α-Functionally Substituted α,β-Unsaturated Aldehydes as Fine Chemicals Reagents: Synthesis and Application
Abstract
1. Introduction
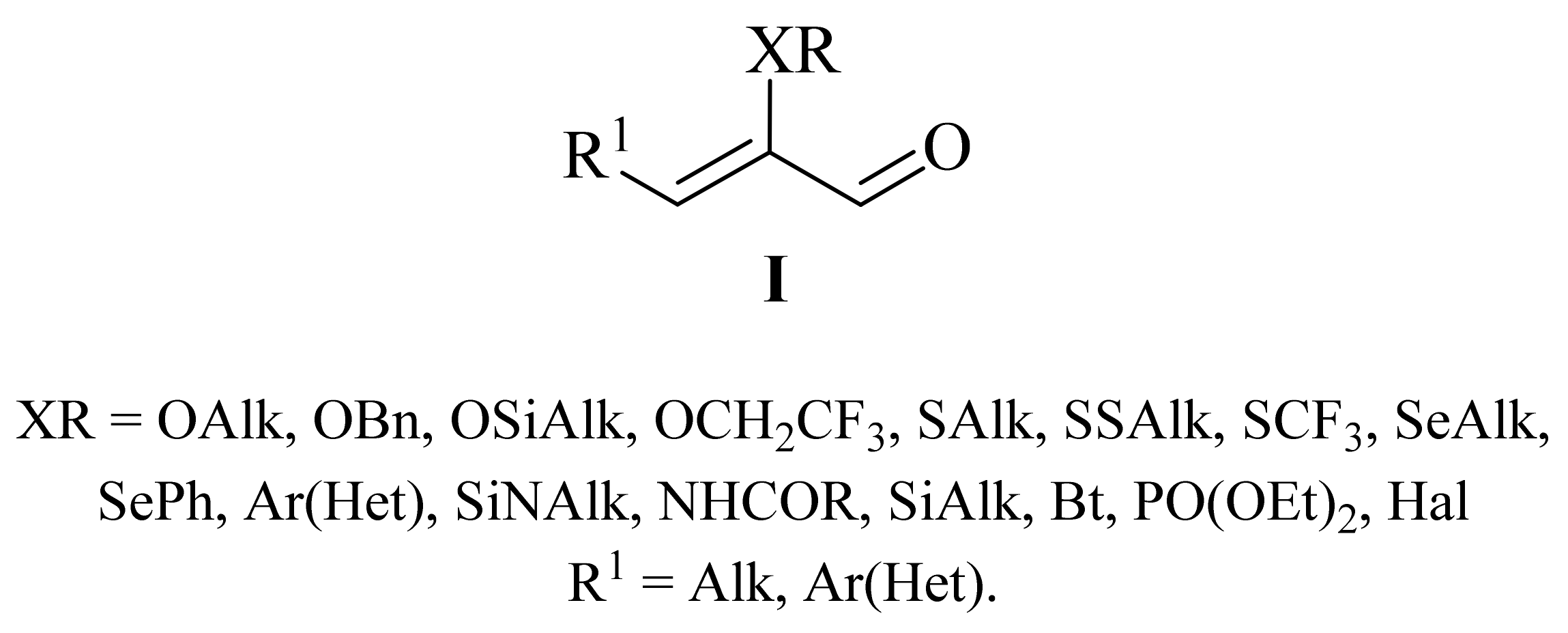
2. Synthesis of α-Functionally Substituted α,β-Unsaturated Aldehydes
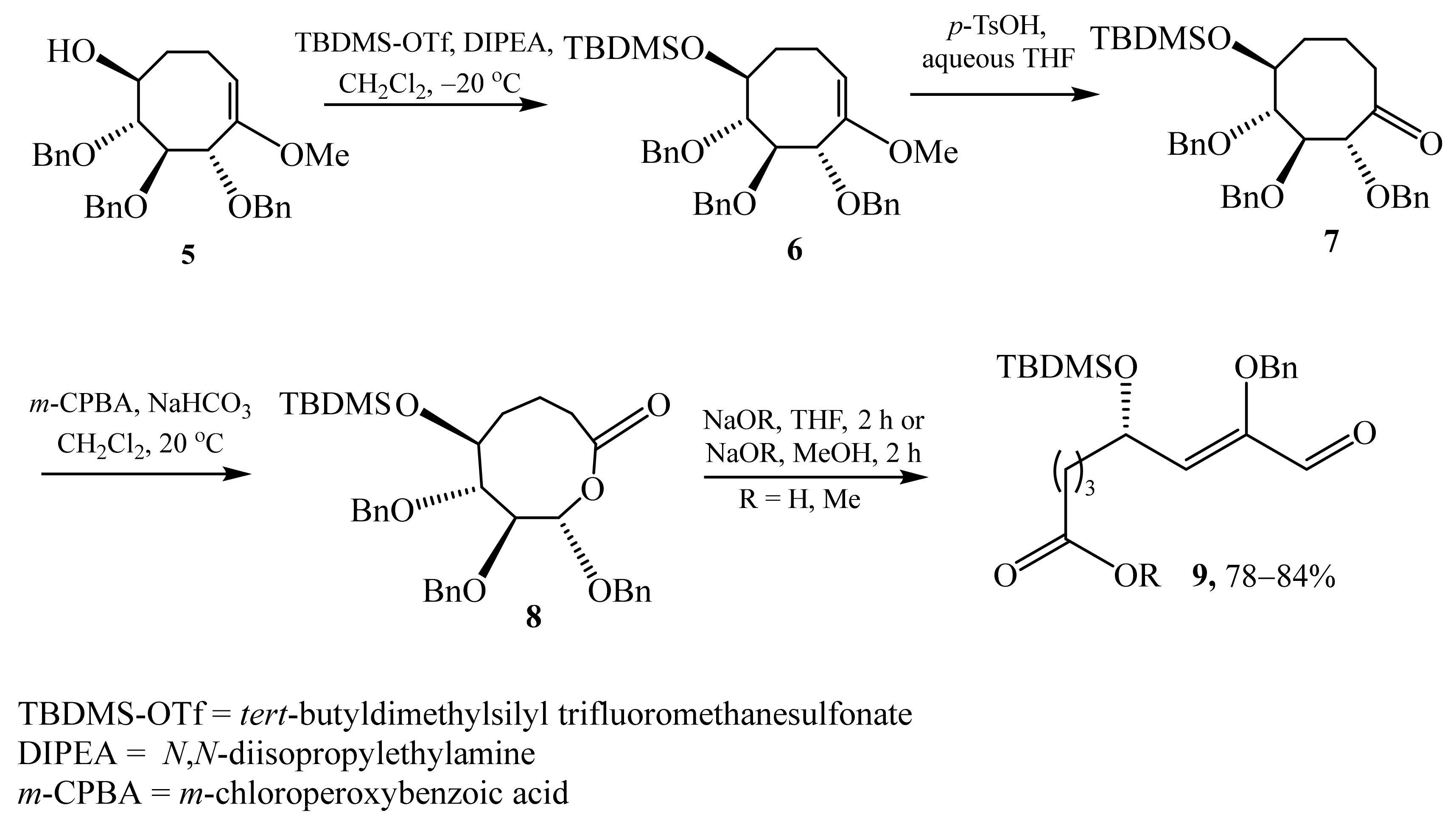
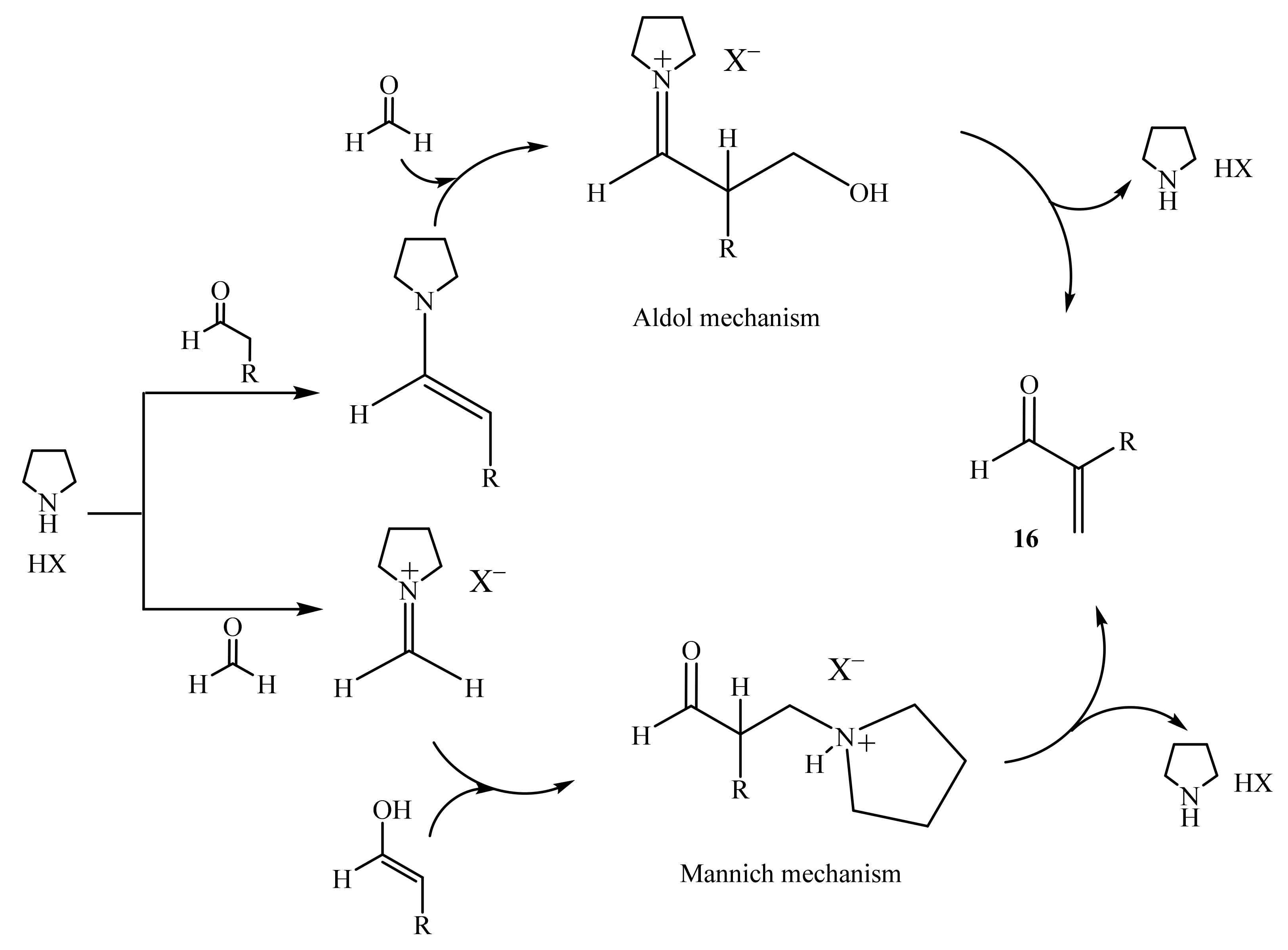
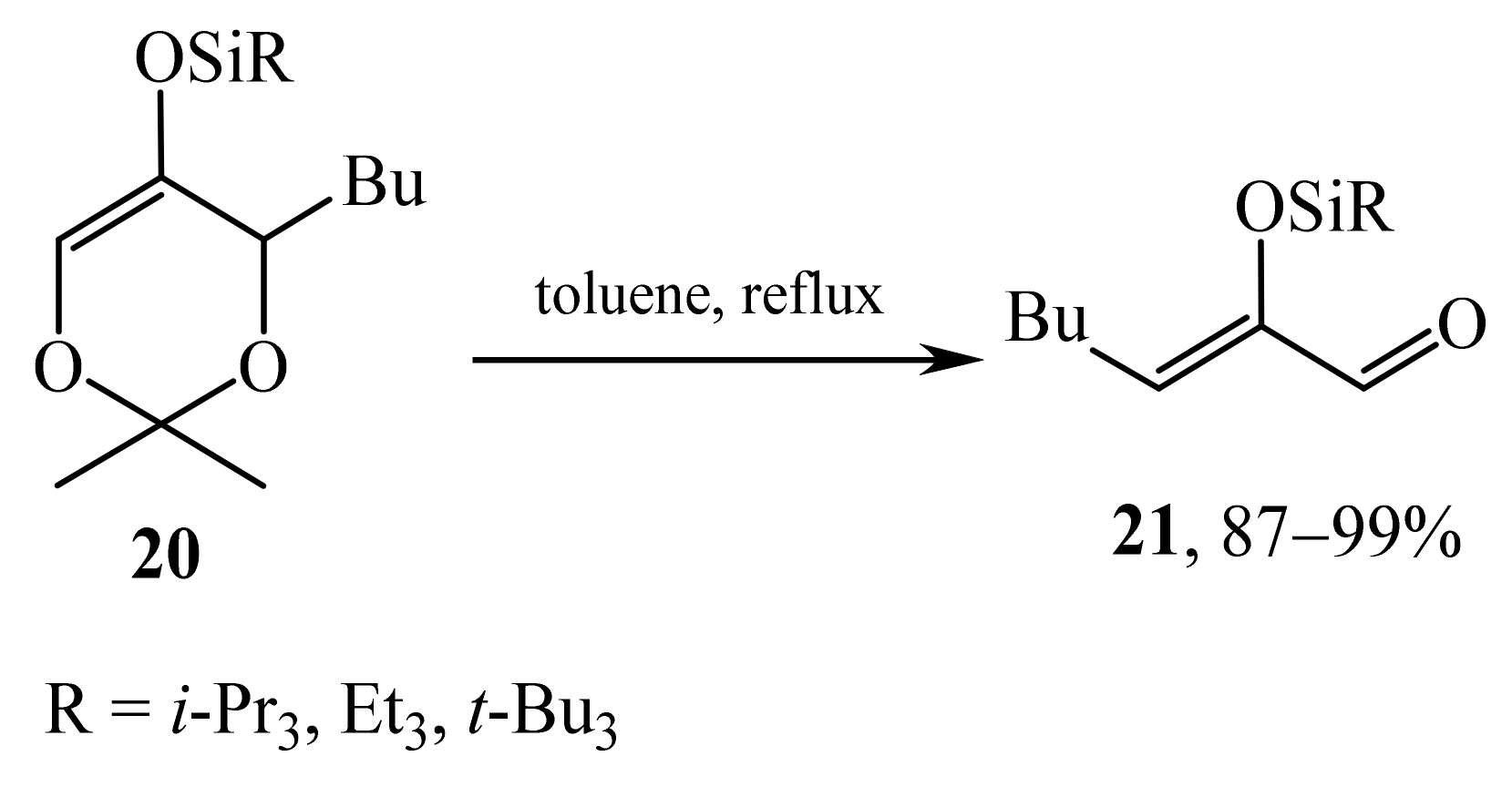
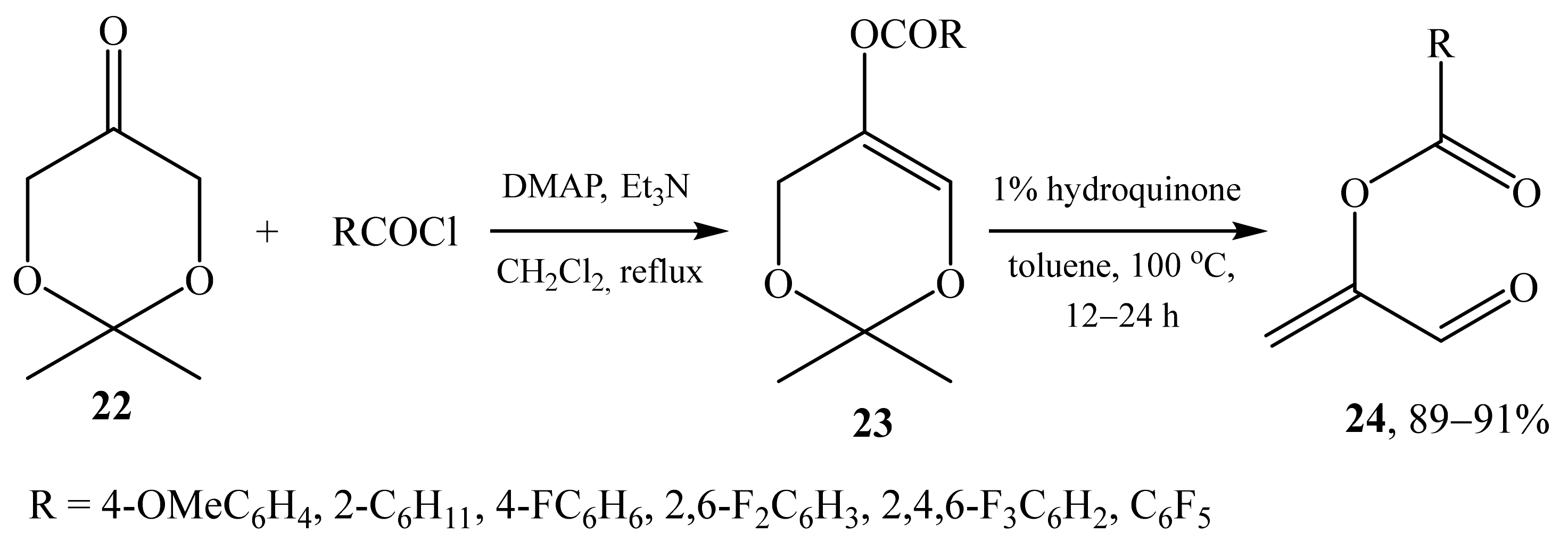
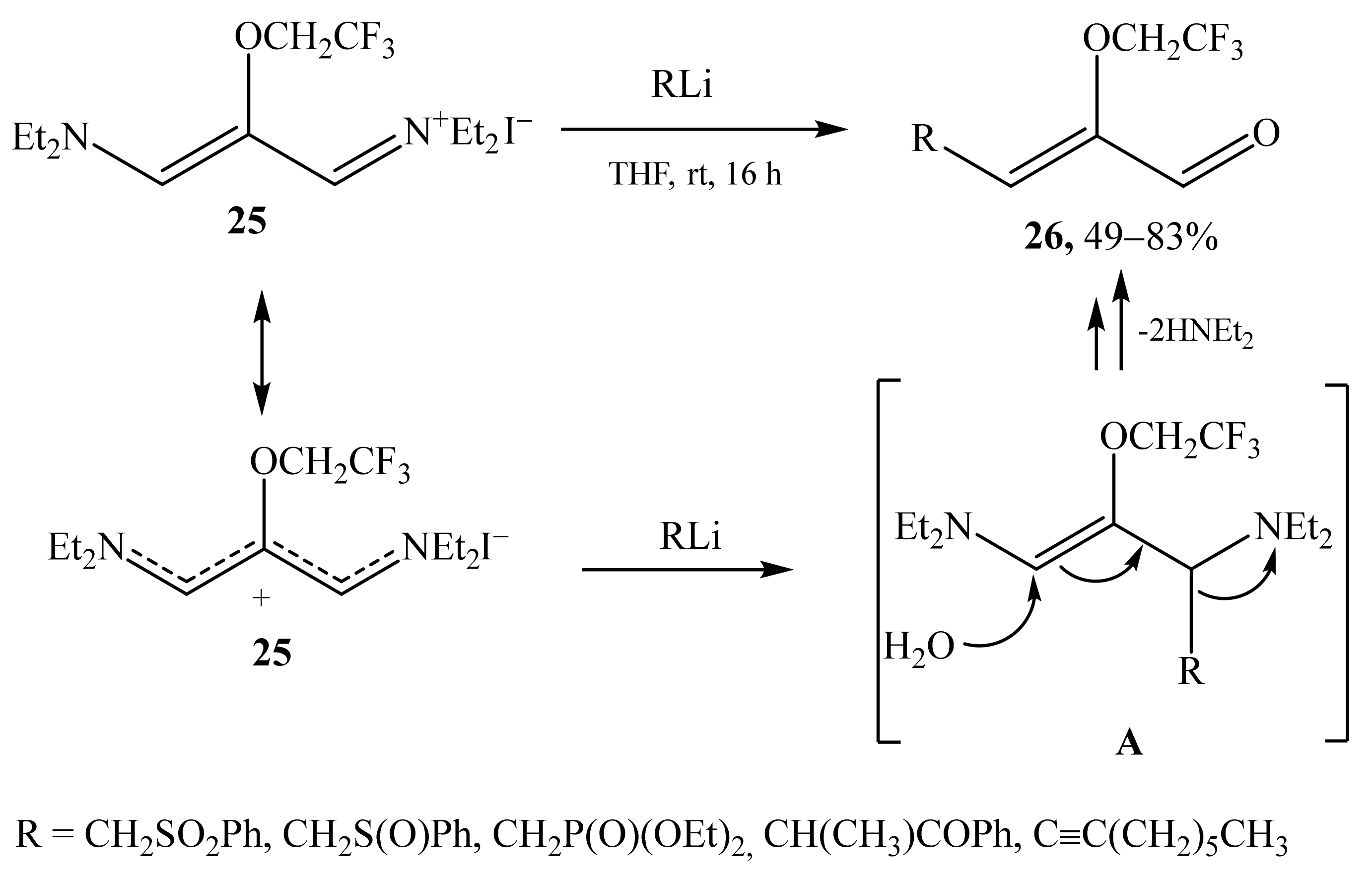
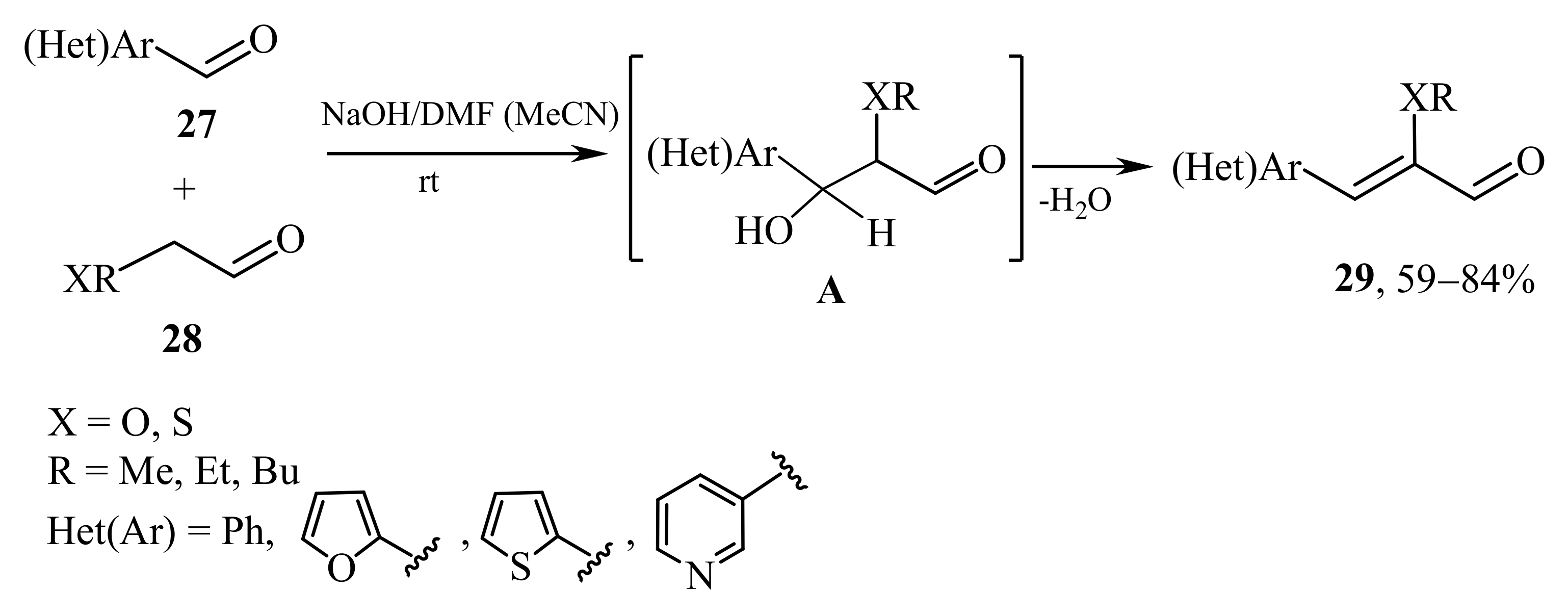
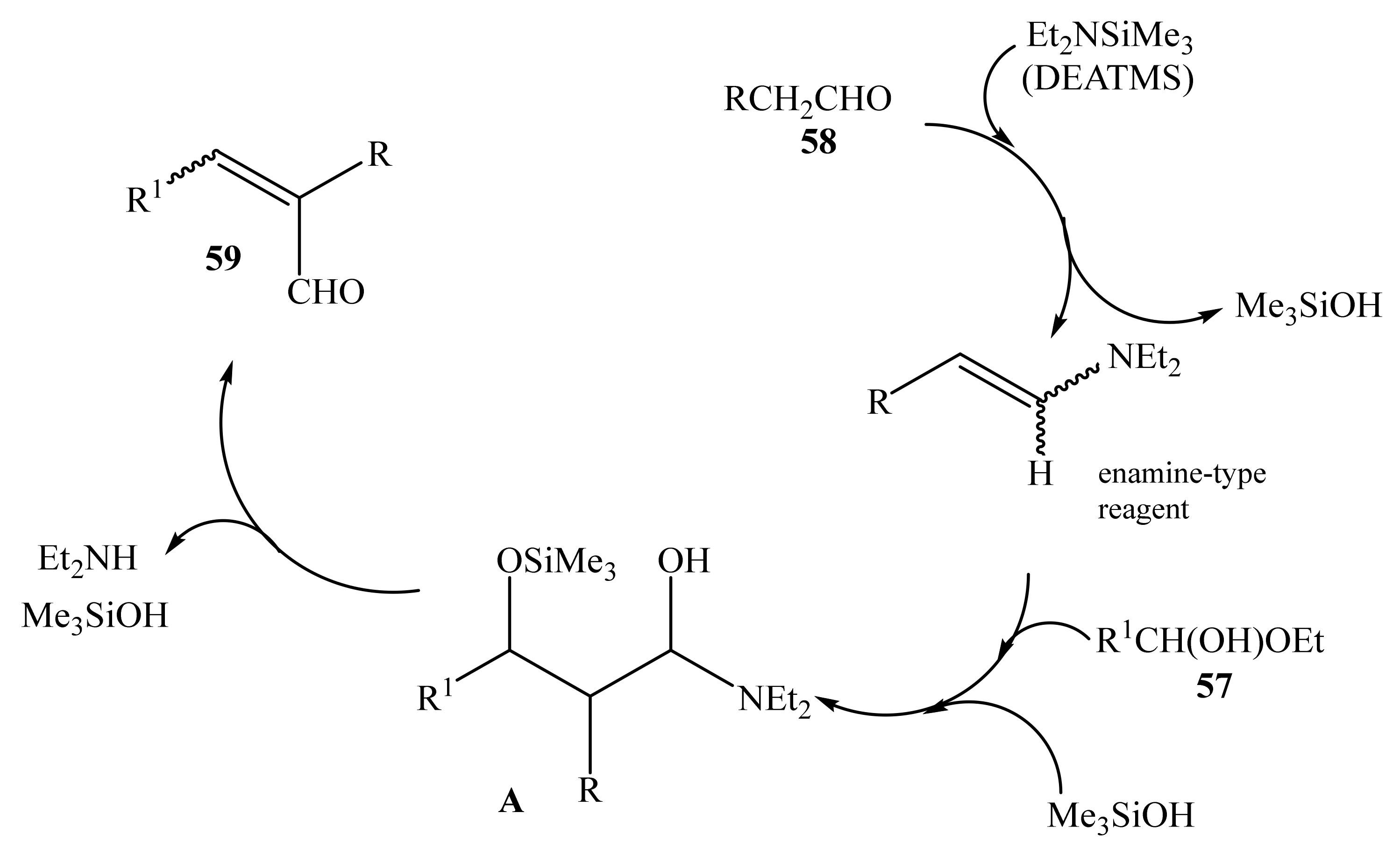
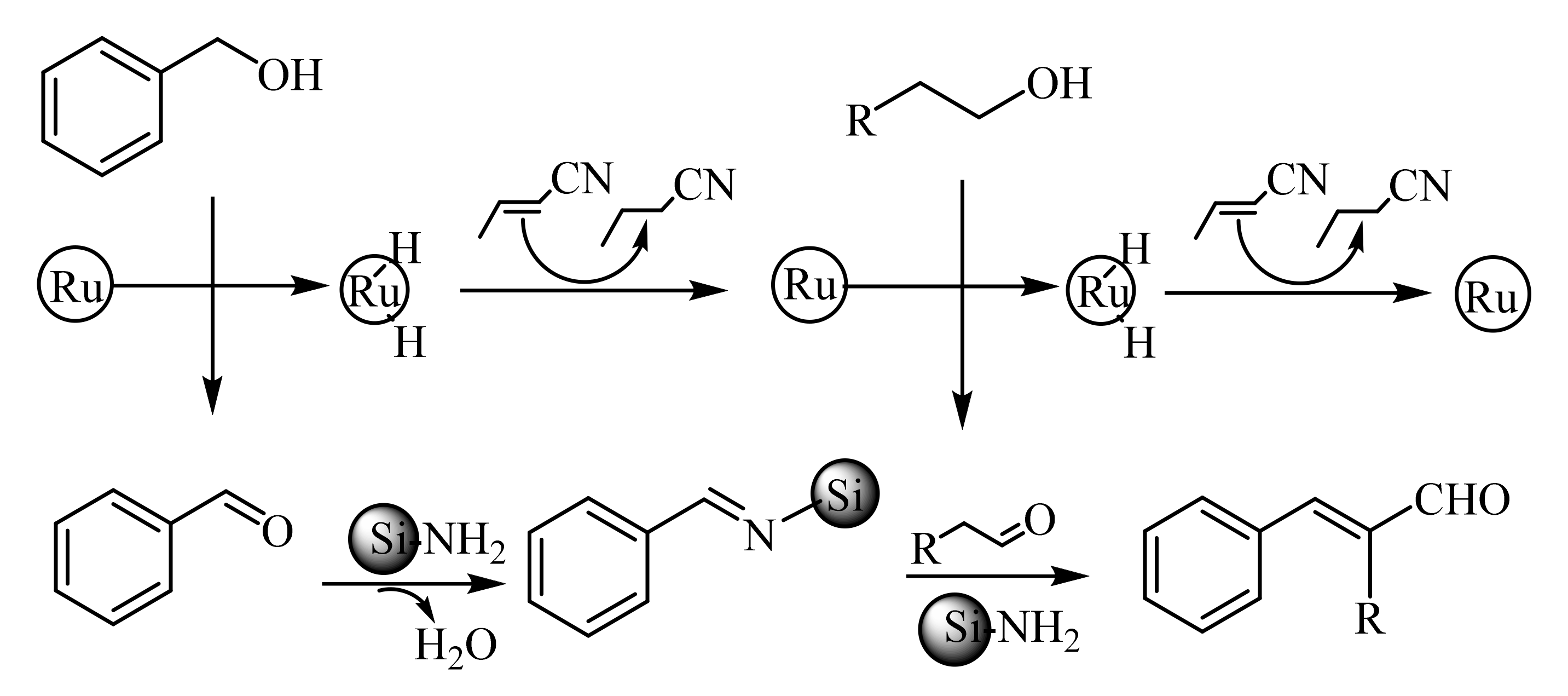
2.1. α-Oxygensubstituted α,β-Unsaturated Aldehydes

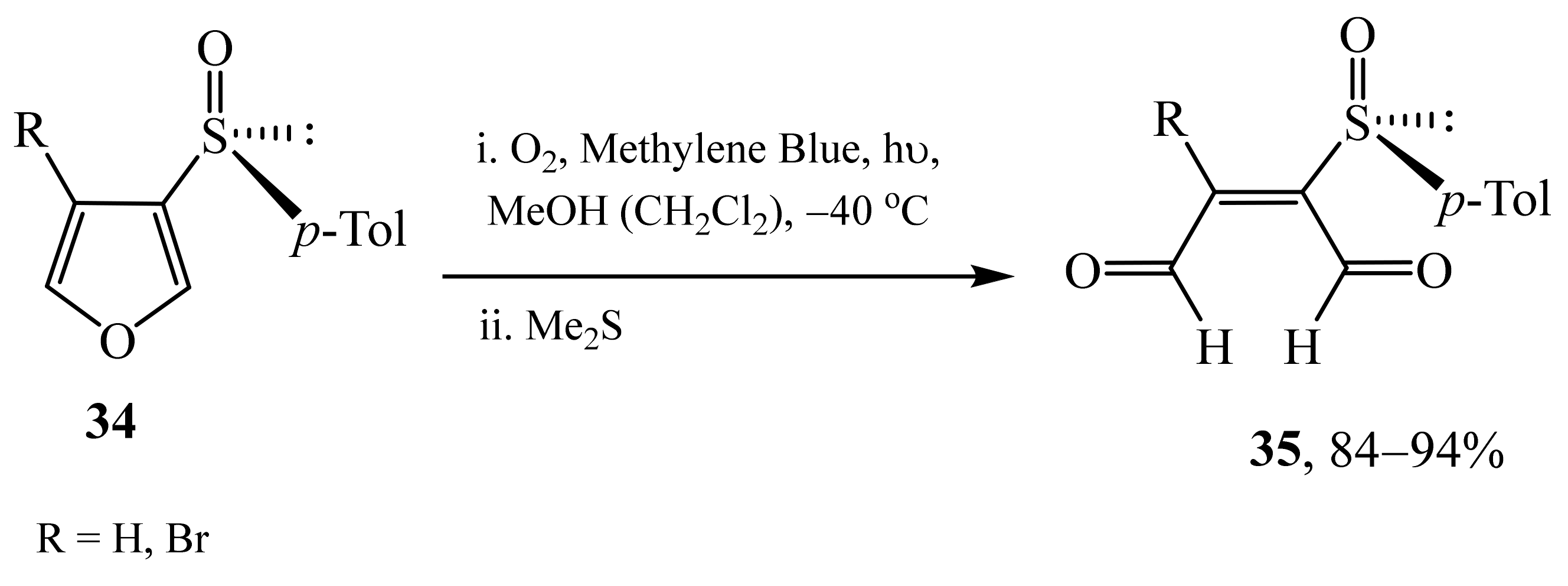
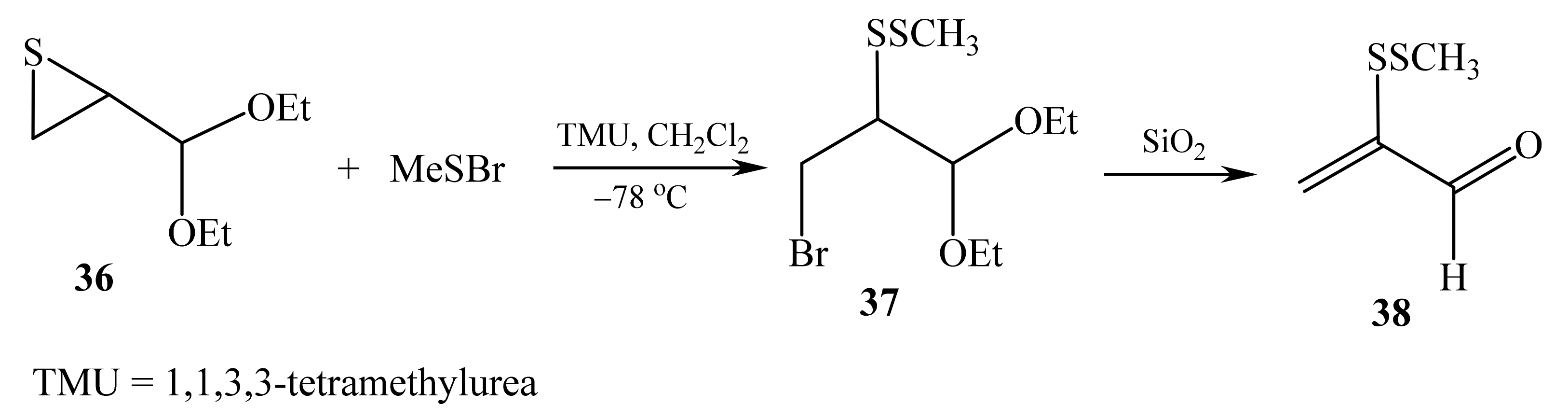
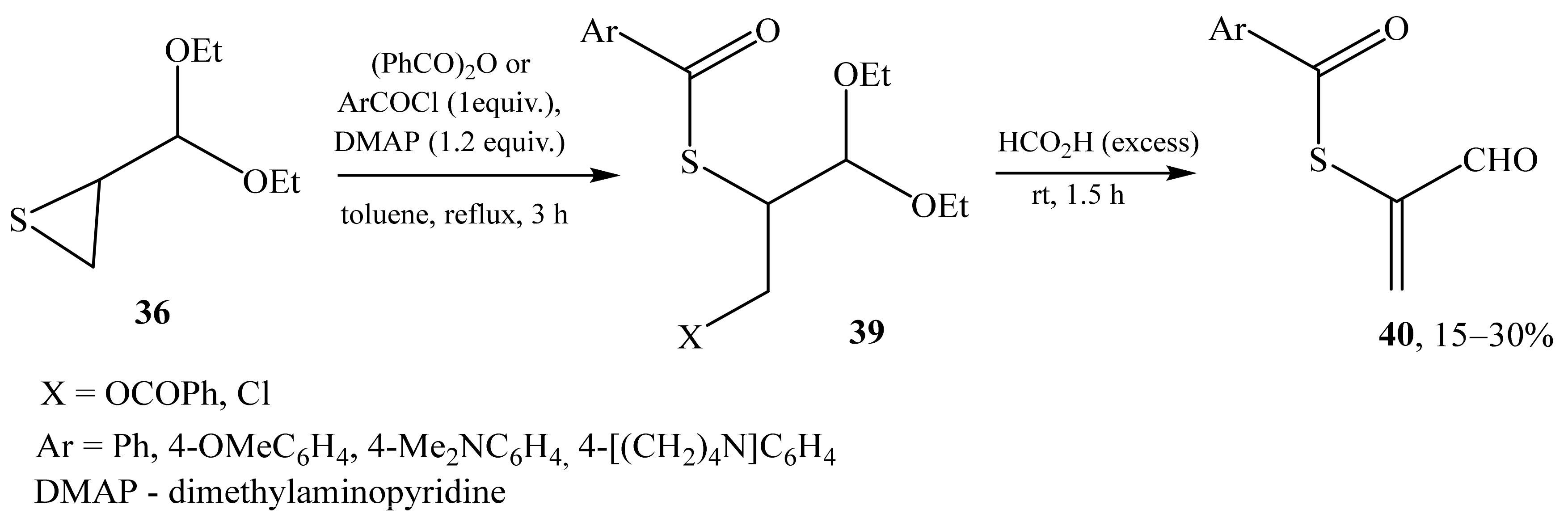
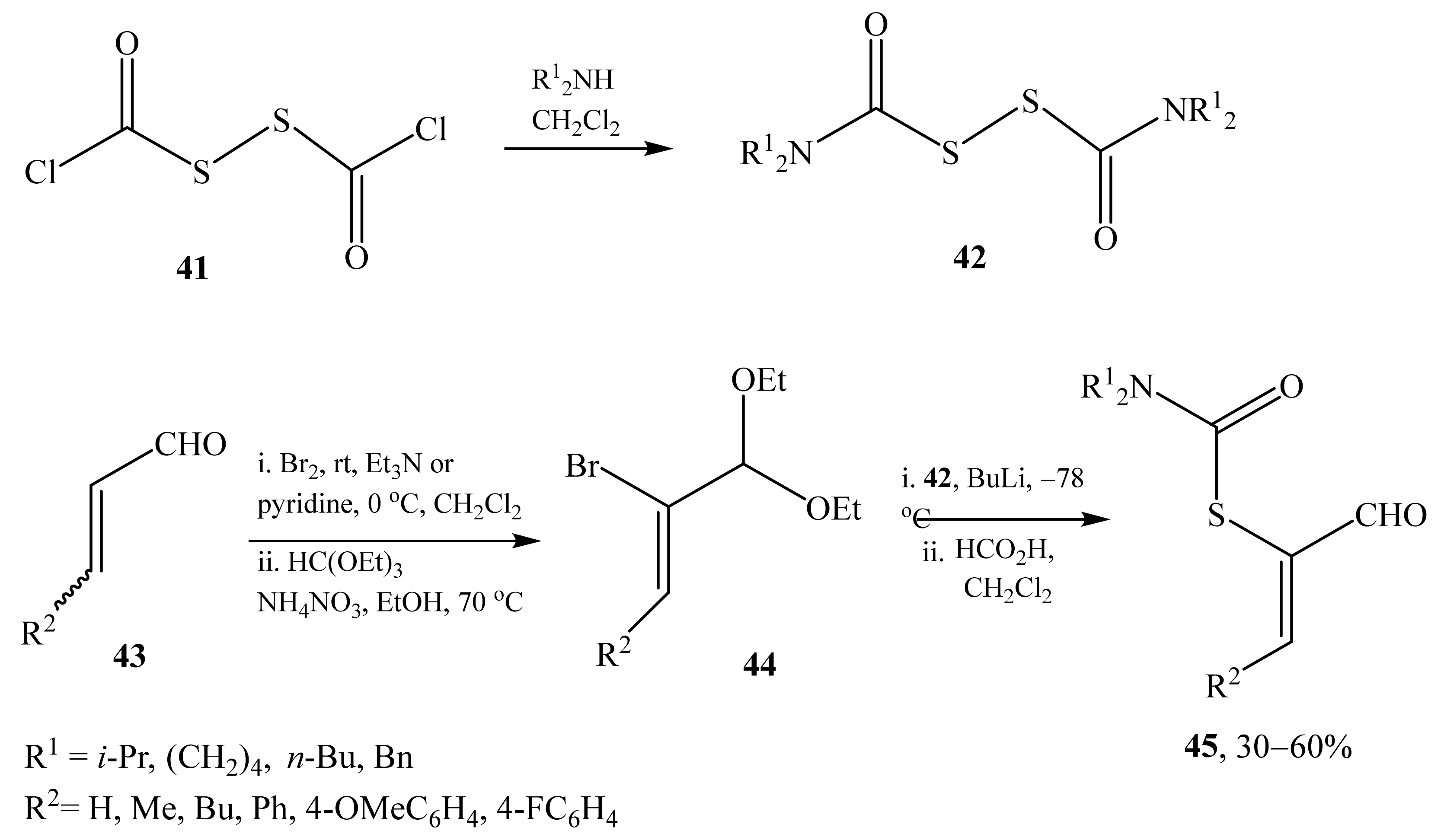
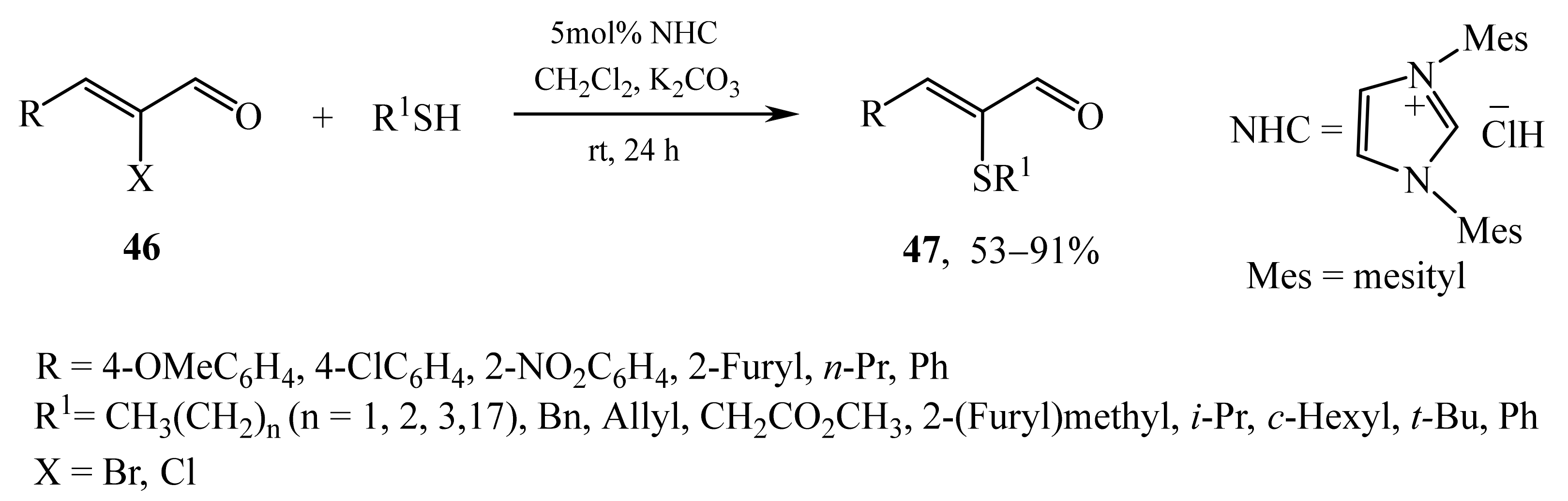
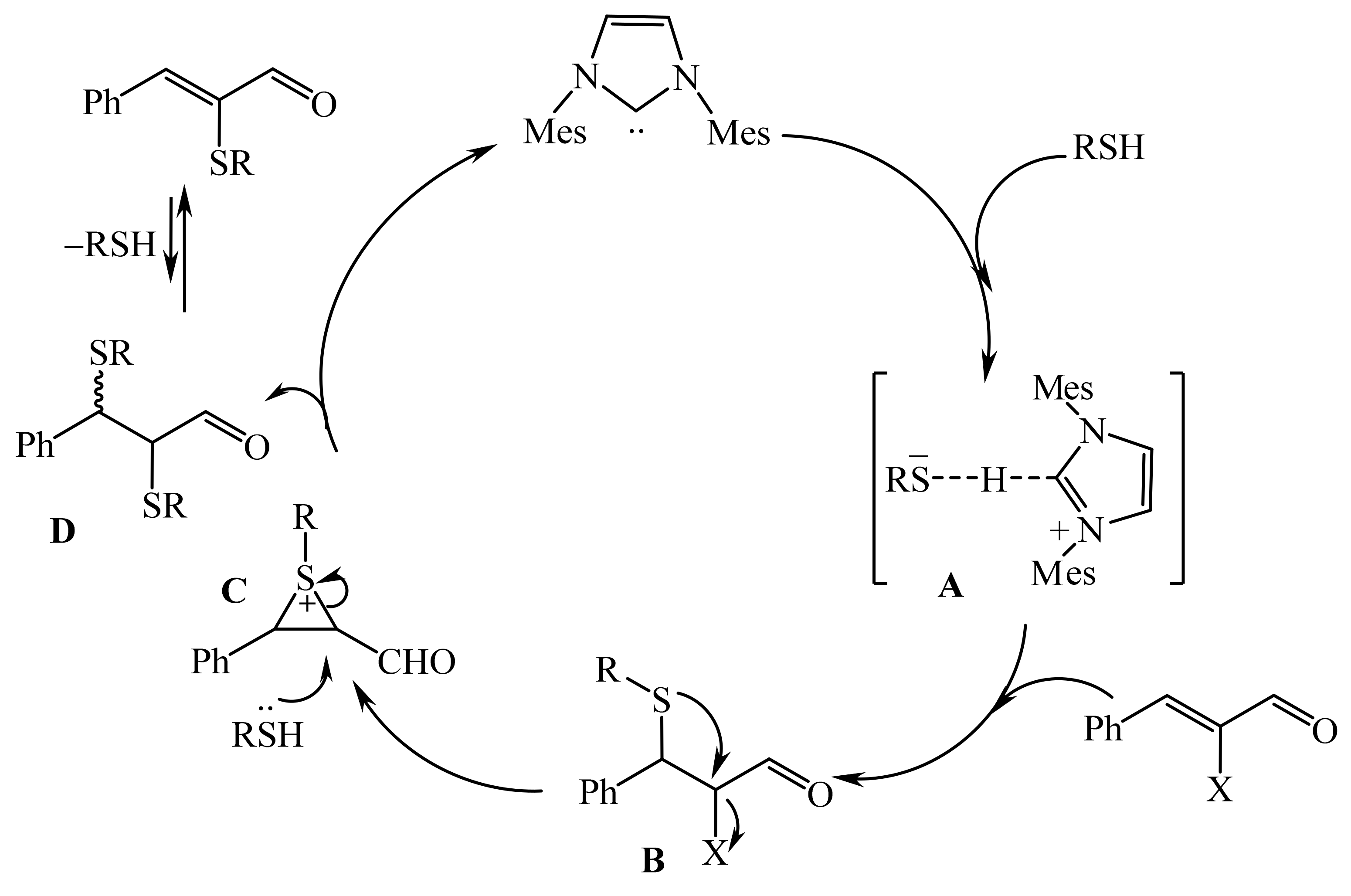
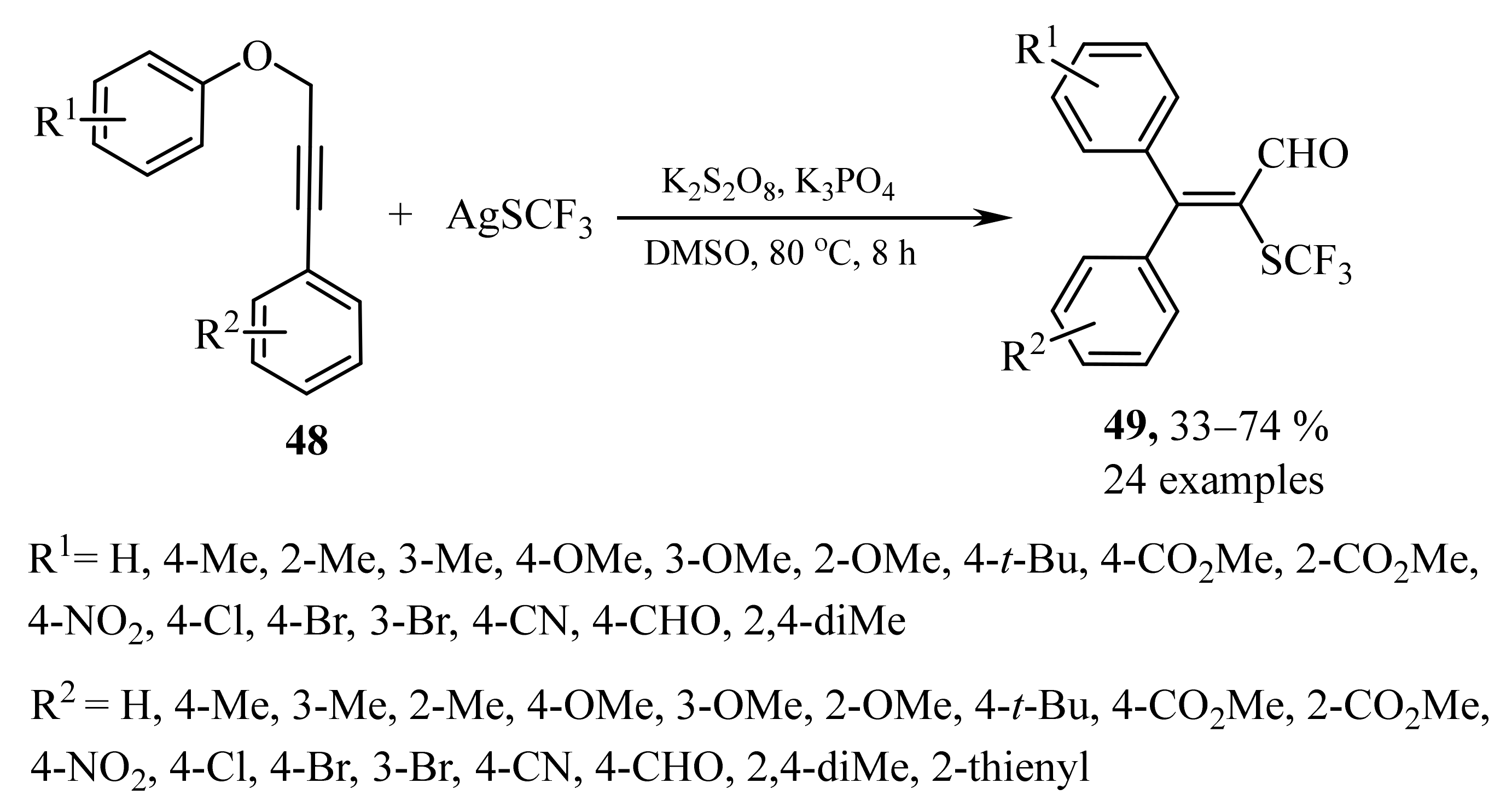
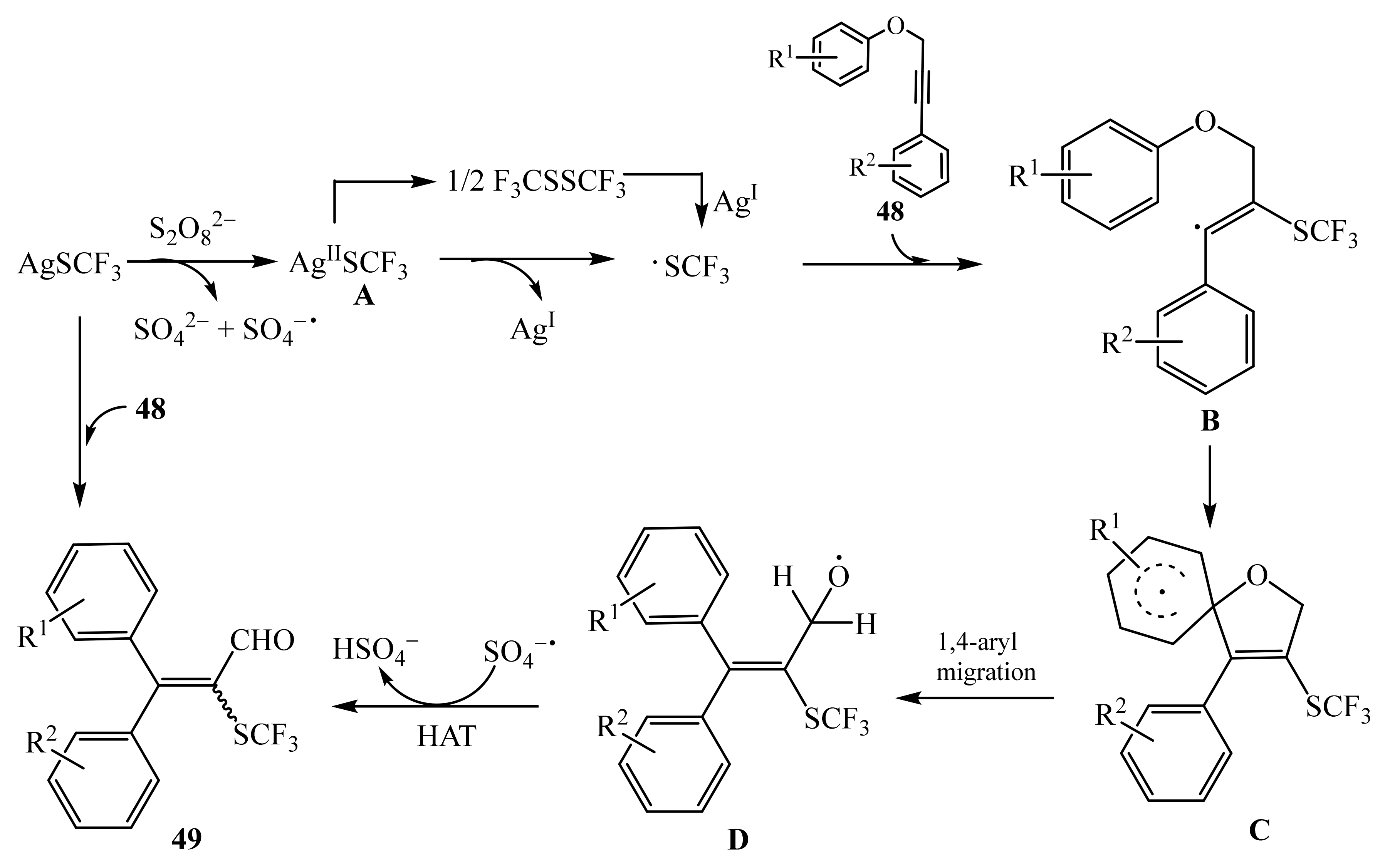
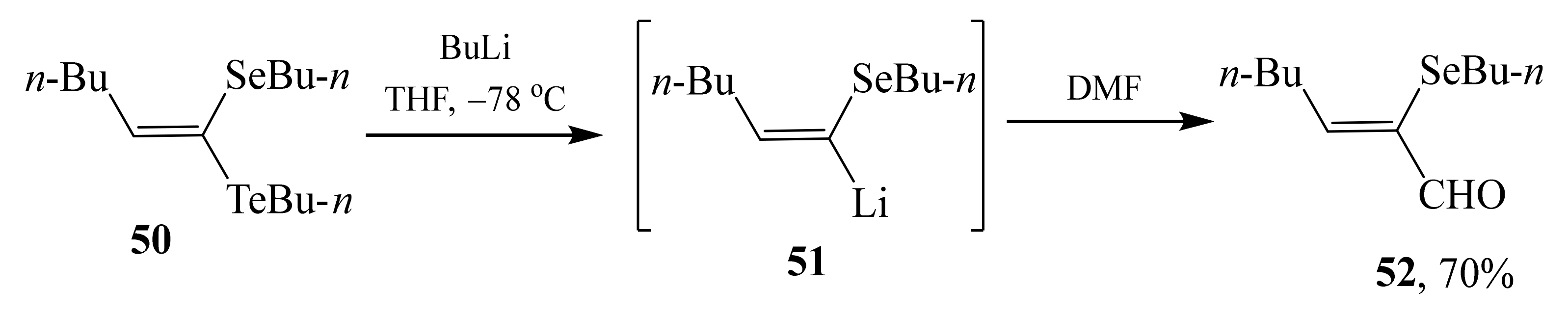
2.2. α-Thio- and α-Seleniumsubstituted α,β-Unsaturated Aldehydes
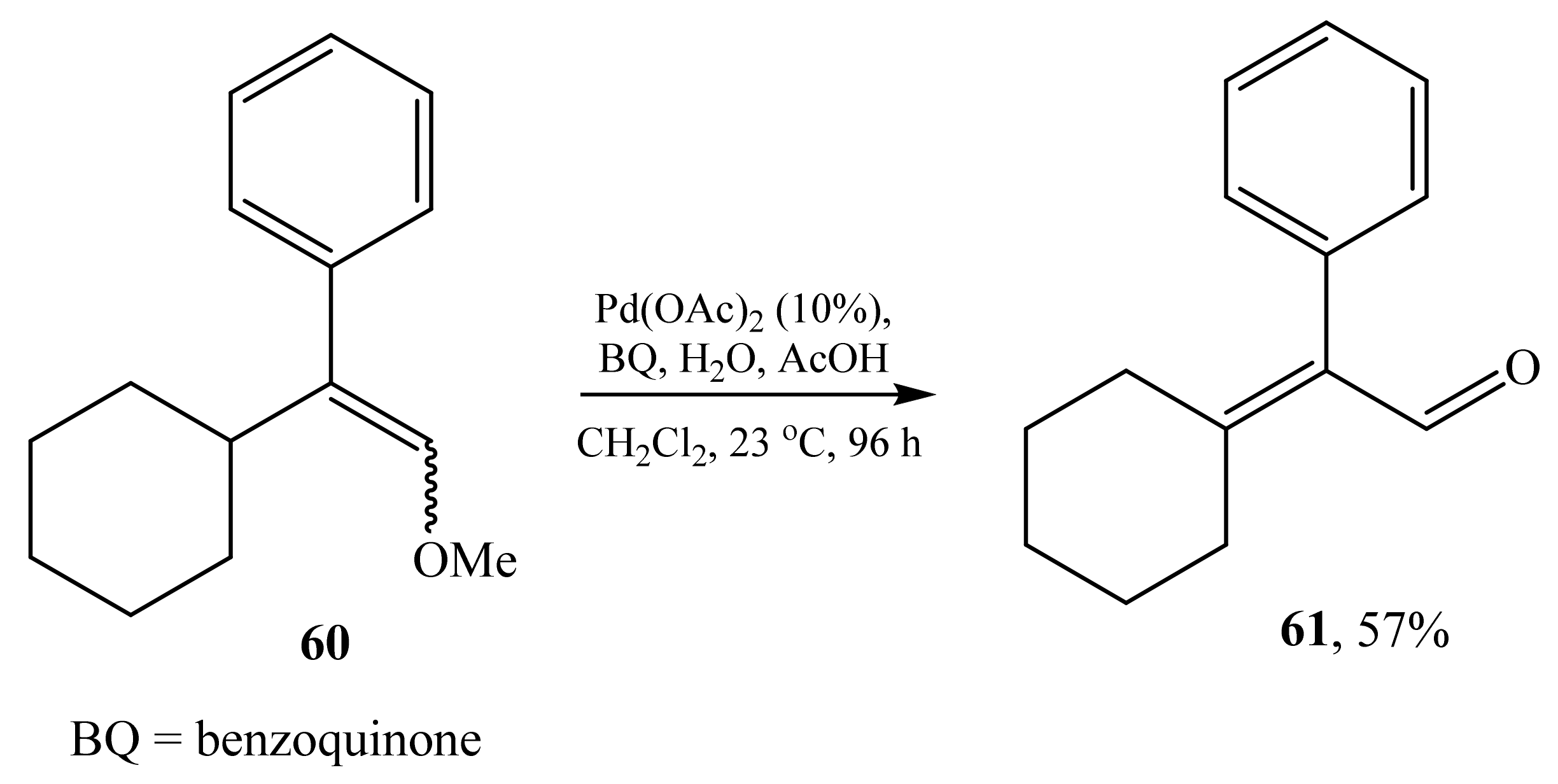
2.3. α-Phenyl(aryl)substituted α,β-Unsaturated Aldehydes
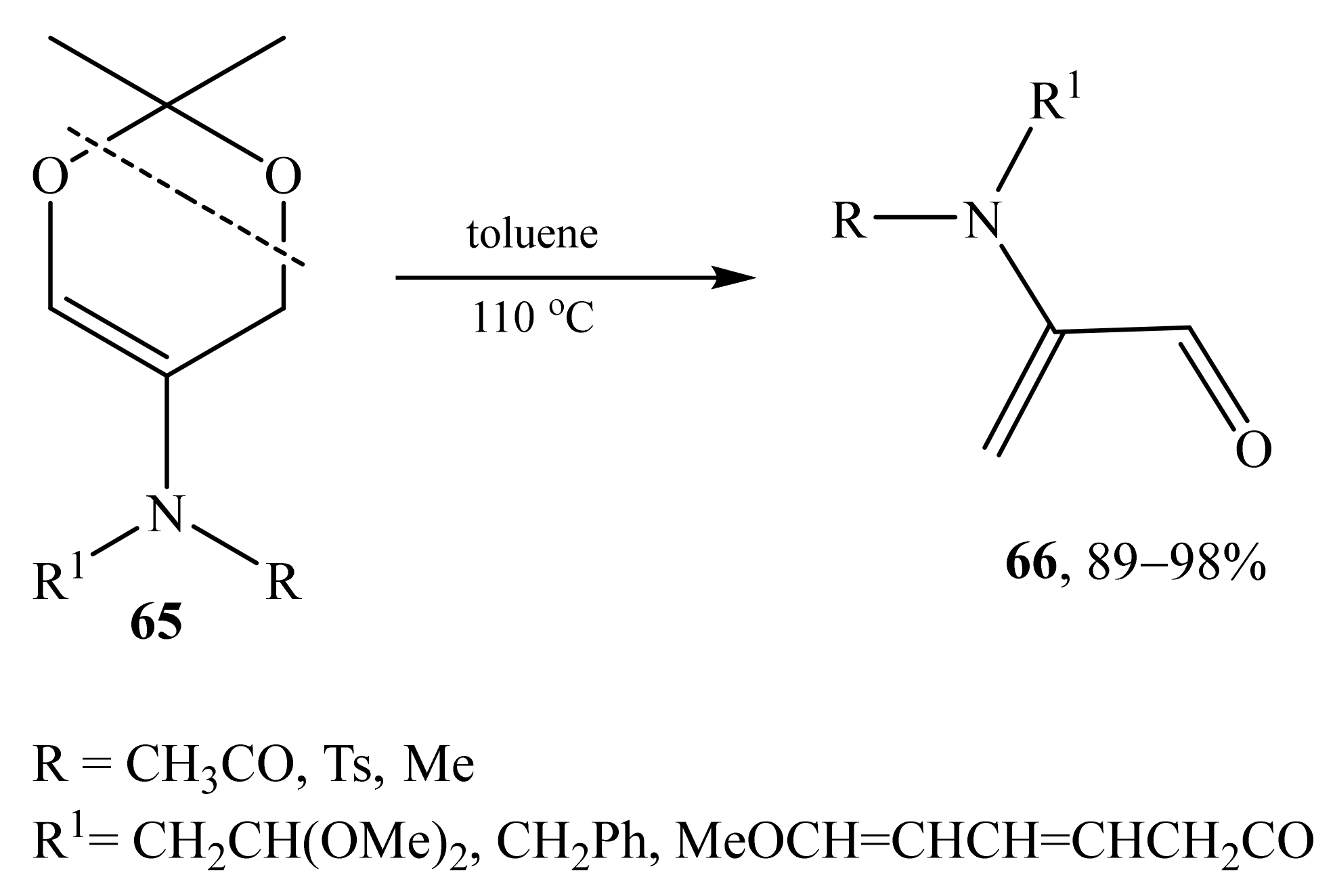
2.4. α-Amino- and α-Amidosubstituted α,β-Unsaturated Aldehydes
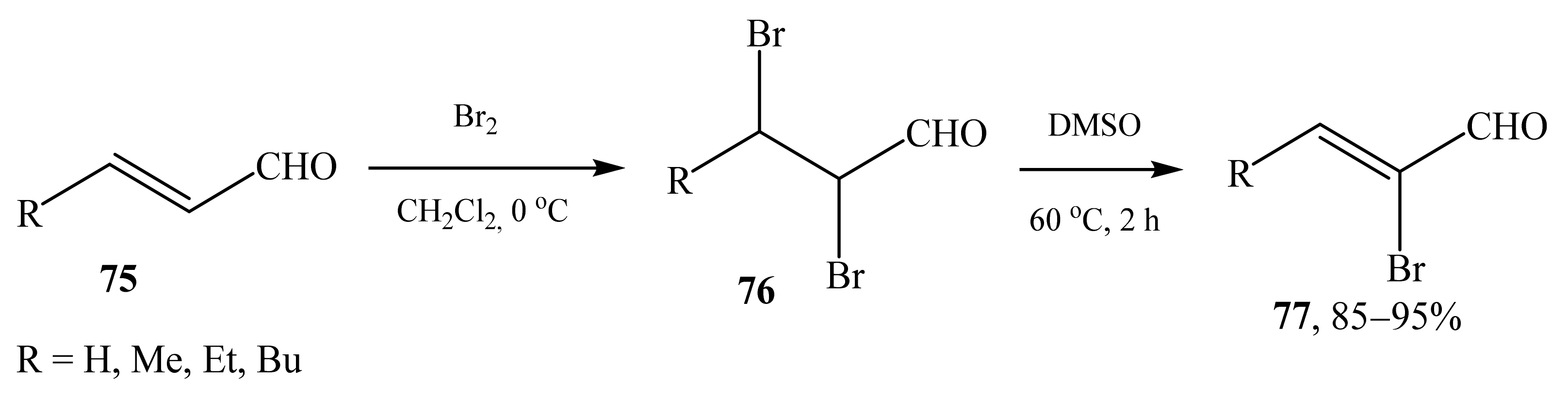
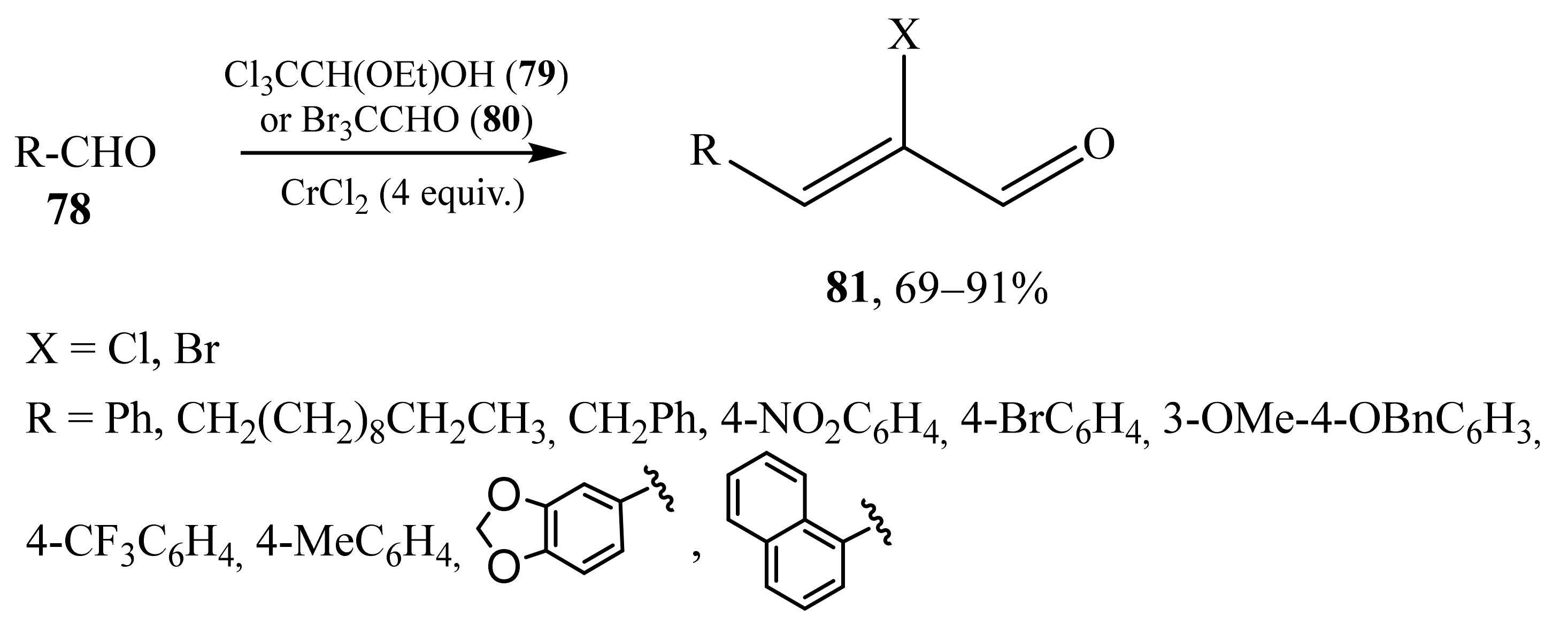
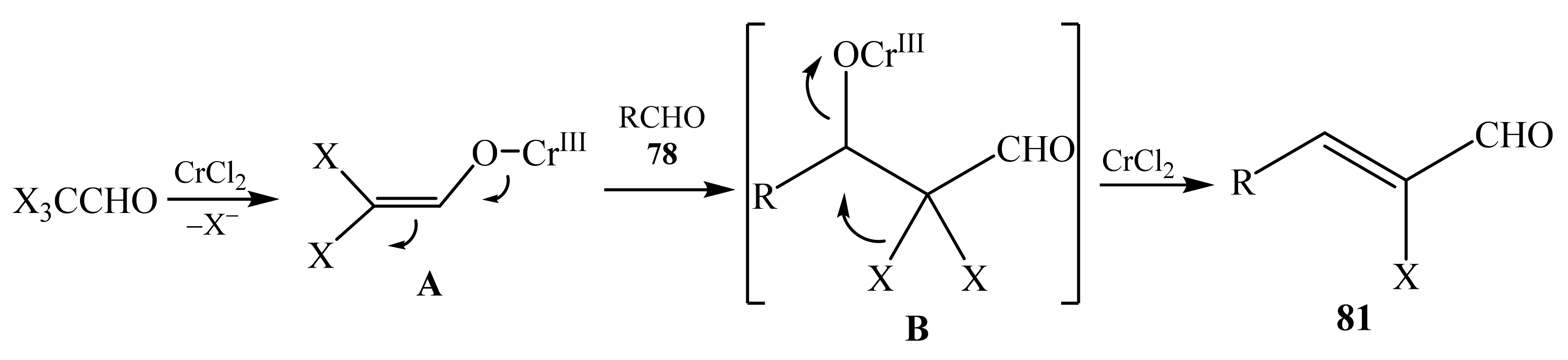
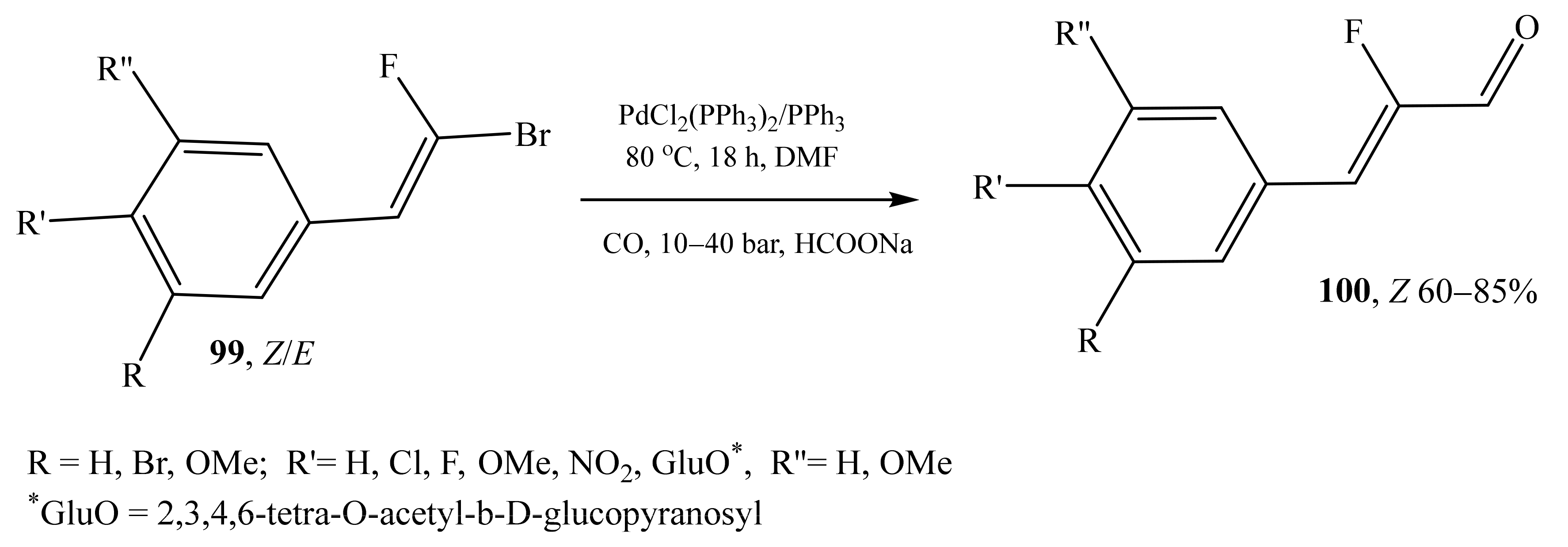
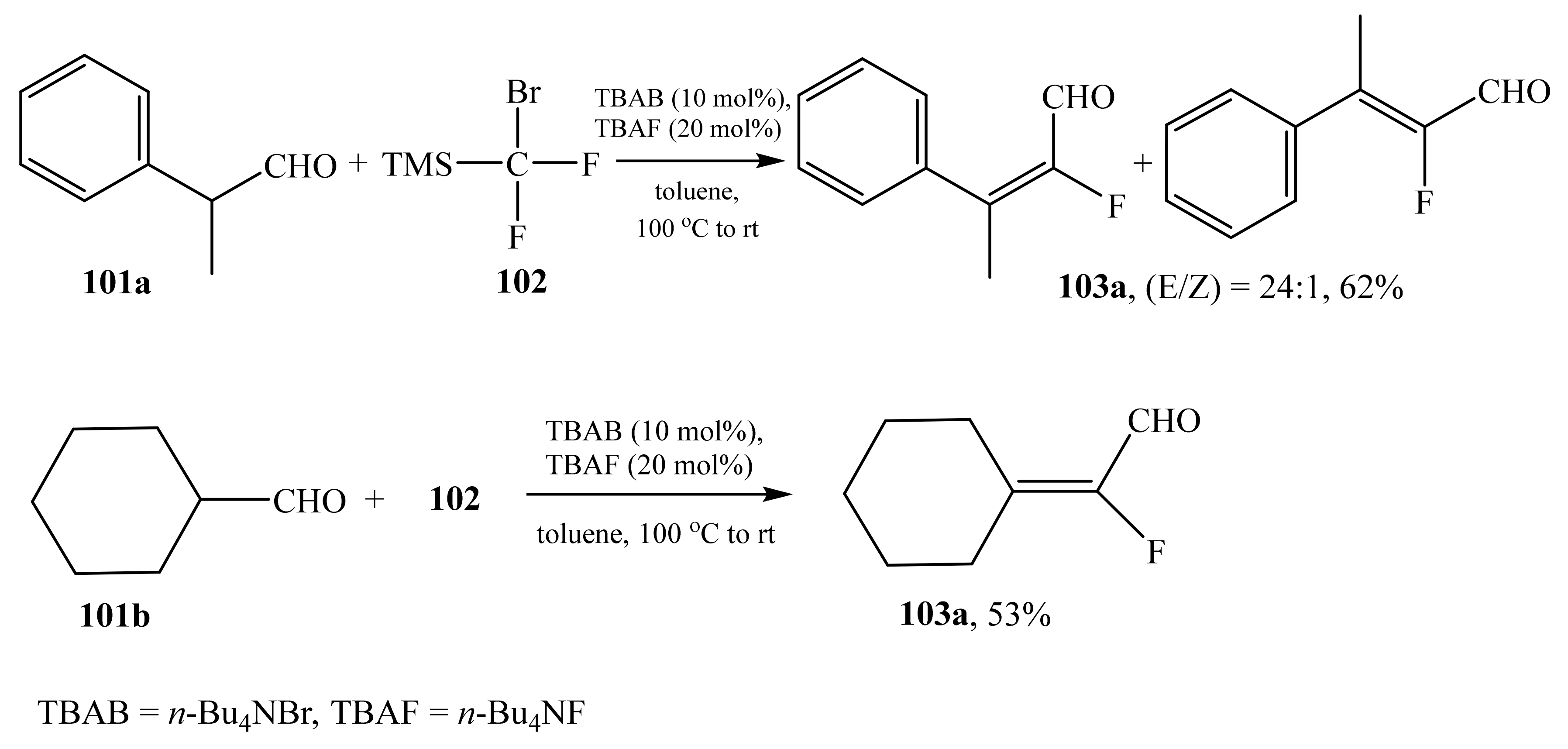
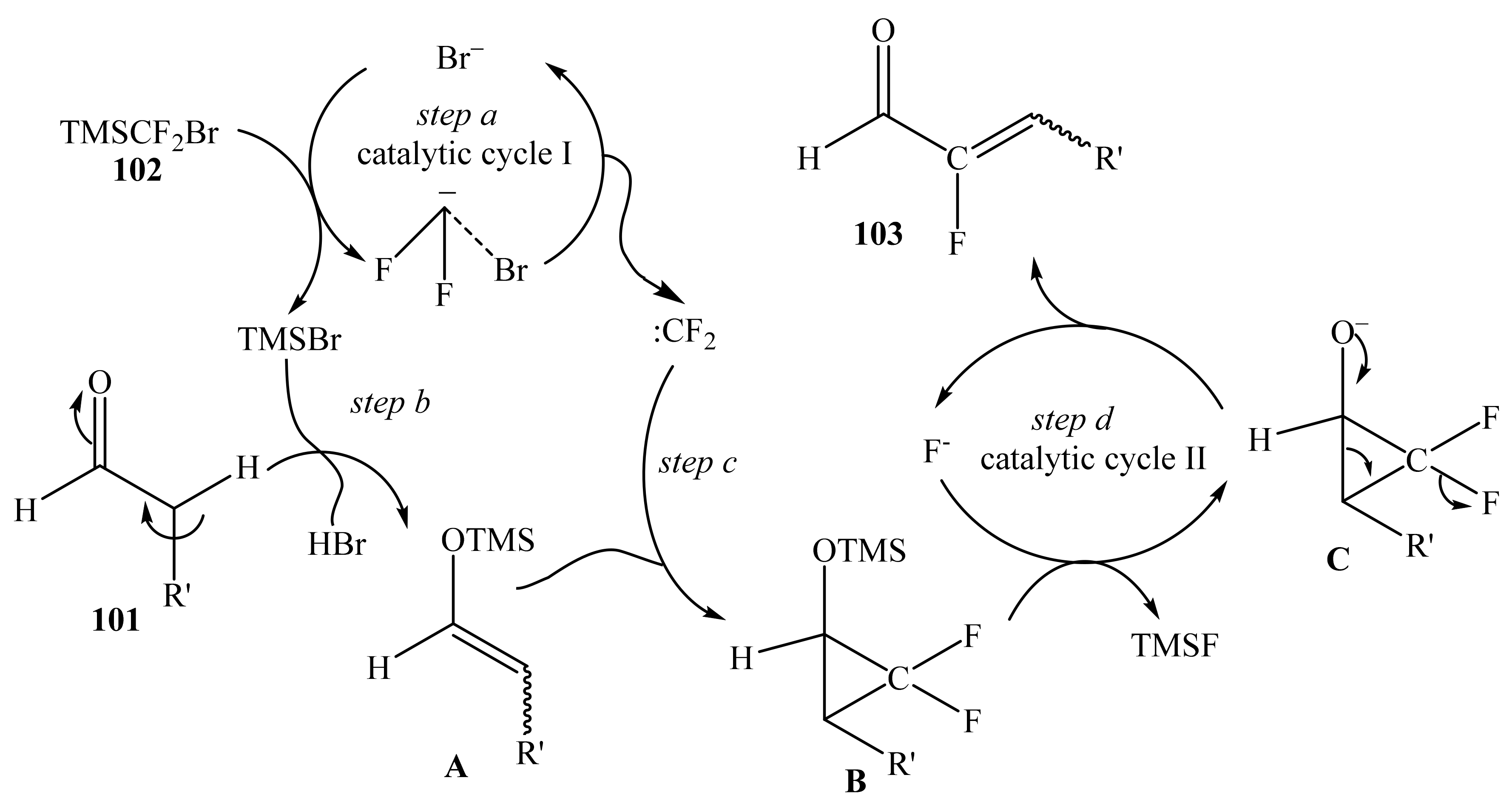
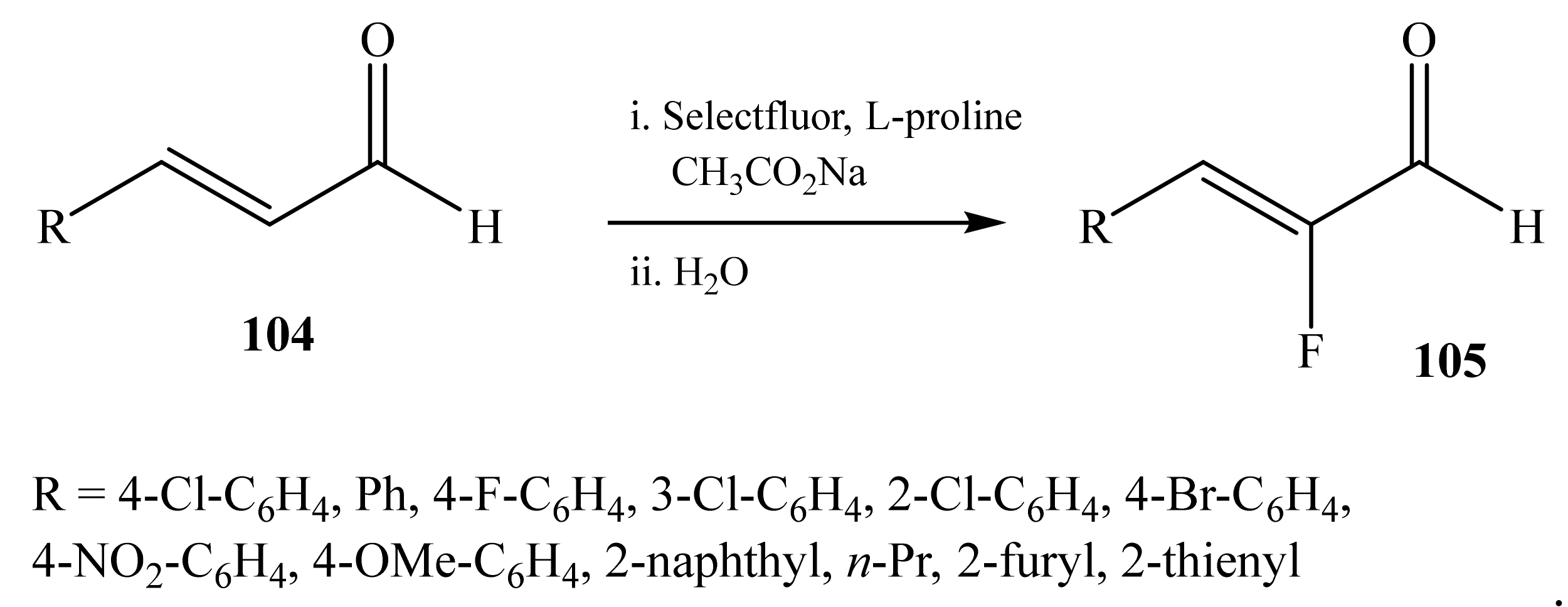
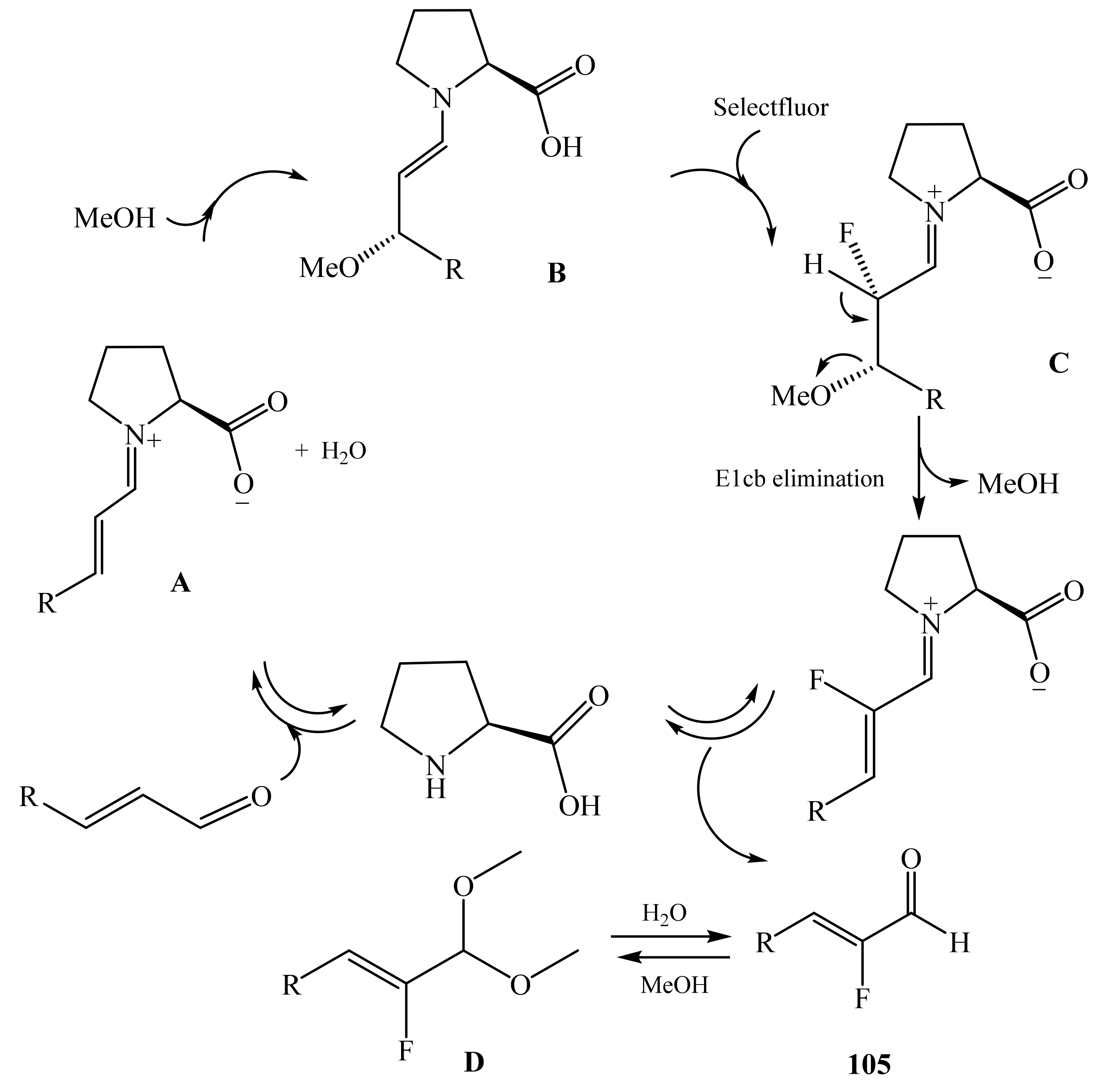
2.5. α-Halosubstituted α,β-Unsaturated Aldehydes
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Keiko, N.A.; Vchislo, N.V. Synthesis of diheteroatomic five-membered heterocyclic compounds from α,β-unsaturated aldehydes. Asian J. Org. Chem. 2016, 5, 1169–1197. [Google Scholar] [CrossRef]
- Keiko, N.A.; Vchislo, N.V. α,β-Unsaturated aldehydes in the synthesis of five-membered heterocyclic compounds with one heteroatom: Recent advances from developments in metal- and organocatalysis. Asian J. Org. Chem. 2016, 5, 439–461. [Google Scholar] [CrossRef]
- Vchislo, N.V.; Verochkina, E.A. Recent advances in total synthesis of alkaloids from α,β-unsaturated aldehydes. ChemistrySelect 2020, 5, 9579–9589. [Google Scholar] [CrossRef]
- Kim, H.; Ralph, J.; Lu, F.; Ralph, S.A. NMR analysis of lignins in CAD-deficient plants. Incorporation of hydroxycinnamaldehydes and hydroxybenzaldehydes into lignins. Org. Biomol. Chem. 2003, 1, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Sy, L.K.; Brown, G.D. Coniferaldehyde derivatives from tissue culture of Artemisia annua and Tanacetum parthenium. Phytochemistry 1999, 50, 781–785. [Google Scholar] [CrossRef]
- Yamamoto, M.; Blaschek, L.; Subbotina, E.; Kajita, S.; Pesquet, E. Importance of lignin coniferaldehyde residues for plant properties and sustainable uses. ChemSusChem 2020, 13, 4400–4408. [Google Scholar] [CrossRef]
- Ralph, J.; Lapierre, C.; Boerjan, W. Lignin structure and its engineering. Curr. Opin. Biotechnol. 2019, 56, 240–249. [Google Scholar] [CrossRef]
- Boerth, D.W.; Eder, E.; Hussain, S.; Hoffman, C. Structures of acrolein-guanine adducts: A semi-empirical self-consistent field and nuclear magnetic resonance spectral study. Chem. Res. Toxicol. 1998, 11, 284–294. [Google Scholar] [CrossRef]
- Jose, T.; Sudheesh, N.; Shukla, R.S. Amino functionalized chitosan as a catalyst for selective solvent-free self-condensation of linear aldehydes. J. Mol. Catal. A Chem. 2010, 333, 158–166. [Google Scholar] [CrossRef]
- Keiko, N.A.; Voronkov, M.G. Methods of synthesis of acrolein and its α-substituted derivatives. Russ. Chem. Rev. 1993, 62, 751–767. [Google Scholar] [CrossRef]
- Mackie, P.R.; Forester, C.E. Aldehydes: α,β-Unsaturated Aldehydes in Comprehensive Organic Functional Group Transformations II; Katritzky, A.R., Taylor, R.J.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2003; Volume 2, Chapter 3.02; pp. 60–89. [Google Scholar]
- Rulev, A.Y. Captodative aminoalkenes. Russ. Chem. Rev. 2002, 71, 195–221. [Google Scholar] [CrossRef]
- Verochkina, E.A. Methods for synthesis of α-alkyl α,β-unsaturated aldehydes. Mini Rev. Org. Chem. 2020, 17, 539–545. [Google Scholar] [CrossRef]
- Fyjita, E.; Nagao, Y. Tumor inhibitors having potential for interaction with mercapto enzymes and/or coenzymes. Bioorg. Chem. 1977, 6, 287–309. [Google Scholar] [CrossRef]
- Keiko, N.A.; Stepanova, L.G.; Voronkov, M.G.; Potapova, G.I.; Gudratov, O.N.; Treshchalina, E.M. Synthesis, DNA-inhibiting activity, and antitumor activity of 2-formyl-2,5-dimethoxy-2,3-dihydro-4H-pyran thiosemicarbazone, a related ethyl analog, and a copper complex. Pharm. Chem. J. 2002, 36, 407–409. [Google Scholar] [CrossRef]
- Kalapos, M.P. Methylglyoxal in living organisms: Chemistry, biochemistry, toxicology and biological implications. Toxicol. Lett. 1999, 110, 145–175. [Google Scholar] [CrossRef]
- Keiko, N.A.; Mamashvili, T.N. New syntheses of the bis-guanylhydrazone and bis-thiosemicarbazone of methylglyoxal. Pharm. Chem. J. 2005, 39, 82–83. [Google Scholar] [CrossRef]
- Mamashvili, T.N.; Keiko, N.A.; Sarapulova, G.I.; Voronkov, M.G. Hydrolysis of 2-alkoxyalk-2-enals. Russ. Chem. Bull. 1998, 47, 2465–2467. [Google Scholar] [CrossRef]
- Brimble, M.A.; Nairn, M.R.; Park, J.S.O. A titanium naphtholate approach for the synthesis of analogues of griseusin A. J. Chem. Soc. Perkin Trans. 1 2000, 5, 697–709. [Google Scholar] [CrossRef]
- Van Hooft, P.A.V.; Litjens, R.E.J.N.; van der Marel, G.A.; van Boeckel, C.A.A.; van Boom, J.H. Stereoselective transformations on D-glucose-derived eight-membered ring carbocycles. Org. Lett. 2001, 3, 731–733. [Google Scholar] [CrossRef]
- Paintner, F.F.; Metz, M.; Bauschke, G. A new, general entry to 3,5-unsubstituted 4-O-alkyl tetramates. Synthesis 2002, 7, 869–874. [Google Scholar] [CrossRef]
- Matiadis, D. Metal catalyzed and metal-mediated approaches to the synthesis and functionalization of tetramic acids. Catalysts 2019, 9, 50. [Google Scholar] [CrossRef]
- Panduwawala, T.D.; Iqbal, S.; Thompson, A.L.; Genov, M.; Pretsch, A.; Pretsch, D.; Liu, S.; Ebright, R.; Howells, A.; Maxwelld, A.; et al. Functionalised bicyclic tetramates derived from cysteine as antibacterial agents. Org. Biomol. Chem. 2019, 17, 5615–5632. [Google Scholar] [CrossRef]
- Erkkilä, A.; Pihko, P.M. Mild organocatalytic α-methylenation of aldehydes. J. Org. Chem. 2006, 71, 2538–2541. [Google Scholar] [CrossRef]
- Erkkilä, A.; Pihko, P.M. Rapid organocatalytic aldehyde-aldehyde condensation reactions. Eur. J. Org. Chem. 2007, 4205–4216. [Google Scholar] [CrossRef]
- Shibata, M.; Fuchigami, R.; Kotaka, R.; Namba, K.; Tanino, K. Acid-catalyzed [4+3] cycloaddition reaction of N-nosyl pyrroles. Tetrahedron 2015, 71, 4495–4499. [Google Scholar] [CrossRef]
- Sasaki, T.; Ishibashi, Y.; Ohn, M. Catalyzed cycloaddition reactions of α-silyloxy-α,β-unsaturated ketone and aldehyde. Tetrahedron Lett. 1982, 23, 1693–1696. [Google Scholar] [CrossRef]
- Harmata, M.; Sharma, U. Synthesis and some cycloaddition reactions of 2-(triisopropylsilyloxy)acrolein. Org. Lett. 2000, 2, 2703–2705. [Google Scholar] [CrossRef]
- Aungst, R.A.; Funk, R.L. Stereoselective preparation of (Z)-2-(trialkylsilyloxy)-2-alkenals by retrocycloaddition reactions of 4H-4-alkyl-5-(trialkylsilyloxy)-1,3-dioxins. Useful reactants for Lewis acid catalyzed [4+3] cyclizations. Org. Lett. 2001, 3, 3553–3555. [Google Scholar] [CrossRef]
- Nilson, M.G.; Funk, R.L. Total synthesis of (±)-Cortistatin J from furan. J. Am. Chem. Soc. 2011, 133, 12451–12453. [Google Scholar] [CrossRef]
- Funk, R.L.; Yost, K.J., III. Preparation and Diels-Alder cycloaddition of 2-acyloxyacroleins. Facile synthesis of functionalized Taxol A-synthons. J. Org. Chem. 1996, 61, 2598–2599. [Google Scholar] [CrossRef]
- Ishihara, K.; Nakano, K. Enantioselective [2+2] cycloaddition of unactivated alkenes with α-acyloxyacroleins catalyzed by chiral organoammonium salts. J. Am. Chem. Soc. 2007, 129, 8930–8931. [Google Scholar] [CrossRef]
- Ishihara, K.; Nakano, K. Design of an organocatalyst for the enantioselective Diels–Alder reaction with α-acyloxyacroleins. J. Am. Chem. Soc. 2005, 127, 10504–10505. [Google Scholar] [CrossRef]
- Sakakura, A.; Suzuki, K.; Nakano, K.; Ishihara, K. Chiral 1,1′-binaphthyl-2,2′-diammonium salt catalysts for the enantioselective Diels–Alder reaction with α-acyloxyacroleins. Org. Lett. 2006, 8, 2229–2232. [Google Scholar] [CrossRef]
- Sakakura, A.; Ishihara, K. Organoammonium salt-catalyzed Enantioselective cycloaddition reactions with α-(acyloxy)- or α-diacylaminoacroleins. Bull. Chem. Soc. Jpn. 2010, 83, 313–322. [Google Scholar] [CrossRef]
- Hayashi, Y.; Bondzic, B.P.; Yamazaki, T.; Gupta, Y.; Ogasawara, S.; Taniguchi, T.; Monde, K. Asymmetric Diels–Alder reaction of α-substituted and β,β-disubstituted α,β-enals via diarylprolinol silyl ether for the construction of all-carbon quaternary stereocenters. Chem. Eur. J. 2016, 22, 15874–15880. [Google Scholar] [CrossRef]
- Sakakura, A.; Yamada, H.; Ishihara, K. Enantioselective Diels-Alder reaction of α-(acylthio)acroleins: A new entry to sulfur-containing chiral quaternary carbons. Org. Lett. 2012, 14, 2972–2975. [Google Scholar] [CrossRef]
- Ishihara, K.; Yamada, H.; Akakura, M. An enantioselective Diels–Alder reaction of 1,2-dihydropyridines with α-acyloxyacroleins catalyzed by a chiral primary ammonium salt. Chem. Commun. 2014, 50, 6357–6360. [Google Scholar] [CrossRef]
- Lin, J.-H.; Xiao, J.-C. Emerging Fluorinated Motifs, 2 Volume Set: Synthesis, Properties and Applications; Cahard, D., Ma, J.-A., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2020; p. 872. [Google Scholar]
- Kase, K.; Katayama, M.; Konno, T.; Ishihara, T.; Yamanaka, H.; Gupton, J.T. Reaction of β-trifluoroethoxy vinamidinium salts with carbon nucleophiles. J. Fluor. Chem. 2003, 120, 33–39. [Google Scholar] [CrossRef]
- Keiko, N.A.; Stepanova, L.G.; Verochkina, E.A.; Larina, L.I. Synthesis and properties of 2-alkoxy- and 2-alkylthio-3-aryl(hetaryl)propenals. Arkivoc 2010, II, 49–60. [Google Scholar] [CrossRef]
- Robert, F.; Heritier, J.; Quiquerez, J.; Simian, H.; Blank, I. Synthesis and sensorial properties of 2-alkylalk-2-enals and 3-(acetylthio)-2-alkyl alkanals. J. Agric. Food. Chem. 2004, 52, 3525–3529. [Google Scholar] [CrossRef] [PubMed]
- Casalea, M.T.; Richmana, A.R.; Elroda, M.J.; Garland, R.M.; Beaver, M.R.; Tolbert, M.A. Kinetics of acid-catalyzed aldol condensation reactions of aliphatic aldehydes. Atmos. Environ. 2007, 41, 6212–6224. [Google Scholar] [CrossRef]
- Mandal, S.; Mandal, S.; Ghosh, S.K.; Ghosh, A.; Saha, R.; Banerjies, S.; Saha, B. Review of the aldol reaction. Synth. Commun. 2016, 46, 1327–1342. [Google Scholar] [CrossRef]
- Vchislo, N.V.; Fedoseeva, V.G.; Novokshonov, V.V.; Larina, L.I.; Rozentsveig, I.B.; Verochkina, E.A. Synthesis of new alkoxy/alkylthiovinylated oxazoles using tosylmethyl isocyanide. Mendeleev Commun. 2020, 30, 350–351. [Google Scholar] [CrossRef]
- Sakakura, A.; Yamada, H.; Ishihara, K. α-Heterosubstituted β-alkylacroleins as useful multisubstituted dienophiles for enantioselective Diels–Alder reactions. Asian J. Org. Chem. 2012, 1, 133–137. [Google Scholar] [CrossRef]
- Clayden, J.; MacLellan, P. Asymmetric synthesis of tertiary thiols and thioethers. Beilstein J. Org. Chem. 2011, 7, 582–595. [Google Scholar] [CrossRef]
- Da Silva, F.J.R.; Williams, R.J.P. The Biological Chemistry of the Elements; Oxford University Press: New York, NY, USA, 2001. [Google Scholar]
- Feng, J.; Lu, G.; Lv, M.; Cai, C. Palladium-catalyzed odorless one-pot synthesis of vinyl sulfides from organohalides, thiourea, and alkynes. Asian J. Org. Chem. 2014, 3, 77–81. [Google Scholar] [CrossRef]
- Ilardi, E.A.; Vitaku, E.; Njardarson, J.T. Data-mining for sulfur and fluorine: An evaluation of pharmaceuticals to reveal opportunities for drug design and discovery. J. Med. Chem. 2014, 57, 2832–2842. [Google Scholar] [CrossRef]
- Arroyo, Y.; Carreno, M.C.; Ruano, J.L.G.; Amo, J.F.R.; Santos, M.; Tejedor, M.A.S. Synthesis and photooxygenation of (S)-p-tolylsulfinylfuran derivatives. Tetrahedron Asymmetry 2000, 11, 1183–1191. [Google Scholar] [CrossRef]
- Silvestri, M.G.; Wong, C.-H. Opening of triiranes: Preparation of orthogonal protected 2-thioglyceraldehyde. J. Org. Chem. 2001, 66, 910–914. [Google Scholar] [CrossRef]
- He, L.; Guo, H.; Li, Y.-Z.; Du, G.-F.; Dai, B. N-heterocyclic carbene-catalyzed formal cross-coupling reaction of α-haloenals with thiols: Organocatalytic construction of sp2 carbon–sulfur bonds. Chem. Commun. 2014, 50, 3719–3721. [Google Scholar] [CrossRef]
- Movassaghi, M.; Schmidt, M.A. N-Heterocyclic carbene-catalyzed amidation of unactivated esters with amino alcohols. Org. Lett. 2005, 7, 2453–2456. [Google Scholar] [CrossRef]
- Phillips, E.M.; Riedrich, M.; Scheidt, K.A. N-Heterocyclic carbene-catalyzed conjugate additions of alcohols. J. Am. Chem. Soc. 2010, 132, 13179–13181. [Google Scholar] [CrossRef]
- Kang, Q.; Zhang, Y. N-Heterocyclic carbene-catalyzed aza-Michael addition. Org. Biomol. Chem. 2011, 9, 6715–6720. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Song, A.; Zhang, F.; Li, H.; Wang, W. Direct stereoselective α-arylation of unmodified enals using an organocatalytic cross-coupling-like reaction. Nat. Commun. 2011, 2, 524–533. [Google Scholar] [CrossRef]
- Kondrashov, E.V.; Romanov, A.R.; Ushakov, I.A.; Rulev, A.Y. Alkyl-and arylsulfanylsubstituted unsaturated carbonyl compounds. J. Sulfur Chem. 2017, 38, 18–33. [Google Scholar] [CrossRef]
- Guo, C.-H.; Chen, D.-Q.; Chen, S.; Liu, X.-Y. Synthesis of tetrasubstituted α,β-unsaturated aldehydes via radical 1,4-aryl migration/trifluoromethylthiolation cascade reaction of aryl propynyl ethers. Adv. Synth. Catal. 2017, 359, 2901–2906. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, Y.; Zhao, J.; Li, Y.; Zhang, Q. Radical cascade reactions of unsaturated C–C bonds involving migration. Sci. China Chem. 2019, 62, 1476–1491. [Google Scholar] [CrossRef]
- Guerrero, P.G.; Dabdoub, M.J.; Baroni, A.C.M. Hydroalumination of selenoacetylenes: A versatile generation and reactions of α-aluminate vinyl selenide intermediates in the highly regio- and stereoselective synthesis of telluro(seleno)ketene acetals. Tetrahedron Lett. 2008, 49, 3872–3876. [Google Scholar] [CrossRef]
- Perin, G.; Lenardão, E.J.; Jacob, R.G.; Panatieri, R.B. Synthesis of vinyl selenides. Chem. Rev. 2009, 109, 1277–1301. [Google Scholar] [CrossRef] [PubMed]
- Yoshimatsu, M.; Oguri, K.; Ikeda, K.; Gotoh, S. The first example of the 1-chalcogene-substituted formylolefination of the ketones and aldehydes using 1-lithio-2-ethoxyvinyl chalcogenides. J. Org. Chem. 1998, 63, 4475–4480. [Google Scholar] [CrossRef]
- Lerouge, P.; Paulmier, C. Synthese D’α-hydroxyallenes α-fonctionnalises a partir D’α-phenylselenoenals. Tetrahedron Lett. 1984, 25, 1987–1990. [Google Scholar] [CrossRef]
- Paulmier, C.; Outurquin, F.; Plaquevent, J.-C. Enantioselective α-selenenylation of 2-phenylpropanal. Tetrahedron Lett. 1988, 29, 5889–5891. [Google Scholar] [CrossRef]
- Uneyama, K.; Takano, K.; Torii, S. Electrochemical hydroxyselenenylation as a new method for one-step preparation of α-arylseleno-α,β-unsaturated aldehydes from 3-hydroxyalkynes. Tetrahedron Lett. 1982, 1161–1164. [Google Scholar] [CrossRef]
- Dabdoub, M.J.; Begnini, M.L.; Guerrero, P.G.; Baroni, A.C.M. Hydrozirconation of lithium alkynylselenolate anions. Generation and reactions of α-zirconated vinyl selenide intermediates. J. Org. Chem. 2000, 65, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Silveira, C.C.; Perin, G.; Braga, A.L.; Dabdoub, M.J.; Jacob, R.G. Phenyltelluroacrylonitriles and phenylselenoacrylonitriles as precursors of (Z)-α-phenylseleno-α,β-unsaturated aldehydes, β-amino-α-phenylselenonitriles and Diels–Alder adducts. Tetrahedron 2001, 57, 5953–5959. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Toader, D.; Chassaing, C.; Aslan, D.C. Masked 2-arylacroleins: Versatile three-carbon units for organic synthesis. J. Org. Chem. 1999, 64, 6080–6084. [Google Scholar] [CrossRef]
- Dumanski, P.G.; Florey, P.; Knettig, M.; Smallridge, A.J.; Trewhella, M.A. The baker’s yeast-mediated reduction of conjugated methylene groups in an organic solvent. J. Mol. Catal. B Enzym. 2001, 11, 905–908. [Google Scholar] [CrossRef]
- Thompson, C.D.; Miller, T.A.; Barthen, M.T.; Dieckhaus, C.M.; Sofia, R.D.; Macdonald, T.L. The synthesis, in vitro reactivity, and evidence for formation in humans of 5-phenyl-1,3-oxazinane-2,4-dione, a metabolite of Felbamate. Drug Metab. Dispos. 2000, 28, 434–439. [Google Scholar]
- Dieckhaus, C.M.; Roller, S.G.; Santos, W.L.; Sofia, R.D.; Macdonald, T.L. Role of glutathione S-transferases A1-1, M1-1, and P1-1 in the detoxification of 2-phenylpropenal, a reactive felbamate metabolite. Chem. Res. Toxicol. 2001, 14, 511–516. [Google Scholar] [CrossRef]
- Fearnley, S.P.; Funk, R.L.; Gregg, R.J. Preparation of 2-alkyl- and 2-acylpropenals from 5-(trifluoromethanesulfonyloxy)-4H-1,3-dioxin: A versatile acrolein α-cation synthon. Tetrahedron 2000, 56, 10275–10281. [Google Scholar] [CrossRef]
- Kitazume, T.; Nagura, H.; Koguchi, S. One step synthesis of 2-substituted 3-tri-(or di-)-fluoromethyl-2-propenalsin an ionic liquid. J. Fluor. Chem. 2004, 125, 79–82. [Google Scholar] [CrossRef]
- Lauer, M.G.; Henderson, W.H.; Awad, A.; Stambuli, J.P. Palladium-Catalyzed Reactions of Enol Ethers: Access to Enals, Furans, and Dihydrofurans. Org. Lett. 2012, 14, 6000–6003. [Google Scholar] [CrossRef]
- Mura, M.G.; Luca, L.D.; Taddei, M.; Williams, J.M.J.; Porcheddu, A. Synthesis of α,β-unsaturated aldehydes based on a one-pot phase-switch dehydrogenative cross-coupling of primary alcohols. Org. Lett. 2014, 16, 2586–2589. [Google Scholar] [CrossRef] [PubMed]
- Funk, R.L.; Belmar, J. Total synthesis of (±)-isophellibiline. Tetrahedron Lett. 2012, 53, 176–178. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kang, S.H.; Kang, S.Y.; Lee, H.-S.; Buglass, A.J. Total synthesis of natural tert-alkylamino hydroxy carboxylic acids. Chem. Rev. 2005, 105, 4537–4558. [Google Scholar] [CrossRef] [PubMed]
- Greshock, T.J.; Funk, R.L. Total synthesis of (±)-Lepadiformine via an amidoacrolein cycloaddition. Org. Lett. 2001, 3, 3511–3514. [Google Scholar] [CrossRef] [PubMed]
- Maeng, J.-H.; Funk, R.L. Total synthesis of (±)-Fasicularin via a 2-amidoacrolein cycloaddition. Org. Lett. 2002, 4, 331–333. [Google Scholar] [CrossRef]
- He, Y.; Funk, R.L. Total syntheses of (±)-β-Erythroidine and (±)-8-oxo-β-Erythroidine by an intramolecular Diels–Alder cycloaddition of a 2-amidoacrolein. Org. Lett. 2006, 8, 3689–3692. [Google Scholar] [CrossRef]
- Fuchs, J.R.; Funk, R.L. Intramolecular electrophilic aromatic substitution reactions of 2-amidoacroleins: A new method for the preparation of tetrahydroisoquinolines, tetrahydro-3-benzazepines, and hexahydro-3-benzazocines. Org. Lett. 2001, 3, 3349–3351. [Google Scholar] [CrossRef]
- Maeng, J.-H.; Funk, R.L. Total synthesis of the Immunosuppressant FR901483 via an amidoacrolein cycloaddition. Org. Lett. 2001, 3, 1125–1128. [Google Scholar] [CrossRef]
- Rulev, A.Y.; Fedorov, S.V.; Chuvashev, Y.A. Captodative formyl- and acyl(amino)alkenes containing a terminal double bond or a weakly basic tertiary amino group. Russ. J. Org. Chem. 2003, 39, 646–649. [Google Scholar] [CrossRef]
- Burk, M.J.; Johnson, N.B.; Lee, J.R. Asymmetric synthesis of β-amino alcohols and 1,2-diamines through DuPHOS-Rh catalyzed hydrogenation. Tetrahedron Lett. 1999, 40, 6685–6688. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Vakulenko, A.V.; Akue-Gedu, R.; Gromova, A.V.; Witek, R.; Rogers, J.W. Regiospecific preparation of 1,4,5-trisubstituted pyrazoles from 2-(1H-1,2,3-benzotriazol-1-yl)-3-(4-aryl)-2-propenals. Arkivoc 2007, 2007, 9–21. [Google Scholar] [CrossRef]
- Brahma, S.; Ray, J.K. Halovinyl aldehydes: Useful tools in organic synthesis. Tetrahedron 2008, 64, 2883–2896. [Google Scholar] [CrossRef]
- Barlow, A.J.; Compton, B.J.; Weavers, R.T. Synthesis of the 3-methylene-2-vinyltetrahydropyran unit; the hallmark of the sesquiterpene, hodgsonox. J.Org. Chem. 2005, 70, 2470–2475. [Google Scholar] [CrossRef] [PubMed]
- Nicolaou, K.C.; Brenzovich, W.E.; Bulger, P.G.; Fransis, T.M. Synthesis of iso-epoxy-amphidinolide N and des-epoxy-caribenolide I structures. Initial forays. Org. Biomol. Chem. 2006, 4, 2119–2157. [Google Scholar] [CrossRef] [PubMed]
- Feutren, S.; McAlonan, H.; Montgomery, D.; Stevenson, P.J. Palladium catalysed formal 6-endo-trig approaches to pumiliotoxin alkaloid: Interception of the elusive cyclopropyl intermediate. J. Chem. Soc. Perkin Trans. 1 2000, 7, 1129–1137. [Google Scholar] [CrossRef]
- Li, W.; Li, J.; Wan, Z.-K.; Wu, J.; Massefski, W. Preparation of α-haloacrylate derivatives via dimethyl sulfoxide-mediated selective dehydrohalogenation. Org. Lett. 2007, 9, 4607–4610. [Google Scholar] [CrossRef]
- Barma, D.K.; Lu, B.; Baati, R.; Mioskowski, C.; Falck, J.R. Convenient preparation of (Z)-α-halo-α,β-unsaturated aldehydes: Synthesis of a Laurencia flexilis toxin. Tetrahedron Lett. 2008, 49, 4359–4361. [Google Scholar] [CrossRef]
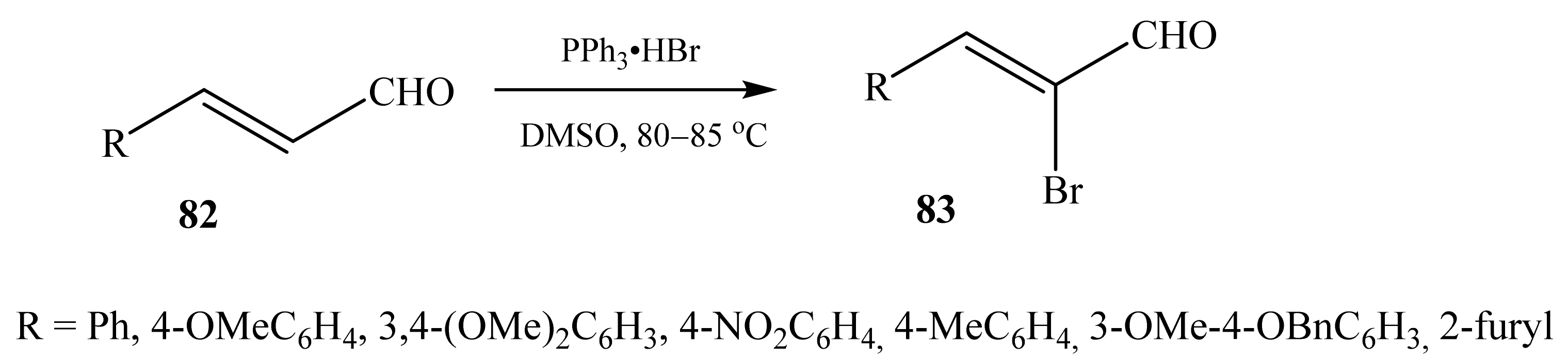
- Mal, K.; Sharma, A.; Maulik, P.R.; Das, I. PPh3·HBr-DMSO Mediated expedient synthesis of γ-substituted β,γ-unsaturated α-ketomethylthioesters and α-bromo enals: Application to the synthesis of 2-methylsulfanyl-3(2H)-furanones. Chem. Eur. J. 2014, 20, 662–667. [Google Scholar] [CrossRef]
- Smart, B.E. Fluorine substituent effects (on bioactivity). J. Fluor. Chem. 2001, 109, 3–11. [Google Scholar] [CrossRef]
- Nenajdenko, V.G.; Muzalevskiy, V.M.; Shastin, A.V. Polyfluorinated ethanes as versatile fluorinated C2-building blocks for organic synthesis. Chem. Rev. 2015, 115, 973–1050. [Google Scholar] [CrossRef] [PubMed]
- Kanai, M.; Percy, J.M. Short, stereoselective synthesis of α-fluoroalkenoate derivatives, α-fluoroenones and α-fluorienals from HFC-134a. Tetrahedron Lett. 2000, 2453–2455. [Google Scholar] [CrossRef]
- Lee, K.; Zhou, W.; Kelley, L.-L.C.; Momany, C.; Chu, C.K. Synthesis of unsaturated fluoride containing D- and L-pyranosyl nucleosides. Tetrahedron Asymmetry 2002, 13, 1589–1598. [Google Scholar] [CrossRef]
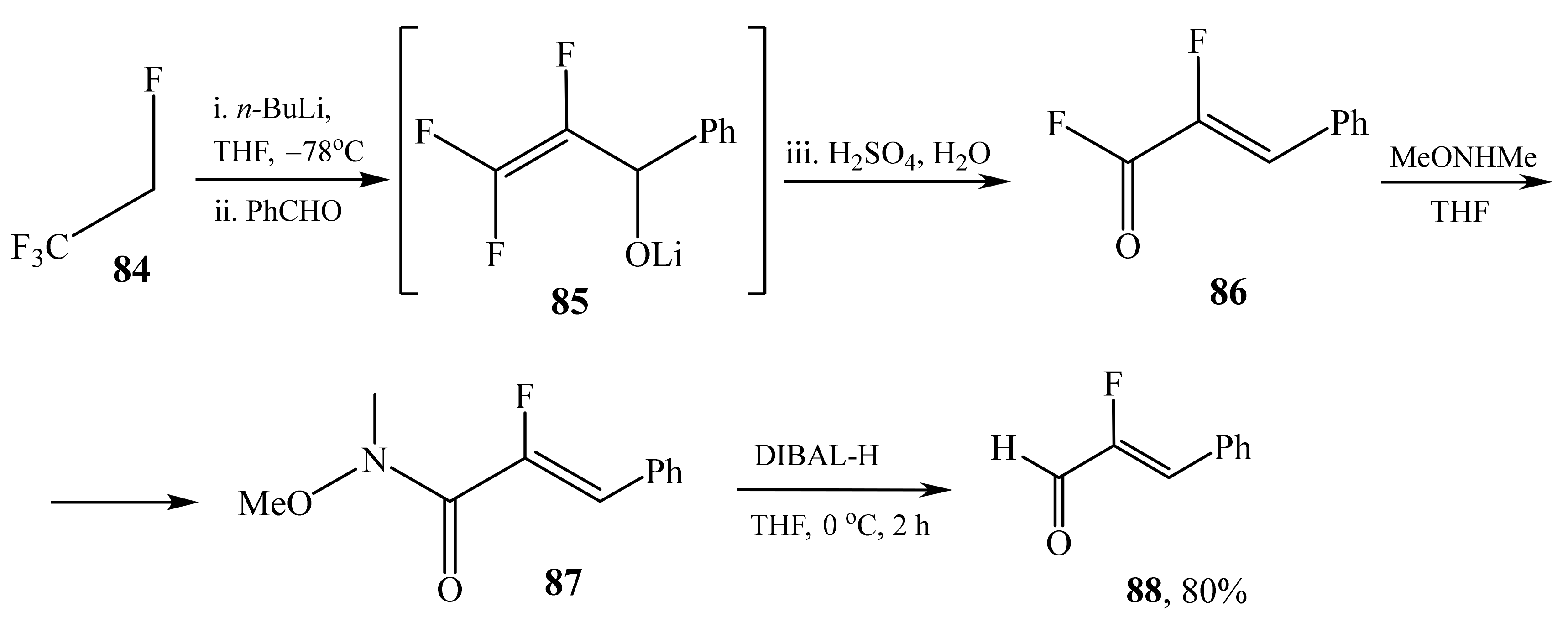
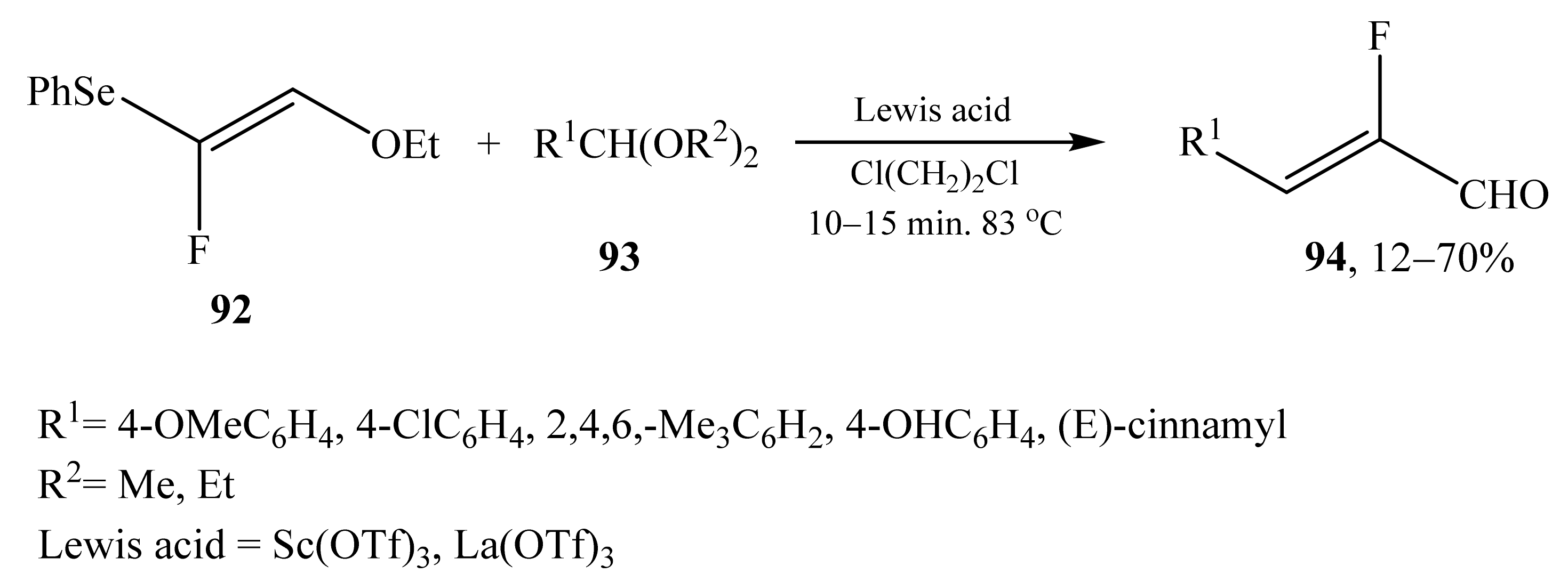
- Hatanaka, F.; Tsuchiya, M.; Yoshimatsu, M. The First synthesis and reaction of β-ethoxy-α-fluoro-α-(phenyl-selanyl)ethene: Scandium or lanthanum triflate catalyzed α-fluoroformylalkenylation of aldehydes. Synlett 2005, 14, 2191–2194. [Google Scholar] [CrossRef]
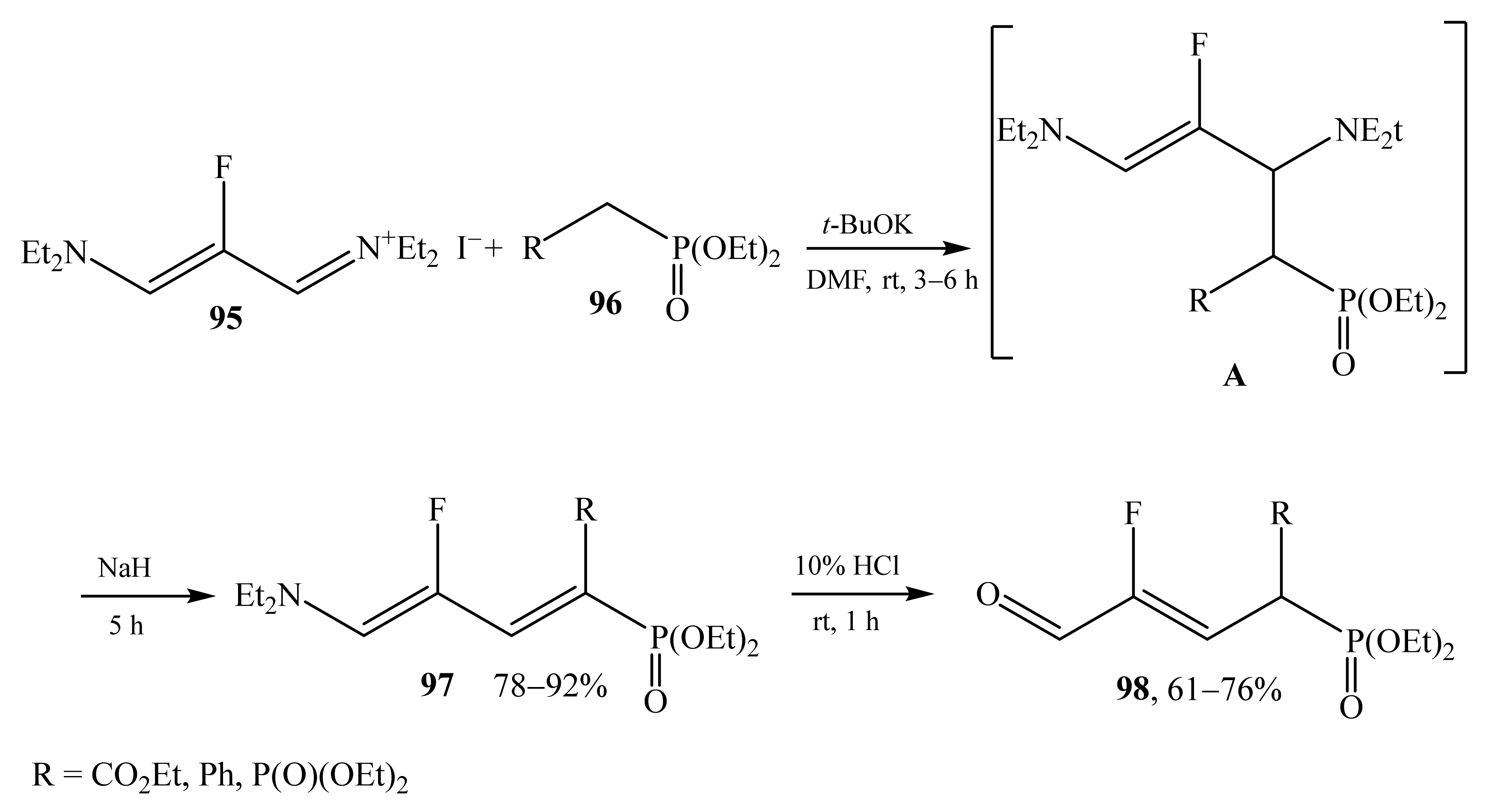
- Arimitsu, S.; Konno, T.; Gupton, J.T.; Ishihara, T.; Yamanaka, H. Synthetic application of fluorinated vinamidinium salts: Synthesis of fluorinated 1,3-butadienylphosphonates by the reaction with Horner–Wadsworth–Emmons reagents. J. Fluor. Chem. 2006, 127, 1235–1241. [Google Scholar] [CrossRef]
- Zemmouri, R.; Kajjout, M.; Castanet, Y.; Eddarir, S.; Rolando, C. Palladium vatalyzed stereoconvergent formylation of (E/Z)-β-bromo-β-fluorostyrenes: Straightforward access to Z)-α-fluorocinnamic aldehydes and (Z)-β-fluorocinnamic alcohols. J. Org. Chem. 2011, 76, 7691–7698. [Google Scholar] [CrossRef]
- Song, X.; Chang, J.; Zhu, D.; Li, J.; Xu, C.; Liu, Q.; Wang, M. Catalytic domino reaction of ketones/aldehydes with Me3SiCF2Br for the synthesis of α-fluoroenones/α-fluoroenals. Org. Lett. 2015, 17, 1712–1715. [Google Scholar] [CrossRef]
- Zhou, J.; Jiang, X.; Jin, C.; Guo, Z.; Su, B.; Su, W. One-pot L-proline-mediated stereoselective α-C(sp2)–H fluorination of α,β-unsaturated aldehydes through methoxyfluorination–elimination. Eur. J. Org. Chem. 2017, 3631–3634. [Google Scholar] [CrossRef]








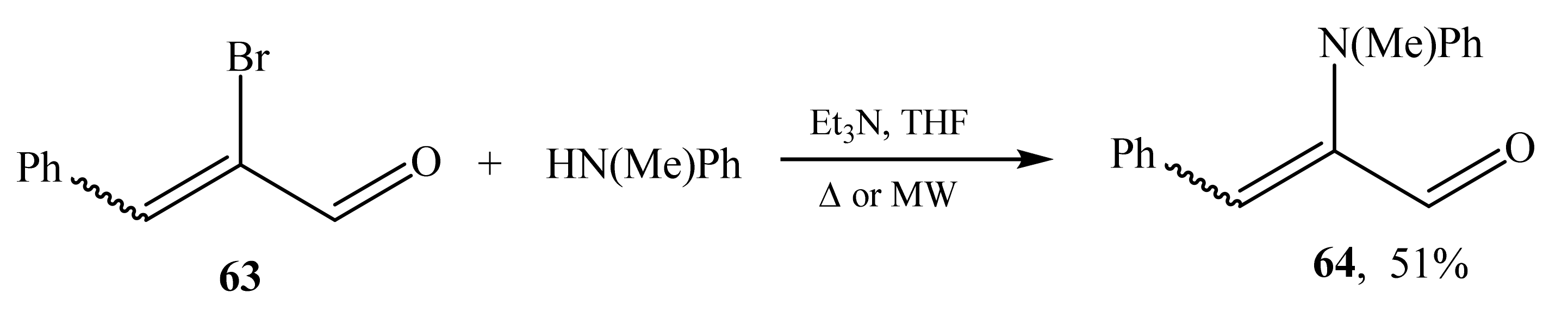



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verochkina, E.A.; Vchislo, N.V.; Rozentsveig, I.B. α-Functionally Substituted α,β-Unsaturated Aldehydes as Fine Chemicals Reagents: Synthesis and Application. Molecules 2021, 26, 4297. https://doi.org/10.3390/molecules26144297
Verochkina EA, Vchislo NV, Rozentsveig IB. α-Functionally Substituted α,β-Unsaturated Aldehydes as Fine Chemicals Reagents: Synthesis and Application. Molecules. 2021; 26(14):4297. https://doi.org/10.3390/molecules26144297
Chicago/Turabian StyleVerochkina, Ekaterina A., Nadezhda Victorovna Vchislo, and Igor B. Rozentsveig. 2021. "α-Functionally Substituted α,β-Unsaturated Aldehydes as Fine Chemicals Reagents: Synthesis and Application" Molecules 26, no. 14: 4297. https://doi.org/10.3390/molecules26144297
APA StyleVerochkina, E. A., Vchislo, N. V., & Rozentsveig, I. B. (2021). α-Functionally Substituted α,β-Unsaturated Aldehydes as Fine Chemicals Reagents: Synthesis and Application. Molecules, 26(14), 4297. https://doi.org/10.3390/molecules26144297




