Content of Carotenoids, Violaxanthin and Neoxanthin in Leaves of Triticum aestivum Exposed to Persistent Environmental Pollutants
Abstract
:1. Introduction
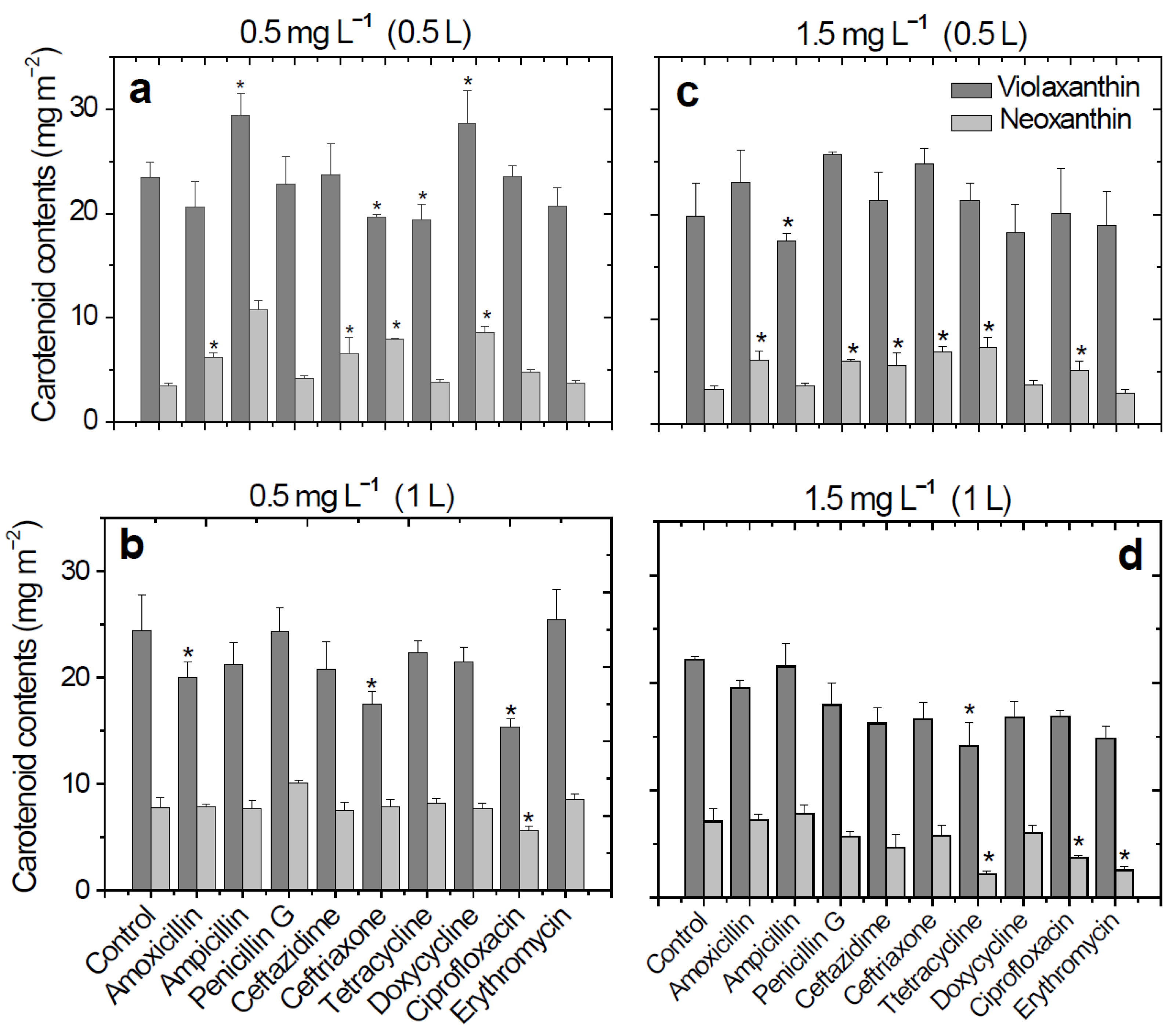
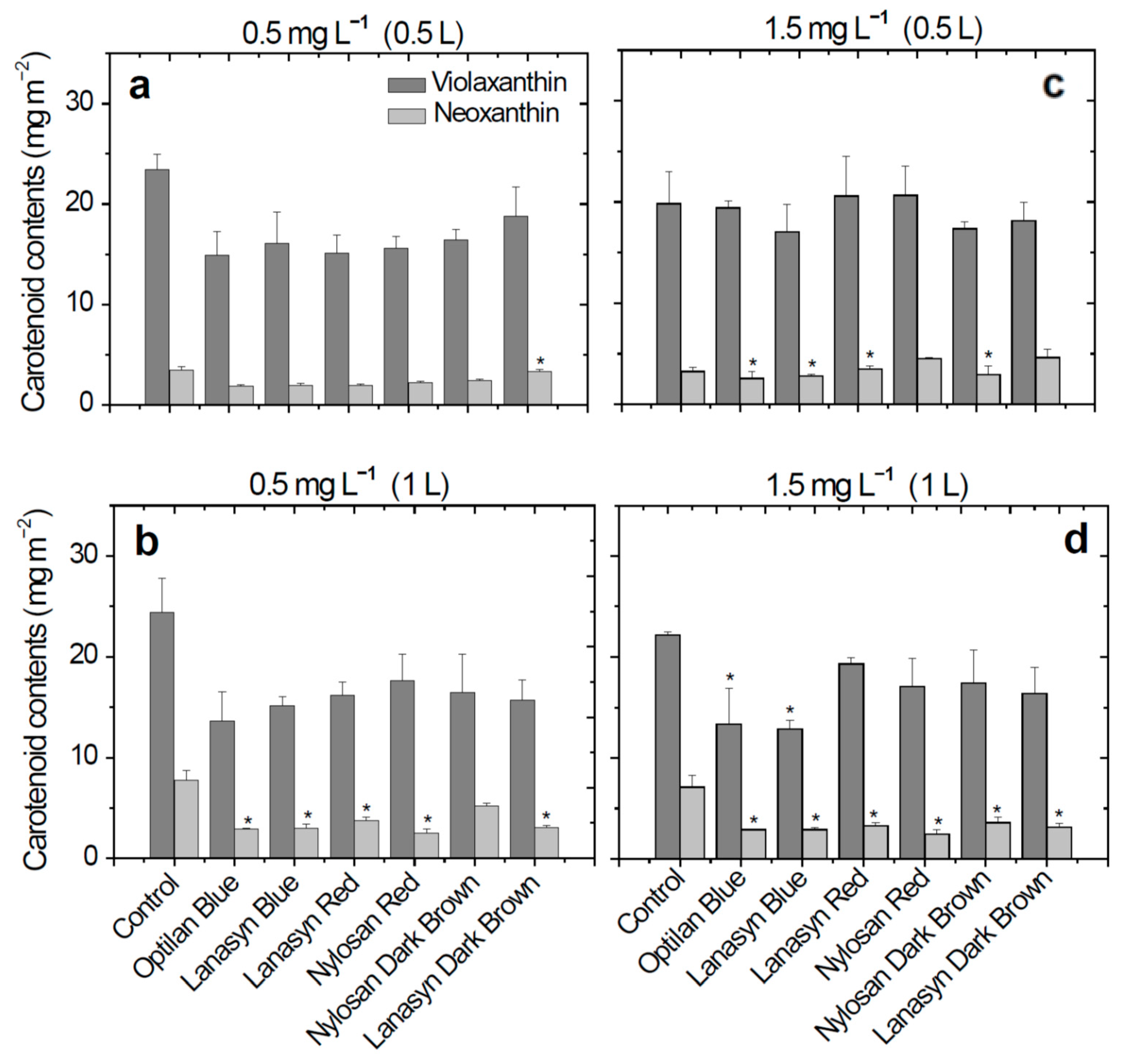
2. Results
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growing Conditions
4.2. Stress Application
4.3. Carotenoids Extraction and Analysis
4.4. Separation of Pigment Standards for HPLC Calibration
4.5. Statistical Analysis and Data Interpretation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Abbas, M.; Adil, M.; Ehtisham-ul-Haque, S.; Munir, B.; Yameen, M.; Ghaffar, A.; Shar, G.A.; Asif Tahir, M.; Iqbal, M. Vibrio fischeri bioluminescence inhibition assay for ecotoxicity assessment: A review. Sci. Total Environ. 2018, 626, 1295–1309. [Google Scholar] [CrossRef] [PubMed]
- Awad, A.M.; Shaikh, S.M.R.; Jalab, R.; Gulied, M.H.; Nasser, M.S.; Benamor, A.; Adham, S. Adsorption of organic pollutants by natural and modified clays: A comprehensive review. Sep. Purif. Technol. 2019, 228, 115719. [Google Scholar] [CrossRef]
- Grenni, P.; Ancona, V.; Barra Caracciolo, A. Ecological effects of antibiotics on natural ecosystems: A review. Microchem. J. 2018, 136, 25–39. [Google Scholar] [CrossRef]
- Kumar, M.; Jaiswal, S.; Sodhi, K.K.; Shree, P.; Singh, D.K.; Agrawal, P.K.; Shukla, P. Antibiotics bioremediation: Perspectives on its ecotoxicity and resistance. Environ. Int. 2019, 124, 448–461. [Google Scholar] [CrossRef] [PubMed]
- Pawlowski, A.C.; Wang, W.; Koteva, K.; Barton, H.A.; McArthur, A.G.; Wright, G.D. A diverse intrinsic antibiotic resistome from a cave bacterium. Nat. Commun. 2016, 7, 13803. [Google Scholar] [CrossRef] [Green Version]
- Kemper, N. Veterinary antibiotics in the aquatic and terrestrial environment. Ecol. Indic. 2008, 8, 1–13. [Google Scholar] [CrossRef]
- Antonkiewicz, J.; Baran, A.; Pełka, R.; Wisła-Świder, A.; Nowak, E.; Konieczka, P. A mixture of cellulose production waste with municipal sewage as new material for an ecological management of wastes. Ecotoxicol. Environ. Saf. 2019, 169, 607–614. [Google Scholar] [CrossRef]
- Tasho, R.P.; Cho, J.Y. Veterinary antibiotics in animal waste, its distribution in soil and uptake by plants: A review. Sci. Total Environ. 2016, 563–564, 366–376. [Google Scholar] [CrossRef]
- Copaciu, F.; Opriş, O.; Coman, V.; Ristoiu, D.; Niinemets, Ü.; Copolovici, L. Diffuse water pollution by anthraquinone and azo dyes in environment importantly alters foliage volatiles, carotenoids and physiology in wheat (Triticum aestivum). Water Air Soil Pollut. 2013, 224, 1478. [Google Scholar] [CrossRef]
- Opriş, O.; Copaciu, F.; Loredana Soran, M.; Ristoiu, D.; Niinemets, Ü.; Copolovici, L. Influence of nine antibiotics on key secondary metabolites and physiological characteristics in Triticum aestivum: Leaf volatiles as a promising new tool to assess toxicity. Ecotoxicol. Environ. Saf. 2013, 87, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Isidori, M.; Lavorgna, M.; Nardelli, A.; Pascarella, L.; Parrella, A. Toxic and genotoxic evaluation of six antibiotics on non-target organisms. Sci. Total Environ. 2005, 346, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Ebert, I.; Bachmann, J.; Kühnen, U.; Küster, A.; Kussatz, C.; Maletzki, D.; Schlüter, C. Toxicity of the fluoroquinolone antibiotics enrofloxacin and ciprofloxacin to photoautotrophic aquatic organisms. Environ. Toxicol. Chem. 2011, 30, 2786–2792. [Google Scholar] [CrossRef]
- Liu, B.-y.; Nie, X.-p.; Liu, W.-q.; Snoeijs, P.; Guan, C.; Tsui, M.T.K. Toxic effects of erythromycin, ciprofloxacin and sulfamethoxazole on photosynthetic apparatus in Selenastrum capricornutum. Ecotoxicol. Environ. Saf. 2011, 74, 1027–1035. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Kahru, A.; Mander, Ü.; Nõges, P.; Nõges, T.; Tuvikene, A.; Vasemägi, A. Interacting environmental and chemical stresses under global change in temperate aquatic ecosystems: Stress responses, adaptation, and scaling. Reg. Environ. Chang. 2017, 17, 2061–2077. [Google Scholar] [CrossRef]
- Minden, V.; Schnetger, B.; Pufal, G.; Leonhardt, S.D. Antibiotic-induced effects on scaling relationships and on plant element contents in herbs and grasses. Ecol. Evol. 2018, 8, 6699–6713. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Z.; Shen, Z.; Ou, Z.; Xu, D.; Yuan, Z.; Zhou, S. Effects of oxytetracycline on growth and chlorophyll fluorescence in rape (Brassica campestris L.). Pol. J. Environ. Stud. 2017, 26, 995–1001. [Google Scholar] [CrossRef]
- Edwards, L.C.; Freeman, H.S.; Claxton, L.D. Developing azo and formazan dyes based on environmental considerations: Salmonella mutagenicity. Mutat. Res. 2004, 546, 17–28. [Google Scholar] [CrossRef]
- Nilratnisakorn, S.; Thiravetyan, P.; Nakbanpote, W. Synthetic reactive dye wastewater treatment by narrow-leaved cattails (Typha angustifolia Linn.): Effects of dye, salinity and metals. Sci. Total Environ. 2007, 384, 67–76. [Google Scholar] [CrossRef]
- Pattnaik, P.; Dangayach, G.S.; Bhardwaj, A.K. A review on the sustainability of textile industries wastewater with and without treatment methodologies. Rev. Environ. Health 2018, 33, 163–203. [Google Scholar] [CrossRef]
- Copaciu, F.; Opriş, O.; Niinemets, Ü.; Copolovici, L. Toxic influence of key organic soil pollutants on the total flavonoid content in wheat leaves. Water Air Soil Pollut. 2016, 227, 196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanagendran, A.; Pazouki, L.; Li, S.; Liu, B.; Kännaste, A.; Niinemets, Ü. Ozone-triggered surface uptake and stress volatile emissions in Nicotiana tabacum ‘Wisconsin’. J. Exp. Bot. 2017, 69, 681–697. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Harley, P.C.; Niinemets, Ü. Ozone-induced foliar damage and release of stress volatiles is highly dependent on stomatal openness and priming by low-level ozone exposure in Phaseolus vulgaris. Plant Cell Environ. 2017, 40, 1984–2003. [Google Scholar] [CrossRef] [Green Version]
- Copaciu, F.; Coman, V.; Vlassa, M.; Opriş, O. Determination of some textile dyes in wastewater by solid phase extraction followed by high-performance thin-layer chromatography. JPC J. Planar Chromatogr. Mod. TLC 2012, 25, 509–515. [Google Scholar] [CrossRef]
- Opriş, O.; Coman, V.; Copaciu, F.; Vlassa, M. Solid phase extraction and high-performance thin-layer chromatography quantification of some antibiotics from surface waters. JPC J. Planar Chromatogr. Mod. TLC 2012, 25, 516–522. [Google Scholar] [CrossRef]
- Pan, X.; Deng, C.; Zhang, D.; Wang, J.; Mu, G.; Chen, Y. Toxic effects of amoxicillin on the photosystem II of Synechocystis sp. characterized by a variety of in vivo chlorophyll fluorescence tests. Aquat. Toxicol. 2008, 89, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Zhou, Q.; Lin, D.; Guo, J.; Bao, Y. Toxic effect of tetracycline exposure on growth, antioxidative and genetic indices of wheat (Triticum aestivum L.). Environ. Sci. Pollut. Res. 2011, 18, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Pilchova, I.; Klacanova, K.; Tatarkova, Z.; Kaplan, P.; Racay, P. The Involvement of Mg2+ in Regulation of Cellular and Mitochondrial Functions. Oxidative Med. Cell. Longev. 2017, 2017, 6797460. [Google Scholar] [CrossRef] [Green Version]
- Brock, N.L.; Dickschat, J.S. Biosynthesis of Terpenoids. In Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes; Ramawat, K.G., Mérillon, J.-M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 2693–2732. [Google Scholar] [CrossRef]
- Sanchez, S.; Demain, A.L. Metabolic regulation and overproduction of primary metabolites. Microb. Biotechnol. 2008, 1, 283–319. [Google Scholar] [CrossRef]
- Esteban, R.; Moran, J.F.; Becerril, J.M.; García-Plazaola, J.I. Versatility of carotenoids: An integrated view on diversity, evolution, functional roles and environmental interactions. Environ. Exp. Bot. 2015, 119, 63–75. [Google Scholar] [CrossRef]
- Aluru, M.R.; Zola, J.; Foudree, A.; Rodermel, S.R. Chloroplast photooxidation-induced transcriptome reprogramming in arabidopsis white leaf sectors. Plant Physiol. 2009, 150, 904–923. [Google Scholar] [CrossRef] [Green Version]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A decimal code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Bilger, W.; Kull, O.; Tenhunen, J.D. Acclimation to high irradiance in temperate deciduous trees in the field: Changes in xanthophyll cycle pool size and in photosynthetic capacity along a canopy light gradient. Plant Cell Environ. 1998, 21, 1205–1218. [Google Scholar] [CrossRef]
- Opriş, O.; Soran, M.-L.; Coman, V.; Copaciu, F.; Ristoiu, D. Determination of some frequently used antibiotics in waste waters using solid phase extraction followed by high performance liquid chromatography with diode array and mass spectrometry detection. Cent. Eur. J. Chem. 2013, 11, 1343–1351. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Opriş, O.; Copaciu, F.; Soran, M.L.; Niinemets, Ü.; Copolovici, L. Content of Carotenoids, Violaxanthin and Neoxanthin in Leaves of Triticum aestivum Exposed to Persistent Environmental Pollutants. Molecules 2021, 26, 4448. https://doi.org/10.3390/molecules26154448
Opriş O, Copaciu F, Soran ML, Niinemets Ü, Copolovici L. Content of Carotenoids, Violaxanthin and Neoxanthin in Leaves of Triticum aestivum Exposed to Persistent Environmental Pollutants. Molecules. 2021; 26(15):4448. https://doi.org/10.3390/molecules26154448
Chicago/Turabian StyleOpriş, Ocsana, Florina Copaciu, Maria Loredana Soran, Ülo Niinemets, and Lucian Copolovici. 2021. "Content of Carotenoids, Violaxanthin and Neoxanthin in Leaves of Triticum aestivum Exposed to Persistent Environmental Pollutants" Molecules 26, no. 15: 4448. https://doi.org/10.3390/molecules26154448
APA StyleOpriş, O., Copaciu, F., Soran, M. L., Niinemets, Ü., & Copolovici, L. (2021). Content of Carotenoids, Violaxanthin and Neoxanthin in Leaves of Triticum aestivum Exposed to Persistent Environmental Pollutants. Molecules, 26(15), 4448. https://doi.org/10.3390/molecules26154448







