New Type of Food Processing Material: The Crystal Structure and Functional Properties of Waxy and Non-Waxy Proso Millet Resistant Starches
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Starch Isolation and Purification
2.3. Preparation of RS
2.4. Analysis of Starch Granule
2.5. Color Characteristics
2.6. Analysis of Starch Amylose Content
2.7. X-ray Diffraction (XRD)
2.8. ATR-FTIR Spectrum Analysis
2.9. Water Solubility and Swelling Power of Starch
2.10. Thermal Properties
2.11. Pasting Properties of Starch
2.12. Rheological Properties of Starch
2.13. Statistical Analysis
3. Results and Discussion
3.1. Analysis of Starch Granules and Color
3.1.1. Scanning Electron Microscopy (SEM) and Size Distribution of Starch Granules
3.1.2. Color Measurement
3.2. Amylose Content
3.3. Crystalline Structure of Starch
3.4. ATR-FTIR Analysis of Starch
3.5. Dissolution Characteristics of Starch
3.6. Differential Scanning Calorimetry (DSC)
3.7. Pasting Properties
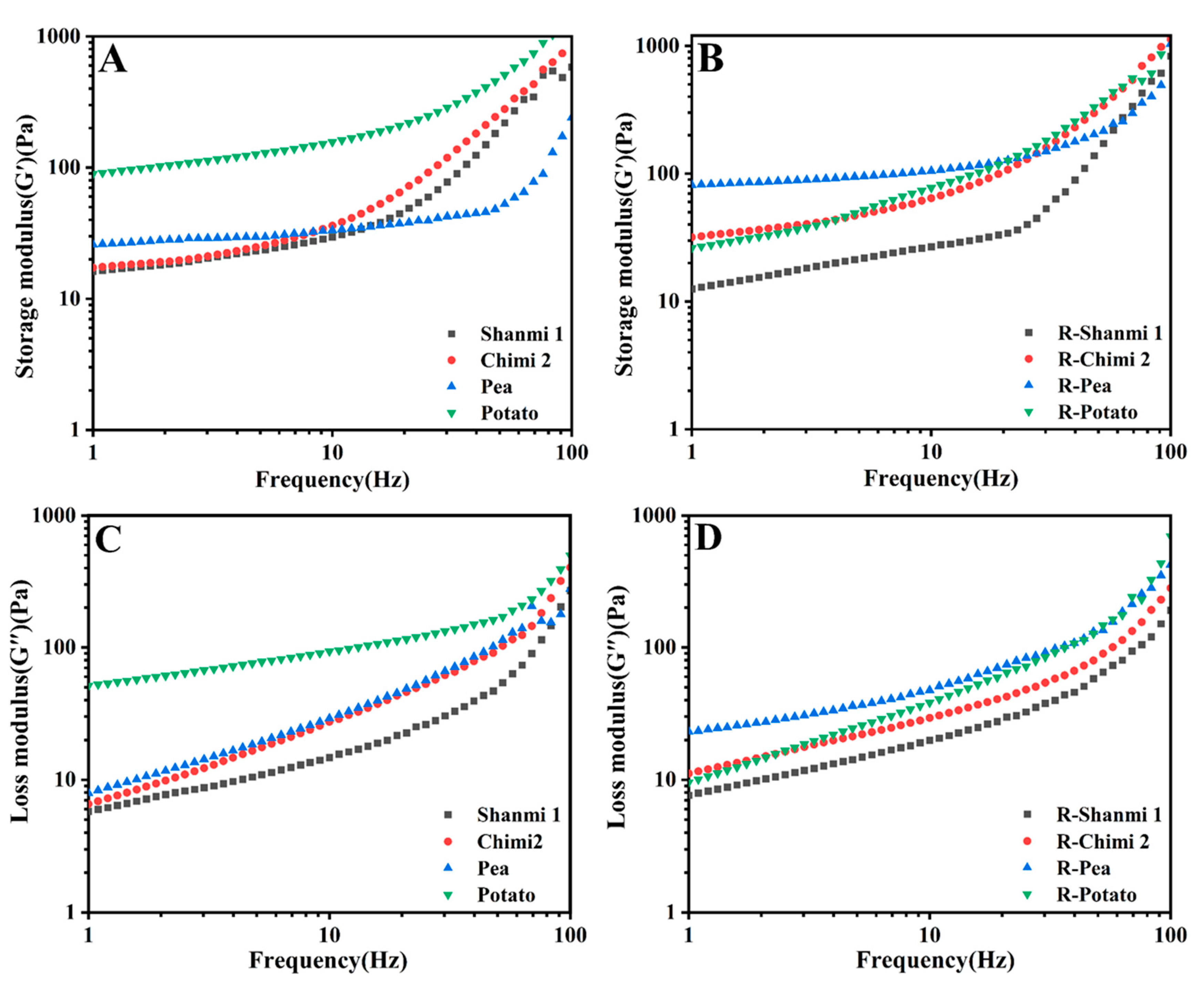
3.8. Dynamic Rheological Properties
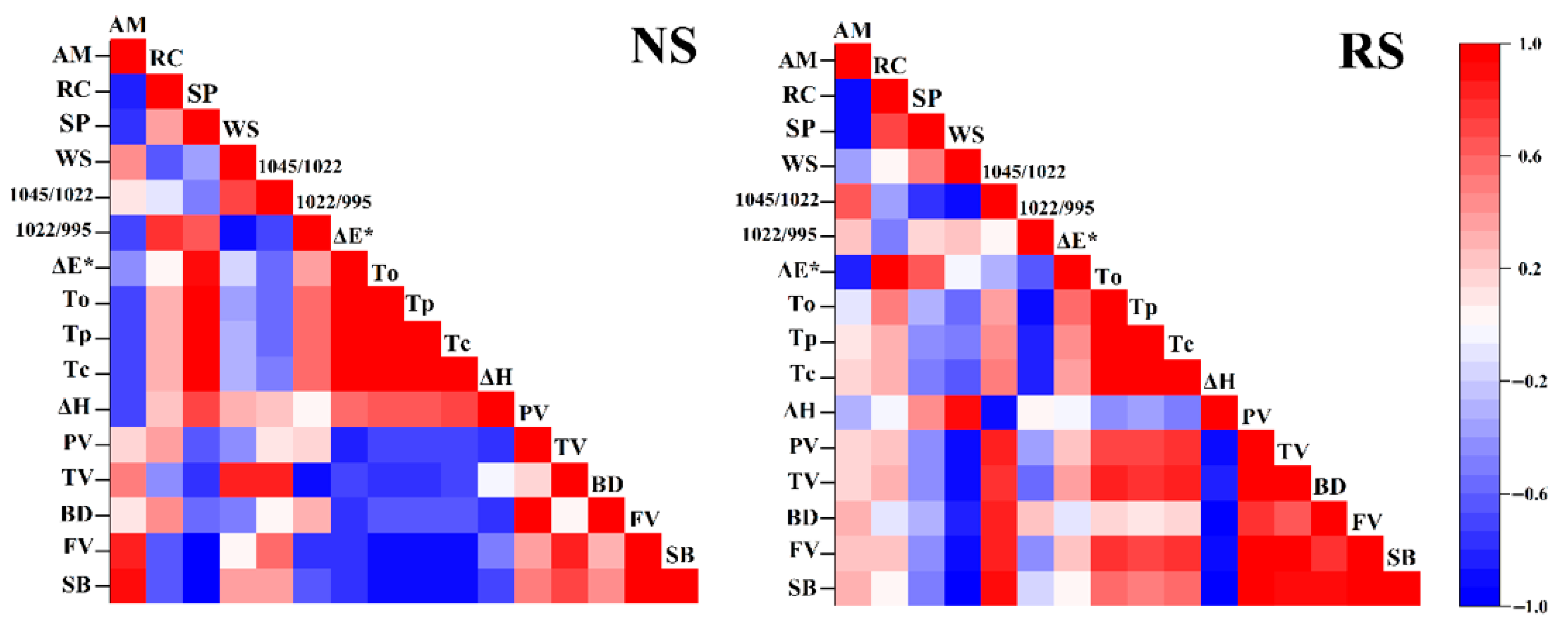
3.9. Correlation Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Lu, H.Y.; Zhang, J.P.; Liu, K.B.; Wu, N.Q.; Li, Y.M. Earliest domestication of common millet (Panicum miliaceum) in East Asia extended to 10,000 years ago. Proc. Natl. Acad. Sci. USA 2009, 106, 7367–7372. [Google Scholar] [CrossRef]
- Zhang, T.Z.; Li, K.H.; Ding, X.H.; Sui, Z.Q.; Yang, Q.Q.; Shah, N.P. Starch properties of high and low amylose proso millet (Panicum miliaceum L.) genotypes are differentially affected by varying salt and pH. Food Chem. 2021, 337, 127784. [Google Scholar] [CrossRef]
- Uarrota, V.G.; Amante, E.R.; Demiate, I.M.; Vieira, F.; Delgadillo, I.; Maraschin, M. Physicochemical, thermal, and pasting properties of flours and starches of eight Brazilian maize landraces (Zea mays L.). Food Hydrocolloid. 2013, 30, 614–624. [Google Scholar] [CrossRef]
- Yang, Q.H.; Zhang, W.L.; Li, J.; Gong, X.W. Physicochemical Properties of Starches in Proso (Non-Waxy and Waxy) and Foxtail Millets (Non-Waxy and Waxy). Molecules 2019, 24, 1743. [Google Scholar] [CrossRef] [PubMed]
- Ashogbon, A.O.; Akintayo, E.T. Recent trend in the physical and chemical modification of starches from different botanical sources: A review. Starch-Starke 2014, 66, 41–57. [Google Scholar] [CrossRef]
- Kapusniak, K.; Nebesny, E. Enzyme-resistant dextrins from potato starch for potential application in the beverage industry. Carbohyd. Polym. 2017, 172, 152–158. [Google Scholar]
- Jeong, O.; Shin, M. Preparation and stability of resistant starch nanoparticles, using acid hydrolysis and cross-linking of waxy rice starch. J. Food Chem. 2018, 256, 77–84. [Google Scholar] [CrossRef]
- Nasrin, T.A.A.; Anal, A.K. Resistant starch III from culled banana and its functional properties in fish oil emulsion. J. Food Hydrocolloid. 2014, 35, 403–409. [Google Scholar] [CrossRef]
- Fan, Y.Y.; Mao, L.K.; Xia, Y.; Jiang, D.J.; Yang, G.M. Comparison of native resistant starch of green banana by using different methods. J. Food Sci. Technol. 2011, 36, 256–259. (In Chinese) [Google Scholar]
- Jiang, F.; Du, C.W.; Jiang, W.Q.; Wang, L.Y.; Du, S.K. The preparation, formation, fermentability, and applications of resistant starch. Int. J. Biol. Macromol. 2020, 150, 1155–1161. [Google Scholar] [CrossRef] [PubMed]
- Nasrin, T.A.A.; Anal, A.K. Enhanced oxidative stability of fish oil by encapsulating in culled banana resistant starch-soy protein isolate based microcapsules in functional bakery products. J. Food Sci. Technol. 2015, 52, 5120–5128. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Regassa, A.; Nyachoti, C.M. Application of resistant starch in swine and poultry diets with particular reference to gut health and function. J. Anim. Nutr. 2018, 4, 305–310. [Google Scholar] [CrossRef]
- Ma, Z.; Yin, X.X.; Hu, X.Z. Structural characterization of resistant starch isolated from Laird lentils (Lens culinaris) seeds subjected to different processing treatments. Food Chem. 2018, 263, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Raigond, P.; Ezekiel, R.; Raigond, B. Resistant starch in food: A review. J. Sci. Food Agric. 2015, 95, 1968–1978. [Google Scholar] [CrossRef]
- Djurle, S.; Andersson, A.A.M.; Andersson, R. Effects of baking on dietary fibre, with emphasis on beta-glucan and resistant starch, in barley breads. J. Cereal Sci. 2018, 79, 449–455. [Google Scholar] [CrossRef]
- Lin, L.S.; Guo, D.W.; Huang, J. Molecular structure and enzymatic hydrolysis properties of starches from high-amylose maize inbred lines and their hybrids. Food Hydrocolloid. 2016, 58, 246–254. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, H.; Wei, T. Differences in physicochemical properties and in vitro digestibility between tartary buckwheat flour and starch modified by heat-moisture treatment. LWT Food Sci. Technol. 2017, 86, 285–292. [Google Scholar] [CrossRef]
- Chao, G.M.; Gao, J.F.; Liu, R.; Wang, L.; Li, C.; Wang, Y. Starch physicochemical properties of waxy proso millet (Panicum miliaceum L.). Starch-Starke 2015, 66, 1005–1012. [Google Scholar] [CrossRef]
- Li, K.H.; Zhang, T.Z.; Narayanamoorthy, S.; Jin, C.; Sui, Z.Q.; Li, Z.J. Diversity analysis of starch physicochemical properties in 95 proso millet (Panicum miliaceum L.) accessions. Food Chem. 2020, 324, 126863. [Google Scholar] [CrossRef]
- Kim, H.R.; Hong, J.S.; Ryu, A.-R. Combination of rice varieties and cooking methods resulting in a high content of resistant starch. Cereal Chem. 2020, 97, 149–157. [Google Scholar] [CrossRef]
- Joshi, M.; Aldred, P.; McKnight, S.; Panozzo, J.F.; Kasapis, S.; Adhikari, R.; Adhikari, B. Physicochemical and functional characteristics of lentil starch. Carbohyd. Polym. 2013, 92, 1484–1496. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Xing, Z.; Tian, Y.; Xu, X.; Jin, Z. Hydrolytic mechanism ofα-maltotriohydrolase on waxy maize starch and retrogradation properties of the hydrolysates. Food Hydrocolloid. 2017, 6, 136–143. [Google Scholar] [CrossRef]
- Gao, L.C.; Xia, M.J.; Li, Z.H. Common buckwheat-resistant starch as a suitable raw material for food production: A structural and physicochemical investigation. Int. J. Biol. Macromol. 2020, 145, 145–153. [Google Scholar] [CrossRef]
- Wang, H.L.; Yang, Q.H.; Gao, L.C.; Gong, X.W.; Qu, Y. Functional and physicochemical properties of flours and starches from different tuber crops. Int. J. Biol. Macromol. 2020, 148, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.H.; Lin, L.S.; Man, J.M. Different structural properties of high-amylose maize starch fractions varying in granule size. J. Agr. Food Chem. 2014, 62, 11711–11721. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; Yang, Q.H.; Ferdinand, U.; Gong, X.W.; Qu, Y.; Gao, W.C.; Ivanistau, A. Isolation and characterization of starch from light yellow, orange, and purple sweet potatoes. Int. J. Biol. Macromol. 2020, 160, 660–668. [Google Scholar] [CrossRef]
- Liu, H.; Wang, L.J.; Cao, R.; Fan, H.H.; Wang, M. In vitro digestibility and changes in physicochemical and structural properties of common buckwheat starch affected by high hydrostatic pressure. Carbohyd. Polym. 2016, 144, 1–8. [Google Scholar] [CrossRef]
- Huang, T.; Zhu, B.; Du, X.Z.; Li, B.; Wu, X.F.; Wang, S.S. Study on gelatinization property and edible quality mechanism of rice. Starch-Starke 2012, 64, 846–854. [Google Scholar] [CrossRef]
- Yang, Q.H.; Zhang, P.P.; Qu, Y.; Gao, X.L.; Liang, J.B.; Yang, P. Comparison of physicochemical properties and cooking edibility of waxy and non-waxy proso millet (Panicum miliaceum L.). Food Chem. 2018, 257, 271–278. [Google Scholar] [CrossRef]
- Kong, X.L.; Kasapis, S.; Bertoft, E.; Corke, H. Rheological properties of starches from grain amaranth and their relationship to starch structure. Starch-Starke 2010, 62, 302–308. [Google Scholar] [CrossRef]
- Yang, Q.H.; Zhang, W.; Luo, Y.; Li, J.; Gao, J.F.; Yang, P. Comparison of structural and physicochemical properties of starches from five coarse grains. Food Chem. 2019, 288, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Gu, B.; Yao, Q.Q.; Li, K.M.; Chen, S.B. Change in physicochemical traits of cassava roots and starches associated with genotypes and environmental factors. Starch-Starke 2013, 65, 253–263. [Google Scholar] [CrossRef]
- Ratnayake, W.S.; David, S.; Jackson, D.S. A new insight into the gelatinization process of native starches. Carbohyd. Polym. 2006, 67, 511–529. [Google Scholar] [CrossRef]
- Abegunde, Q.K.; Mu, T.H.; Chen, W.T. Physicochemical characterization of sweet potato starches popularly used in Chinese starch industry. Food Hydrocolloid. 2013, 33, 169–177. [Google Scholar] [CrossRef]
- Fuentes-Zaragoza, E.; Riquelme-Navarrete, M.J.; Sanchez-Zapata, E.; Perez-Alvarez, J.A. Resistant starch as functional ingredient: A review. Food Res. Int. 2010, 43, 931–942. [Google Scholar] [CrossRef]
- Patras, A.; Brunton, N.; Da Pieve, S.; Butler, F.; Downey, G. Effect of thermal and high pressure processing on antioxidant activity and instrumental colour of tomato and carrot purees. Innov. Food Sci. Emerg. 2009, 10, 16–22. [Google Scholar] [CrossRef]
- Riley, C.K.; Wheatley, A.O.; Hassan, I.; Ahmad, M.H.; Morrison, E.Y.S.A.; Asemota, H.N. In vitro digestibility of raw starches extracted fromfive yam (Dioscorea spp.) species grown in Jamaica. Starch-Starke 2004, 56, 69–73. [Google Scholar] [CrossRef]
- Huang, J.R.; Schols, H.A.; van Soest, J.J.G.; Jin, Z.Y.; Sulmann, E.; Voragen, A.G.J. Physicochemical properties and amylopectin chain profiles of cowpea, chickpea and yellow pea starches. Food Chem. 2007, 101, 1338–1345. [Google Scholar] [CrossRef]
- Qin, R.B.; Wang, S.J.; Xiang, F.J. Structural changes and in vitro enzymatic digestibility of starch-lipid complexes altered by high hydrostatic pressure. Food Res. Dev. 2021, 42, 25–30. (In Chinese) [Google Scholar]
- Sevenou, O.; Hill, S.E.; Farhat, I.A.; Mitchell, J.R. Organisation of the external region of the starch granule as determined by infrared spectroscopy. Int. J. Biol. Macromol. 2002, 31, 79–85. [Google Scholar] [CrossRef]
- Vermeylen, R.; Goderis, B.; Delcour, J.A. An X-ray study of hydrothermally treated potato starch. Carbohyd. Polym. 2005, 64, 364–375. [Google Scholar] [CrossRef]
- Cai, J.W.; Man, J.M.; Huang, J.; Liu, Q.Q.; Wei, W.X.; Wei, C.X. Relationship between structure and functional properties of normal rice starches with different amylose contents. Carbohyd. Polym. 2015, 125, 35–44. [Google Scholar] [CrossRef]
- Waduge, R.N.; Hoover, R.; Vasanthan, T.; Gao, J.; Li, J. Effect of annealing on the structure and physicochemical properties of barley starches of varying amylose content. Food Res. Int. 2005, 39, 59–77. [Google Scholar] [CrossRef]
- Zavareze, E.D.; Storck, C.R.; de Castro, L.A.S.; Schirmer, M.A.; Dias, A.R.G. Effect of heat-moisture treatment on rice starch of varying amylose content. Food Chem. 2010, 121, 358–365. [Google Scholar] [CrossRef]
- Hormdok, R.; Noomhorm, A. Hydrothermal treatments of rice starch for improvement of rice noodle quality. LWT Food Sci. Technol. 2006, 40, 1723–1731. [Google Scholar] [CrossRef]
- Chung, H.J.; Liu, Q.; Hoover, R. Impact of annealing and heat-moisture treatment on rapidly digestible, slowly digestible and resistant starch levels in native and gelatinized corn, pea and lentil starches. Carbohyd. Polym. 2009, 75, 436–447. [Google Scholar] [CrossRef]
- Liu, C.; Song, M.K.; Liu, L. Effect of heat-moisture treatment on the structure and physicochemical properties of ball mill damaged starches from different botanical sources. Int. J. Biol. Macromol. 2020, 156, 403–410. [Google Scholar] [CrossRef]
- Englyst, H.N.; Kingman, S.M.; Cummings, J.H. Classification and measurement of nutritionally important starch fractions. J. Eur. J. Clin. Nutr. 1992, 46, S33–S50. [Google Scholar]
- Yu, Z.J.; Ji, S.J. Research Progress of Resistant Starch. Agric. Sci. Equip. 2010, 32, 14–17. (In Chinese) [Google Scholar]
- Kim, Y.Y.; Woo, K.S.; Chung, H.J. Starch characteristics of cowpea and mungbean cultivars grown in Korea. Food Chem. 2018, 263, 104–111. [Google Scholar] [CrossRef]
- Hoover, R.; Vasanthan, T. Effect of heat-moisture treatment on the structure and physicochemical properties of cereal, legume, and tuber starches. Carbohyd. Res. 1994, 252, 33–53. [Google Scholar] [CrossRef]
- Barichello, V.; Yada, R.Y.; Coffin, R.H.; Stanley, D.W. Low Temperature Sweetening in Susceptible and Resistant Potatoes: Starch Structure and Composition. J. Food Sci. 1990, 55, 1054–1059. [Google Scholar] [CrossRef]
- Ye, F.Y.; Li, J.F.; Zhao, G.H. Physicochemical properties of different-sized fractions of sweet potato starch and their contributions to the quality of sweet potato starch. Food Hydrocolloid. 2020, 108, 106023. [Google Scholar] [CrossRef]
- Gunaratne, A.; Hoover, R. Effect of heat–moisture treatment on the structure and physicochemical properties of tuber and root starches. Carbohyd. Polym. 2002, 49, 425–437. [Google Scholar] [CrossRef]
- Sharma, M.; Yadav, D.N.; Singh, A.K.; Tomar, S.K. Rheological and functional properties of heat moisture treated pearl millet starch. J. Food Sci. Tech. Mys. 2015, 52, 6502–6510. [Google Scholar] [CrossRef]
- Rezaei, R.; Khomeiri, M.; Kashaninejad, M.; Mazaheri-Tehrani, M.; Aalami, M. Effect of resistant starch and aging conditions on the physicochemical properties of frozen soy yogurt. J. Food Sci. Tech. Mys. 2015, 52, 8164–8171. [Google Scholar] [CrossRef] [PubMed]
- Cornejo, F.; Novillo, G.; Villacres, E.; Rosell, C.M. Evaluation of the physicochemical and nutritional changes in two amaranth species (Amaranthus quitensis and Amaranthus caudatus) after germination. Food Res. Int. 2019, 121, 933–939. [Google Scholar] [CrossRef]
- Singh, J.; Singh, N. Studies on the morphological, thermal and rheological properties of starch separated from some Indian potato cultivars. Food Chem. 2001, 75, 67–77. [Google Scholar] [CrossRef]

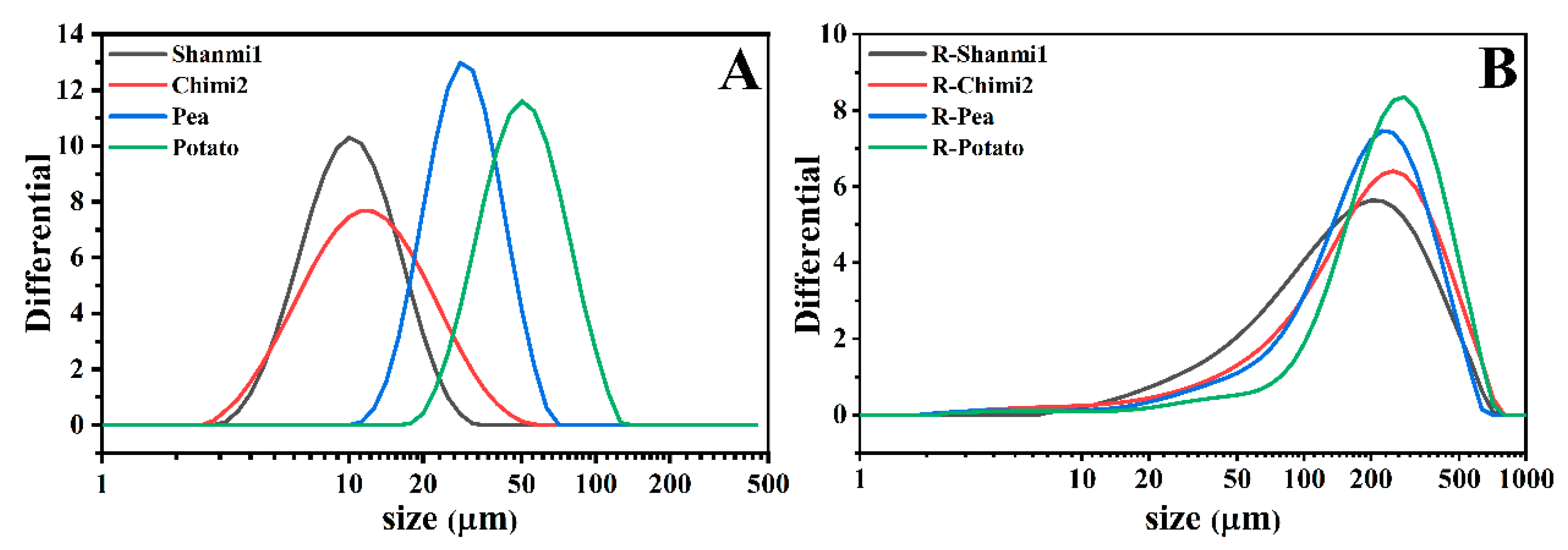


| Varieties and Lines | Granule Size b | Color Values c | |||||
|---|---|---|---|---|---|---|---|
| d (0.5) µm | D (3, 2) µm | D (4, 3) µm | L* | a* | b* | ΔE* | |
| Shanmi1 | 9.91 ± 0.35 g | 9.14 ± 0.31 g | 10.73 ± 0.49 g | 95.05 ± 0.14 b | 0.20 ± 0.01 b | 1.34 ± 0.08 d | 95.06 ± 0.14 b |
| Chimi2 | 11.22 ± 0.23 g | 9.60 ± 0.16 g | 12.92 ± 0.09 g | 95.42 ± 0.01 a | 0.13 ± 0.01 c | 1.59 ± 0.01 c | 95.43 ± 0.01 a |
| Pea | 26.95 ± 0.37 f | 25.44 ± 0.32 f | 28.39 ± 0.36 f | 93.28 ± 0.16 c | 0.29 ± 0.04 a | 3.15 ± 0.02 a | 93.33 ± 0.16 c |
| Potato | 47.39 ± 0.14 e | 44.36 ± 0.08 e | 50.25 ± 0.36 e | 92.75 ± 0.02 d | 0.07 ± 0.01 d | 2.37 ± 0.01 b | 92.78 ± 0.02 d |
| R-Shanmi1 | 159.53 ± 3.87 d | 86.52 ± 3.80 b | 184.33 ± 8.67 d | 89.11 ± 0.10 a | 1.01 ± 0.01 b | 5.65 ± 0.02 c | 89.29 ± 0.10 a |
| R-Chimi2 | 183.32 ± 2.78 b | 74.06 ± 0.91 d | 205.38 ± 4.49 b | 82.58 ± 0.18 c | 2.54 ± 0.02 a | 11.78 ± 0.03 a | 83.46 ± 0.18 c |
| R-Pea | 179.08 ± 3.36 c | 80.75 ± 2.72 c | 195.12 ± 2.93 c | 81.86 ± 0.17 d | 2.57 ± 0.08 a | 11.56 ± 0.10 b | 82.72 ± 0.15 d |
| R-Potato | 232.50 ± 1.73 a | 117.53 ± 4.95 a | 262.65 ± 5.09 a | 87.72 ± 0.13 b | 0.36 ± 0.01 c | 5.14 ± 0.03 d | 87.87 ± 0.13 b |
| Varieties and Lines | Amylose Content (%) | Relative Crystallinity (%) | IR Ratio | |
|---|---|---|---|---|
| 1045/1022 cm−1 | 1022/995 cm−1 | |||
| Shanmi 1 | 4.04 ± 0.19 h | 30.42 ± 0.12 c | 78.55 ± 0.88 b | 75.72 ± 1.25 d |
| Chimi 2 | 31.44 ± 0.60 e | 28.64 ± 0.08 g | 73.02 ± 0.28 c | 74.05 ± 0.86 e |
| Pea | 37.85 ± 0.14 b | 28.34 ± 0.08 h | 83.21 ± 2.82 a | 66.79 ± 0.44 f |
| Potato | 27.88 ± 0.90 f | 29.96 ± 0.08 e | 77.82 ± 0.55 b | 74.90 ± 0.66 de |
| R-Shanmi 1 | 7.55 ± 0.15 g | 32.44 ± 0.06 a | 66.08 ± 0.65 e | 82.55 ± 0.98 b |
| R-Chimi 2 | 36.41 ± 0.40 c | 30.14 ± 0.06 d | 71.03 ± 0.76 cd | 86.82 ± 0.92 a |
| R-Pea | 46.77 ± 0.70 a | 29.57 ± 0.09 f | 69.52 ± 0.72 d | 83.14 ± 0.46 b |
| R-Potato | 33.08 ± 0.78 d | 31.66 ± 0.09 b | 72.75 ± 0.04 c | 80.85 ± 0.92 c |
| Varieties | To (°C) | Tp (°C) | Tc (°C) | ΔH (J/g) |
|---|---|---|---|---|
| Shanmi 1 | 74.70 ± 0.14 d | 78.61 ± 0.12 e | 88.82 ± 1.50 e | 17.59 ± 0.48 a |
| Chimi 2 | 72.77 ± 0.06 e | 76.45 ± 0.38 f | 86.24 ± 0.46 f | 13.10 ± 0.15 c |
| Pea | 61.05 ± 0.10 f | 64.76 ± 0.07 g | 74.62 ± 0.44 g | 14.33 ± 0.16 b |
| Potato | 61.10 ± 0.34 f | 64.68 ± 0.35 g | 73.55 ± 0.32 g | 10.60 ± 0.14 d |
| R-Shanmi 1 | 132.24 ± 0.52 b | 132.30 ± 0.54 c | 133.79 ± 0.63 c | 4.73 ± 0.01 e |
| R-Chimi 2 | 128.69 ± 0.84 c | 129.13 ± 0.90 d | 131.47 ± 0.66 d | 2.45 ± 0.26 f |
| R-Pea | 131.86 ± 0.07 b | 133.78 ± 0.19 b | 135.39 ± 0.37 b | 4.38 ± 0.07 e |
| R-Potato | 137.64 ± 0.09 a | 138.35 ± 0.51 a | 140.37 ± 0.22 a | 1.41 ± 0.17 g |
| Varieties | PV (cP) | BD (cP) | FV (cP) | SB (cP) | PTM (°C) | PT (min) |
|---|---|---|---|---|---|---|
| Shanmi1 | 2574 ± 19.78 d | 1285 ± 31.75 c | 1633 ± 5.72 e | 348 ± 6.24 f | 77.04 ± 0.07 d | 4.13 ± 0.00 g |
| Chimi2 | 1658 ± 13.53 e | 630 ± 6.77 d | 2984 ± 87.95 d | 1957 ± 81.18 c | 81.68 ± 0.03 a | 4.30 ± 0.03 e |
| Pea | 4464 ± 42.15 b | 1719 ± 33.31 b | 6208 ± 186.83 a | 3392 ± 59.33 a | 79.92 ± 0.03 b | 4.67 ± 0.00 d |
| Potato | 17562 ± 52.50 a | 16582 ± 192.05 a | 4652 ± 80.22 b | 2944 ± 227.42 b | 67.89 ± 0.05 c | 2.87 ± 0.00 h |
| R-Shanmi1 | 415 ± 32.27 h | 25 ± 5.20 e | 547 ± 5.20 h | 159 ± 32.27 g | 83.78 ± 1.28 a | 4.20 ± 0.00 f |
| R-Chimi2 | 1190 ± 8.85 f | 536 ± 10.93 d | 1406 ± 1.04 f | 752 ± 1.04 e | 80.81 ± 0.84 bc | 6.75 ± 0.07 b |
| R-Pea | 489 ± 8.85 g | 47 ± 1.56 e | 747 ± 6.24 g | 305 ± 1.04 fg | 83.19 ± 0.45 a | 8.00 ± 0.00 a |
| R-Potato | 2842 ± 7.29 c | 525 ± 20.82 d | 3528 ± 1.56 c | 1217 ± 15.09 d | 68.20 ± 0.44 e | 5.03 ± 0.04 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, M.; Dang, K.; Wang, J.; Gao, L.; Wang, H.; Ivanistau, A.; Yang, Q.; Feng, B. New Type of Food Processing Material: The Crystal Structure and Functional Properties of Waxy and Non-Waxy Proso Millet Resistant Starches. Molecules 2021, 26, 4283. https://doi.org/10.3390/molecules26144283
Han M, Dang K, Wang J, Gao L, Wang H, Ivanistau A, Yang Q, Feng B. New Type of Food Processing Material: The Crystal Structure and Functional Properties of Waxy and Non-Waxy Proso Millet Resistant Starches. Molecules. 2021; 26(14):4283. https://doi.org/10.3390/molecules26144283
Chicago/Turabian StyleHan, Mengru, Ke Dang, Jiale Wang, Licheng Gao, Honglu Wang, Aliaksandr Ivanistau, Qinghua Yang, and Baili Feng. 2021. "New Type of Food Processing Material: The Crystal Structure and Functional Properties of Waxy and Non-Waxy Proso Millet Resistant Starches" Molecules 26, no. 14: 4283. https://doi.org/10.3390/molecules26144283
APA StyleHan, M., Dang, K., Wang, J., Gao, L., Wang, H., Ivanistau, A., Yang, Q., & Feng, B. (2021). New Type of Food Processing Material: The Crystal Structure and Functional Properties of Waxy and Non-Waxy Proso Millet Resistant Starches. Molecules, 26(14), 4283. https://doi.org/10.3390/molecules26144283






