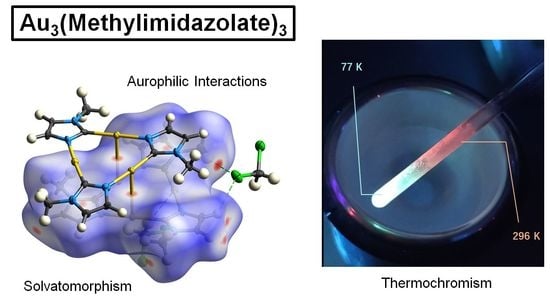
Investigation of Solvatomorphism and Its Photophysical Implications for Archetypal Trinuclear Au3(1-Methylimidazolate)3
Abstract
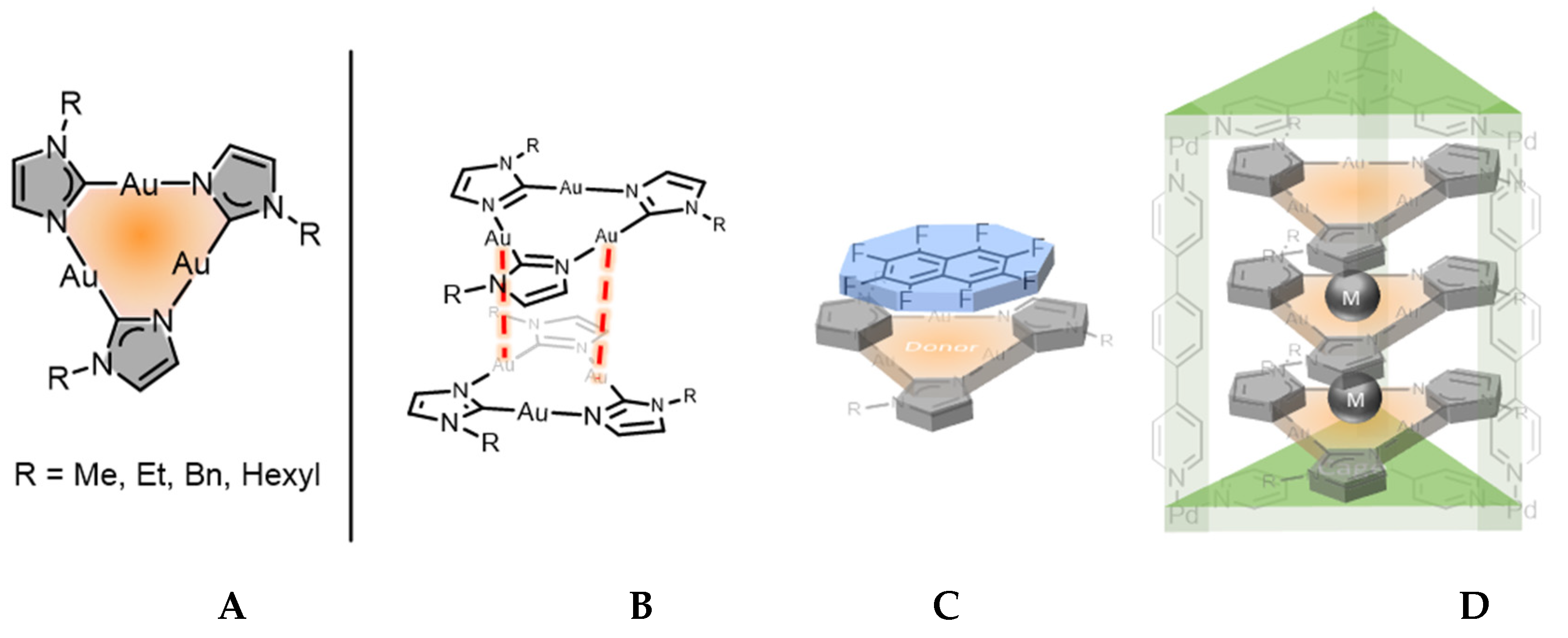
:1. Introduction
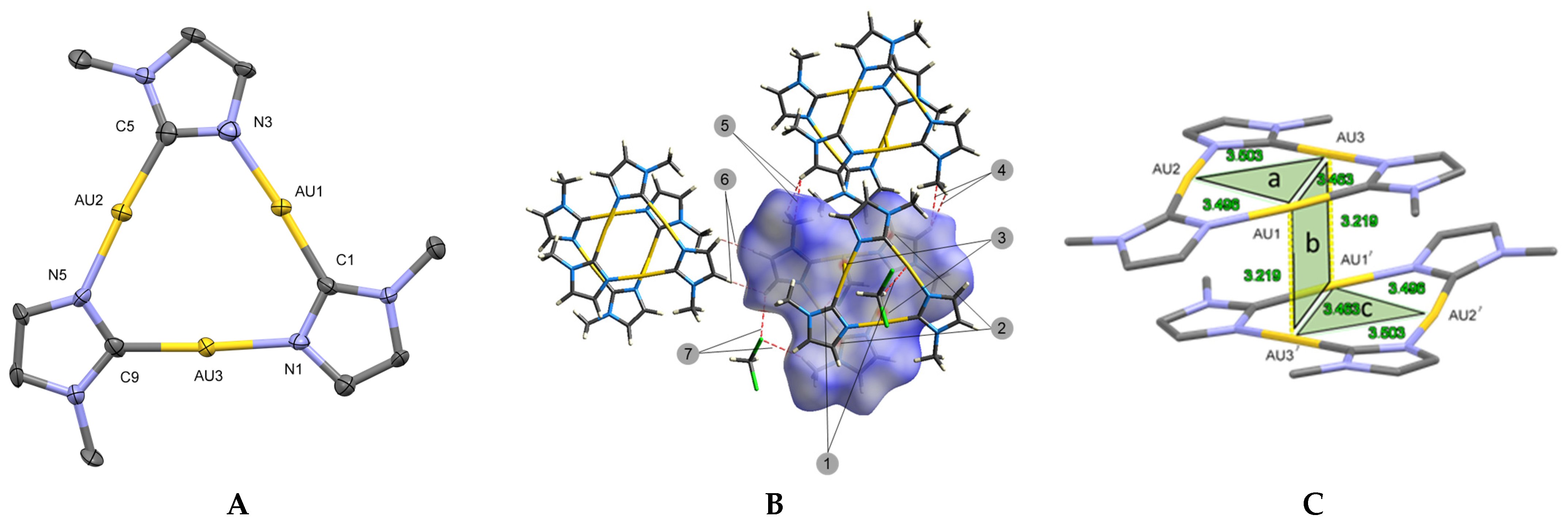
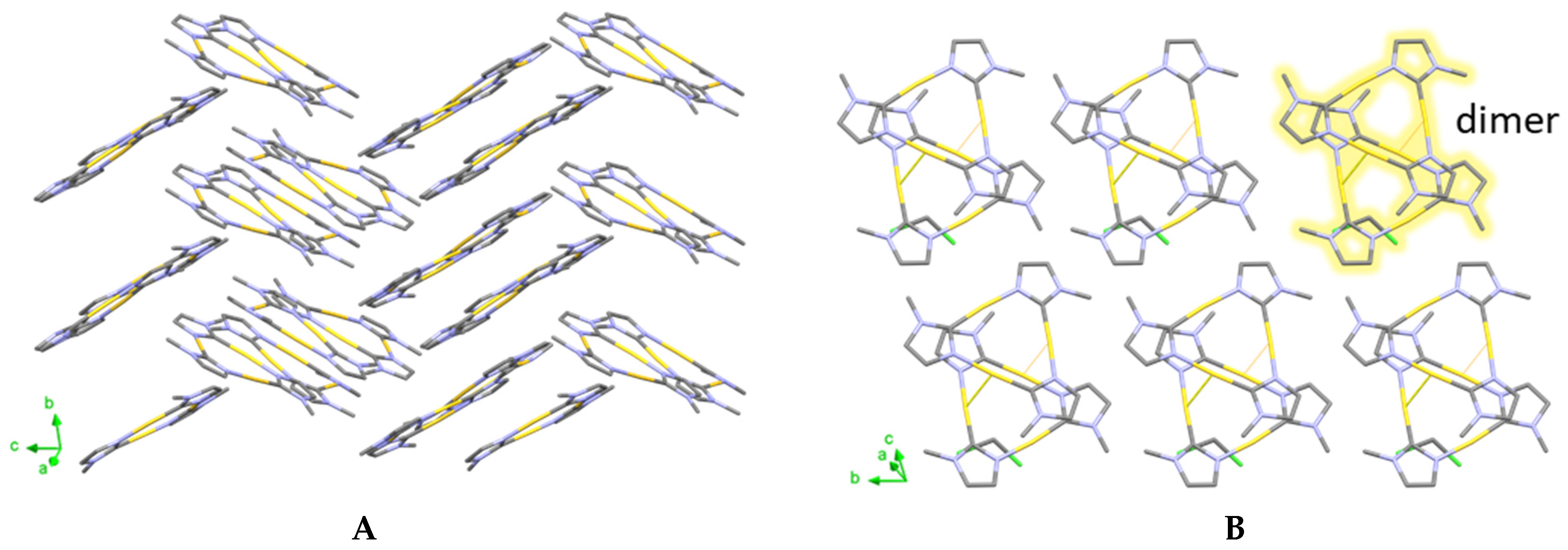
2. Results and Discussion
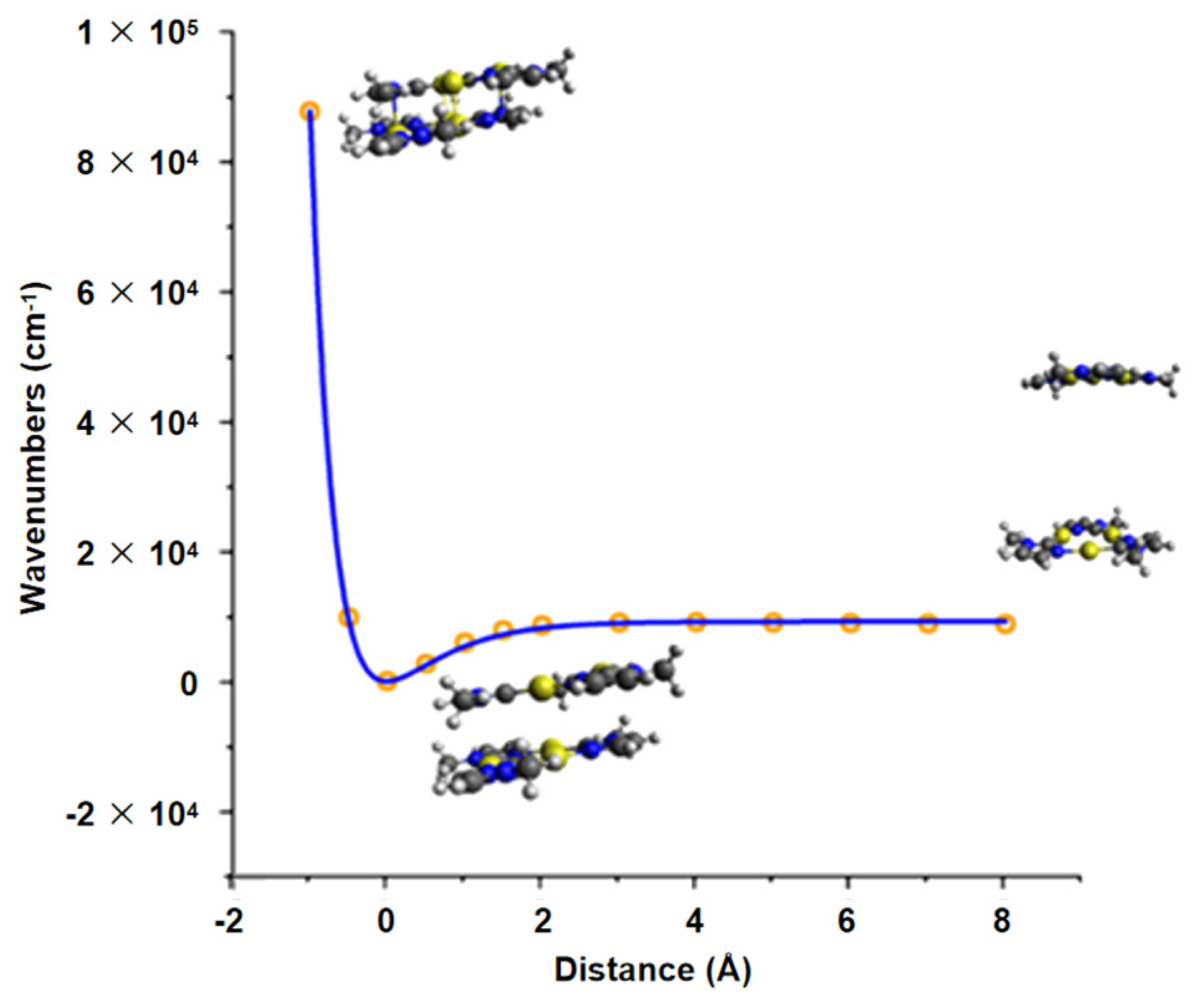
2.1. DFT Studies
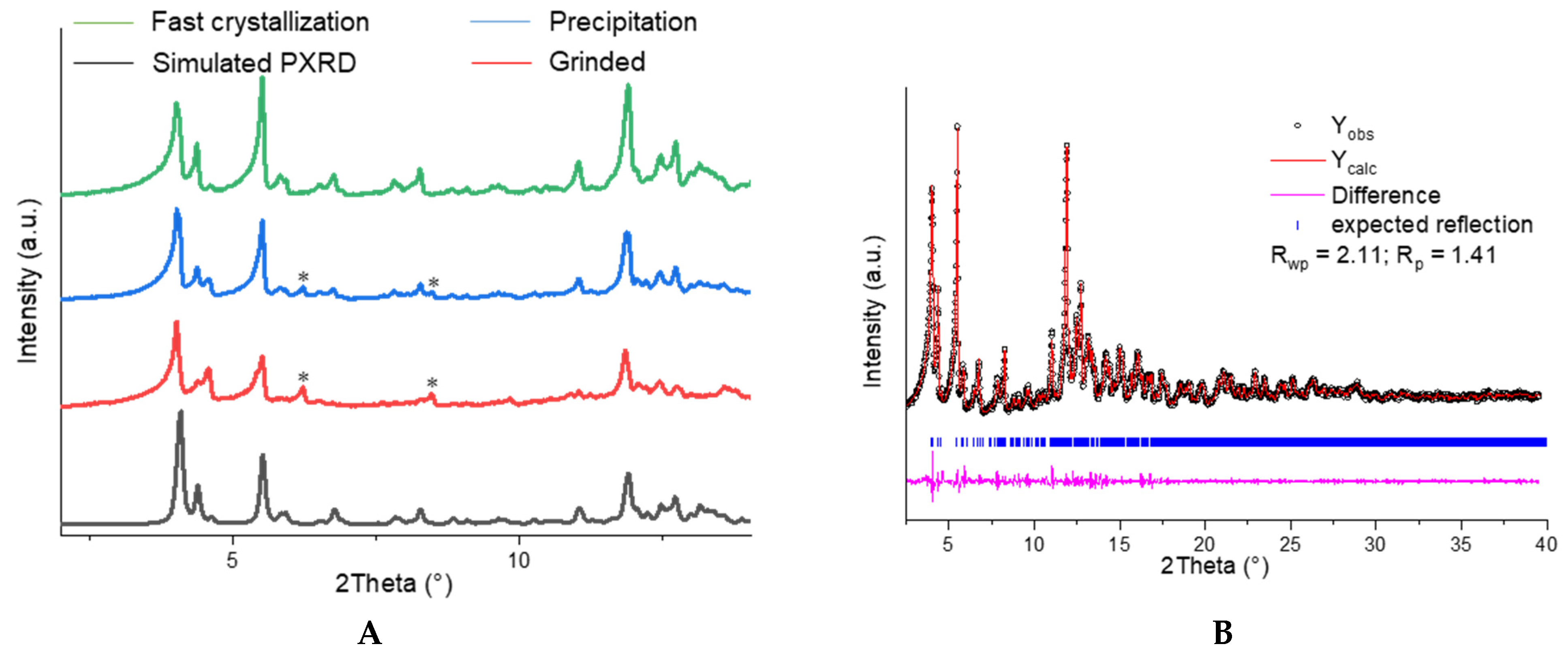
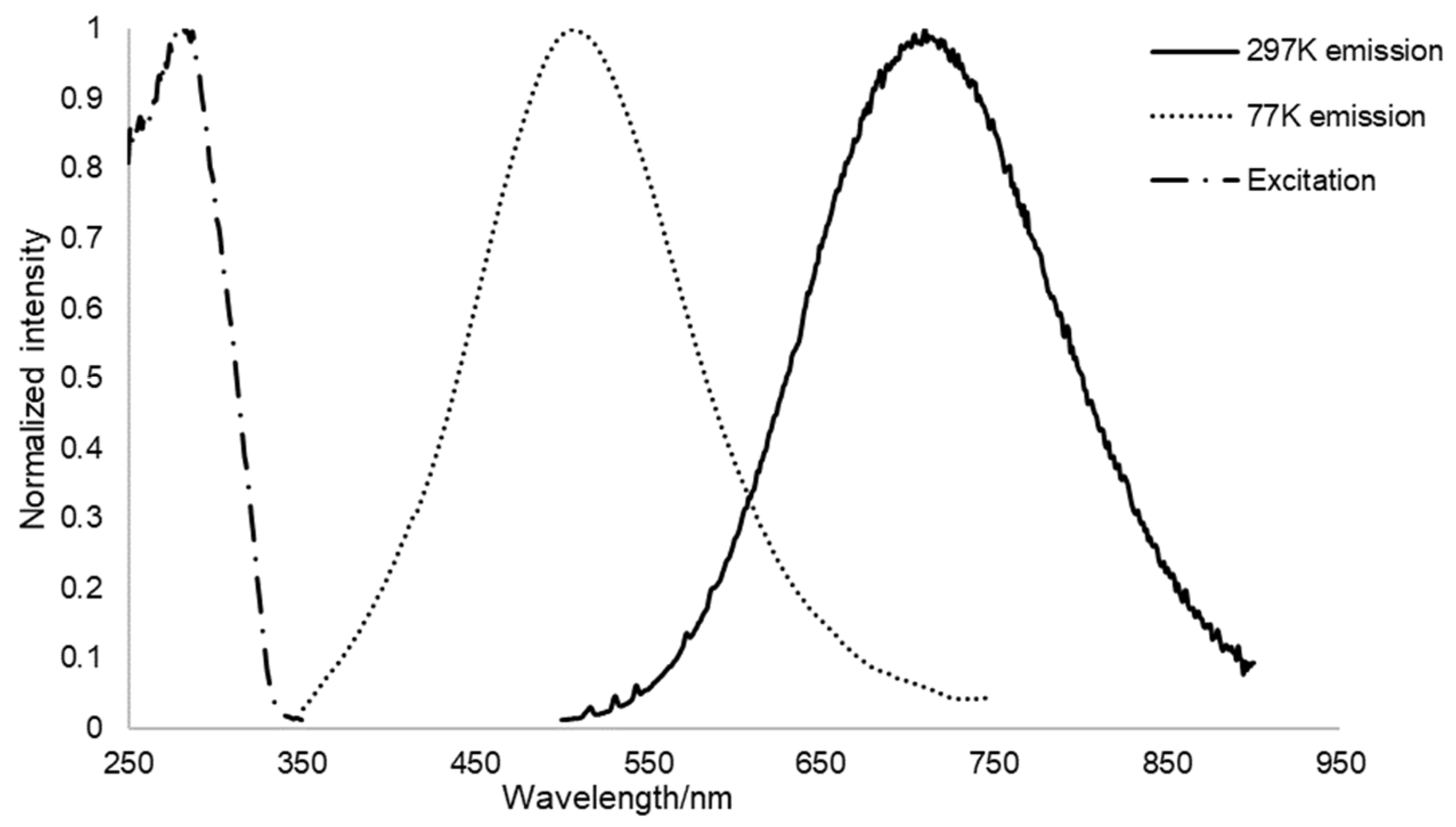
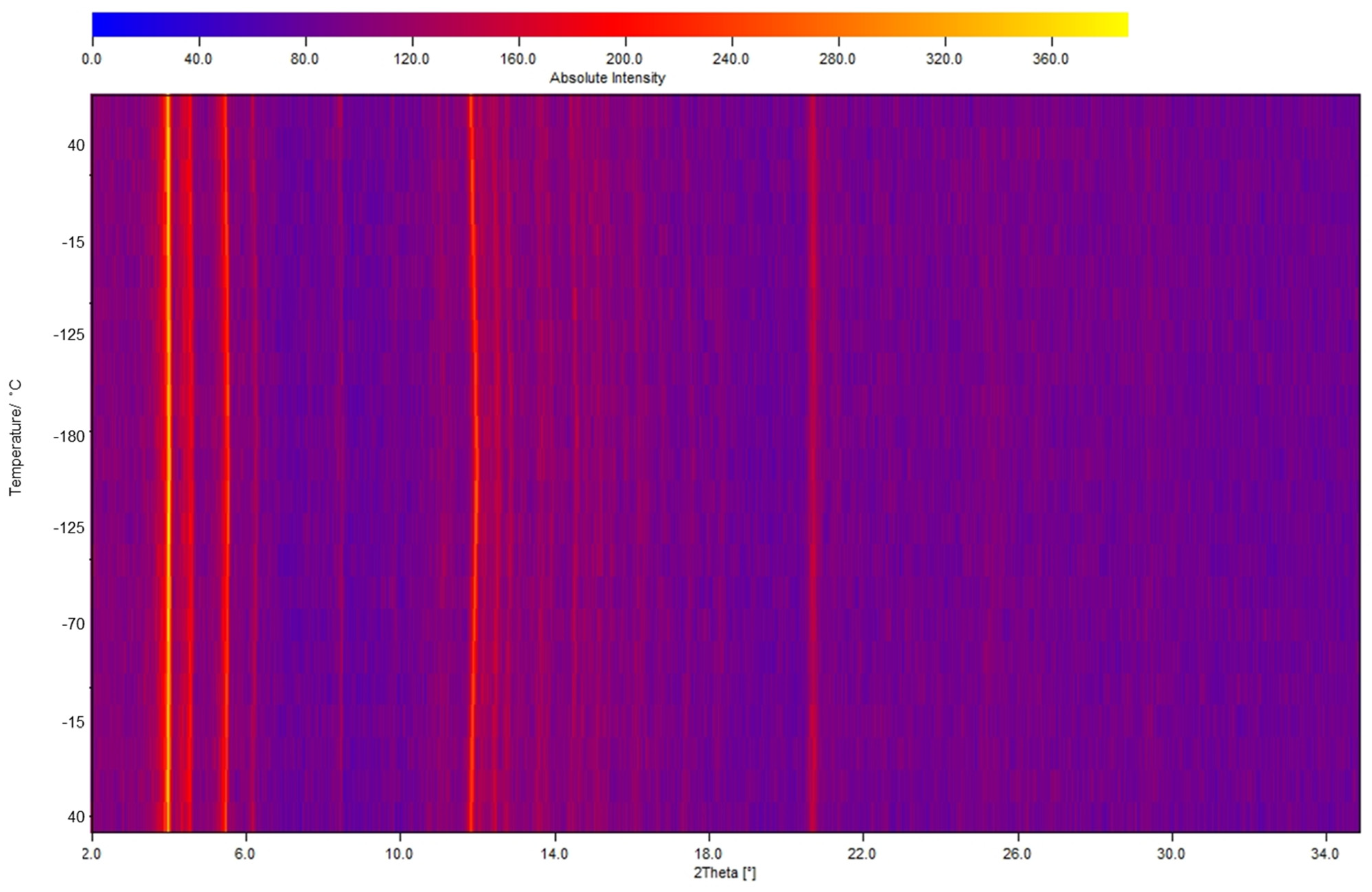
2.2. Qualitative Phase Analysis and Photophysical Studies
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Gimeno, M.C.; Johnson, A. Yldiide Ligands as Building Blocks for Polynuclear Coinage Metal Complexes: Metal Squares, Triangles and Chains. Chem. A Eur. J. 2020, 26, 11256–11265. [Google Scholar] [CrossRef] [PubMed]
- Gade, L.H. “Hyt was of Gold, and Shon so Bryghte…”: Luminescent Gold (I) Compounds. Angew. Chem. Int. Ed. Engl. 1997, 36, 1171–1173. [Google Scholar] [CrossRef]
- Ghimire, M.M.; Nesterov, V.N.; Omary, M.A. Remarkable aurophilicity and photoluminescence thermochromism in a homoleptic cyclic trinuclear gold (I) imidazolate complex. Inorg. Chem. 2017, 56, 12086–12089. [Google Scholar] [CrossRef]
- Rawashdeh-Omary, M.A.; Omary, M.A.; Fackler, J.P.; Galassi, R.; Pietroni, B.R.; Burini, A. Chemistry and optoelectronic properties of stacked supramolecular entities of trinuclear gold (I) complexes sandwiching small organic acids. J. Am. Chem. Soc. 2001, 123, 9689–9691. [Google Scholar] [CrossRef] [PubMed]
- Olmstead, M.M.; Jiang, F.; Attar, S.; Balch, A.L. Alteration of the Aurophilic Interactions in Trimeric Gold (I) Compounds through Charge Transfer. Behavior of Solvoluminescent Au3(MeN COMe)3 in the Presence of Electron Acceptors. J. Am. Chem. Soc. 2001, 123, 3260–3267. [Google Scholar] [CrossRef] [PubMed]
- Burini, A.; Fackler, J.P., Jr.; Galassi, R.; Grant, T.A.; Omary, M.A.; Rawashdeh-Omary, M.A.; Pietroni, B.R.; Staples, R.J. Supramolecular chain assemblies formed by interaction of a π molecular acid complex of mercury with π-base trinuclear gold complexes. J. Am. Chem Soc. 2000, 122, 11264–11265. [Google Scholar] [CrossRef]
- Bonati, F.; Burini, A.; Pietroni, B.R.; Bovio, B. Reactions of C-imidazolyllithium derivatives with group Ib compounds: Tris[μ-(1-alkylimidazolato-N3, C2)] tri-gold (I) and-silver (I). Crystal structure of bis (1-benzylimidazolin-2-yliden) gold (I) chloride. J. Organomet. Chem. 1989, 375, 147–160. [Google Scholar] [CrossRef]
- Schmidbaur, H.; Schier, A. Aurophilic interactions as a subject of current research: An up-date. Chem. Soc. Rev. 2012, 41, 370–412. [Google Scholar] [CrossRef]
- Schmidbaur, H.; Schier, A. A briefing on aurophilicity. Chem. Soc. Rev. 2008, 37, 1931–1951. [Google Scholar] [CrossRef]
- Tekarli, S.M.; Cundari, T.R.; Omary, M.A. Rational design of macrometallocyclic trinuclear complexes with superior π-acidity and π-basicity. J. Am. Chem. Soc. 2008, 130, 1669–1675. [Google Scholar] [CrossRef] [Green Version]
- Vickery, J.C.; Olmstead, M.M.; Fung, E.Y.; Balch, A.L. Solvent-Stimulated Luminescence from the Supramolecular Aggregation of a Trinuclear Gold (i) Complex that Displays Extensive Intermolecular AuċAu Interactions. Angew. Chem. Int. Ed. Engl. 1997, 36, 1179–1181. [Google Scholar] [CrossRef]
- Hayashi, A.; Olmstead, M.M.; Attar, S.; Balch, A.L. Crystal chemistry of the Gold (I) Trimer, Au3(NC5H4)3: Formation of hourglass figures and self-association through aurophilic attraction. J. Am. Chem. Soc. 2002, 124, 5791–5795. [Google Scholar] [CrossRef]
- Omary, M.A.; Elbjeirami, O.; Gamage, C.S.P.; Sherman, K.M.; Dias, H.R. Sensitization of Naphthalene monomer phosphorescence in a sandwich adduct with an electron-poor trinuclear Silver (I) pyrazolate complex. Inorg. Chem. 2009, 48, 1784–1786. [Google Scholar] [CrossRef]
- Osuga, T.; Murase, T.; Fujita, M. Triple-Decker Au3-Ag-Au3-Ag-Au3 Ion Cluster Enclosed in a Self-Assembled Cage. Angew. Chem. 2012, 124, 12365–12367. [Google Scholar] [CrossRef]
- Kiguchi, M.; Inatomi, J.; Takahashi, Y.; Tanaka, R.; Osuga, T.; Murase, T.; Fujita, M.; Tada, T.; Watanabe, S. Highly Conductive [3 × n] Gold-Ion Clusters Enclosed within Self-Assembled Cages. Angew. Chem. 2013, 125, 6322–6325. [Google Scholar] [CrossRef]
- Osuga, T.; Murase, T.; Hoshino, M.; Fujita, M. A Tray-Shaped, PdII-Clipped Au3 Complex as a Scaffold for the Modular Assembly of [3 × n] Au Ion Clusters. Angew. Chem. Int. Ed. 2014, 53, 11186–11189. [Google Scholar] [CrossRef] [PubMed]
- Bovio, B.; Calogero, S.; Wagner, F. A study of reaction products of trimerie 1-benzyl-2-gold (Ⅰ)-imidazole leading to Au^Ⅰ carbene or Au^Ⅰ imidazoline complexes and trinuclear Au^Ⅲ imidazolyl derivatives X-ray crystal structure of [{(μ-1-benzylimidazolato-N3, C2) Au} 312]. J. Organomet. Chem. 1994, 470, 275. [Google Scholar] [CrossRef]
- Hahn, R.; Bohle, F.; Fang, W.; Walther, A.; Grimme, S.; Esser, B. Raising the Bar in Aromatic Donor–Acceptor Interactions with Cyclic Trinuclear Gold (I) Complexes as Strong π-Donors. J. Am. Chem. Soc. 2018, 140, 17932–17944. [Google Scholar] [CrossRef]
- Burini, A.; Bravi, R.; Fackler Jr, J.P.; Galassi, R.; Grant, T.A.; Omary, M.A.; Pietroni, B.R.; Staples, R.J. Luminescent Chains Formed from Neutral, Triangular Gold Complexes Sandwiching TlI and AgI. Structures of {Ag ([Au(μ-C2,N3-bzim)]3)2} BF4·CH2Cl2, {Tl ([Au (μ-C2,N3-bzim)]3)2} PF6·0.5 THF (bzim= 1-Benzylimidazolate), and {Tl ([Au (μ-C (OEt) NC6H4CH3)]3)2} PF6·THF, with MAu6 (M = Ag+, Tl+) Cluster Cores. Inorg. Chem. 2000, 39, 3158–3165. [Google Scholar] [PubMed]
- Ruiz, J.; Sol, D.; Mateo, M.A.; Vivanco, M.; Badía-Laiño, R. A new approach to the synthesis of trinuclear gold (i) imidazolate complexes and their silver (i)-induced photoluminescence behavior. Dalton Trans. 2020, 49, 6561–6565. [Google Scholar] [CrossRef]
- Jandl, C.; Öfele, K.; Pöthig, A. A Pd Halide Cluster from 1964: Pd6Cl8 Capped by Ring-Opened C3Ph3 Ligands from Oxidative Addition of Cyclopropenium Ions. Organometallics 2017, 36, 4348–4350. [Google Scholar] [CrossRef]
- Jandl, C.; Pankhurst, J.R.; Love, J.B.; Pöthig, A. Rational Synthesis and Electronic Structure of Functionalized Trinuclear Pd Metal Sheet Sandwich Complexes. Organometallics 2017, 36, 2772–2783. [Google Scholar] [CrossRef]
- Altmann, P.J.; Pöthig, A. Pillarplexes: A metal–organic class of supramolecular hosts. J. Am. Chem. Soc. 2016, 138, 13171–13174. [Google Scholar] [CrossRef] [PubMed]
- Guan, S.; Pickl, T.; Jandl, C.; Schuchmann, L.; Zhou, X.; Altmann, P.J.; Pöthig, A. Triazolate-based pillarplexes: Shape-adaptive metallocavitands via rim modification of macrocyclic ligands. Org. Chem. Front. 2021. [Google Scholar] [CrossRef]
- Schmidbaur, H.; Graf, W.; Müller, G. Weak Intramolecular Bonding Relationships: The Conformation-Determining Attractive Interaction between Gold (I) Centers. Angew. Chem. Int. Ed. Engl. 1988, 27, 417–419. [Google Scholar] [CrossRef]
- Davey, R.J. Polymorphism in Molecular Crystals Joel Bernstein; Oxford University Press: New York, NY, USA, 2002; Volume 2, pp. 675–676. ISBN 0198506058. [Google Scholar]
- Lefebvre, J.; Batchelor, R.J.; Leznoff, D.B. Cu[Au(CN)2]2(DMSO)2: Golden Polymorphs That Exhibit Vapochromic Behavior. J. Am. Chem. Soc. 2004, 126, 16117–16125. [Google Scholar] [CrossRef]
- White-Morris, R.L.; Olmstead, M.M.; Balch, A.L. Aurophilic interactions in cationic gold complexes with two isocyanide ligands. Polymorphic yellow and colorless forms of [(cyclohexyl isocyanide) 2AuI](PF6) with distinct luminescence. J. Am. Chem Soc. 2003, 125, 1033–1040. [Google Scholar] [CrossRef]
- He, X.; Yam, V.W.-W. Luminescent gold(I) complexes for chemosensing. Coord. Chem. Rev. 2011, 255, 2111–2123. [Google Scholar] [CrossRef]
- Vaughan, L.G. Organogold chemistry. III. 2-pyridylgold (I). J. Am. Chem. Soc. 1970, 92, 730–731. [Google Scholar] [CrossRef]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Schmidbaur, H.; Raubenheimer, H.G.; Dobrzańska, L. The gold–hydrogen bond, Au–H, and the hydrogen bond to gold, Au⋯ H–X. Chem. Soc. Rev. 2014, 43, 345–380. [Google Scholar] [CrossRef]
- Kryachko, E.; Karpfen, A.; Remacle, F. Nonconventional hydrogen bonding between clusters of gold and hydrogen fluoride. J. Phys. Chem. A 2005, 109, 7309–7318. [Google Scholar] [CrossRef]
- Rigoulet, M.; Massou, S.; Carrizo, E.D.S.; Mallet-Ladeira, S.; Amgoune, A.; Miqueu, K.; Bourissou, D. Evidence for genuine hydrogen bonding in gold (I) complexes. Proc. Natl. Acad. Sci. USA 2019, 116, 46–51. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Schultz, N.; Truhlar, D.G. Design of density functionals by combining the method of constraint satisfaction with parametrization for thermochemistry, thermochemical kinetics, and noncovalent interactions. J. Chem. Theory Comput. 2006, 2, 364–382. [Google Scholar]
- Cundari, T.R.; Stevens, W.J. Effective core potential methods for the lanthanides. J. Chem. Phys. 1993, 98, 5555–5565. [Google Scholar] [CrossRef]
- Galassi, R.; Ghimire, M.M.; Otten, B.M.; Ricci, S.; McDougald, R.N.; Almotawa, R.M.; Alhmoud, D.; Ivy, J.F.; Rawashdeh, A.-M.M.; Nesterov, V.N. Cupriphication of gold to sensitize d10–d10 metal–metal bonds and near-unity phosphorescence quantum yields. Proc. Natl. Acad. Sci. USA 2017, 114, E5042–E5051. [Google Scholar] [PubMed] [Green Version]
- Galassi, R.; Ricci, S.; Burini, A.; Macchioni, A.; Rocchigiani, L.; Marmottini, F.; Tekarli, S.M.; Nesterov, V.N.; Omary, M.A. Solventless supramolecular chemistry via vapor diffusion of volatile small molecules upon a new trinuclear silver (I)-nitrated pyrazolate macrometallocyclic solid: An experimental/theoretical investigation of the dipole/quadrupole chemisorption phenomena. Inorg. Chem. 2013, 52, 14124–14137. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. Density functionals with broad applicability in chemistry. Acc. Chem. Res. 2008, 41, 157–167. [Google Scholar] [CrossRef]
- Jayaratna, N.B.; Olmstead, M.M.; Kharisov, B.I.; Dias, H.R. Coinage Metal Pyrazolates [(3, 5-(CF3) 2Pz) M] 3 (M = Au, Ag, Cu) as Buckycatchers. Inorg. Chem. 2016, 55, 8277–8280. [Google Scholar] [CrossRef]
- Coulson, C.A. The electronic structure of some polyenes and aromatic molecules. VII. Bonds of fractional order by the molecular orbital method. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci. 1939, 169, 413–428. [Google Scholar]
- Giambiagi, M.; Giambiagi, M.; Grempel, D.R.; Heymann, C.D. Sur la définition d’un indice de liaison (TEV) pour des bases non orthogonales. Propriétés et applications. J. Chim. Phys. 1975, 72, 15–22. [Google Scholar] [CrossRef]
- Mayer, I.; Salvador, P. Overlap populations, bond orders and valences for ‘fuzzy’atoms. Chem. Phys. Lett. 2004, 383, 368–375. [Google Scholar] [CrossRef]
- Schmidbaur, H.; Raubenheimer, H.G. Excimer and Exciplex Formation in Gold (I) Complexes Preconditioned by Aurophilic Interactions. Angew. Chem. 2020, 59, 14748–14771. [Google Scholar] [CrossRef]
- Vellé, A.; Rodríguez-Santiago, L.; Sodupe, M.; Sanz Miguel, P.J. Enhanced Metallophilicity in Metal–Carbene Systems: Stronger Character of Aurophilic Interactions in Solution. Chem. A Eur. J. 2020, 26, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Messerschmidt, M.; Coppens, P.; Omary, M.A. Trinuclear gold (I) triazolates: A new class of wide-band phosphors and sensors. Inorg. Chem. 2006, 45, 6592–6594. [Google Scholar] [CrossRef] [PubMed]
- Omary, M.A.; Rawashdeh-Omary, M.A.; Gonser, M.A.; Elbjeirami, O.; Grimes, T.; Cundari, T.R.; Diyabalanage, H.V.; Gamage, C.S.P.; Dias, H.R. Metal Effect on the Supramolecular Structure, Photophysics, and Acid–Base Character of Trinuclear Pyrazolato Coinage Metal Complexes. Inorg. Chem. 2005, 44, 8200–8210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodall, C.H.; Christensen, J.; Skelton, J.M.; Hatcher, L.E.; Parlett, A.; Raithby, P.R.; Walsh, A.; Parker, S.C.; Beavers, C.M.; Teat, S.J. Observation of a re-entrant phase transition in the molecular complex tris (μ2-3, 5-diisopropyl-1, 2, 4-triazolato-κ2N1: N2) trigold (I) under high pressure. IUCrJ 2016, 3, 367–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Experimental Data | Calculated Results | |
|---|---|---|
| Au-N bond/Å | 2.030(9), 2.036(9), 2.043(8) | 2.091 |
| Au-Ccarb bond/Å | 1.986(11), 1.987(11), 1.988(11) | 2.038 |
| Ccarb-Au-N angle/° | 173.6(4), 175.3(4), 175.8(4) | 175.13 |
| intratrimer Au1-Au2/Å | 3.4961(9) | 3.567 |
| intratrimer Au1-Au3/Å | 3.4636(7) | 3.555 |
| intratrimer Au2-Au3/Å | 3.5032(8) | 3.584 |
| WBO | MBO | FBO | BCP | |
|---|---|---|---|---|
| Au1-Au2 (intra) | 0.135 | 0.062 | 0.190 | no |
| Au1-Au3 (intra) | 0.135 | 0.079 | 0.192 | no |
| Au1-Au2-Au3 (intra) | --- | 0.035 (normalized multi-center BO) | --- | yes |
| Au1-Au3 (inter) | 0.269 | 0.156 | 0.428 | yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, S.; Mayer, D.C.; Jandl, C.; Weishäupl, S.J.; Casini, A.; Pöthig, A. Investigation of Solvatomorphism and Its Photophysical Implications for Archetypal Trinuclear Au3(1-Methylimidazolate)3. Molecules 2021, 26, 4404. https://doi.org/10.3390/molecules26154404
Guan S, Mayer DC, Jandl C, Weishäupl SJ, Casini A, Pöthig A. Investigation of Solvatomorphism and Its Photophysical Implications for Archetypal Trinuclear Au3(1-Methylimidazolate)3. Molecules. 2021; 26(15):4404. https://doi.org/10.3390/molecules26154404
Chicago/Turabian StyleGuan, Shengyang, David C. Mayer, Christian Jandl, Sebastian J. Weishäupl, Angela Casini, and Alexander Pöthig. 2021. "Investigation of Solvatomorphism and Its Photophysical Implications for Archetypal Trinuclear Au3(1-Methylimidazolate)3" Molecules 26, no. 15: 4404. https://doi.org/10.3390/molecules26154404
APA StyleGuan, S., Mayer, D. C., Jandl, C., Weishäupl, S. J., Casini, A., & Pöthig, A. (2021). Investigation of Solvatomorphism and Its Photophysical Implications for Archetypal Trinuclear Au3(1-Methylimidazolate)3. Molecules, 26(15), 4404. https://doi.org/10.3390/molecules26154404