The Effect of the Molecular Weight of Polyvinylpyrrolidone and the Model Drug on Laser-Induced In Situ Amorphization
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Effect of the Molecular Weight of Polyvinylpyrrolidone on In Situ Amorphization
2.1.1. The Determination of the Temperature Thresholds, TOnset and TMin
2.1.2. The Rate of Amorphization upon Exposure to Laser Radiation
2.2. The Influence of the Model Drug on In Situ Amorphization
2.2.1. Solubility of Celecoxib, Indomethacin and Naproxen in Polyvinylpyrrolidone
2.2.2. The Determination of the Temperature Thresholds, TOnset and TMin
2.2.3. The Rate of Amorphization upon Exposure to Laser Radiation
2.3. Chemical Stability upon In Situ Amorphization
3. Materials and Methods
3.1. Materials
3.2. Plasmonic Nanoaggregate Synthesis
3.3. Compact Preparation
3.4. In Situ Amorphization
3.5. Qualitative Analysis of Crystallinity by Solid-State Analysis
3.6. Thermal Analysis and Quantification of Crystallinity
3.6.1. Quantification of the Residual Drug Crystallinity
3.6.2. Determination of the (Predicted) Solubility of Naproxen in Polyvinylpyrrolidone
3.6.3. Glass Transition Temperatures of the Polymers
3.6.4. Determination of the Temperature Thresholds, TOnset and TMin
3.7. Determination of the Water Content
3.8. Determination of the Drug Molecule Size
3.9. Drug Degradation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Qiang, W.; Lobmann, K.; McCoy, C.P.; Andrews, G.P.; Zhao, M. Microwave-Induced In Situ Amorphization: A New Strategy for Tackling the Stability Issue of Amorphous Solid Dispersions. Pharmaceutics 2020, 12, 655. [Google Scholar] [CrossRef]
- Priemel, P.A.; Laitinen, R.; Grohganz, H.; Rades, T.; Strachan, C.J. In situ amorphisation of indomethacin with Eudragit(R) E during dissolution. Eur. J. Pharm. BioPharm. 2013, 85, 1259–1265. [Google Scholar] [CrossRef]
- Hempel, N.J.; Knopp, M.M.; Berthelsen, R.; Lobmann, K. Convection-Induced vs. Microwave Radiation-Induced in situ Drug Amorphization. Molecules 2020, 25, 1068. [Google Scholar] [CrossRef] [Green Version]
- Hempel, N.J.; Merkl, P.; Asad, S.; Knopp, M.M.; Berthelsen, R.; Bergstrom, C.A.S.; Teleki, A.; Sotiriou, G.A.; Lobmann, K. Utilizing Laser Activation of Photothermal Plasmonic Nanoparticles to Induce On-Demand Drug Amorphization inside a Tablet. Mol. Pharm. 2021. [Google Scholar] [CrossRef]
- Holm, T.P.; Knopp, M.M.; Lobmann, K.; Berthelsen, R. Microwave induced in situ amorphisation facilitated by crystalline hydrates. Eur. J. Pharm. Sci. 2021, 163, 105858. [Google Scholar] [CrossRef]
- Hempel, N.J.; Knopp, M.M.; Berthelsen, R.; Zeitler, J.A.; Lobmann, K. The influence of drug and polymer particle size on the in situ amorphization using microwave irradiation. Eur. J. Pharm. BioPharm. 2020, 149, 77–84. [Google Scholar] [CrossRef]
- Grant, E.; Halstead, B.J. Dielectric parameters relevant to microwave dielectric heating. Chem. Soc. Rev. 1998, 27, 213–224. [Google Scholar] [CrossRef]
- Kravets, V.G.; Kabashin, A.V.; Barnes, W.L.; Grigorenko, A.N. Plasmonic Surface Lattice Resonances: A Review of Properties and Applications. Chem. Rev. 2018, 118, 5912–5951. [Google Scholar] [CrossRef]
- Kim, M.; Lee, J.H.; Nam, J.M. Plasmonic Photothermal Nanoparticles for Biomedical Applications. Adv. Sci. 2019, 6, 1900471. [Google Scholar] [CrossRef] [Green Version]
- Sotiriou, G.A.; Pratsinis, S.E. Antibacterial activity of nanosilver ions and particles. Environ. Sci. Technol. 2010, 44, 5649–5654. [Google Scholar] [CrossRef]
- Boken, J.; Khurana, P.; Thatai, S.; Kumar, D.; Prasad, S. Plasmonic nanoparticles and their analytical applications: A review. Appl. Spectrosc. Rev. 2017, 52, 774–820. [Google Scholar] [CrossRef]
- Merkl, P.; Zhou, S.; Zaganiaris, A.; Shahata, M.; Eleftheraki, A.; Thersleff, T.; Sotiriou, G.A. Plasmonic Coupling in Silver Nanoparticle Aggregates and Their Polymer Composite Films for Near-Infrared Photothermal Biofilm Eradication. ACS Appl. Nano Mater. 2021. [Google Scholar] [CrossRef]
- Sotiriou, G.A.; Blattmann, C.O.; Deligiannakis, Y. Nanoantioxidant-driven plasmon enhanced proton-coupled electron transfer. Nanoscale 2016, 8, 796–803. [Google Scholar] [CrossRef]
- Moseson, D.E.; Taylor, L.S. The application of temperature-composition phase diagrams for hot melt extrusion processing of amorphous solid dispersions to prevent residual crystallinity. Int. J. Pharm. 2018, 553, 454–466. [Google Scholar] [CrossRef]
- Moseson, D.E.; Parker, A.S.; Gilpin, C.J.; Stewart, A.A.; Beaudoin, S.P.; Taylor, L.S. Dissolution of Indomethacin Crystals into a Polymer Melt: Role of Diffusion and Fragmentation. Cryst. Growth Des. 2019, 19, 3315–3328. [Google Scholar] [CrossRef]
- Tian, Y.; Caron, V.; Jones, D.S.; Healy, A.-M.; Andrews, G.P. Using Flory-Huggins phase diagrams as a pre-formulation tool for the production of amorphous solid dispersions: A comparison between hot-melt extrusion and spray drying. J. Pharm. Pharmacol. 2014, 66, 256–274. [Google Scholar] [CrossRef]
- Roudaut, G.; Simatos, D.; Champion, D.; Contreras-Lopez, E.; Le Meste, M. Molecular mobility around the glass transition temperature: A mini review. Innov. Food Sci. Emerg. Technol. 2004, 5, 127–134. [Google Scholar] [CrossRef]
- Hancock, B.C.; Shamblin, S.L.; Zografi, G. Molecular Mobility of Amorphous Pharmaceutical Solids Below Their Glass Transition Temperatures. Pharm. Res. 1995, 12, 799–806. [Google Scholar] [CrossRef]
- Hilden, L.R.; Morris, K.R. Physics of amorphous solids. J. Pharm. Sci. 2004, 93, 3–12. [Google Scholar] [CrossRef]
- Noyes, A.A.; Whitney, W.R. The Rate of Solution of Solid Substances in Their Own Solutions. J. Am. Chem. Soc. 2002, 19, 930–934. [Google Scholar] [CrossRef] [Green Version]
- Edward, J.T. Molecular volumes and the Stokes-Einstein equation. J. Chem. Educ. 1970, 47, 261. [Google Scholar] [CrossRef]
- Hempel, N.J.; Dao, T.; Knopp, M.M.; Berthelsen, R.; Lobmann, K. The Influence of Temperature and Viscosity of Polyethylene Glycol on the Rate of Microwave-Induced In Situ Amorphization of Celecoxib. Molecules 2020, 26, 110. [Google Scholar] [CrossRef]
- Doreth, M.; Lobmann, K.; Priemel, P.; Grohganz, H.; Taylor, R.; Holm, R.; Lopez de Diego, H.; Rades, T. Influence of PVP molecular weight on the microwave assisted in situ amorphization of indomethacin. Eur. J. Pharm. BioPharm. 2018, 122, 62–69. [Google Scholar] [CrossRef]
- Knopp, M.M.; Olesen, N.E.; Holm, P.; Langguth, P.; Holm, R.; Rades, T. Influence of Polymer Molecular Weight on Drug-Polymer Solubility: A Comparison between Experimentally Determined Solubility in PVP and Prediction Derived from Solubility in Monomer. J. Pharm. Sci. 2015, 104, 2905–2912. [Google Scholar] [CrossRef] [Green Version]
- Knopp, M.M.; Gannon, N.; Porsch, I.; Rask, M.B.; Olesen, N.E.; Langguth, P.; Holm, R.; Rades, T. A Promising New Method to Estimate Drug-Polymer Solubility at Room Temperature. J. Pharm. Sci. 2016, 105, 2621–2624. [Google Scholar] [CrossRef] [Green Version]
- Baird, J.A.; Van Eerdenbrugh, B.; Taylor, L.S. A Classification System to Assess the Crystallization Tendency of Organic Molecules from Undercooled Melts. J. Pharm. Sci. 2010, 99, 3787–3806. [Google Scholar] [CrossRef]
- Nunes, R.W.; Martin, J.R.; Johnson, J.F. Influence of molecular weight and molecular weight distribution on mechanical properties of polymers. Polym. Eng. Sci. 1982, 22, 205–228. [Google Scholar]
- Knopp, M.M.; Olesen, N.E.; Holm, P.; Lobmann, K.; Holm, R.; Langguth, P.; Rades, T. Evaluation of drug-polymer solubility curves through formal statistical analysis: Comparison of preparation techniques. J. Pharm. Sci. 2015, 104, 44–51. [Google Scholar] [CrossRef]
- Mädler, L.; Kammler, H.K.; Mueller, R.; Pratsinis, S.E. Controlled synthesis of nanostructured particles by flame spray pyrolysis. J. Aerosol. Sci. 2002, 33, 369–389. [Google Scholar] [CrossRef]
- Sotiriou, G.A.; Teleki, A.; Camenzind, A.; Krumeich, F.; Meyer, A.; Panke, S.; Pratsinis, S.E. Nanosilver on nanostructured silica: Antibacterial activity and Ag surface area. Chem. Eng. J. 2011, 170, 547–554. [Google Scholar] [CrossRef] [Green Version]
- Marsac, P.J.; Shamblin, S.L.; Taylor, L.S. Theoretical and practical approaches for prediction of drug-polymer miscibility and solubility. Pharm. Res. 2006, 23, 2417–2426. [Google Scholar] [CrossRef]
- Marsac, P.J.; Li, T.; Taylor, L.S. Estimation of drug-polymer miscibility and solubility in amorphous solid dispersions using experimentally determined interaction parameters. Pharm. Res. 2009, 26, 139–151. [Google Scholar] [CrossRef]
- Knopp, M.M.; Tajber, L.; Tian, Y.; Olesen, N.E.; Jones, D.S.; Kozyra, A.; Löbmann, K.; Paluch, K.; Brennan, C.M.; Holm, R.; et al. Comparative Study of Different Methods for the Prediction of Drug–Polymer Solubility. Mol. Pharm. 2015, 12, 3408–3419. [Google Scholar] [CrossRef] [Green Version]
- Palmelund, H.; Andersson, M.P.; Asgreen, C.J.; Boyd, B.J.; Rantanen, J.; Lobmann, K. Tailor-made solvents for pharmaceutical use? Experimental and computational approach for determining solubility in deep eutectic solvents (DES). Int. J. Pharm. X 2019, 1, 100034. [Google Scholar] [CrossRef]
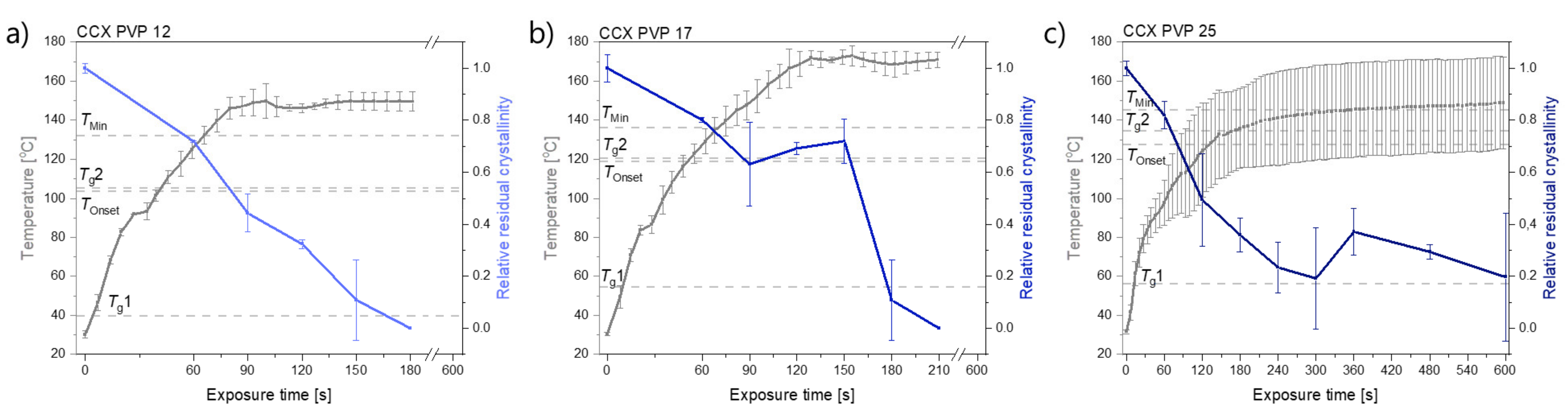
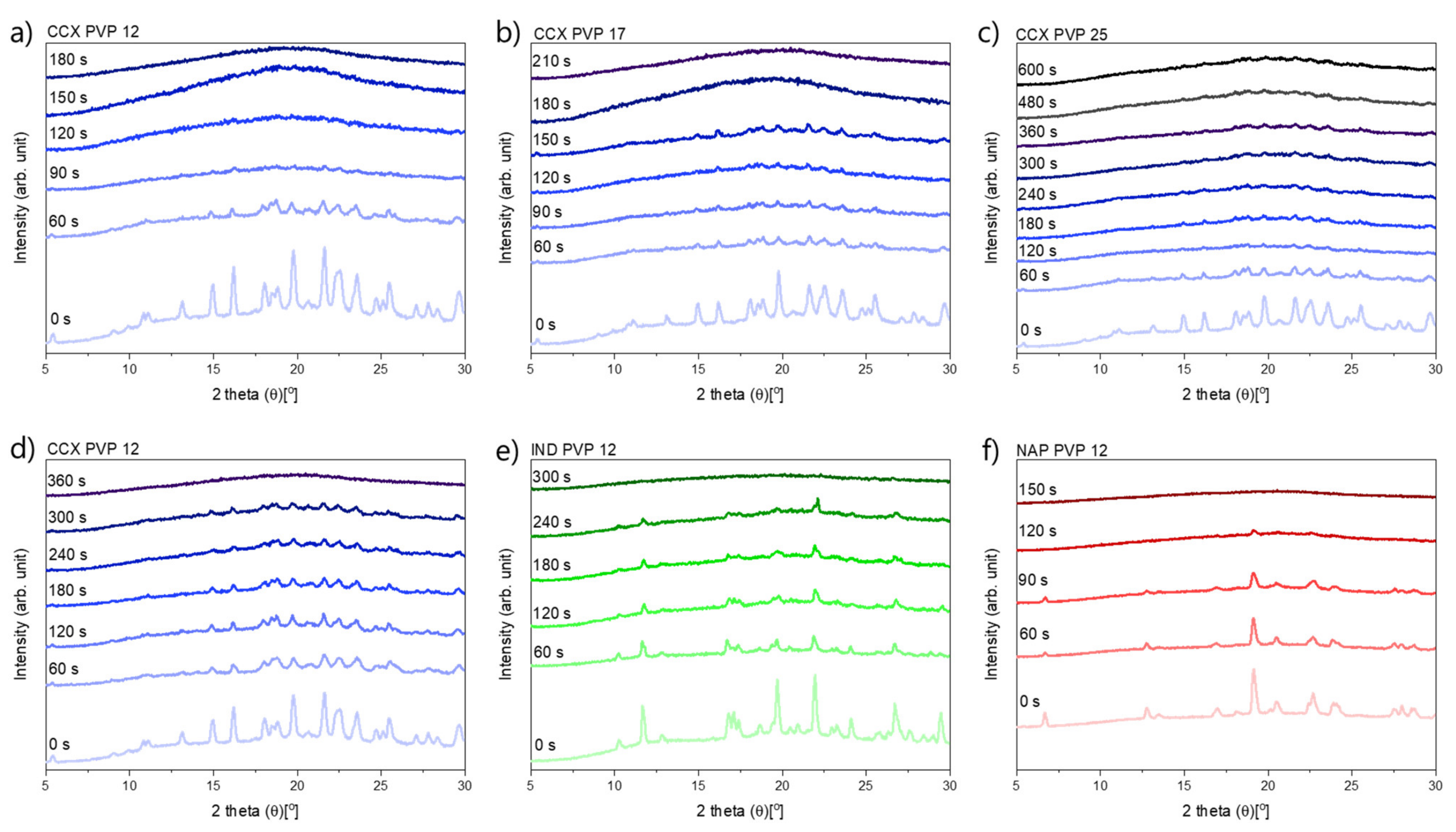
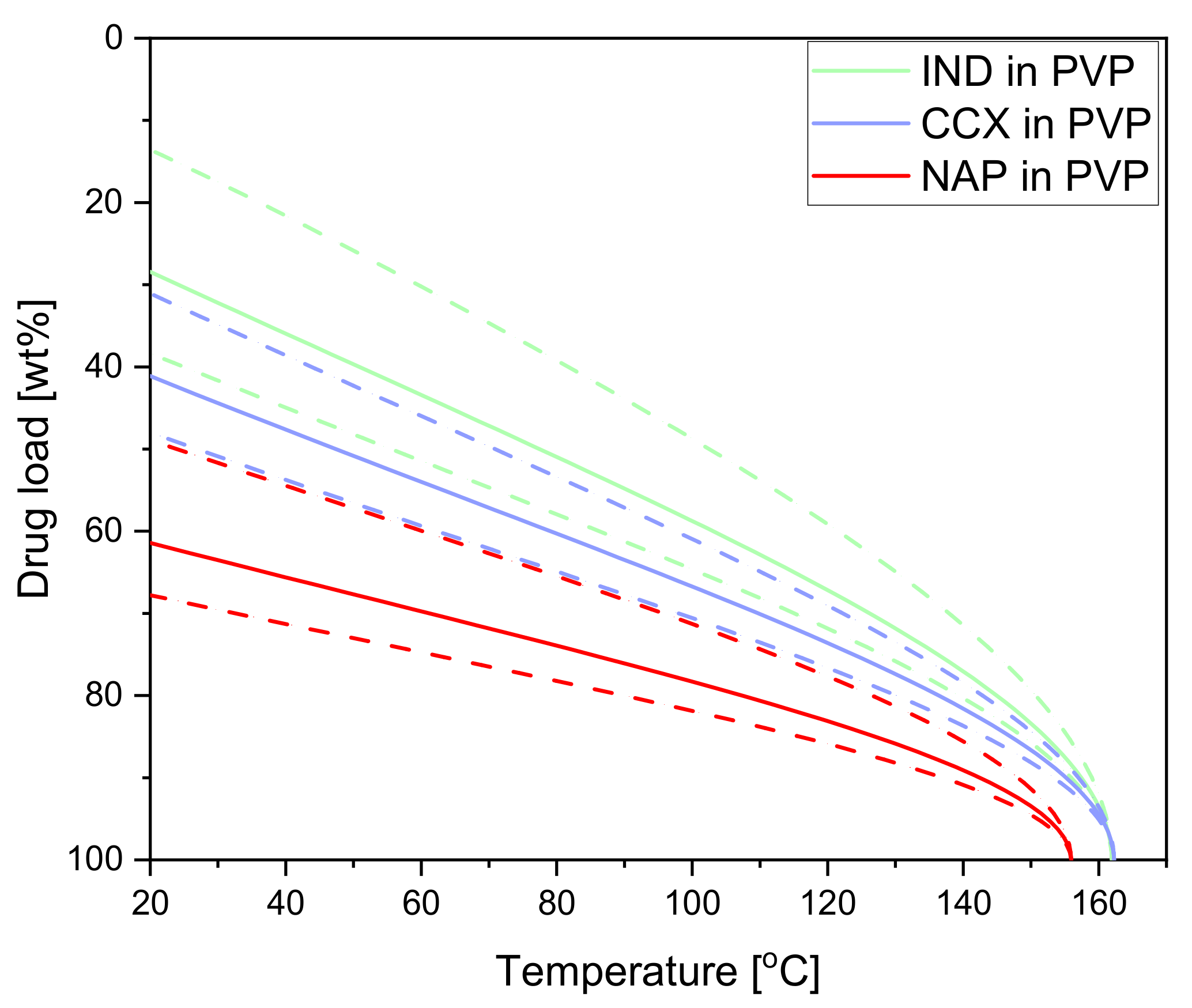
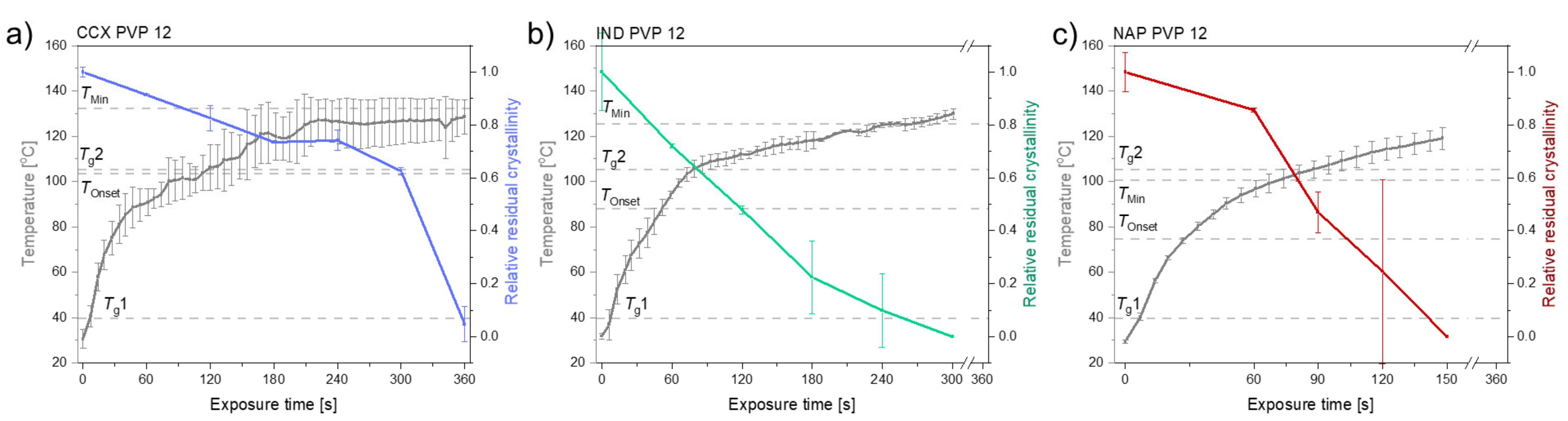
| Compact Composition | TOnset [°C] | TMin [°C] | Polymer | Tg1 (Bulk) [°C] | Tg2 (Water-Free) [°C] |
|---|---|---|---|---|---|
| 30 wt% CCX PVP12 | 103.6 | 132.2 | PVP12 | 39.7 ± 0.2 | 105.3 ± 0.5 |
| 30 wt% CCX PVP17 | 119.0 | 136.2 | PVP17 | 54.5 ± 2.9 | 120.6 ± 0.1 |
| 30 wt% CCX PVP25 | 127.8 | 145.4 | PVP25 | 56.1 ± 0.6 | 134.5 ± 1.5 |
| 30 wt% IND PVP12 | 88.0 | 125.6 | PVP12 | 39.7 ± 0.2 | 105.3 ± 0.5 |
| 30 wt% NAP PVP12 | 74.5 | 100.5 | PVP12 | 39.7 ± 0.2 | 105.3 ± 0.5 |
| Compact Composition | 60 s | 90 s | 120 s | 150 s | 180 s | 210 s | 240 s | 300 s | 360 s | 480 s | 600 s |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CCX PVP12 | c | c | c | c | a | ||||||
| CCX PVP17 | c | c | c | c | c | a | |||||
| CCX PVP25 | c | c | c | c | c | c | c | c | |||
| CCX PVP12 | c | c | c | c | c | c | c | c | a | ||
| IND PVP12 | c | c | c | c | c | c | c | a | |||
| NAP PVP12 | c | c | c | a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hempel, N.-J.; Merkl, P.; Knopp, M.M.; Berthelsen, R.; Teleki, A.; Hansen, A.K.; Sotiriou, G.A.; Löbmann, K. The Effect of the Molecular Weight of Polyvinylpyrrolidone and the Model Drug on Laser-Induced In Situ Amorphization. Molecules 2021, 26, 4035. https://doi.org/10.3390/molecules26134035
Hempel N-J, Merkl P, Knopp MM, Berthelsen R, Teleki A, Hansen AK, Sotiriou GA, Löbmann K. The Effect of the Molecular Weight of Polyvinylpyrrolidone and the Model Drug on Laser-Induced In Situ Amorphization. Molecules. 2021; 26(13):4035. https://doi.org/10.3390/molecules26134035
Chicago/Turabian StyleHempel, Nele-Johanna, Padryk Merkl, Matthias Manne Knopp, Ragna Berthelsen, Alexandra Teleki, Anders Kragh Hansen, Georgios A. Sotiriou, and Korbinian Löbmann. 2021. "The Effect of the Molecular Weight of Polyvinylpyrrolidone and the Model Drug on Laser-Induced In Situ Amorphization" Molecules 26, no. 13: 4035. https://doi.org/10.3390/molecules26134035
APA StyleHempel, N.-J., Merkl, P., Knopp, M. M., Berthelsen, R., Teleki, A., Hansen, A. K., Sotiriou, G. A., & Löbmann, K. (2021). The Effect of the Molecular Weight of Polyvinylpyrrolidone and the Model Drug on Laser-Induced In Situ Amorphization. Molecules, 26(13), 4035. https://doi.org/10.3390/molecules26134035







