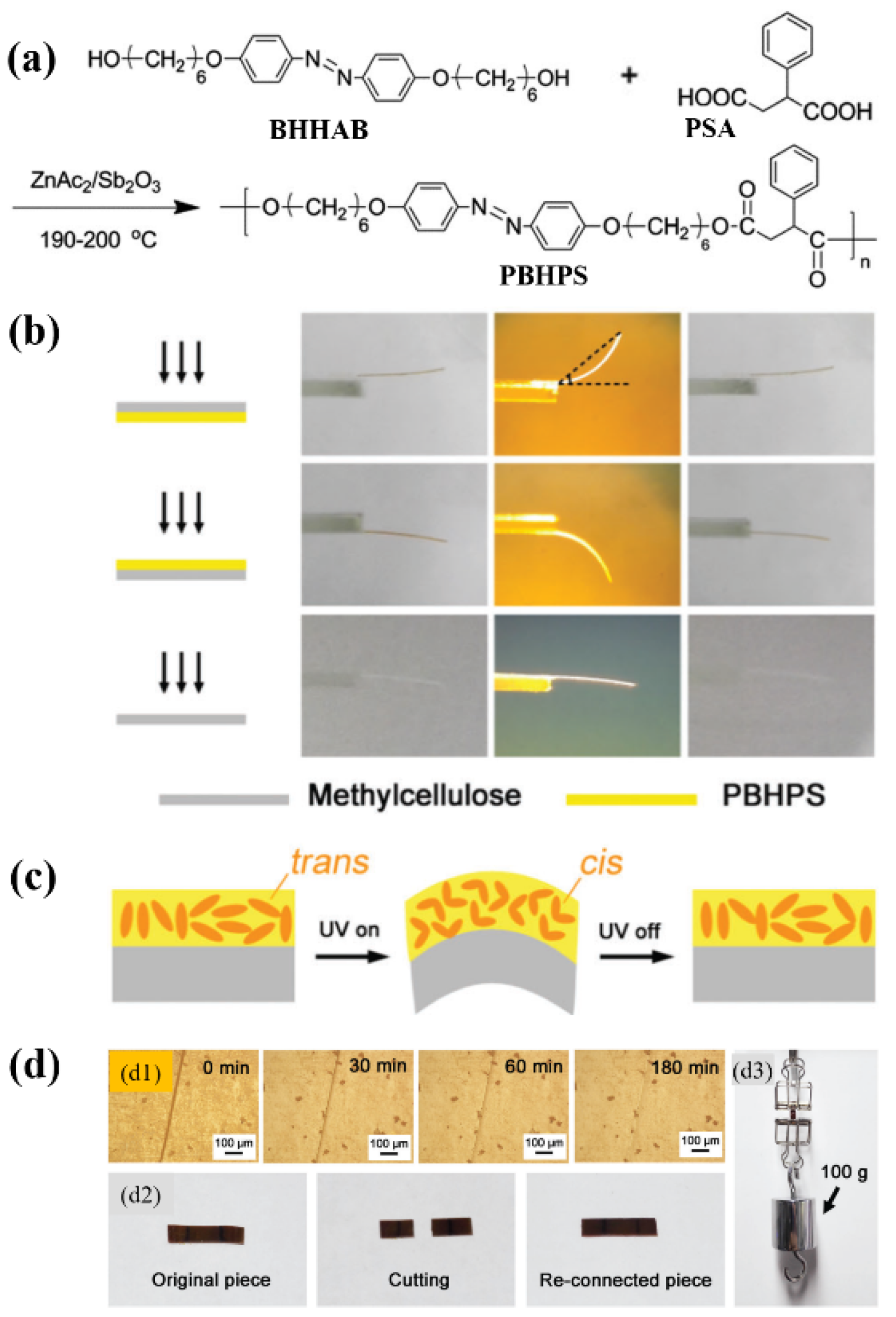
2.1. Hydrogen Bonding (H-bonding) Interactions
2.1.1. H-Bond-Crosslinked Photodeformable Side-Chain Azo Polymers
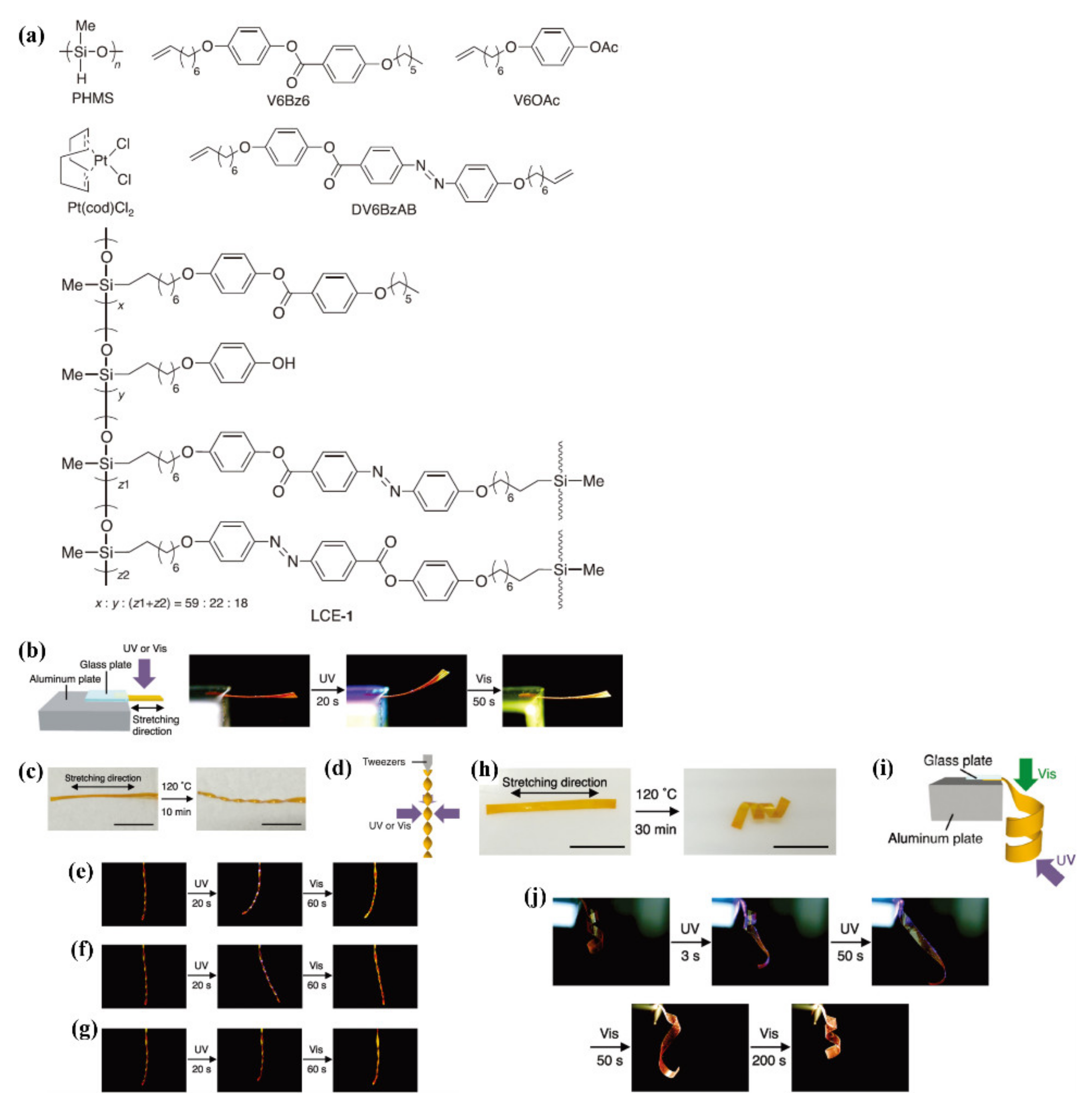
2.1.2. H-Bond-Crosslinked Photodeformable Main-Chain Azo Polymers
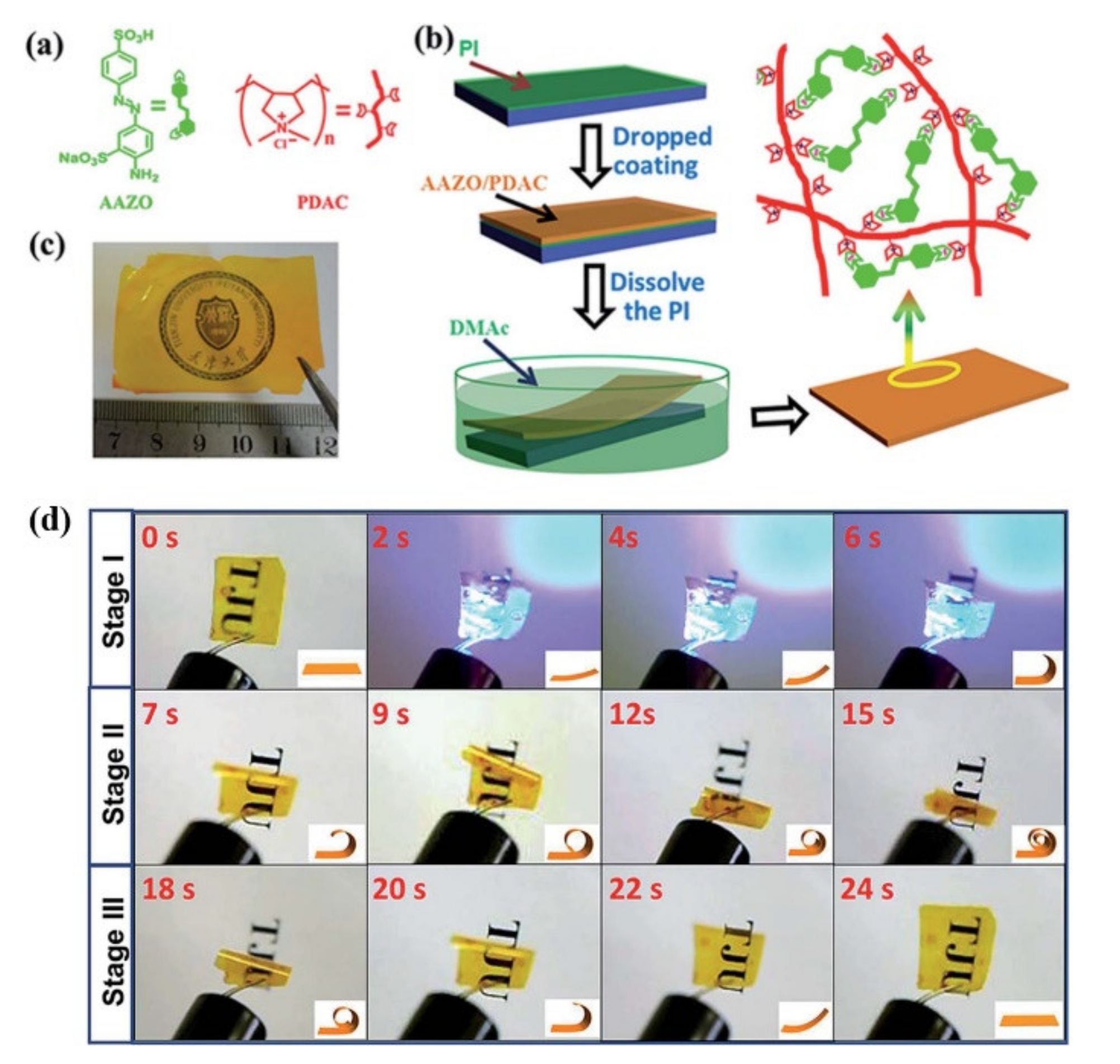
2.2. Electrostatic Interaction
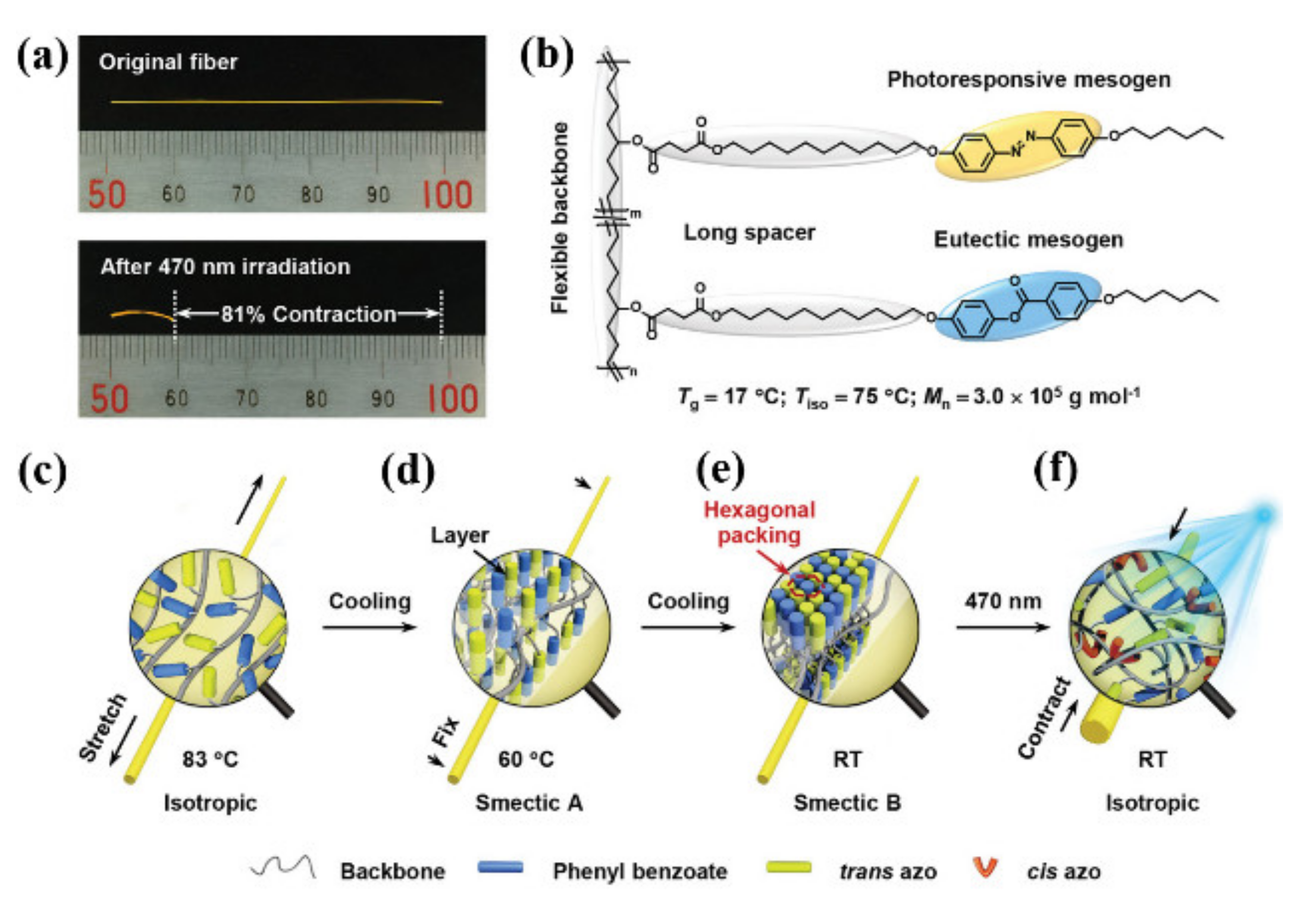
2.3. π–π Interaction
2.4. Self-Assembly-Induced Physical Crosslinking
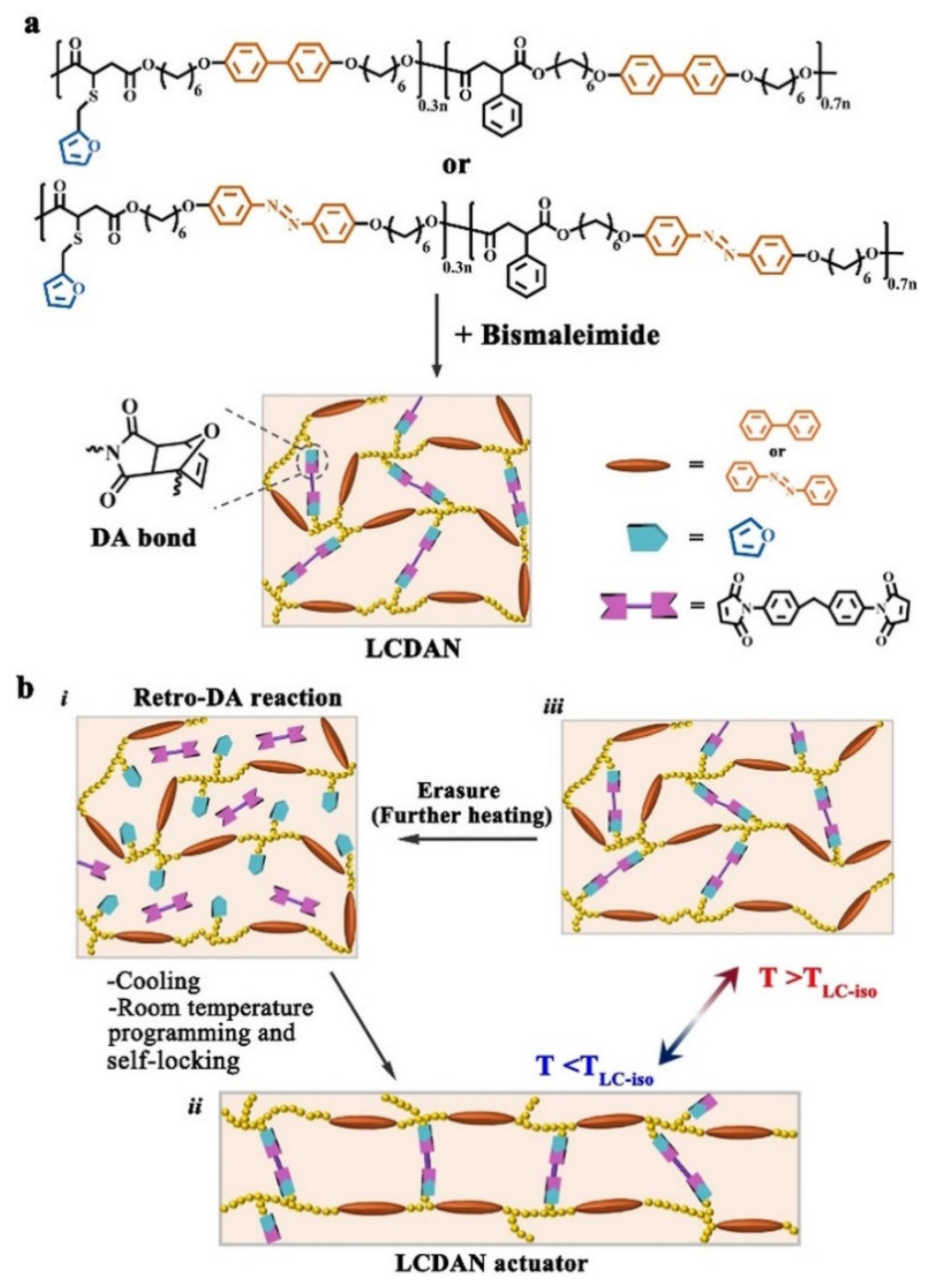
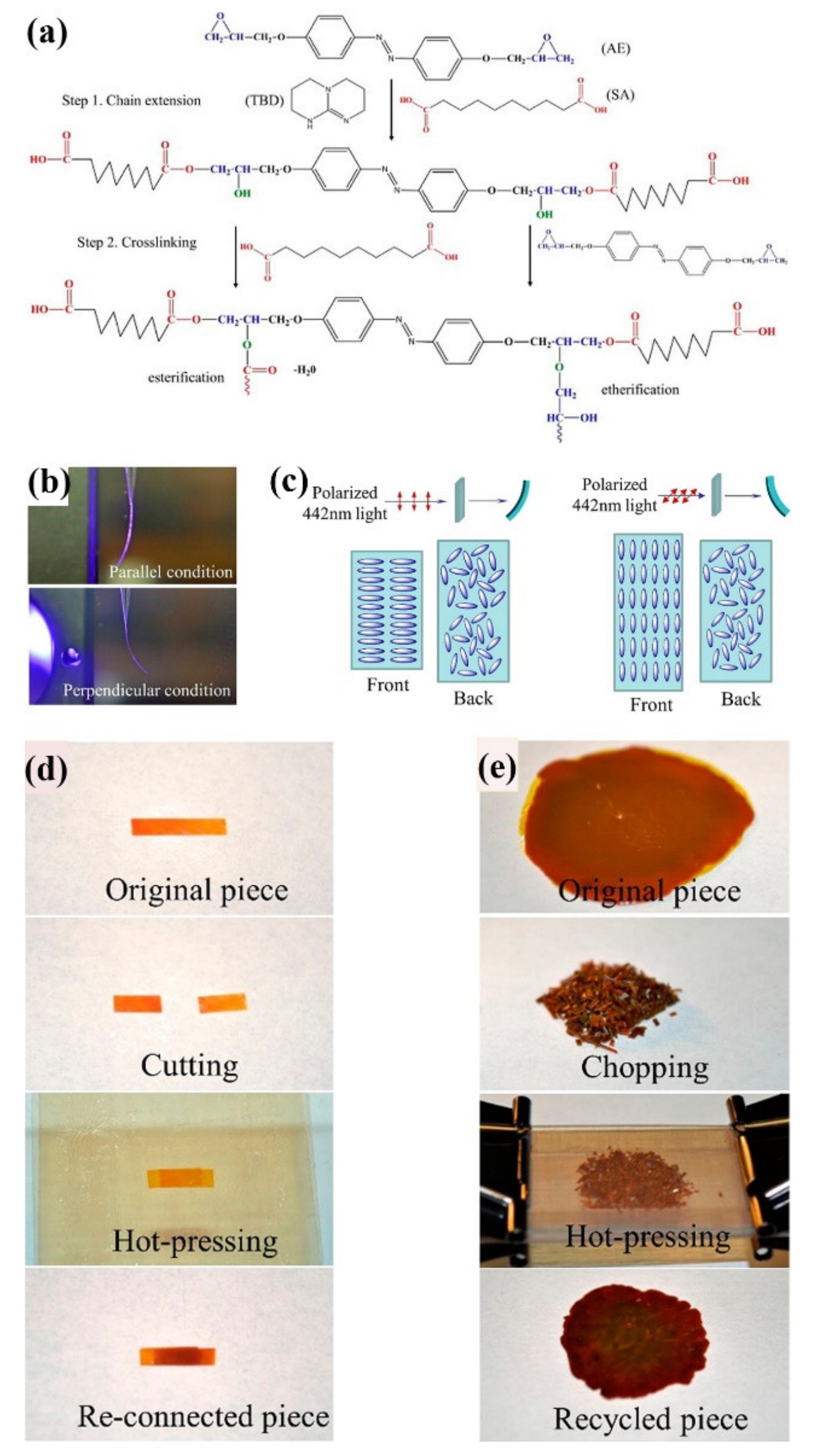
3. Reprocessable Photodeformable Azo Polymers with Dynamic Covalent Bond (DCB)-Crosslinked Networks
4. Some Other Reprocessable Uncrosslinked Photodeformable Azo Polymers
5. Conclusions and Outlook
Funding
Conflicts of Interest
References
- Li, M.-H.; Keller, P. Artificial muscles based on liquid crystal elastomers. Philos. Trans. R. Soc. Lond. Ser. A Math. Phys. Eng. Sci. 2006, 364, 2763–2777. [Google Scholar] [CrossRef]
- Ikeda, T.; Mamiya, J.-I.; Yu, Y. Photomechanics of liquid-crystalline elastomers and other polymers. Angew. Chem. Int. Ed. 2007, 46, 506–528. [Google Scholar] [CrossRef]
- Ohm, C.; Brehmer, M.; Zentel, R. Liquid crystalline elastomers as actuators and sensors. Adv. Mater. 2010, 22, 3366–3387. [Google Scholar] [CrossRef]
- Ikeda, T.; Ube, T. Photomobile polymer materials: From nano to macro. Mater. Today 2011, 14, 480–487. [Google Scholar] [CrossRef]
- Yu, H.; Ikeda, T. Photocontrollable liquid-crystalline actuators. Adv. Mater. 2011, 23, 2149–2180. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Ye, G.; Wang, X.; Keller, P. Micron-sized liquid crystalline elastomer actuators. Soft Matter 2011, 7, 815–823. [Google Scholar] [CrossRef]
- Jiang, H.; Li, C.; Huang, X. Actuators based on liquid crystalline elastomer materials. Nanoscale 2013, 5, 5225–5240. [Google Scholar] [CrossRef] [PubMed]
- Ube, T.; Ikeda, T. Photomobile polymer materials with crosslinked liquid-crystalline structures: Molecular design, fabrication, and functions. Angew. Chem. Int. Ed. 2014, 53, 10290–10299. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Broer, D.J. Programmable and adaptive mechanics with liquid crystal polymer networks and elastomers. Nat. Mater. 2015, 14, 1087–1098. [Google Scholar] [CrossRef]
- Bushuyev, O.S.; Aizawa, M.; Shishido, A.; Barrett, C.J. Shape-shifting azo dye polymers: Towards sun-light-driven molecular devices. Macromol. Rapid Commun. 2018, 39, 1700253. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.-C.; Xiao, Y.; Zhao, Y. Shining light on liquid crystal polymer networks: Preparing, reconfiguring, and driving soft actuators. Adv. Opt. Mater. 2019, 7, 1900262. [Google Scholar] [CrossRef]
- Ube, T.; Ikeda, T. Photomobile polymer materials with complex 3D deformation, continuous motions, self-regulation, and enhanced processability. Adv. Opt. Mater. 2019, 7, 1900380. [Google Scholar] [CrossRef]
- Pang, X.; Lv, J.; Zhu, C.; Qin, L.; Yu, Y. Photodeformable azobenzene-containing liquid crystal polymers and soft actuators. Adv. Mater. 2019, 31, 1904224. [Google Scholar] [CrossRef]
- McCracken, J.M.; Donovan, B.R.; White, T.J. Materials as machines. Adv. Mater. 2020, 32, 1906564. [Google Scholar] [CrossRef]
- Chen, M.; Liang, S.; Liu, C.; Liu, Y.; Wu, S. Reconfigurable and recyclable photoactuators based on azobenzene-containing polymers. Front. Chem. 2020, 8, 706. [Google Scholar] [CrossRef]
- Kumar, G.S.; Neckers, D.C. Photochemistry of azobenzene-containing polymers. Chem. Rev. 1989, 89, 1915–1925. [Google Scholar] [CrossRef]
- Chang, V.Y.; Fedele, C.; Priimagi, A.; Shishido, A.; Barrett, C.J. Photoreversible soft azo dye materials: Toward optical control of bio-interfaces. Adv. Opt. Mater. 2019, 7, 1900091. [Google Scholar] [CrossRef]
- Merian, E. Steric factor influencing the dyeing of hydrophobic fibers. Text. Res. J. 1966, 36, 612–618. [Google Scholar] [CrossRef]
- Matĕjka, L.; Dušek, K.; Ilavský, M. The thermal effect in the photomechanical conversion of a photochromic polymer. Polym. Bull. 1979, 1, 659–664. [Google Scholar] [CrossRef]
- Eisenbach, C.D. Isomerization of aromatic azo chromophores in poly(ethyl acrylate) networks and photomechanical effect. Polymer 1980, 21, 1175–1179. [Google Scholar] [CrossRef]
- Finkelmann, H.; Nishikawa, E.; Pereira, G.G.; Warner, M. A new opto-mechanical effect in solids. Phys. Rev. Lett. 2001, 87, 015501. [Google Scholar] [CrossRef]
- Yu, Y.; Nakano, M.; Ikeda, T. Photomechanics: Directed bending of a polymer film by light. Nature 2003, 425, 145. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Nakano, M.; Shishido, A.; Shiono, T.; Ikeda, T. Effect of cross-linking density on photoinduced bending behavior of oriented liquid-crystalline network films containing azobenzene. Chem. Mater. 2004, 16, 1637–1643. [Google Scholar] [CrossRef]
- De Gennes, P.-G.; Hébert, M.; Kant, R. Artificial muscles based on nematic gels. Macromol. Symp. 1997, 113, 39–49. [Google Scholar] [CrossRef]
- Bublitz, D.; Helgert, M.; Fleck, B.; Wenke, L.; Hvilstedt, S.; Ramanujam, P.S. Photoinduced deformation of azobenzene polyester films. Appl. Phys. B 2000, 70, 863–865. [Google Scholar] [CrossRef]
- Priimagi, A.; Shimamura, A.; Kondo, M.; Hiraoka, T.; Kubo, S.; Mamiya, J.I.; Kinoshita, M.; Ikeda, T.; Shishido, A. Location of the azobenzene moieties within the cross-linked liquid-crystalline polymers can dictate the direction of photoinduced bending. ACS Macro Lett. 2012, 1, 96–99. [Google Scholar] [CrossRef]
- Petrova, T.; Toshchevikov, V.; Saphiannikova, M. Light-induced deformation of polymer networks containing azobenzene chromophores and liquid crystalline mesogens. Soft Matter 2015, 11, 3412–3423. [Google Scholar] [CrossRef]
- Toshchevikov, V.; Petrova, T.; Saphiannikova, M. Kinetics of light-induced ordering and deformation in LC azobenzene-containing materials. Soft Matter 2017, 13, 2823–2835. [Google Scholar] [CrossRef]
- Fang, L.; Zhang, H.T.; Li, Z.; Zhang, Y.; Zhang, Y.Y.; Zhang, H. Synthesis of reactive azobenzene main-chain liquid crystalline polymers via Michael addition polymerization and photomechanical effects of their supramolecular hydrogen-bonded fibers. Macromolecules 2013, 46, 7650–7660. [Google Scholar] [CrossRef]
- Fang, L.; Han, G.; Zhang, J.; Zhang, H.T.; Zhang, H. Synthesis of well-defined easily crosslinkable azobenzene side-chain liquid crystalline polymers via reversible addition-fragmentation chain transfer polymerization and photomechanical properties of their post-crosslinked fibers. Eur. Polym. J. 2015, 69, 592–604. [Google Scholar] [CrossRef]
- Nie, J.; Liu, X.; Yan, Y.; Zhang, H. Supramolecular hydrogen-bonded photodriven actuators based on an azobenzene-containing main-chain liquid crystalline poly(ester-amide). J. Mater. Chem. C 2017, 5, 10391–10398. [Google Scholar] [CrossRef]
- Dong, H.; Liu, G.; Zhang, H. Preparation of photodeformable azobenzene polymer fibers by postcrosslinking strategy: Understanding the structure-property relationship. Eur. Polym. J. 2020, 135, 109863. [Google Scholar] [CrossRef]
- Mamiya, J.; Yoshitake, A.; Kondo, M.; Yu, Y.; Ikeda, T. Is chemical crosslinking necessary for the photoinduced bending of polymer films? J. Mater. Chem. 2008, 18, 63–65. [Google Scholar] [CrossRef]
- Ozawa, T.; Kondo, M.; Mamiya, J.; Ikeda, T. Enhancement of mechanical stability in hydrogenbonded photomobile materials with chemically modified single-walled carbon nanotubes. J. Mater. Chem. C 2014, 2, 2313–2315. [Google Scholar] [CrossRef]
- Wang, J.; Huang, S.; Zhang, Y.; Liu, J.; Yu, M.; Yu, H. Hydrogen bond enhances photomechanical swing of liquid-crystalline polymer bilayer films. ACS Appl. Mater. Interfaces 2021, 13, 6585–6596. [Google Scholar] [CrossRef]
- Yan, M.; Tang, J.; Xie, H.-L.; Ni, B.; Zhang, H.-L.; Chen, E.-Q. Self-healing and phase behavior of liquid crystalline elastomer based on a block copolymer constituted of a side-chain liquid crystalline polymer and a hydrogen bonding block. J. Mater. Chem. C 2015, 3, 8526–8534. [Google Scholar] [CrossRef]
- Ni, B.; Xie, H.-L.; Tang, J.; Zhang, H.-L.; Chen, E.-Q. A self-healing photoinduced-deformable material fabricated by liquid crystalline elastomers using multivalent hydrogen bonds as cross-linkers. Chem. Commun. 2016, 52, 10257–10260. [Google Scholar] [CrossRef]
- Si, Q.; Feng, Y.; Yang, W.; Fu, L.; Yan, Q.; Dong, L.; Long, P.; Feng, W. Controllable and stable deformation of a self-healing photo-responsive supramolecular assembly for an optically actuated manipulator arm. ACS Appl. Mater. Interfaces 2018, 10, 29909–29917. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.-Y.; Mu, J.-H.; Fu, Y.-L.; Zhang, Y.-C.; Han, J.-S.; Zhao, R.-Y.; Zhao, J.; Wang, Z.-H.; Zhao, Z.-C.; Li, W.-J.; et al. Azobenzene based Photo-responsive mechanical actuator fabricated by intermolecular H-bond interaction. Chin. J. Polym. Sci. 2021, 39, 417–424. [Google Scholar] [CrossRef]
- Cheng, Z.; Wang, T.; Li, X.; Zhang, Y.; Yu, H. NIR-vis-UV light-responsive actuator films of polymer-dispersed liquid crystal/graphene oxide nanocomposites. ACS Appl. Mater. Interfaces 2015, 7, 27494–27501. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, Q.; Lv, X.; Gao, L.; Fang, S.; Yu, H. Photoinduced triple shape memory polyurethane enabled by doping with azobenzene and GO. J. Mater. Chem. C 2016, 4, 9993–9997. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhang, L.; Weis, P.; Naumov, P.; Wu, S. A solar actuator based on hydrogen-bonded azopolymers for electricity generation. J. Mater. Chem. A 2018, 6, 3361–3366. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.-Z.; Zhang, H.-Q. Synthesis of an azobenzene-containing main-chain crystalline polymer and photodeformation behaviors of its supramolecular hydrogen-bonded fibers. Chin. J. Polym. Sci. 2020, 38, 37–44. [Google Scholar] [CrossRef]
- Wie, J.J.; Wang, D.H.; Lee, K.M.; White, T.J.; Tan, L.-S. The contribution of hydrogen bonding to the photomechanical response of azobenzene-functionalized polyamides. J. Mater. Chem. C 2018, 6, 5964–5974. [Google Scholar] [CrossRef]
- Ban, J.; Mu, L.; Yang, J.; Chen, S.; Zhuo, H. New stimulus-responsive shape-memory polyurethanes capable of UV light-triggered deformation, hydrogen bond-mediated fixation, and thermal-induced recovery. J. Mater. Chem. A 2017, 5, 14514–14518. [Google Scholar] [CrossRef]
- Li, S.; Han, G.; Zhang, W. Concise synthesis of photoresponsive polyureas containing bridged azobenzenes as visible-light-driven actuators and reversible photopatterning. Macromolecules 2018, 51, 4290–4297. [Google Scholar] [CrossRef]
- Li, S.; Tu, Y.; Bai, H.; Hibi, Y.; Wiesner, L.W.; Pan, W.; Wang, K.; Giannelis, E.P.; Shepherd, R.F. Simple synthesis of elastomeric photomechanical switches that self-heal. Macromol. Rapid Commun. 2019, 40, 1800815. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Feng, Y.; Luo, W.; Cao, C.; Hu, W.; Feng, W. A supramolecular assembly of cross-linked azobenzene/polymers for a high-performance light-driven actuator. J. Mater. Chem. A 2015, 3, 16453–16460. [Google Scholar] [CrossRef]
- Qin, C.; Feng, Y.; An, H.; Han, J.; Cao, C.; Feng, W. Tetracarboxylated azobenzene/polymer supramolecular assemblies as high-performance multiresponsive actuators. ACS Appl. Mater. Interfaces 2017, 9, 4066–4073. [Google Scholar] [CrossRef]
- Zhong, H.-Y.; Chen, L.; Yang, R.; Meng, Z.-Y.; Ding, X.-M.; Liu, X.-F.; Wang, Y.-Z. Azobenzene-containing liquid crystalline polyester with π-π interactions: Diverse thermo- and photo-responsive behaviours. J. Mater. Chem. C 2017, 5, 3306–3314. [Google Scholar] [CrossRef]
- Zhong, H.-Y.; Chen, L.; Liu, X.-F.; Yang, R.; Wang, Y.-Z. Novel liquid crystalline copolyester containing amphi-mesogenic units toward multiple stimuliresponse behaviors. J. Mater. Chem. C 2017, 5, 9702–9711. [Google Scholar] [CrossRef]
- Choi, H.J.; Jeong, K.-U.; Chien, L.-C.; Lee, M.-H. Photochromic 3-dimensional actuator based on an uncrosslinked liquid crystal elastomer. J. Mater. Chem. 2009, 19, 7124–7129. [Google Scholar] [CrossRef]
- Kim, D.-Y.; Lee, S.-A.; Choi, H.J.; Chien, L.-C.; Lee, M.-H.; Jeong, K.-U. Reversible actuating and writing behaviours of a head-to-side connected main-chain photochromic liquid crystalline polymer. J. Mater. Chem. C 2013, 1, 1375–1382. [Google Scholar] [CrossRef]
- Kim, D.-Y.; Shin, S.; Yoon, W.-J.; Choi, Y.-J.; Hwang, J.-K.; Kim, J.-S.; Lee, C.-R.; Choi, T.-L.; Jeong, K.-U. From smart denpols to remote-controllable actuators: Hierarchical superstructures of azobenzene-based polynorbornenes. Adv. Funct. Mater. 2017, 27, 1606294. [Google Scholar] [CrossRef]
- Hosono, N.; Kajitani, T.; Fukushima, T.; Ito, K.; Sasaki, S.; Takata, M.; Aida, T. Large-area three-dimensional molecular ordering of a polymer brush by one-step processing. Science 2010, 330, 808–811. [Google Scholar] [CrossRef] [Green Version]
- Lv, J.A.; Liu, Y.; Wei, J.; Chen, E.; Qin, L.; Yu, Y. Photocontrol of fluid slugs in liquid crystal polymer microactuators. Nature 2016, 537, 179–184. [Google Scholar] [CrossRef]
- Xu, B.; Zhu, C.; Qin, L.; Wei, J.; Yu, Y. Light-directed liquid manipulation in flexible bilayer microtubes. Small 2019, 15, 1901847. [Google Scholar] [CrossRef]
- Pang, X.; Qin, L.; Xu, B.; Liu, Q.; Yu, Y. Ultralarge contraction directed by light-driven unlocking of prestored strain energy in linear liquid crystal polymer fibers. Adv. Funct. Mater. 2020, 30, 2002451. [Google Scholar] [CrossRef]
- Chen, M.; Yao, B.; Kappl, M.; Liu, S.; Yuan, J.; Berger, R.; Zhang, F.; Butt, H.-J.; Liu, Y.; Wu, S. Entangled azobenzene-containing polymers with photoinduced reversible solid-to-liquid transitions for healable and reprocessable photoactuators. Adv. Funct. Mater. 2020, 30, 1906752. [Google Scholar] [CrossRef]
- Petr, M.; Katzman, B.-A.; DiNatale, W.; Hammond, P.T. Synthesis of a new, low-Tg siloxane thermoplastic elastomer with a functionalizable backbone and its use as a rapid, room temperature photoactuator. Macromolecules 2013, 46, 2823–2832. [Google Scholar] [CrossRef]
- Han, G.; Nie, J.; Zhang, H. Facile preparation of recyclable photodeformable azobenzene polymer fibers with chemically crosslinked networks. Polym. Chem. 2016, 7, 5088–5092. [Google Scholar] [CrossRef]
- Guo, C.; Gao, J.; Ma, S.; Zhang, H. Efficient preparation of chemically crosslinked recyclable photodeformable azobenzene polymer fibers with high processability and reconstruction ability via a facile post-crosslinking method. Eur. Polym. J. 2020, 139, 109998. [Google Scholar] [CrossRef]
- Jiang, Z.-C.; Xiao, Y.-Y.; Yin, L.; Han, L.; Zhao, Y. “Self-lockable” liquid crystalline Diels–Alder dynamic network actuators with room temperature programmability and solution reprocessability. Angew. Chem. Int. Ed. 2020, 59, 4925–4931. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, K.; Ube, T.; Ikeda, T. Remoldable crosslinked liquid-crystalline polysiloxane with side chain mesogens based on exchangeable crosslinks. Mol. Cryst. Liq. Cryst. 2015, 614, 62–66. [Google Scholar] [CrossRef]
- Ube, T.; Kawasaki, K.; Ikeda, T. Photomobile liquid-crystalline elastomers with rearrangeable networks. Adv. Mater. 2016, 28, 8212–8217. [Google Scholar] [CrossRef] [PubMed]
- Tsunoda, H.; Kawasaki, K.; Ube, T.; Ikeda, T. Liquid-crystalline elastomer photoactuator with photorearrangeable network structures. Mol. Cryst. Liq. Cryst. 2018, 662, 61–67. [Google Scholar] [CrossRef]
- Matsushita, M.; Kawasaki, K.; Ube, T.; Ikeda, T. Remolding of photoresponsive polymer materials by means of dynamic covalent bonds in a main chain. Mol. Cryst. Liq. Cryst. 2018, 676, 17–23. [Google Scholar] [CrossRef]
- Li, Y.; Rios, O.; Keum, J.K.; Chen, J.; Kessler, M.R. Photoresponsive liquid crystalline epoxy networks with shape memory behavior and dynamic ester bonds. ACS Appl. Mater. Interfaces 2016, 8, 15750–15757. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Rios, O.; Keum, J.K.; Kessler, M.R. Photo-responsive liquid crystalline epoxy networks with exchangeable disulfide bonds. RSC Adv. 2017, 7, 37248–37254. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.; Guo, S.; Tong, X.; Xia, H.; Zhao, Y. Tunable photocontrolled motions using stored strain energy in malleable azobenzene liquid crystalline polymer actuators. Adv. Mater. 2017, 29, 1606467. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, H.; Fei, G.; Yu, B.; Tong, X.; Xia, H.; Zhao, Y. Liquid-crystalline dynamic networks doped with gold nanorods showing enhanced photocontrol of actuation. Adv. Mater. 2018, 30, 1706597. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.M.; Wang, D.H.; Koerner, H.; Vaia, R.A.; Tan, L.-S.; White, T.J. Enhancement of photogenerated mechanical force in azobenzene-functionalized polyimides. Angew. Chem. Int. Ed. 2012, 51, 4117–4121. [Google Scholar] [CrossRef] [PubMed]
- White, T.J. Light to work transduction and shape memory in glassy, photoresponsive macromolecular systems: Trends and opportunities. J. Polym. Sci. Part B Polym. Phys. 2012, 50, 877–880. [Google Scholar] [CrossRef]
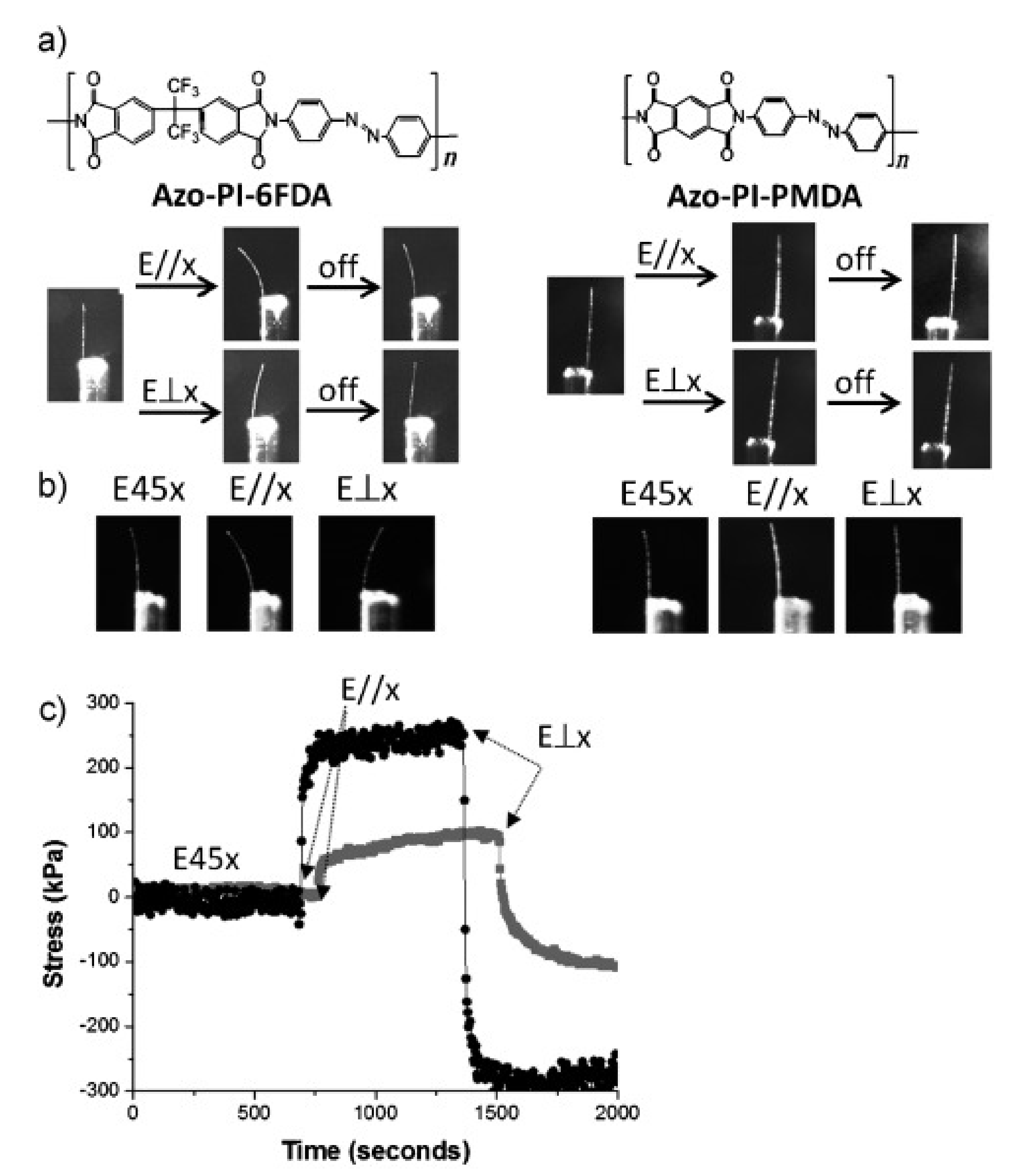
- Wang, D.H.; Wie, J.J.; Lee, K.M.; White, T.J.; Tan, L.-S. Impact of backbone rigidity on the photomechanical response of glassy, azobenzene-functionalized polyimides. Macromolecules 2014, 47, 659–667. [Google Scholar] [CrossRef]
- Wie, J.J.; Wang, D.H.; Lee, K.M.; Tan, L.-S.; White, T.J. Molecular engineering of azobenzene-functionalized polyimides to enhance both photomechanical work and motion. Chem. Mater. 2014, 26, 5223–5230. [Google Scholar] [CrossRef]
- Baczkowski, M.L.; Wang, D.H.; Lee, D.H.; Lee, K.M.; Smith, M.L.; White, T.J.; Tan, L.-S. Photomechanical deformation of azobenzene-functionalized polyimides synthesized with bulky substituents. ACS Macro Lett. 2017, 6, 1432–1437. [Google Scholar] [CrossRef]
- Skandani, A.A.; Chatterjee, S.; Wang, D.H.; Tan, L.-S.; White, T.J.; Shankar, M.R.; Smith, M.L. Relaxation dynamics and strain persistency of azobenzene-functionalized polymers and actuators. Macromol. Mater. Eng. 2017, 302, 1700256. [Google Scholar] [CrossRef]
- Li, Y.; He, Y.; Tong, X.; Wang, X.G. Photoinduced deformation of amphiphilic azo polymer colloidal spheres. J. Am. Chem. Soc. 2005, 127, 2402–2403. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.B.; He, Y.N.; Tong, X.L.; Wang, X.G. Stretching effect of linearly polarized Ar+ laser single-beam on azo polymer colloidal spheres. Langmuir 2006, 22, 2288–2291. [Google Scholar] [CrossRef]
- Liu, J.P.; He, Y.N.; Wang, X.G. Azo polymer colloidal spheres containing different amounts of functional groups and their photoinduced deformation behavior. Langmuir 2008, 24, 678–682. [Google Scholar] [CrossRef]
- Liu, J.P.; He, Y.N.; Wang, X.G. Size-dependent light-driven effect observed for azo polymer colloidal spheres with different average diameters. Langmuir 2009, 25, 5974–5979. [Google Scholar] [CrossRef]
- Liu, J.P.; He, Y.N.; Wang, X.G. Influence of chromophoric electronwithdrawing groups on photoinduced deformation of azo polymer colloids. Polymer 2010, 51, 2879–2886. [Google Scholar] [CrossRef]
- Wang, D.R.; Ye, G.; Wang, X.G. Synthesis of aminoazobenzene-containing diblock copolymer and photoinduced deformation behavior of its micelle-like aggregates. Macromol. Rapid Commun. 2007, 28, 2237–2243. [Google Scholar] [CrossRef]
- Wang, D.R.; Ye, G.; Zhu, Y.; Wang, X.G. Photoinduced mass-migration behavior of two amphiphilic side-chain azo diblock copolymers with different length flexible spacers. Macromolecules 2009, 42, 2651–2657. [Google Scholar] [CrossRef]
- Wang, D.R.; Liu, J.P.; Ye, G.; Wang, X.G. Amphiphilic block copolymers bearing strong push-pull azo chromophores: Synthesis, micelle formation and photoinduced shape deformation. Polymer 2009, 50, 418–427. [Google Scholar] [CrossRef]
- Wang, D.; Wang, X.G. Amphiphilic azo polymers: Molecular engineering, self-assembly and photoresponsive properties. Prog. Polym. Sci. 2013, 38, 271–301. [Google Scholar] [CrossRef]
- Natansohn, A.; Rochon, P. Photoinduced motions in azo-containing polymers. Chem. Rev. 2002, 102, 4139–4175. [Google Scholar] [CrossRef]
- Lee, S.; Kang, H.S.; Park, J.-K. Directional photofluidization lithography: Micro/nanostructural evolution by photofluidic motions of azobenzene materials. Adv. Mater. 2012, 24, 2069–2103. [Google Scholar] [CrossRef]
- Seki, T. Meso- and microscopic motions in photoresponsive liquid crystalline polymer films. Macromol. Rapid Commun. 2014, 35, 271–290. [Google Scholar] [CrossRef]
- Brunsveld, L.; Folmer, B.J.B.; Meijer, E.W.; Sijbesma, R.P. Supramolecular polymers. Chem. Rev. 2001, 101, 4071–4097. [Google Scholar] [CrossRef]
- Broer, D.J.; Bastiaansen, C.M.W.; Debije, M.G.; Schenning, A.P.H.J. Functional organic materials based on polymerized liquid-crystal monomers: Supramolecular hydrogen-bonded systems. Angew. Chem. Int. Ed. 2012, 51, 7102–7109. [Google Scholar] [CrossRef]
- Vapaavuori, J.; Bazuin, C.G.; Priimagi, A. Supramolecular design principles for efficient photoresponsive polymer-azobenzene complexes. J. Mater. Chem. C 2018, 6, 2168–2188. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.-T.; Tang, J.-W.; Feng, Y.-Y.; Feng, W. Structural design and application of azo-based supramolecular polymer systems. Chin. J. Polym. Sci. 2019, 37, 1183–1199. [Google Scholar] [CrossRef]
- Kopyshev, A.; Galvin, C.J.; Genzer, J.; Lomadze, N.; Santer, S. Opto-mechanical scission of polymer chains in photosensitive diblock-copolymer brushes. Langmuir 2013, 29, 13967–13974. [Google Scholar] [CrossRef] [PubMed]
- Yadavalli, N.S.; Linde, F.; Kopyshev, A.; Santer, S. Soft matter beats hard matter: Rupturing of thin metallic films induced by mass transport in photosensitive polymer films. ACS Appl. Mater. Interfaces 2013, 5, 7743–7747. [Google Scholar] [CrossRef] [PubMed]
- Di Florio, G.; Brundermann, E.; Yadavalli, N.S.; Santer, S.; Havenith, M. Graphene multilayer as nanosized optical strain gauge for polymer surface relief gratings. Nano Lett. 2014, 14, 5754–5760. [Google Scholar] [CrossRef] [PubMed]
- Mitus, A.; Saphiannikova, M.; Radosz, W.; Toshchevikov, V.; Pawlik, G. Modeling of nnlinear otical penomena in host-guest systems using bond fluctuation Monte Carlo model: A review. Materials 2021, 14, 1454. [Google Scholar] [CrossRef] [PubMed]
- Patrick, J.F.; Robb, M.J.; Sottos, N.R.; Moore, J.S.; White, S.R. Polymers with autonomous life-cycle control. Nature 2016, 540, 363–370. [Google Scholar] [CrossRef]
- Wang, S.; Urban, M.W. Self-healing polymers. Nat. Rev. Mater. 2020, 5, 562–583. [Google Scholar] [CrossRef]
- Li, C.-H.; Zuo, J.-L. Self-healing polymers based on coordination bonds. Adv. Mater. 2020, 32, 1903762. [Google Scholar] [CrossRef]
- Song, Y.; Liu, Y.; Qi, T.; Li, G.L. Towards dynamic but supertough healable polymers through biomimetic hierarchical hydrogen-bonding interactions. Angew. Chem. Int. Ed. 2018, 57, 13838–13842. [Google Scholar] [CrossRef]
- Qin, B.; Zhang, S.; Sun, P.; Tang, B.; Yin, Z.; Cao, X.; Chen, Q.; Xu, J.-F.; Zhang, X. Tough and multi-recyclable cross-linked supramolecular polyureas via incorporating noncovalent bonds into main-chains. Adv. Mater. 2020, 32, 2000096. [Google Scholar] [CrossRef]
- Kondo, M.; Yu, Y.; Ikeda, T. How does the initial alignment of mesogens affect the photoinduced bending behavior of liquid-crystalline elastomers? Angew. Chem. Int. Ed. 2006, 45, 1378–1382. [Google Scholar] [CrossRef]
- Yu, Y.; Maeda, T.; Mamiya, J.; Ikeda, T. Photomechanical effects of ferroelectric liquid-crystalline elastomers containing azobenzene chromophores. Angew. Chem. Int. Ed. 2007, 46, 881–883. [Google Scholar] [CrossRef]
- Yoshino, T.; Kondo, M.; Mamiya, J.; Kinoshita, M.; Yu, Y.; Ikeda, T. Three-dimensional photomobility of crosslinked azobenzene liquid-crystalline polymer fibers. Adv. Mater. 2010, 22, 1361–1363. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wang, W.; Qiu, L.; Guo, W.; Yu, Y.; Peng, H. Unusual reversible photomechanical actuation in polymer/nanotube composites. Angew. Chem. Int. Ed. 2012, 51, 8520–8524. [Google Scholar] [CrossRef]
- Siewertsen, R.; Neumann, H.; Buchheim-Stehn, B.; Herges, R.; Näther, C.; Renth, F.; Temps, F. Highly efficient reversible Z-E photoisomerization of a bridged azobenzene with visible light through resolved S1(nπ*) absorption bands. J. Am. Chem. Soc. 2009, 131, 15594–15595. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.P.; Rong, M.Z.; Zhang, M.Q. Polymer engineering based on reversible covalent chemistry: A promising innovative pathway towards new materials and new functionalities. Prog. Polym. Sci. 2018, 80, 39–93. [Google Scholar] [CrossRef]
- Chakma, P.; Konkolewicz, D. Dynamic covalent bonds in polymeric materials. Angew. Chem. Int. Ed. 2019, 58, 9682–9695. [Google Scholar] [CrossRef] [PubMed]
- Podgórski, M.; Fairbanks, B.D.; Kirkpatrick, B.E.; McBride, M.; Martinez, A.; Dobson, A.; Bongiardina, N.J.; Bowman, C.N. Toward stimuli-responsive dynamic thermosets through continuous development and improvements in covalent adaptable networks (CANs). Adv. Mater. 2020, 32, 1906876. [Google Scholar] [CrossRef] [PubMed]
- Van Zee, N.J.; Nicolaÿ, R. Vitrimers: Permanently crosslinked polymers with dynamic network topology. Prog. Polym. Sci. 2020, 104, 101233. [Google Scholar] [CrossRef]
- Montarnal, D.; Capelot, M.; Tournilhac, F.; Leibler, L. Silica-like malleable materials from permanent organic networks. Science 2011, 334, 965–968. [Google Scholar] [CrossRef] [PubMed]
- Capelot, M.; Montarnal, D.; Tournilhac, F.; Leibler, L. Metal-catalyzed transesterification for healing and assembling of thermosets. J. Am. Chem. Soc. 2012, 134, 7664–7667. [Google Scholar] [CrossRef] [PubMed]
- Pei, Z.; Yang, Y.; Chen, Q.; Terentjev, E.M.; Wei, Y.; Ji, Y. Mouldable liquid-crystalline elastomer actuators with exchangeable covalent bonds. Nat. Mater. 2014, 13, 36–41. [Google Scholar] [CrossRef]
- Chen, Q.-M.; Yang, Y.; Wei, Y.; Ji, Y. Liquid crystalline polymer actuators with exchangeable dynamic covalent bonds. Acta Polym. Sin. 2019, 50, 451–468. [Google Scholar]
- Wang, Z.; Cai, S. Recent progress in dynamic covalent chemistries for liquid crystal elastomers. J. Mater. Chem. B 2020, 8, 6610–6623. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H. Reprocessable Photodeformable Azobenzene Polymers. Molecules 2021, 26, 4455. https://doi.org/10.3390/molecules26154455
Zhang H. Reprocessable Photodeformable Azobenzene Polymers. Molecules. 2021; 26(15):4455. https://doi.org/10.3390/molecules26154455
Chicago/Turabian StyleZhang, Huiqi. 2021. "Reprocessable Photodeformable Azobenzene Polymers" Molecules 26, no. 15: 4455. https://doi.org/10.3390/molecules26154455
APA StyleZhang, H. (2021). Reprocessable Photodeformable Azobenzene Polymers. Molecules, 26(15), 4455. https://doi.org/10.3390/molecules26154455





