An Analysis of Oxidative Changes and the Fatty Acid Profile in Stored Poultry Sausages with Liquid and Microencapsulated Fish Oil Additives
Abstract
:1. Introduction
2. Results and Discussion
2.1. Basic Analyses
2.2. Sensory Assessment
2.3. Fatty Acid Profile
2.4. Fatty Acid Profile in Sample Subjected to In Vitro Digestion
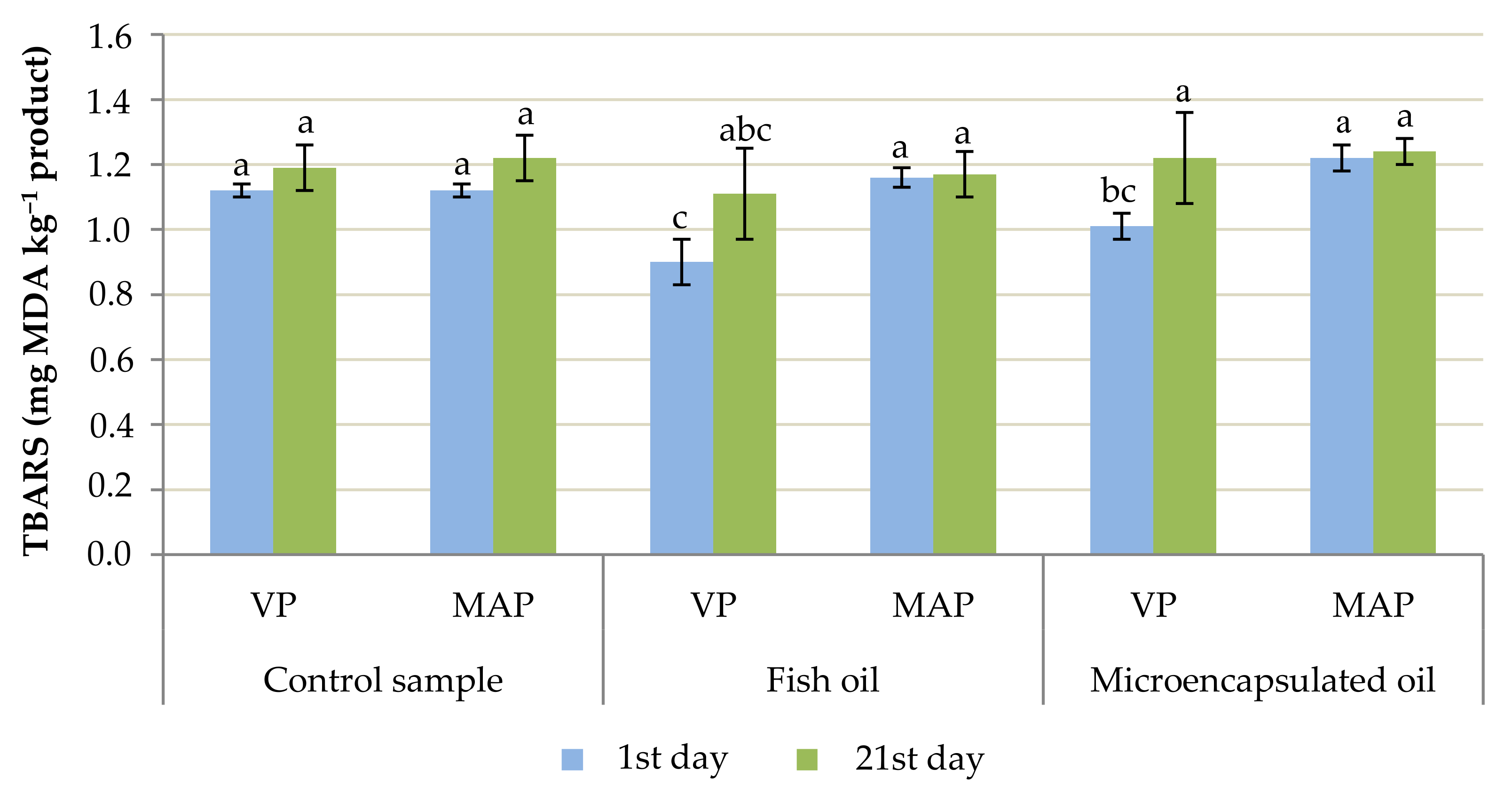
2.5. TBARS Analysis
3. Materials and Methods
3.1. Sample Preparation
3.2. Basic Chemical Composition
3.3. Sensory Assessment
3.4. Fatty Acid Analysis
3.5. In Vitro Digestion Conditions
- pepsin from porcine gastric mucosa (P7000, Sigma-Aldrich, St Louis, MO, USA);
- pancreatin from porcine pancreas (P1750, Sigma-Aldrich, St Louis, MO, USA);
- bovine bile (Sigma B-8381, Sigma-Aldrich, St Louis, MO, USA).
3.6. TBARS Analysis
3.7. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Granato, D.; Barba, F.J.; Bursać Kovačević, D.; Lorenzo, J.M.; Cruz, A.G.; Putnik, P. Functional Foods: Product Development, Technological Trends, Efficacy Testing, and Safety. Annu. Rev. Food Sci. Technol. 2020, 11, 93–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jędrusek-Golińska, A.; Górecka, D.; Buchowski, M.; Wieczorowska-Tobis, K.; Gramza-Michałowska, A.; Szymandera-Buszka, K. Recent progress in the use of functional foods for older adults: A narrative review. Compr. Rev. Food Sci. 2020, 19, 835–856. [Google Scholar] [CrossRef] [PubMed]
- Pintado, T.; Delgado-Pando, G. Towards More Sustainable Meat Products: Extenders as a Way of Reducing Meat Content. Foods 2020, 9, 1044. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.; Verbeke, W.; de Kok, T.M. Stakeholder and consumer reactions towards innovative processed meat products: Insights from a qualitative study about nitrite reduction and phytochemical addition. Food Control. 2016, 60, 690–698. [Google Scholar] [CrossRef]
- De Piano, A.; Masquio, D.C.L.; Damaso, A.R. The effects of soy products and isoflavones in metabolic syndrome and nonalcoholic fatty liver disease. In Bioactive Food as Dietary Interventions for Diabetes; Watson, R., Preedy, V.R., Eds.; Academic Press, Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 121–136. [Google Scholar]
- Roshan, H.; Ghaedi, E.; Rahmani, J.; Barati, M.; Najafi, M.; Karimzedeh, M.; Nikpayam, O. Effects of probiotics and synbiotic supplementation on antioxidant status: A meta-analysis of randomized clinical trials. Clin. Nutr. 2019, 30, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Ejtahed, H.S.; Angoorani, P.; Soroush, A.R.; Atlasi, R.; Hasani-Ranjbar, S.; Mortazavian, A.M.; Larijani, B. Probiotics supplementation for the obesity management; a systematic review of animal studies and clinical trials. J. Funct. Foods 2019, 52, 228–242. [Google Scholar] [CrossRef]
- Salami, M.; Kouchaki, E.; Asemi, Z.; Tamtaji, O.R. How probiotic bacteria influence the motor and mental behaviors as well as immunological and oxidative biomarkers in multiple sclerosis? A double blind clinical trial. J. Funct. Foods 2019, 52, 8–13. [Google Scholar] [CrossRef]
- Pourashouri, P.; Shabanpour, B.; Razavi, S.H.; Jafari, S.M.; Shabani, A.; Aubourg, S.P. Oxidative stability of spray-dried microencapsulated fish oils with different wall materials. J. Aquat. Food Prod. Technol. 2014, 23, 567–578. [Google Scholar] [CrossRef]
- Botrel, D.A.; de Barros Fernandes, R.V.; Borges, S.V.; Yoshida, M.I. Influence of wall matrix systems on the properties of spray-dried microparticles containing fish oil. Food Res. Int. 2014, 62, 344–352. [Google Scholar] [CrossRef]
- Molfino, A.; Gioia, G.; Rossi Fanelli, F.; Muscaritoli, M. The role for dietary omega-3 fatty acids supplementation in older adults. Nutrients 2014, 6, 4058–4073. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.T.; Xu, J.; Wang, Y.M.; Xue, C.H. Health benefits of dietary marine DHA/EPA-enriched glycerophospholipids. Prog. Lipid Res. 2019, 75, 100997. [Google Scholar] [CrossRef] [PubMed]
- Ellulu, M.S.; Khaza’ai, H.; Abed, Y.; Rahmat, A.; Ismail, P.; Ranneh, Y. Role of fish oil in human health and possible mechanism to reduce the in flammation. Inflammopharmacology 2015, 23, 79–89. [Google Scholar] [CrossRef]
- Stonehouse, W.; Conlon, C.A.; Podd, J.; Hill, S.; Minihane, A.M.; Haskell, C.; Kennedy, D. DHA supplementation improved both memory and reaction time in healthy young adults: A randomized controlled trial. Am. J. Clin. Nutr. 2013, 97, 1114–1134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lauritzen, L.; Brambilla, P.; Mazzocchi, A.; Harsløf, L.B.; Ciappolino, V.; Agostoni, C. DHA Effects in Brain Development and Function. Nutrients 2016, 8, 6. [Google Scholar] [CrossRef] [Green Version]
- López-Pedrouso, M.; Lorenzo, J.M.; Gullón, B.; Bastianello Campagnol, P.C.; Franco, D. Novel strategy for developing healthy meat products replacing saturated fat with oleogels. Curr. Opin. Food Sci. 2020, 40, 40–45. [Google Scholar] [CrossRef]
- Piacentini, E.; Giorno, L.; Dragosavac, M.M.; Vladisavljević, G.T.; Holdich, R.G. Microencapsulation of oil droplets using cold water fish gelatine/gum Arabic complex coacervation by membrane emulsification. Food Res. Int. 2013, 53, 362–372. [Google Scholar] [CrossRef] [Green Version]
- Heck, R.T.; Lorenzo, J.L.; Santos, B.A.D.; Cichoski, A.J.; de Menezes, C.R.; Campagnol, P.C.B. Microencapsulation of healthier oils: An efficient strategy to improve the lipid profile of meat products. Curr. Opin. Food Sci. 2021, 40, 6–12. [Google Scholar] [CrossRef]
- Whelan, J.; Rust, C. Innovative dietary sources of n-3 fatty acids. Annu. Rev. Nutr. 2006, 26, 75–103. [Google Scholar] [CrossRef] [PubMed]
- Bou, R.; Codony, R.; Tres, A.; Decker, E.A.; Guardiola, F. Dietary strategies to improve nutritional value, oxidative stability, and sensory properties of poultry products. Crit. Rev. Food Sci. Nutr. 2009, 49, 800–822. [Google Scholar] [CrossRef] [PubMed]
- Josquin, N.M.; Linssen, J.P.H.; Houben, J.H. Quality characteristics of Dutch-style fermented sausages manufactured with partial replacement of pork back-fat with pure, pre-emulsified or encapsulated fish oil. Meat Sci. 2012, 90, 81–86. [Google Scholar] [CrossRef]
- Stangierski, J.; Rezler, R.; Kawecki, K.; Peplińska, B. Effect of microencapsulated fish oil powder on selected quality characteristics of chicken sausages. J. Sci. Food Agric. 2020, 100, 2043–2051. [Google Scholar] [CrossRef] [PubMed]
- Kawecki, K.; Rezler, R.; Baranowska, H.M.; Stangierski, J. Influence of fish oil and microencapsulated fish oil additives on water binding and the rheological properties of poultry sausage batters. J. Sci. Food Agric. 2021, 101, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Kawecki, K.; Stangierski, J.; Cegielska-Radziejewska, R. The Influence of Packing Methods and Storage Time of Poultry Sausages with Liquid and Microencapsulated Fish Oil Additives on Their Physicochemical, Microbial and Sensory Properties. Sensors 2021, 21, 2653. [Google Scholar] [CrossRef] [PubMed]
- Aquilani, C.; Pérez-Palacios, T.; Sirtori, F.; Jiménez-Martín, E.; Antequera, T.; Franci, O.; Acciaioli, A.; Bozzi, R.; Pugliese, C. Enrichment of Cinta Senese burgers with omega-3 fatty acids. Effect of type of addition and storage conditions on quality characteristics. Grasas Aceites 2018, 69, e235. [Google Scholar] [CrossRef] [Green Version]
- Lorenzo, J.M.; Munekata, P.; Pateiro, M.; Campagnol, P.C.B.; Dominguez, R. Healthy Spanish salchicho´n enriched with encapsulated n-3 long chain fatty acids in konjac glucomannan matrix. Food Res. Int. 2016, 89, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Martín, E.; Pérez-Palacios, T.; Carrascal, J.R.; Rojas, T.A. Enrichment of chicken nuggets with microencapsulated omega-3 fish oil: Effect of frozen storage time on oxidative stability and sensory quality. Food Bioproc. Technol. 2016, 9, 285–297. [Google Scholar] [CrossRef]
- Solomando, J.C.; Antequera, T.; Perez-Palacios, T. Evaluating the use of fish oil microcapsules as omega-3 vehicle in cooked and dry-cured sausages as affected by their processing, storage and cooking. Meat Sci. 2020, 162, 108031. [Google Scholar] [CrossRef]
- Raeisi, S.; Ojagh, S.M.; Pourashouri, P.; Salaün, F.; Quek, S.Y. Shelf-life and quality of chicken nuggets fortified with encapsulated fish oil and garlic essential oil during refrigerated storage. J. Food Sci. Technol. 2021, 58, 121–128. [Google Scholar] [CrossRef]
- Solomando, J.C.; Antequera, T.; Perez-Palacios, T. Lipid digestion and oxidative stability in ω-3-enriched meat model systems: Effect of fish oil microcapsules and processing or culinary cooking. Food Chem. 2020, 328, 127125. [Google Scholar] [CrossRef]
- Solomando, J.C.; Antequera, T.; Pérez-Palacios, T. Study on fish oil microcapsules as neat and added to meat model systems: Enrichment and bioaccesibility of EPA and DHA. LWT Food Sci. Technol. 2020, 120, 108946. [Google Scholar] [CrossRef]
- Solomando, J.C.; Antequera, T.; Perez-Palacios, T. Lipid digestion products in meat derivatives enriched with fish oil microcapsules. J. Funct. Foods. 2020, 68, 103916. [Google Scholar] [CrossRef]
- Calvo, P.; Lozano, M.; Espinosa-Mansilla, A.; González-Gómez, D. In-vitro evaluation of the availability of ω-3 and ω-6 fatty acids and tocopherols from microencapsulated walnut oil. Food Res. Int. 2012, 48, 316–321. [Google Scholar] [CrossRef]
- Berton, A.; Rouvellac, S.; Robert, B.; Rousseau, F.; Lopez, C.; Crenon, I. Effect of the size and interface composition of milk fat globules on their in vitro digestion by the human pancreatic lipase: Native versus homogenized milk fat globules. Food Hydrocoll. 2012, 29, 123–134. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Agregán, R.; Lorenzo, J.M. Effect of the partial replacement of pork backfat by microencapsulated fish oil or mixed fish and olive oil on the quality of frankfurter type sausage. J. Food Sci. Technol. 2017, 54, 26–37. [Google Scholar] [CrossRef] [Green Version]
- PN-ISO 1442:2000: Meat and Meat Products—The Determination of Moisture Content (Reference Method); Polish Committee for Standardization: Warsaw, Poland.
- PN-75/A-04018+Az3:2002 Food and Agricultural Products—Protein Determination by the Kjeldahl Method and Conversion to Protein Content; Polish Committee for Standardization: Warsaw, Poland.
- PN-ISO 1444:2000 Meat and Meat Products—Determination of Free Fat Content; Polish Committee for Standardization: Warsaw, Poland.
- PN-ISO 936:2000 Meat and Meat Products—Determination of Total Ash Content; Polish Committee for Standardization: Warsaw, Poland.
- PN-EN ISO 8586:2014–03 Sensory Analysis—General Guidelines for the Selection, Training and Monitoring of Selected Assessors and Expert Sensory Assessors; Polish Committee for Standardization: Warsaw, Poland.
- PN–ISO 4121: 1998. Sensory Analysis—Methodology—Evaluation of Food Products by Methods Using Scales; Polish Committee for Standardization: Warsaw, Poland.
- Folch, J.; Lees, M.; Sloane-Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Hoebler, C.; Lecannu, G.; Belleville, C.; Devaux, M.F.; Popineau, Y.; Barry, J.L. Development of an in vitro system simulating bucco-gastric digestion to assess the physical and chemical changes of food. Int. J. Food Sci. Nutr. 2002, 53, 389–402. [Google Scholar] [CrossRef]
- Gumienna, M.; Goderska, K.; Nowak, J.; Czarnecki, Z. Changes in the antioxidative activities of bean products and intestinal microflora in the model of the gastrointestinal tract “in vitro”. Polish J. Food Nutr. Sci. 2006, 15, 29–32. [Google Scholar]
- Salih, A.M.; Smith, D.M.; Price, J.F.; Dawson, L.E. Modified extraction 2-thiobarbituric acid method for measuring lipid oxidation in poultry. Poult. Sci. 1987, 66, 1483–1488. [Google Scholar] [CrossRef]

| Chemical | Type of Sample | ||
|---|---|---|---|
| Composition (%) | Control Sample (CO) | Fish Oil (FO) | Microencapsulated Oil (MC) |
| Moisture | 67.2 ± 0.6 | 66.9 ± 0.4 | 66.6 ± 0.6 |
| Protein | 18.8 ± 0.4 | 18.6 ± 0.3 | 19.1 ± 0.4 |
| Fat | 11.5 ± 0.3 | 12.1 ± 0.2 | 11.7 ± 0.3 |
| Ash | 2.4 ± 0.0 | 2.3 ± 0.0 | 2.4 ± 0.0 |
| Sensory Characteristics | Type of Sample | |||||
|---|---|---|---|---|---|---|
| Control Sample (CO) | Fish Oil (FO) | Microencapsulated Oil (MC) | ||||
| 1st Day | 21st Day | 1st Day | 21st Day | 1st Day | 21st Day | |
| External appearance | 4.1 ± 0.4 | 4.0 ± 0.4 | 4.1 ± 0.3 | 3.9 ± 0.4 | 4.1 ± 0.3 | 4.0 ± 0.3 |
| External colour | 4.1 ± 0.3 | 4.0 ± 0.3 | 4.1 ± 0.4 | 4.0 ± 0.4 | 4.2 ± 0.3 | 4.0 ± 0.3 |
| Cross-sectional appearance | 4.2 ± 0.3 | 4.2 ± 0.3 | 4.2 ± 0.3 | 4.2 ± 0.3 | 4.2 ± 0.3 | 4.3 ± 0.3 |
| Cross-sectional colour | 4.2 ± 0.4 | 4.1 ± 0.4 | 4.1 ± 0.3 | 4.1 ± 0.3 | 4.3 ± 0.3 | 4.1 ± 0.3 |
| Smell of cold product | 4.3 ± 0.3 | 4.2 ± 0.3 | 4.2 ± 0.3 | 4.1 ± 0.3 | 4.3 ± 0.3 | 4.1 ± 0.3 |
| Taste of cold product | 4.3 ± 0.4 | 4.1 ± 0.3 | 4.2 ± 0.4 | 4.0 ± 0.4 | 4.2 ± 0.4 | 4.0 ± 0.4 |
| Consistency of cold product | 4.5 ± 0.2 | 4.4 ± 0.3 | 4.4 ± 0.3 | 4.4 ± 0.3 | 4.5 ± 0.2 | 4.4 ± 0.3 |
| Smell of heated product | 4.2 ± 0.4 | 4.2 ± 0.4 | 4.2 ± 0.4 | 4.2 ± 0.4 | 4.2 ± 0.4 | 4.2 ± 0.4 |
| Taste of heated product | 4.3 ± 0.3 | 4.3 ± 0.3 | 4.2 ± 0.4 | 4.2 ± 0.4 | 4.2 ± 0.3 | 4.2 ± 0.3 |
| Consistency of heated product | 4.5 ± 0.2 | 4.4 ± 0.2 | 4.4 ± 0.3 | 4.4 ± 0.3 | 4.5 ± 0.2 | 4.5 ± 0.2 |
| Fatty Acids | Control Sample (CO) | Fish Oil (FO) | Microencapsulated (MC) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MAP | VP | MAP | VP | MAP | VP | |||||||
| 1st Day | 21st Day | 1st Day | 21st Day | 1st Day | 21st Day | 1st Day | 21st Day | 1st Day | 21st Day | 1st Day | 21st Day | |
| C12:0 (lauric acid) | 2.40 ± 0.06 a | 2.01 ± 0.08 a | 2.03 ± 0.12 a | 1.85 ± 0.08 b | 2.00 ± 0.05 a | 1.83 ± 0.04 b | 2.08 ± 0.05 a | 1.74 ± 0.04 cb | 2.15 ± 0.13 a | 1.84 ± 0.09 b | 2.30 ± 0.03 a | 1.82 ± 0.13 b |
| C14:0 (myristic acid) | 1.98 ± 0.06 a | 2.02 ± 0.10 ad | 2.03 ± 0.03 ad | 1.97 ± 0.10 a | 2.40 ± 0.02 b | 2.23 ± 0.07 c | 2.40 ± 0.03 b | 2.19 ± 0.08 c | 2.12 ± 0.02 cd | 1.95 ± 0.11 a | 2.01 ± 0.05 a | 1.93 ± 0.15 a |
| C15:0 (pentadecanoic acid) | 0.08 ± 0.01 a | 0.09 ± 0.01 be | 0.08 ± 0.01 ae | 0.08 ± 0.01 ae | 0.13 ± 0.01 c | 0.14 ± 0.01 d | 0.13 ± 0.01 c | 0.14 ± 0.00 cd | 0.09 ± 0.01 ef | 0.10 ± 0.00 bf | 0.10 ± 0.01 bf | 0.09 ± 0.01 ae |
| C16:0 (palmitic acid) | 19.90 ± 0.07 a | 20.26 ± 1.56 a | 20.05 ± 0.20 a | 20.30 ± 1.59 a | 20.17 ± 0.20 a | 19.29 ± 1.04 a | 20.05 ± 0.14 a | 19.50 ± 1.17 a | 20.74 ± 0.31 a | 19.87 ± 1.21 a | 19.62 ± 0.52 a | 19.64 ± 1.82 a |
| C16:1n-7 (palmitolenic acid) | 5.77 ± 0.06 a | 5.95 ± 0.36 ac | 5.74 ± 0.11 a | 5.94 ± 0.49 ad | 5.65 ± 0.13 a | 6.38 ± 0.48 bcde | 5.72 ± 0.03 a | 5.93 ± 0.25 a | 5.68 ± 0.11 a | 5.82 ± 0.41 a | 5.64 ± 0.06 a | 6.10 ± 0.76 ae |
| C18:0 (stearic acid) | 5.07 ± 0.11 a | 3.91 ± 0.25 b | 4.91 ± 0.07 a | 3.59 ± 0.30 bc | 3.65 ± 0.03 bd | 3.24 ± 0.41 c | 3.60 ± 0.10 bc | 3.51 ± 0.13 a | 3.80 ± 0.21 b | 3.36 ± 0.17 cd | 3.30 ± 0.27 cd | 3.32 ± 0.64 cd |
| C18:1n-9 + n-7 (cis-oleic acid) | 37.65 ± 0.35 adf | 38.08 ± 0.38 adf | 37.39 ± 0.14 acf | 38.84 ± 0.50 b | 36.47 ± 0.04 ce | 37.44 ± 0.28 df | 36.52 ± 0.32 a | 37.82 ± 0.27 ad | 37.32 ± 0.11 f | 38.65 ± 0.29 bg | 38.44 ± 0.25 bg | 38.76 ± 0.83 bg |
| C18:2n-6 (linoleic acid) | 23.44 ± 0.27 ac | 24.16 ± 0.91 a | 23.89 ± 0.02 a | 23.98 ± 0.94 a | 22.42 ± 0.17 b | 23.18 ± 0.74 ab | 22.62 ± 0.18 b | 23.17 ± 0.86 ab | 23.38 ± 0.11 ab | 23.98 ± 0.90 a | 24.11 ± 0.31 a | 24.13 ± 1.22 a |
| C18:3n-3 (α-linolenic acid) | 1.41 ± 0.01 a | 1.39 ± 0.15 a | 1.43 ± 0.04 a | 1.40 ± 0.15 a | 1.40 ± 0.05 b | 1.39 ± 0.10 a | 1.42 ± 0.05 b | 1.45 ± 0.12 ad | 1.31 ± 0.10 bc | 1.34 ± 0.13 bcd | 1.28 ± 0.03 bc | 1.33 ± 0.02 abd |
| C18:3n-6 (ɣ-linolenic acid) | nd | nd | nd | nd | 0.19 ± 0.02 a | 0.23 ± 0.05 b | 0.23 ± 0.03 b | 0.21 ± 0.04 ab | 0.21 ± 0.02 ab | 0.10 ± 0.02 c | 0.20 ± 0.03 ab | 0.06 ± 0.03 d |
| C20:1 (eicosenoic acid) | 0.37 ± 0.04 ab | 0.34 ± 0.04 a | 0.37 ± 0.03 ab | 0.46 ± 0.01 b | 0.57 ± 0.03 c | 0.57 ± 0.01 c | 0.57 ± 0.01 c | 0.59 ± 0.02 c | 0.36 ± 0.09 ab | 0.39 ± 0.19ab | 0.37 ± 0.02 ab | 0.41 ± 0.03 ab |
| C20:2n-6 (eicosadienic acid) | 0.25 ± 0.01 a | 0.18 ± 0.03 b | 0.26 ± 0.03 a | 0.20 ± 0.01 bc | 0.22 ± 0.02 cd | 0.20 ± 0.01 be | 0.23 ± 0.02 ad | 0.19 ± 0.01 be | 0.24 ± 0.03 ad | 0.21 ± 0.01 bc | 0.22 ± 0.03 cde | 0.20 ± 0.01 bc |
| C20:4n-6 (arachidonic acid) | 0.77 ± 0.12 ac | 0.51 ± 0.12 b | 0.78 ± 0.05 ac | 0.45 ± 0.02 b | 0.82 ± 0.01 a | 0.54 ± 0.02 b | 0.82 ± 0.02 a | 0.50 ± 0.01 b | 0.72 ± 0.01 c | 0.48 ± 0.03 b | 0.70 ± 0.12 c | 0.49 ± 0.01 b |
| C20:5n-3 (eicosapentaenoic acid; EPA) | nd | nd | nd | nd | 0.76 ± 0.01 a | 0.66 ± 0.02 b | 0.73 ± 0.03 c | 0.63 ± 0.01 d | 0.08 ± 0.01 e | 0.06 ± 0.01 f | 0.09 ± 0.01 e | 0.05 ± 0.01 f |
| C22:6n-3 (docosahexaenoic acid; DHA) | nd | nd | nd | nd | 1.07 ± 0.06 a | 0.86 ± 0.05 b | 1.09 ± 0.13 a | 0.81 ± 0.01 c | 0.18 ± 0.01 d | 0.13 ± 0.02 de | 0.17 ± 0.02 d | 0.09 ± 0.01 e |
| Σ EPA and DHA | - | - | - | - | 1.82 ± 0.06 a | 1.52 ± 0.06 b | 1.82 ± 0.16 a | 1.44 ± 0.01 c | 0.26 ± 0.02 d | 0.19 ± 0.03 de | 0.25 ± 0.02 d | 0.13 ± 0.02 e |
| Σ EPA and DHA (g∙100 g−1 product) | - | - | - | - | 0.22 ± 0.01 a | 0.18 ± 0.01 b | 0.22 ± 0.02 a | 0.17 ± 0.00 c | 0.03 ± 0.00 d | 0.02 ± 0.00 d | 0.03 ± 0.00 d | 0.02 ± 0.00 d |
| EPA (g∙100 g−1 product) | - | - | - | - | 0.09 ± 0.00 a | 0.08 ± 0.00 b | 0.09 ± 0.00 a | 0.08 ± 0.00 c | 0.01 ± 0.00 d | 0.01 ± 0.00de | 0.01 ± 0.00 d | 0.01 ± 0.00 e |
| DHA (g∙100 g−1 product) | - | - | - | - | 0.13 ± 0.01 a | 0.10 ± 0.01 b | 0.13 ± 0.01 a | 0.09 ± 0.00 c | 0.02 ± 0.00d | 0.01 ± 0.00 de | 0.02 ± 0.00d | 0.01 ± 0.00 e |
| Fatty Acids | Type of Sample | Packaging Method | Storage Time (day) | Type of Sample × Packaging Method | Type of Sample × Storage Time (day) | Packaging Method × Storage Time (day) | Type of Sample × Packaging Method × Storage Time (day) |
|---|---|---|---|---|---|---|---|
| C12:0 | 0.035 * | 0.020 | <0.001 | 0.265 | 0.007 | 0.097 | 0.020 |
| C14:0 | <0.001 | 0.136 | <0.001 | 0.459 | 0.003 | 0.667 | 0.148 |
| C15:0 | <0.001 | 0.278 | 0.018 | 0.355 | 0.007 | 0.018 | 0.369 |
| C16:0 | 0.518 | 0.510 | 0.299 | 0.418 | 0.278 | 0.487 | 0.749 |
| C16:1n-7 | 0.625 | 0.742 | 0.001 | 0.377 | 0.458 | 0.741 | 0.170 |
| C18:0 | <0.001 | 0.507 | <0.001 | 0.451 | 0.359 | 0.050 | 0.168 |
| C18:1n-9 + n-7 | <0.001 | <0.001 | <0.001 | 0.164 | 0.411 | 0.549 | <0.001 |
| C18:2n-6 | <0.001 | 0.213 | 0.012 | 0.678 | 0.712 | 0.184 | 0.866 |
| C18:3n-3 | 0.002 | 0.658 | 0.733 | 0.687 | 0.542 | 0.665 | 0.906 |
| C18:3n-6 | <0.001 | 0.609 | <0.001 | 0.036 | <0.001 | 0.022 | 0.212 |
| C20:1 | <0.001 | 0.102 | 0.138 | 0.425 | 0.876 | 0.187 | 0.451 |
| C20:2n-6 | 0.158 | 0.965 | <0.001 | 0.184 | 0.010 | 0.895 | 0.261 |
| C20:4n-6 | 0.003 | 0.415 | <0.001 | 0.827 | 0.131 | 0.415 | 0.451 |
| C20:5n-3 | <0.001 | 0.006 | <0.001 | 0.014 | <0.001 | 0.309 | 0.468 |
| C22:6n-3 | 0.002 | 0.012 | <0.001 | 0.003 | <0.001 | 0.098 | 0.735 |
| Σ EPA and DHA | <0.001 | 0.102 | <0.001 | 0.479 | <0.001 | 0.122 | 0.495 |
| Σ EPA and DHA (g∙100 g−1 product) | <0.001 | 0.006 | <0.001 | 0.014 | <0.001 | 0.309 | 0.468 |
| Fatty Acids | Before Digestion | After Stomach Stage | pH 2.0–7.4 (Initial Section of Small Intestine) | 2 h in Small Intestine | pH 7.4–8.0 (Initial Section of Large Intestine) | 18 h pH 8.0 Large Intestine |
|---|---|---|---|---|---|---|
| C12:0 | 2.11 ± 0.24 a | 1.71 ± 0.18 b | 1.78 ± 0.15 b | 1.47 ± 0.20 c | 1.55 ± 0.11 c | 1.09 ± 0.09 d |
| C14:0 | 1.51 ± 0.08 a | 1.21 ± 0.12 b | 2.81 ± 0.31 c | 2.88 ± 0.21 c | 2.72 ± 0.19 c | 2.03 ± 0.14 d |
| C15:0 | 0.32 ± 0.09 a | 0.24 ± 0.07 a | 0.28 ± 0.07 a | 0.25 ± 0.05 a | 0.21 ± 0.08 a | 0.17 ± 0.07 a |
| C16:0 | 23.60 ± 1.34 a | 21.39 ±1.27 b | 26.35 ± 1.41 c | 27.93 ± 1.38 d | 25.49 ± 1.51 c | 27.75 ± 1.39 d |
| C16:1n-7 | 3.61 ± 0.23 a | 3.25 ± 0.29 b | 3.81 ± 0.19 a | 3.13 ± 0.22 b | 2.46 ± 0.16 c | 2.25 ± 0.23 c |
| C18:0 | 3.53 ± 0.22 a | 2.93 ± 0.17 b | 4.29 ± 0.37 c | 5.62 ± 0.48 d | 4.82 ± 0.41 e | 5.14 ± 0.45 d |
| C18:1n-9 + n-7 | 35.34 ± 2.15 a | 38.13 ± 2.34 b | 31.99 ± 2.29 c | 31.90 ± 2.53 c | 33.04 ± 1.99 c | 33.35 ± 2.19 c |
| C18:2n-6 | 27.39 ± 1.64 a | 29.06 ± 1.51 b | 24.79 ± 1.42 c | 23.53 ± 1.41 c | 26.80 ± 1.37 c | 25.68 ± 1.44 c |
| C18:3n-3 | 0.13 ± 0.03 a | 0.10 ± 0.03 a | 0.26 ± 0.07 b | 0.17 ± 0.05 c | 0.08 ± 0.01 a | 0.13 ± 0.09 a |
| C18:3n-6 | 0.10 ± 0.02 a | 0.06 ± 0.03 b | 0.35 ± 0.09 c | 0.15 ± 0.06 a | 0.09 ± 0.02 ab | 0.10 ± 0.05 ab |
| C20:1 | 2.11 ± 0.17 a | 1.74 ± 0.12 b | 1.33 ± 0.09 c | 1.33 ± 0.11 c | 1.20 ± 0.10 d | 1.00 ± 0.10 d |
| C20:2n-6 | 0.08 ± 0.02 a | 0.09 ± 0.02 a | 0.32 ± 0.11 b | 0.35 ± 0.12 b | 0.27 ± 0.11 b | 0.28 ± 0.14 b |
| C20:4n-6 | 0.04 ± 0.00 a | 0.04 ± 0.01 a | 0.03 ± 0.01 a | 0.16 ± 0.09 b | 0.18 ± 0.08 b | 0.15 ± 0.10 b |
| C20:5n-3 | 0.13 ± 0.09 a | 0.05 ± 0.01 b | 0.35 ± 0.12 c | 0.38 ± 0.10 c | 0.50 ± 0.13 d | 0.48 ± 0.15 d |
| C22:6n-3 | nd | nd | 1.26 ± 0.15 a | 0.75 ± 0.09 b | 0.59 ± 0.12 c | 0.40 ± 0.11 d |
| Σ EPA and DHA | 0.13 ± 0.05 a | 0.05 ± 0.01 b | 1.61 ± 0.21 c | 1.13 ± 0.19 d | 1.09 ± 0.14 d | 0.88 ± 0.12 e |
| Σ EPA and DHA (g∙100 g−1 product) | 0.01 ± 0.01 a | 0.01 ± 0.00 a | 0.17 ± 0.04 b | 0.12 ± 0.03 c | 0.11 ± 0.04 c | 0.09 ± 0.03 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawecki, K.; Stangierski, J.; Konieczny, P. An Analysis of Oxidative Changes and the Fatty Acid Profile in Stored Poultry Sausages with Liquid and Microencapsulated Fish Oil Additives. Molecules 2021, 26, 4293. https://doi.org/10.3390/molecules26144293
Kawecki K, Stangierski J, Konieczny P. An Analysis of Oxidative Changes and the Fatty Acid Profile in Stored Poultry Sausages with Liquid and Microencapsulated Fish Oil Additives. Molecules. 2021; 26(14):4293. https://doi.org/10.3390/molecules26144293
Chicago/Turabian StyleKawecki, Krzysztof, Jerzy Stangierski, and Piotr Konieczny. 2021. "An Analysis of Oxidative Changes and the Fatty Acid Profile in Stored Poultry Sausages with Liquid and Microencapsulated Fish Oil Additives" Molecules 26, no. 14: 4293. https://doi.org/10.3390/molecules26144293
APA StyleKawecki, K., Stangierski, J., & Konieczny, P. (2021). An Analysis of Oxidative Changes and the Fatty Acid Profile in Stored Poultry Sausages with Liquid and Microencapsulated Fish Oil Additives. Molecules, 26(14), 4293. https://doi.org/10.3390/molecules26144293






