Benzo[f]indole-4,9-dione Derivatives Effectively Inhibit the Growth of Triple-Negative Breast Cancer
Abstract
1. Introduction
2. Results
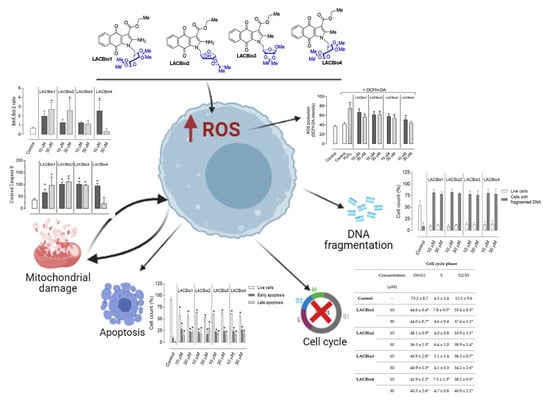
2.1. Benzo[f]indole-4,9-dione Derivatives Induced Apoptosis in MDA-MB 231 Cells
2.2. Benzo[f]indole-4,9-dione Derivatives Caused DNA Fragmentation in MDA-MB 231 Cells
2.3. Effects of Benzo[f]indole-4,9-dione Derivatives on Cell Cycle Distribution
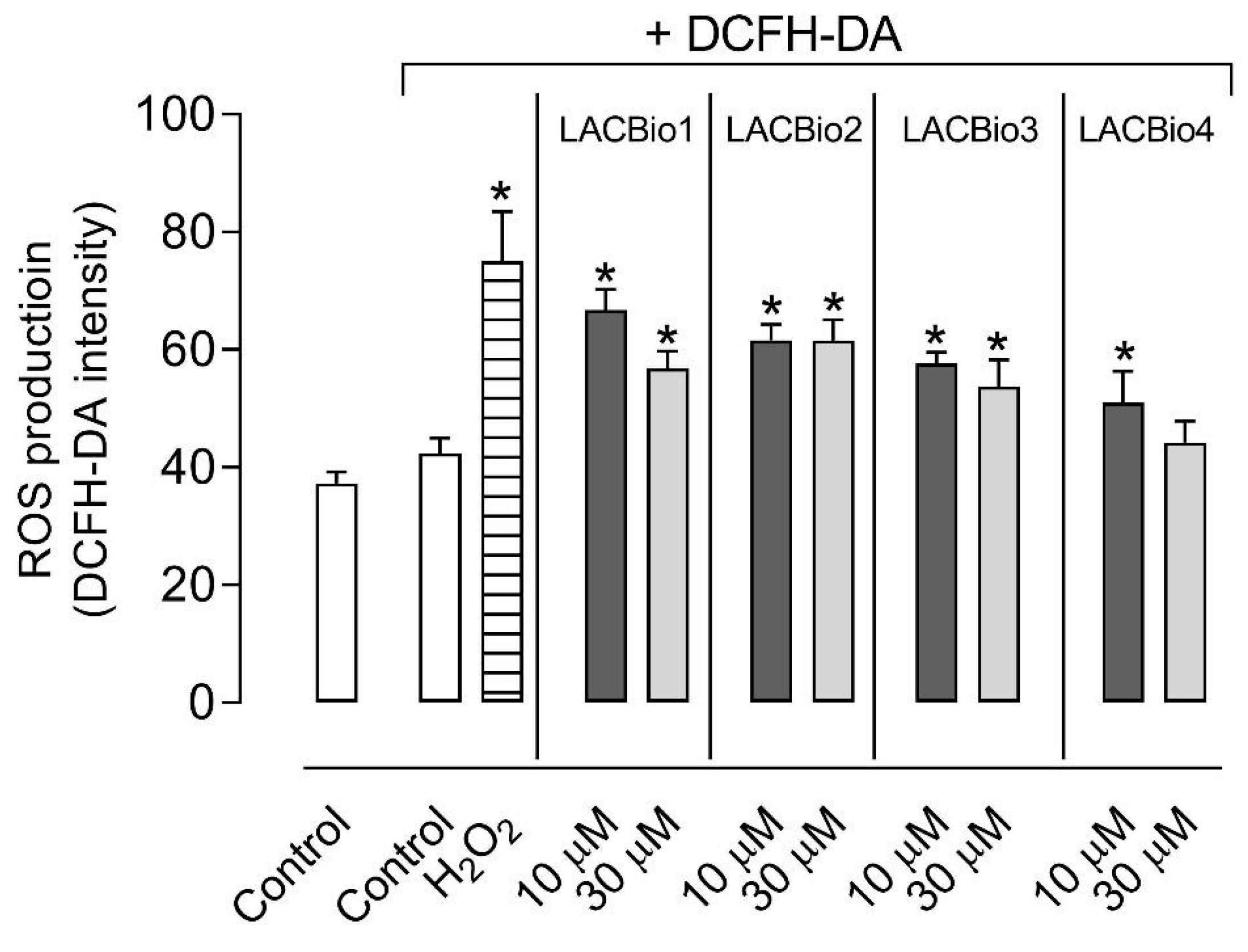
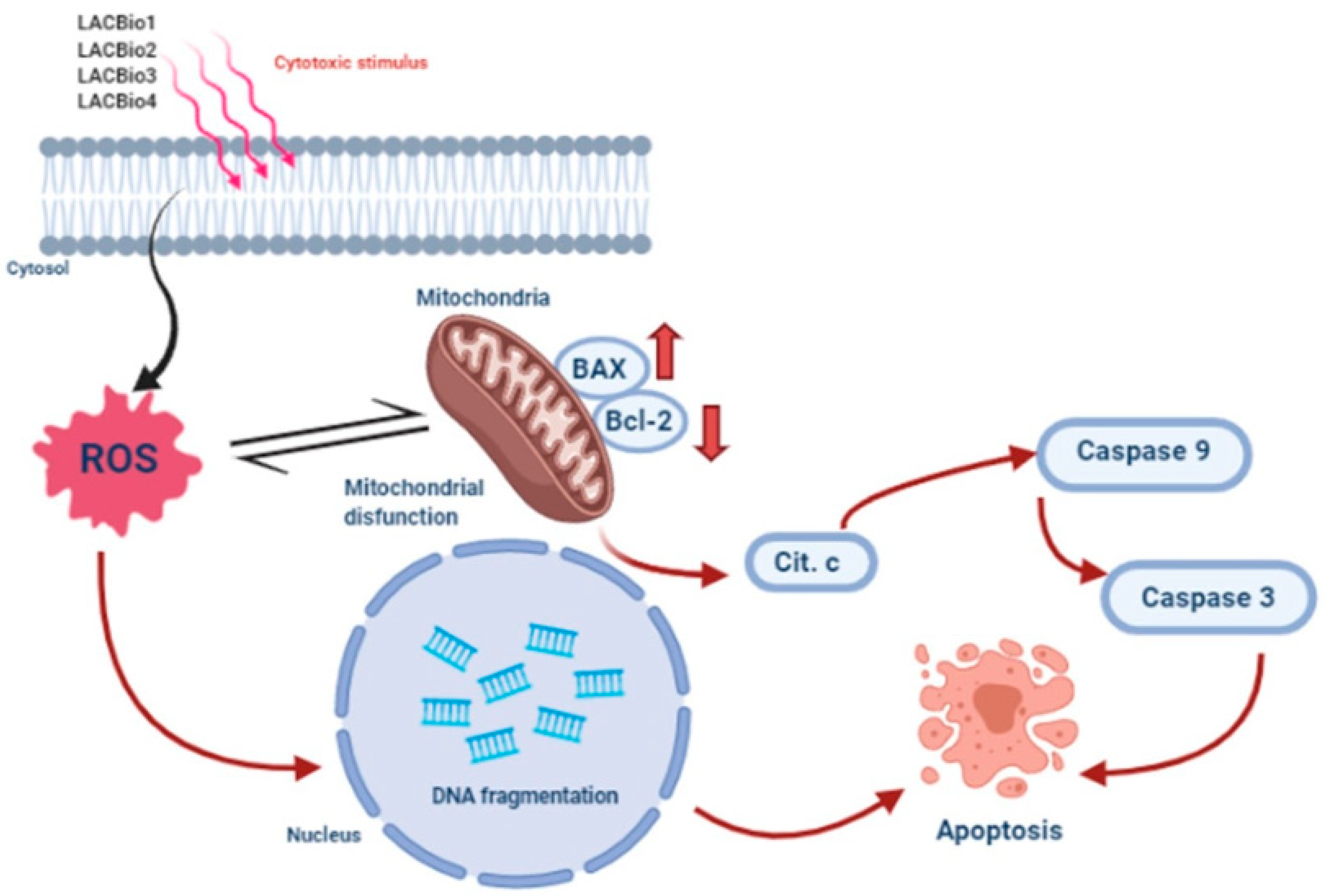
2.4. Benzo[f]indole-4,9-dione Derivatives Increased Intracellular Reactive Oxygen Species (ROS) Levels
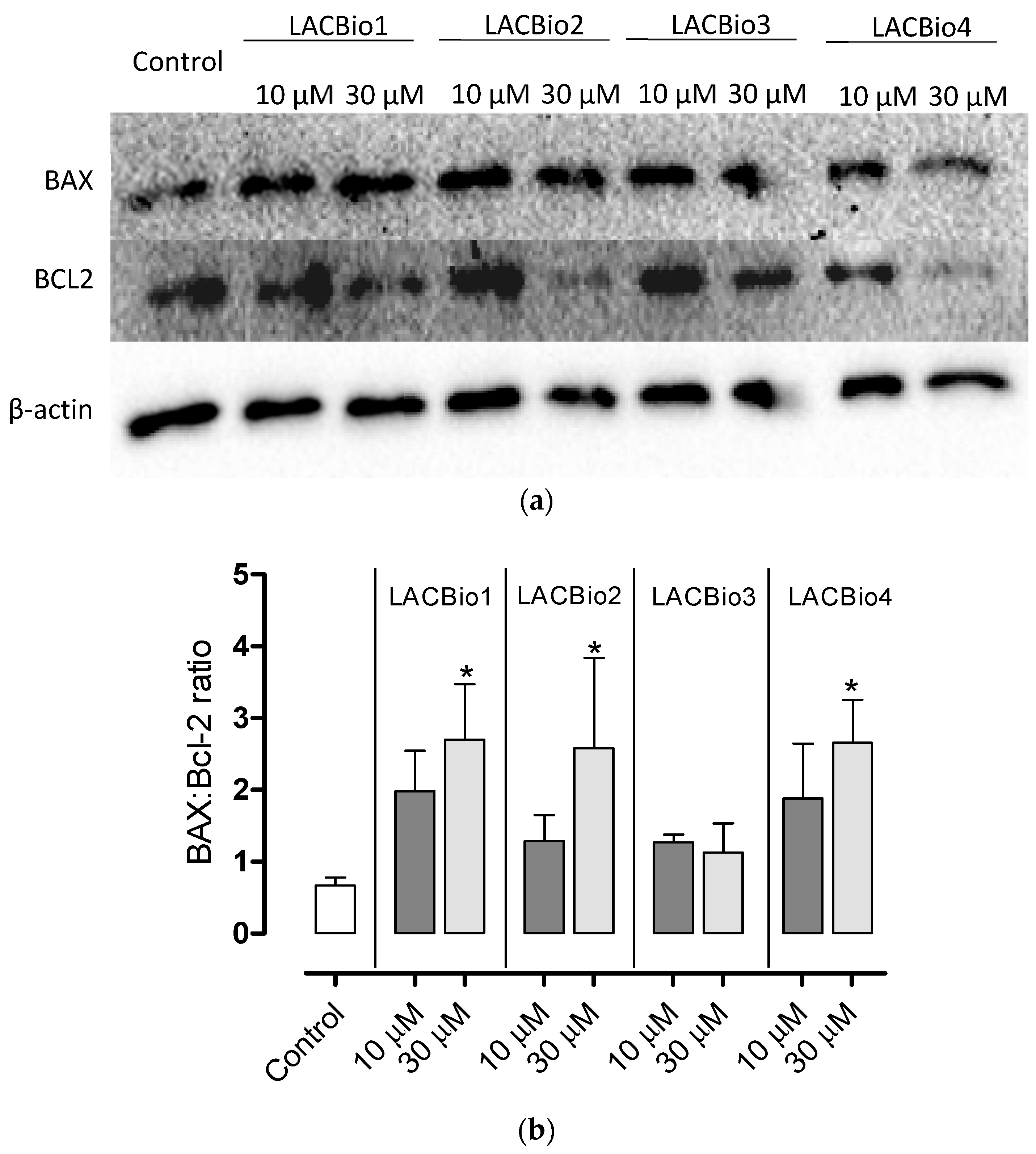
2.5. Benzo[f]indole-4,9-dione Derivatives Increased the Bax:Bcl-2 Ratio
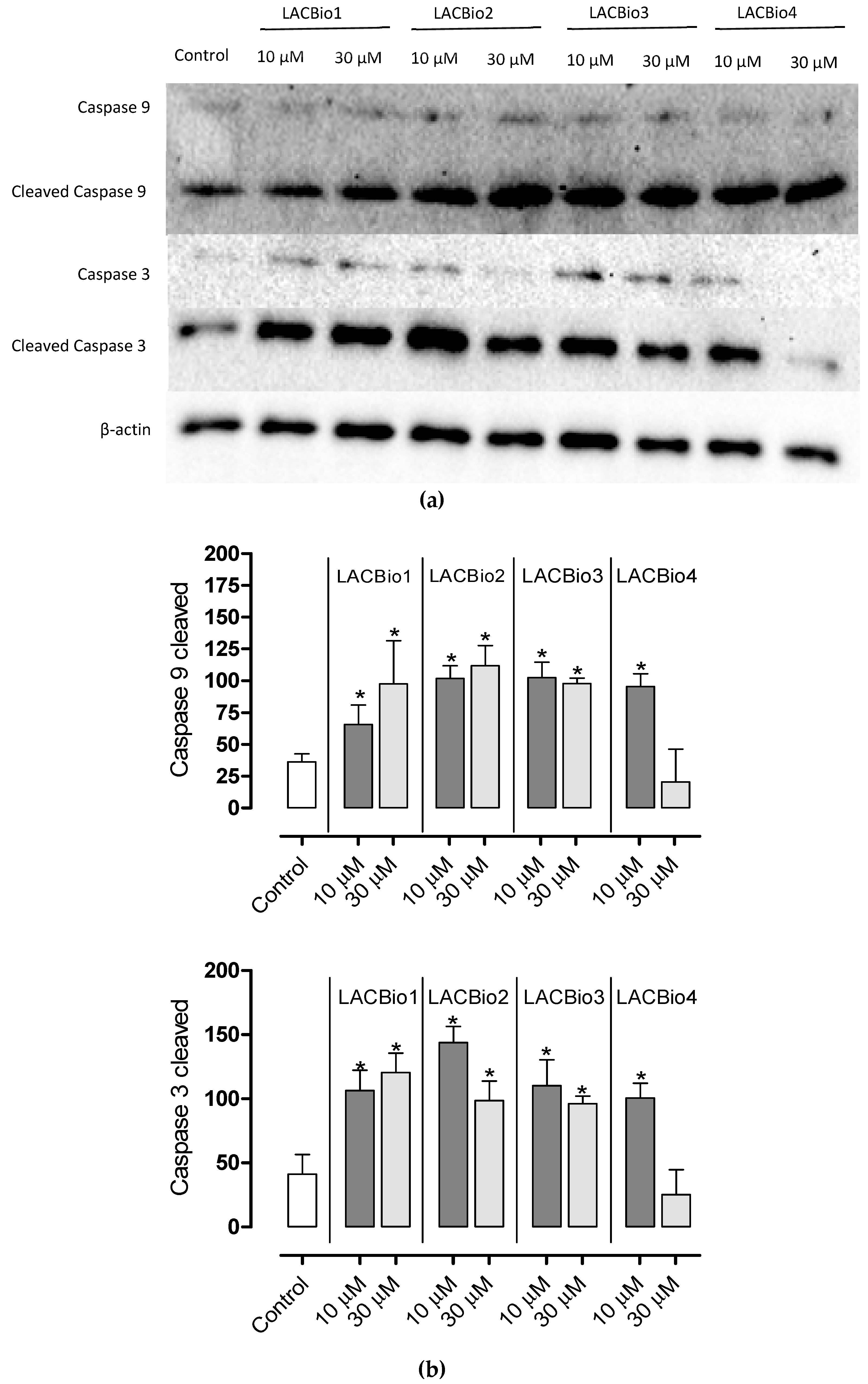
2.6. Benzo[f]indole-4,9-dione Derivatives Induced Activation of the Intrinsic Caspase Cascade
3. Discussion
4. Materials and Methods
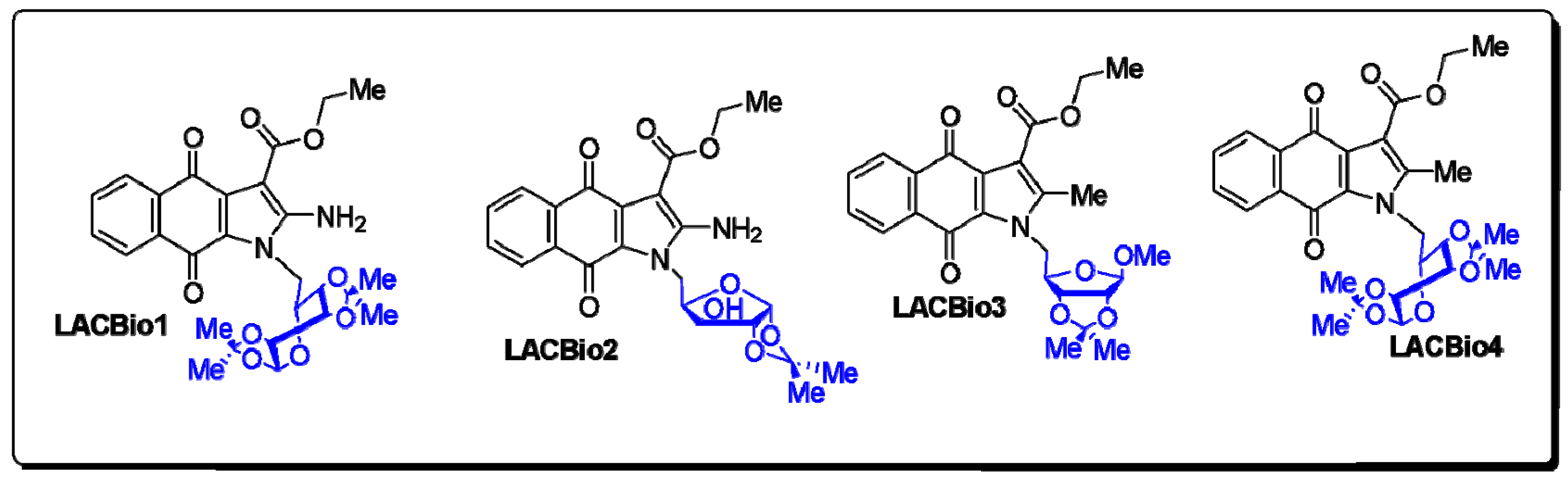
4.1. Synthesis of Benzo[f]indole-4,9-dione Derivatives
4.2. Cell Culture and Treatment
4.3. Apoptosis Assay
4.4. DNA Fragmentation Analyses
4.5. Cell Cycle Analysis
4.6. ROS Generation Assay
4.7. Western Blotting Analysis
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Bertucci, F.; Houlgatte, R.; Benziane, A.; Granjeaud, S.; Adélaïde, J.; Tagett, R.; Loriod, B.; Jacquemier, J.; Viens, P.; Jordan, B.; et al. Gene expression profiling of primary breast carcinomas using arrays of candidate genes. Hum. Mol. Genet. 2000, 9, 2981–2991. [Google Scholar] [CrossRef]
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Portmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast cancer. Nat. Rev. Dis. Primers 2019, 19, 66. [Google Scholar] [CrossRef] [PubMed]
- Marotti, J.D.; Abreu, F.B.; Wells, W.A.; Tsongalis, G.J. Triple-negative breast cancer: Next-generation sequencing for target identification. Am. J. Pathol. 2017, 187, 2133–2138. [Google Scholar] [CrossRef] [PubMed]
- Shilpi, S.; Shivvedi, R.; Khatri, K. Triple Negative Breast Cancer (TNBC): A challenge for current cancer therapy. J. Hum. Virol. Retrovirol. 2018, 6, 00189. [Google Scholar]
- Tomao, F.; Papa, A.; Zaccarelli, E.; Rossi, L.; Caruso, D.; Minozzi, M.; Vici, P.; Frati, L.; Tomao, S. Triple-negative breast cancer: New perspectives for targeted therapies. OncoTargets Ther. 2015, 8, 177–193. [Google Scholar] [CrossRef] [PubMed]
- Benites, J.; Valderrama, J.A.; Rivera, F.; Rojo, L.; Campos, N.; Pedro, M.; José Nascimento, M.S. Studies on quinones. Part 42: Synthesis of furylquinone and hydroquinones with antiproliferative activity against human tumor cell lines. Bioorg. Med. Chem. 2008, 16, 862–868. [Google Scholar] [CrossRef] [PubMed]
- Tsao, Y.C.; Chang, Y.J.; Wang, C.H.; Chen, L. Discovery of isoplumbagin as a novel NQ01 substrate and anti-cancer quinone. Int. J. Mol. Sci. 2020, 21, 4378. [Google Scholar] [CrossRef]
- Colucci, M.A.; Moody, C.J.; Couch, G.D. Natural and synthetic quinones and their reduction by the quinone reductase enzyme NQO1: From synthetic organic chemistry to compounds with anticancer potential. Org. Biomol. Chem. 2008, 6, 637–656. [Google Scholar] [CrossRef]
- Seacat, A.M.; Kuppusamy, P.; Zweier, J.L.; Yager, J.D. ESR identification of free radicals formed from the oxidation of catechol estrogens by Cu2+. Arch. Biochem. Bioph. 1997, 347, 45–52. [Google Scholar] [CrossRef]
- Fukuda, M.; Qianjun, L.; Kishikawa, N.; Ohyama, K.; Kuroda, N. Development of ultrafast colorimetric microplate assay method for ubiquinone utilizing the redox cycle of the quinone. Microchem. J. 2019, 150, 104. [Google Scholar] [CrossRef]
- Kannan, K.; Jain, S.K. Oxidative stress and apoptosis. Pathophysiology 2000, 7, 153–163. [Google Scholar] [CrossRef]
- Forman, H.J.; Torres, M.; Fukuto, J. Redox signaling. Mol. Cell. Biochem. 2002, 234, 49–62. [Google Scholar] [CrossRef]
- Wang, X.; Qian, J.; Zhu, P.; Hua, R.; Liu, J.; Hang, J.; Meng, C.; Shan, W.; Miao, J.; Ling, Y. Novel phenylmethylenecyclohexenone derivatives as poten TrxR inhibitors display high antiproliferative activity and induce ROS, apoptosis, and DNA damage. Chem. Med. Chem. 2020, 16, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Dias, F.R.F.; Guerra, F.S.; Lima, F.A.; Castro, Y.K.C.; Ferreira, V.F.; Campos, V.R.; Fernandes, P.D.; Cunha, A.C. Synthesis and biological evaluation of Benzo[f]indole-4,9-diones N-linked to carbohydrate chains as new type of antitumor agents. J. Braz. Chem. Soc. 2020, in press.. [Google Scholar] [CrossRef]
- Loo, D.T. In situ detection of apoptosis by the TUNEL assay: An overview of techniques. Methods Mol. Biol. 2011, 682, 3–13. [Google Scholar]
- Kumar, P.; Aggarwal, R. An overview of triple-negative breast cancer. Arch. Gynecol. Obstet. 2016, 293, 247–269. [Google Scholar] [CrossRef]
- Matassov, D.; Kagan, T.; Leblanc, J.; Sikorska, M.; Zakeri, Z. Measurement of apoptosis by DNA fragmentation. In Apoptosis Methods and Protocols; Humana Press Inc.: Totowa, NJ, USA, 2004; Volume 282, pp. 1–17. [Google Scholar]
- Gach, K.; Modranka, J.; Szymański, J.; Pomorska, D.; Krajewska, D.; Mirowski, M.; Janecki, T.; Janecka, A. Anticancer properties of new synthetic hybrid molecules combining naphtho[2,3-b]furan-4,9-dione or benzo[f]indole-4,9-dione motif with phosphonate subunit. Eur. J. Med. Chem. 2016, 120, 51–63. [Google Scholar] [CrossRef]
- Chan, K.T.; Meng, F.Y.; Li, Q.; Ho, C.Y.; Lam, T.S.; To, Y.; Lee, W.H.; Li, M.; Chu, K.H.; Toh, M. Cucurbitacin B induces apoptosis and S phase cell cycle arrest in BEL-7402 human hepatocellular carcinoma cells and is effective via oral administration. Cancer Lett. 2010, 294, 118–124. [Google Scholar] [CrossRef]
- Noroozi, M.K.; Mahmoodi, M.; Jafarzadeh, A.; Darehkordi, A.; Hajizadeh, M.R.; Khorramdelazad, H.; Sayadi, A.R.; Falahati-pour, S.K.; Hassanshahi, G. Indole itself and its novel derivative affect PML cells proliferation via controlling the expression of cell cycle genes. Cell Mol. Biol. 2019, 65, 41–47. [Google Scholar] [CrossRef]
- Hartwell, L.H.; Weinert, T.A. Checkpoints: Controls that ensure the order of cell cycle events. Science 1989, 246, 629–634. [Google Scholar] [CrossRef]
- Taylor, W.R.; Sark, G.R. Regulation of the G2/M transition by p53. Oncogene 2001, 20, 1803–1815. [Google Scholar] [CrossRef]
- Srinivas, U.S.; Tan, B.W.Q.; Vellayappan, B.A.; Jeyasekharan, A.D. ROS and the DNA damage response in câncer. Redox Biol. 2019, 25, 101084. [Google Scholar] [CrossRef] [PubMed]
- Conklin, K.A. Chemotherapy-associated oxidative stress: Impact on chemotherapeutic effectiveness. Integr. Cancer Ther. 2004, 3, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Marullo, R.; Werner, E.; Degtyareva, N.; Moore, B.; Altavilla, G.; Ramalingam, S.S.; Doetsch, P.W. Cisplatin induces a mitochondrial-ROS response that contributes to cytotoxicity depending on mitochondrial redox status and bioenergetic functions. PLoS ONE 2013, 8, e81162. [Google Scholar]
- Valderrama, J.A.; Ibacache, J.A.; Arancibia, V.; Rodriguez, J.; Theoduloz, C. Studies on quinones. Part 45: Novel 7-aminoisoquinoline-5,8-quinone derivatives with antitumor properties on cancer cell lines. Bioorg. Med. Chem. 2009, 17, 2894–2901. [Google Scholar] [CrossRef] [PubMed]
- Vaux, D.L.; Korsmeyer, S.J. Cell death in development. Cell 1999, 96, 245–254. [Google Scholar] [CrossRef]
- Quast, S.-A.; Berger, A.; Eberle, J. ROS-dependent phosphorylation of Bax by wortmannin sensitizes melanoma cells for TRAIL-induced apoptosis. Cell Death Dis. 2013, 4, 3829. [Google Scholar] [CrossRef] [PubMed]
- Desagher, S.; Martinou, J.C. Mitochondria as the central control point of apoptosis. Trends Cell Biol. 2000, 10, 369–377. [Google Scholar] [CrossRef]
- Hochenbery, D.M.; Oltvai, Z.N.; Yin, X.M.; Milliman, C.L.; Korsmeyer, S.J. Bcl-2 function in an antioxidant pathway to prevent apoptosis. Cell 1992, 75, 241–251. [Google Scholar] [CrossRef]
- Jurgensmeier, J.M.; Xie, Z.H.; Deveraux, Q.; Ellerby, L.; Bredesen, D.; Reed, J.C. Bax directly induces release of cytochrome c from isolated mitochondria. Proc. Nat. Acad. Sci. USA 1998, 95, 4997–5002. [Google Scholar] [CrossRef]
- Hu, H.; Kavanagh, J.J. Anticancer therapy targeting the apoptotic pathway. Lancet Oncol. 2003, 4, 721–729. [Google Scholar] [CrossRef]
- Muñoz-Pinedo, C.; El Mjiyad, N.; Ricci, J.-E. Cancer metabolism: Current perspectives and future directions. Cell Death. Dis. 2012, 3, e248. [Google Scholar] [CrossRef]
- Caltabiano, R.; Leonardi, R.; Musumeci, G.; Bartoloni, G.; Rusu, M.C.; Almeida, L.E.; Loreto, C. Apoptosis in temporomandibular joint disc with internal derangement involves mitochondrial-dependent pathways. An in vivo study. Acta Odontol. Scand. 2013, 71, 577–583. [Google Scholar] [CrossRef]
- Bratton, S.B.; Salvesen, G.S. Regulation of the Apaf-1-caspase-9 apoptosome. J. Cell Sci. 2010, 123, 3209–3214. [Google Scholar] [CrossRef] [PubMed]
- Franco, C.F.J.; Jordão, A.K.; Ferreira, V.F.; Pinto, A.C.; de Souza, M.C.B.V.; Resende, J.A.L.C.; Cunha, A.C. Synthesis of new 2-aminocarbohydrate-1,4-naphthoquinone derivatives promoted by ultrasonic irradiation. J. Braz. Chem. Soc. 2011, 22, 187–193. [Google Scholar] [CrossRef][Green Version]
- Mohan, C.; Long, K.; Mutneja, M.; Ma, J. Detection of end-stage apoptosis by ApopTag® TUNEL technique. In Apoptosis and Cancer; Humana Press: New York, NY, USA, 2015; pp. 43–56. [Google Scholar]
- Eruslanov, E.; Kusmartsev, S. Identification of ROS using oxidized DCFDA and flow-cytometry. Methods Mol. Biol. 2010, 594, 57–72. [Google Scholar] [PubMed]


| Cell Cycle Phase | ||||
|---|---|---|---|---|
| Concentration (µM) | G0/G1 | S | G2/M | |
| Control | - | 73.2 ± 8.7 | 4.3 ± 2.4 | 11.5 ± 9.6 |
| LACBio1 | 10 | 44.6 ± 0.4 * | 7.8 ± 0.5 * | 33.4 ± 0.3 * |
| 30 | 44.0 ± 0.7 * | 4.6 ± 0.4 | 37.4 ± 1.1 * | |
| LACBio2 | 10 | 48.1 ± 0.9 * | 4.0 ± 0.8 | 33.9 ± 3.1 * |
| 30 | 36.5 ± 1.5 * | 6.4 ± 1.0 | 38.9 ± 1.4 * | |
| LACBio3 | 10 | 40.9 ± 2.8 * | 5.1 ± 1.4 | 38.3 ± 0.7 * |
| 30 | 46.9 ± 1.3 * | 4.1 ± 0.3 | 34.1 ± 2.6 * | |
| LACBio4 | 10 | 41.9 ± 1.3 * | 7.5 ± 1.3 * | 38.1 ± 0.5 * |
| 30 | 40.5 ± 2.6 * | 4.7 ± 0.8 | 40.9 ± 2.1 * | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerra, F.S.; Dias, F.R.F.; Cunha, A.C.; Fernandes, P.D. Benzo[f]indole-4,9-dione Derivatives Effectively Inhibit the Growth of Triple-Negative Breast Cancer. Molecules 2021, 26, 4414. https://doi.org/10.3390/molecules26154414
Guerra FS, Dias FRF, Cunha AC, Fernandes PD. Benzo[f]indole-4,9-dione Derivatives Effectively Inhibit the Growth of Triple-Negative Breast Cancer. Molecules. 2021; 26(15):4414. https://doi.org/10.3390/molecules26154414
Chicago/Turabian StyleGuerra, Fabiana Sélos, Flaviana Rodrigues Fintelman Dias, Anna Claudia Cunha, and Patricia Dias Fernandes. 2021. "Benzo[f]indole-4,9-dione Derivatives Effectively Inhibit the Growth of Triple-Negative Breast Cancer" Molecules 26, no. 15: 4414. https://doi.org/10.3390/molecules26154414
APA StyleGuerra, F. S., Dias, F. R. F., Cunha, A. C., & Fernandes, P. D. (2021). Benzo[f]indole-4,9-dione Derivatives Effectively Inhibit the Growth of Triple-Negative Breast Cancer. Molecules, 26(15), 4414. https://doi.org/10.3390/molecules26154414