Interaction between a Novel Oligopeptide Fragment of the Human Neurotrophin Receptor TrkB Ectodomain D5 and the C-Terminal Fragment of Tetanus Neurotoxin
Abstract
1. Introduction
1.1. The Clostridium Neurotoxins
1.2. The Neurotrophin Receptor Family
1.3. The Established Receptor Mechanism
1.4. The Inconsistent Dual-Receptor Mechanism
1.5. The Proposed Receptor Mechanism
2. Results
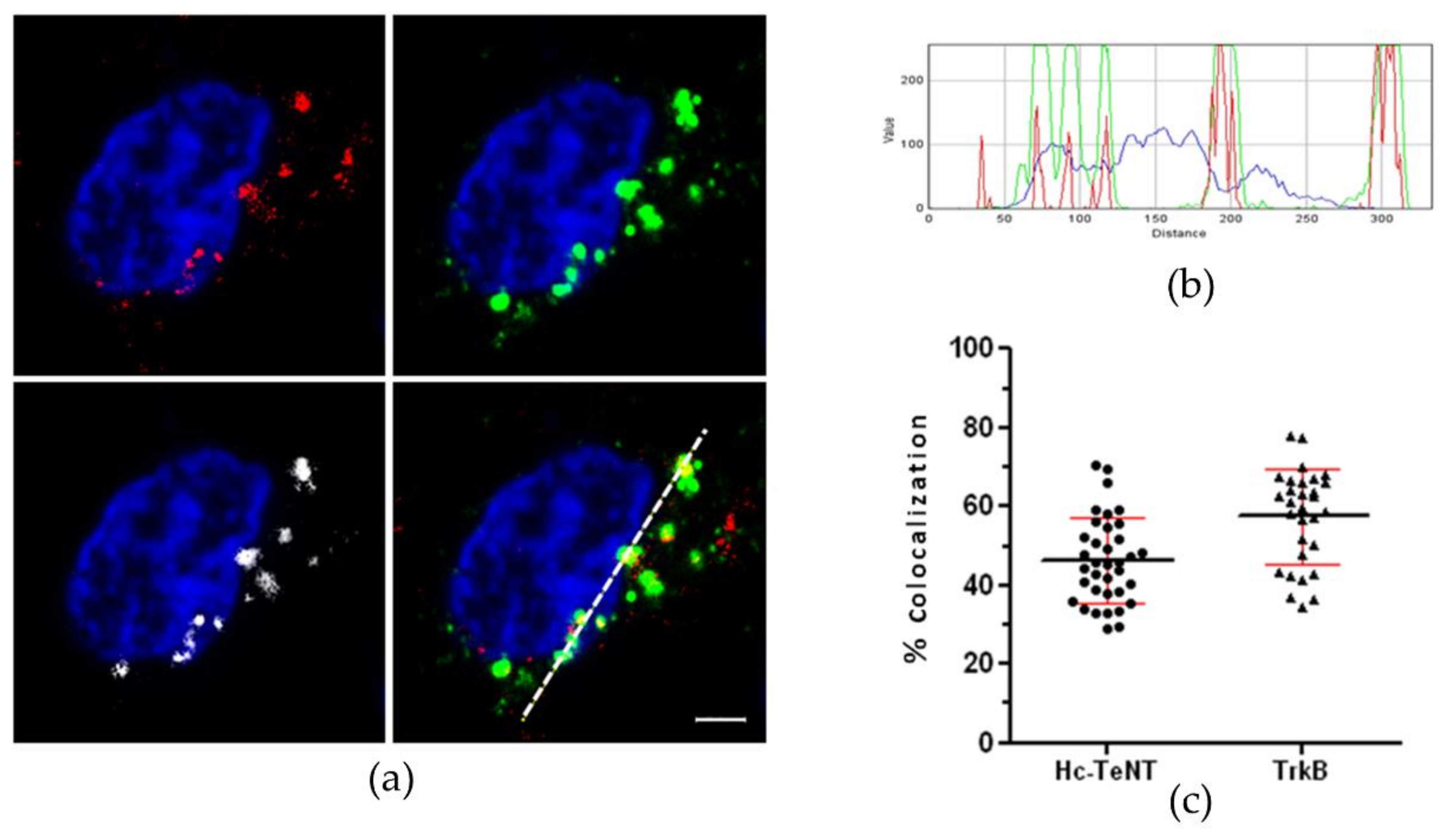
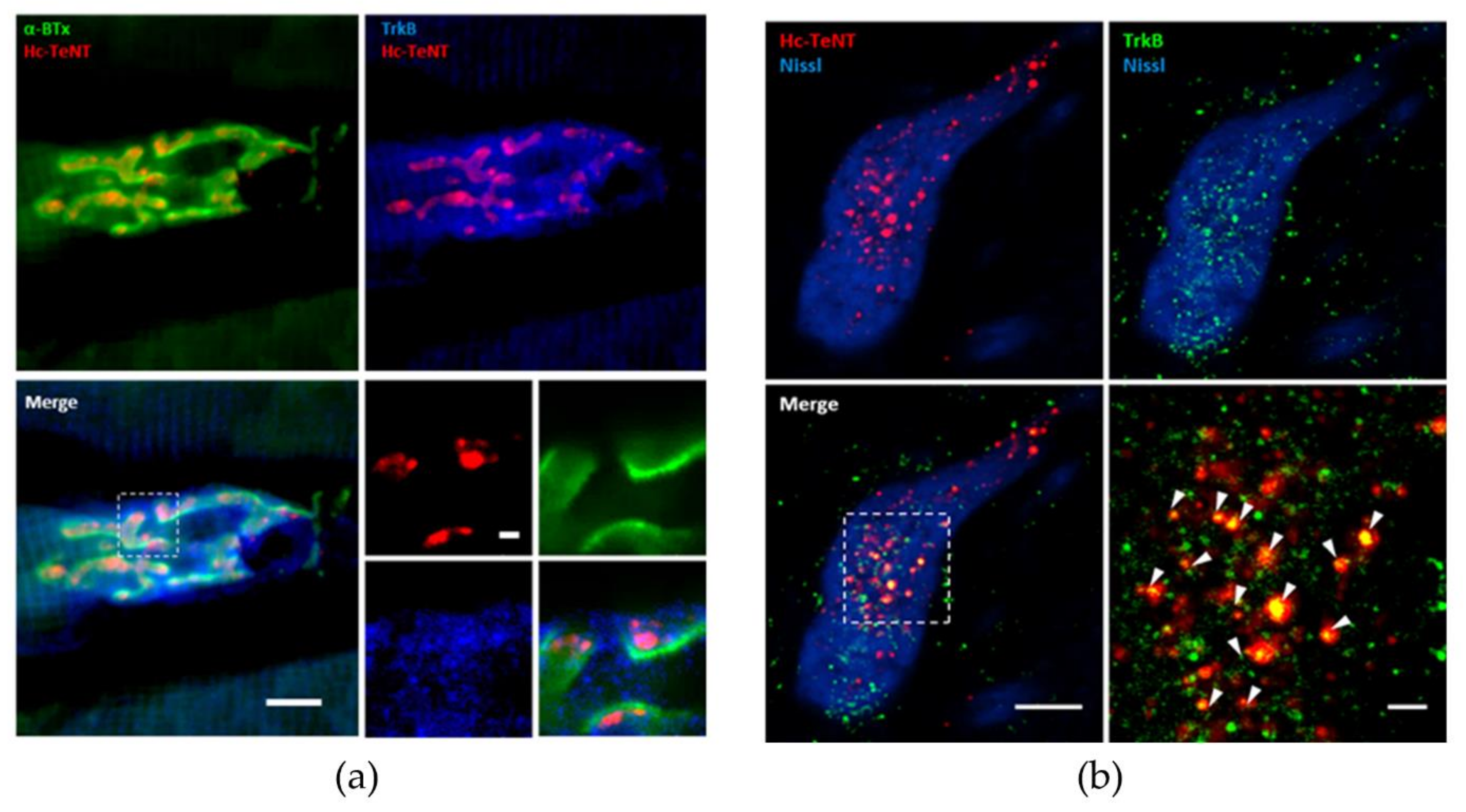
2.1. Hc-TeNT Interaction with Target TrkB Receptor
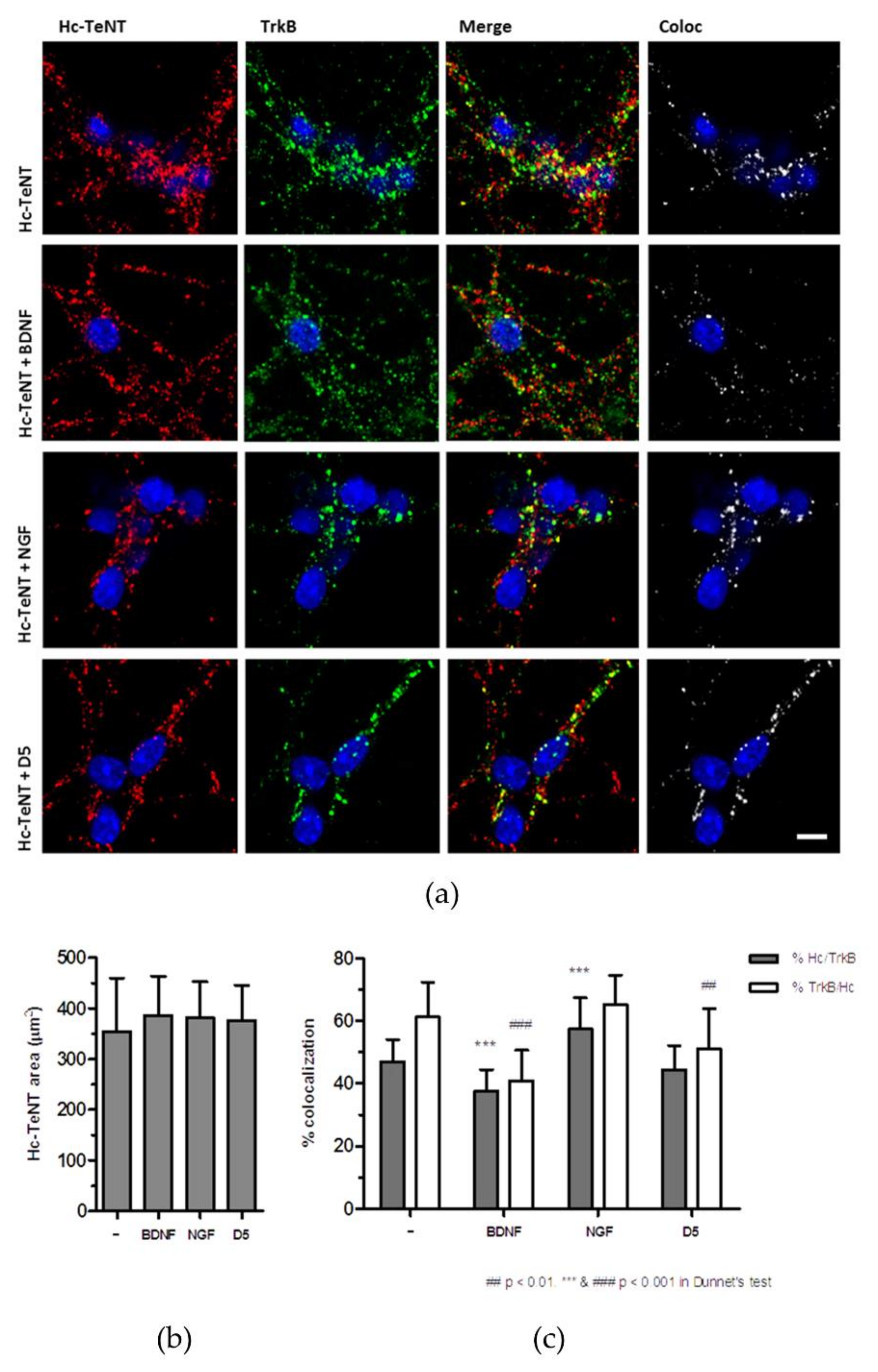
2.2. Hc-TeNT Competes with BDNF for Binding and Internalization in Cultured Neurons
2.3. Mutations in Predicted Binding Site Affect Binding and Internalization Ability
2.4. Computed Results at the Predicted Binding Site
3. Discussion
4. Materials and Methods
4.1. The Peptide Design to Evaluate the Protein-Protein Interface
4.2. Synthesis of the Cyclic Peptide and Negative Control Sequences
4.3. Expression and Purification of Hc-TeNT
4.4. Cloning and Expression of Hc-Mut with a Triple Mutation (Y266A-K311A-E343A)
4.5. Cerebellar Granule Neurons (CGNs) Cultures
4.6. Western Blot Analysis
4.7. Dot-Blot Analysis
4.8. In Vivo Hc-TeNT Intramuscular Injections
4.9. Immunohystochemistry and Image Analysis
4.10. Hc-TeNT Endocytic Assay and Uptake Competition Assays in CGNs
4.11. Binding Competition Assays
4.12. Flow Cytometry in Hc-TeNT Binding and Uptake Assays in CGNs
4.13. CGNs Immunocytofluorescence
4.14. Molecular Modeling and Bioinformatics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Calvo, A.C.; Oliván, S.; Manzano, R.; Zaragoza, P.; Aguilera, J.; Osta, R. Fragment C of Tetanus Toxin: New Insights into Its Neuronal Signaling Pathway. Int. J. Mol. Sci. 2012, 13, 6883–6901. [Google Scholar] [CrossRef] [PubMed]
- Gill, D.M. Bacterial Toxins: A Table of Lethal Amounts. Microbiol. Rev. 1982, 46, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Holmgren, J.; Elwing, H.; Fredman, P.; Svennerholm, L. Polystyrene-Adsorbed Gangliosides for Investigation of the Structure of the Tetanus-Toxin Receptor. Eur. J. Biochem. 1980, 106, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Fu, Z.; Kim, J.-J.P.; Barbieri, J.T.; Baldwin, M.R. Gangliosides as High Affinity Receptors for Tetanus Neurotoxin. J. Biol. Chem. 2009, 284, 26569–26577. [Google Scholar] [CrossRef]
- Yavin, E.; Yavin, Z.; Kohn, L.D. Temperature-Mediated Interaction of Tetanus Toxin with Cerebral Neuron Cultures: Characterization of a Neuraminidase-Insensitive Toxin-Receptor Complex. J. Neurochem. 1983, 40, 1212–1219. [Google Scholar] [CrossRef]
- Brunger, A.T.; Rummel, A. Receptor and Substrate Interactions of Clostridial Neurotoxins. Toxicon 2009, 54, 550–560. [Google Scholar] [CrossRef]
- Schwab, M.E.; Thoenen, H. Retrograde Axonal and Transsynaptic Transport of Macromolecules: Physiological and Pathophysiological Importance. Agents Actions 1977, 7, 361–368. [Google Scholar] [CrossRef]
- Lalli, G.; Herreros, J.; Osborne, S.L.; Montecucco, C.; Rossetto, O.; Schiavo, G. Functional Characterisation of Tetanus and Botulinum Neurotoxins Binding Domains. J. Cell. Sci. 1999, 112 Pt 16, 2715–2724. [Google Scholar] [CrossRef]
- Cubí, R.; Candalija, A.; Ortega, A.; Gil, C.; Aguilera, J. Tetanus Toxin Hc Fragment Induces the Formation of Ceramide Platforms and Protects Neuronal Cells against Oxidative Stress. PLoS ONE 2013, 8, e68055. [Google Scholar] [CrossRef]
- Martin-Zanca, D.; Hughes, S.H.; Barbacid, M. A Human Oncogene Formed by the Fusion of Truncated Tropomyosin and Protein Tyrosine Kinase Sequences. Nature 1986, 319, 743–748. [Google Scholar] [CrossRef]
- Bertrand, T.; Kothe, M.; Liu, J.; Dupuy, A.; Rak, A.; Berne, P.F.; Davis, S.; Gladysheva, T.; Valtre, C.; Crenne, J.Y.; et al. The Crystal Structures of TrkA and TrkB Suggest Key Regions for Achieving Selective Inhibition. J. Mol. Biol. 2012, 423, 439–453. [Google Scholar] [CrossRef]
- Banfield, M.J.; Naylor, R.L.; Robertson, A.G.S.; Allen, S.J.; Dawbarn, D.; Brady, R.L. Specificity in Trk Receptor: Neurotrophin Interactions. Structure 2001, 9, 1191–1199. [Google Scholar] [CrossRef]
- Robertson, A.G.S.; Banfield, M.J.; Allen, S.J.; Dando, J.A.; Mason, G.G.F.; Tyler, S.J.; Bennett, G.S.; Brain, S.D.; Clarke, A.R.; Naylor, R.L.; et al. Identification and Structure of the Nerve Growth Factor Binding Site on TrkA. Biochem. Biophys. Res. Commun. 2001, 282, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Schneider, R.; Schweiger, M. A Novel Modular Mosaic of Cell Adhesion Motifs in the Extracellular Domains of the Neurogenic Trk and TrkB Tyrosine Kinase Receptors. Oncogene 1991, 6, 1807–1811. [Google Scholar]
- Holden, P.H.; Asopa, V.; Robertson, A.G.S.; Clarke, A.R.; Tyler, S.; Bennett, G.S.; Brain, S.D.; Wilcock, G.K.; Allen, S.J.; Smith, S.K.F.; et al. Immunoglobulin-like Domains Define the Nerve Growth Factor Binding Site of the TrkA Receptor. Nat. Biotechnol. 1997, 15, 668–672. [Google Scholar] [CrossRef] [PubMed]
- Pérez, P.; Coll, P.M.; Hempstead, B.L.; Martı́n-Zanca, D.; Chao, M.V. NGF Binding to the Trk Tyrosine Kinase Receptor Requires the Extracellular Immunoglobulin-like Domains. Mol. Cell. Neurosci. 1995, 6, 97–105. [Google Scholar] [CrossRef]
- Urfer, R.; Tsoulfas, P.; O’Connell, L.; Presta, L.G. Specificity Determinants in Neurotrophin-3 and Design of Nerve Growth Factor-Based TrkC Agonists by Changing Central Beta-Strand Bundle Residues to Their Neurotrophin-3 Analogs. Biochemistry 1997, 36, 4775–4781. [Google Scholar] [CrossRef]
- Ultsch, M.H.; Wiesmann, C.; Simmons, L.C.; Henrich, J.; Yang, M.; Reilly, D.; Bass, S.H.; de Vos, A.M. Crystal Structures of the Neurotrophin-Binding Domain of TrkA, TrkB and TrkC 1 1Edited by I. A. Wilson. J. Mol. Biol. 1999, 290, 149–159. [Google Scholar] [CrossRef]
- Wiesmann, C.; Ultsch, M.H.; Bass, S.H.; de Vos, A.M. Crystal Structure of Nerve Growth Factor in Complex with the Ligand-Binding Domain of the TrkA Receptor. Nature 1999, 401, 184–188. [Google Scholar] [CrossRef]
- Montecucco, C. How Do Tetanus and Botulinum Toxins Bind to Neuronal Membranes? Trends Biochem. Sci. 1986, 11, 314–317. [Google Scholar] [CrossRef]
- Karlsson, K.A. On the Character and Functions of Sphingolipids. Acta Biochim. Pol. 1998, 45, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Herreros, J.; Ng, T.; Schiavo, G. Lipid Rafts Act as Specialized Domains for Tetanus Toxin Binding and Internalization into Neurons. MBoC 2001, 12, 2947–2960. [Google Scholar] [CrossRef] [PubMed]
- Rummel, A.; Mahrhold, S.; Bigalke, H.; Binz, T. The HCC-Domain of Botulinum Neurotoxins A and B Exhibits a Singular Ganglioside Binding Site Displaying Serotype Specific Carbohydrate Interaction: Ganglioside Binding of Botulinum Neurotoxins. Mol. Microbiol. 2003, 51, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Emsley, P.; Fotinou, C.; Black, I.; Fairweather, N.F.; Charles, I.G.; Watts, C.; Hewitt, E.; Isaacs, N.W. The Structures of the HC Fragment of Tetanus Toxin with Carbohydrate Subunit Complexes Provide Insight into Ganglioside Binding. J. Biol. Chem. 2000, 275, 8889–8894. [Google Scholar] [CrossRef]
- Umland, T.C.; Wingert, L.M.; Swaminathan, S.; Furey, W.F.; Schmidt, J.J.; Sax, M. Structure of the Receptor Binding Fragment HC of Tetanus Neurotoxin. Nat. Struct. Mol. Biol. 1997, 4, 788–792. [Google Scholar] [CrossRef]
- Fotinou, C.; Emsley, P.; Black, I.; Ando, H.; Ishida, H.; Kiso, M.; Sinha, K.A.; Fairweather, N.F.; Isaacs, N.W. The Crystal Structure of Tetanus Toxin Hc Fragment Complexed with a Synthetic GT1b Analogue Suggests Cross-Linking between Ganglioside Receptors and the Toxin. J. Biol. Chem. 2001, 276, 32274–32281. [Google Scholar] [CrossRef]
- Knapp, M.; Segelke, B.; Rupp, B. The 1.61 Angstrom Structure of the Tetanus Toxin Ganglioside Binding Region: Solved by MAD and Mir Phase Combination. Am. Cryst. Assoc. Abstr. Pap. Annu. Meet. 1998, 25. [Google Scholar] [CrossRef]
- Jayaraman, S.; Eswaramoorthy, S.; Kumaran, D.; Swaminathan, S. Common Binding Site for Disialyllactose and Tri-Peptide in C-Fragment of Tetanus Neurotoxin. Proteins 2005, 61, 288–295. [Google Scholar] [CrossRef]
- Binz, T.; Rummel, A. Cell Entry Strategy of Clostridial Neurotoxins. J. Neurochem. 2009, 109, 1584–1595. [Google Scholar] [CrossRef]
- Candalija, A.; Cubí, R.; Ortega, A.; Aguilera, J.; Gil, C. Trk Receptors Need Neutral Sphingomyelinase Activity to Promote Cell Viability. FEBS Lett. 2014, 588, 167–174. [Google Scholar] [CrossRef]
- Rummel, A.; Häfner, K.; Mahrhold, S.; Darashchonak, N.; Holt, M.; Jahn, R.; Beermann, S.; Karnath, T.; Bigalke, H.; Binz, T. Botulinum Neurotoxins C, E and F Bind Gangliosides via a Conserved Binding Site Prior to Stimulation-Dependent Uptake with Botulinum Neurotoxin F Utilising the Three Isoforms of SV2 as Second Receptor. J. Neurochem. 2009, 110, 1942–1954. [Google Scholar] [CrossRef]
- Bigalke, H.; Müller, H.; Dreyer, F. Botulinum A Neurotoxin Unlike Tetanus Toxin Acts via a Neuraminidase Sensitive Structure. Toxicon 1986, 24, 1065–1074. [Google Scholar] [CrossRef]
- Marxen, P.; Bigalke, H. Tetanus Toxin: Inhibitory Action in Chromaffin Cells Is Initiated by Specified Types of Gangliosides and Promoted in Low Ionic Strength Solution. Neurosci. Lett. 1989, 107, 261–266. [Google Scholar] [CrossRef]
- Pierce, E.J.; Davison, M.D.; Parton, R.G.; Habig, W.H.; Critchley, D.R. Characterization of Tetanus Toxin Binding to Rat Brain Membranes. Evidence for a High-Affinity Proteinase-Sensitive Receptor. Biochem. J. 1986, 236, 845–852. [Google Scholar] [CrossRef]
- Lazarovici, P.; Yavin, E. Affinity-Purified Tetanus Neurotoxin Interaction with Synaptic Membranes: Properties of a Protease-Sensitive Receptor Component. Biochemistry 1986, 25, 7047–7054. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.J.; Chiu, I.M. Bacterial Signaling to the Nervous System through Toxins and Metabolites. J. Mol. Biol. 2017, 429, 587–605. [Google Scholar] [CrossRef]
- Netzahualcoyotzi, C.; Tapia, R. Tetanus Toxin C-Fragment Protects against Excitotoxic Spinal Motoneuron Degeneration In Vivo. Sci. Rep. 2018, 8, 16584. [Google Scholar] [CrossRef]
- Megighian, A.; Pirazzini, M.; Fabris, F.; Rossetto, O.; Montecucco, C. Tetanus and Tetanus Neurotoxin: From Peripheral Uptake to Central Nervous Tissue Targets. J. Neurochem. 2021. [Google Scholar] [CrossRef]
- Matak, I.; Bölcskei, K.; Bach-Rojecky, L.; Helyes, Z. Mechanisms of Botulinum Toxin Type A Action on Pain. Toxins 2019, 11, 459. [Google Scholar] [CrossRef]
- Reyes-Long, S.; Alfaro-Rodríguez, A.; Cortes-Altamirano, J.L.; LaraPadilla, E.; Herrera-Maria, E.; Romero-Morelos, P.; Salcedo, M.; Bandala, C. The Mechanisms of Action of Botulinum Toxin Type A in Nociceptive and Neuropathic Pathways in Cancer Pain. Curr. Med. Chem. 2020. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, S.; Eswaramoorthy, S. Structural Analysis of the Catalytic and Binding Sites of Clostridium Botulinum Neurotoxin B. Nat. Struct. Biol. 2000, 7, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Gil, C.; Chaı̈b-Oukadour, I.; Pelliccioni, P.; Aguilera, J. Activation of Signal Transduction Pathways Involving TrkA, PLCγ-1, PKC Isoforms and ERK-1/2 by Tetanus Toxin. FEBS Lett. 2000, 481, 177–182. [Google Scholar] [CrossRef]
- Gil, C.; Chaib-Oukadour, I.; Blasi, J.; Aguilera, J. HC Fragment (C-Terminal Portion of the Heavy Chain) of Tetanus Toxin Activates Protein Kinase C Isoforms and Phosphoproteins Involved in Signal Transduction. Biochem. J. 2001, 356, 97–103. [Google Scholar] [CrossRef]
- Gil, C.; Chaib-Oukadour, I.; Aguilera, J. C-Terminal Fragment of Tetanus Toxin Heavy Chain Activates Akt and MEK/ERK Signalling Pathways in a Trk Receptor-Dependent Manner in Cultured Cortical Neurons. Biochem. J. 2003, 373, 613–620. [Google Scholar] [CrossRef]
- Chaib-Oukadour, I.; Gil, C.; Aguilera, J. The C-Terminal Domain of the Heavy Chain of Tetanus Toxin Rescues Cerebellar Granule Neurones from Apoptotic Death: Involvement of Phosphatidylinositol 3-Kinase and Mitogen-Activated Protein Kinase Pathways. J. Neurochem. 2004, 90, 1227–1236. [Google Scholar] [CrossRef]
- Chaïb-Oukadour, I.; Gil, C.; Rodríguez-Alvarez, J.; Ortega, A.; Aguilera, J. Tetanus Toxin HC Fragment Reduces Neuronal MPP+ Toxicity. Mol. Cell. Neurosci. 2009, 41, 297–303. [Google Scholar] [CrossRef]
- Gong, Y.; Cao, P.; Yu, H.; Jiang, T. Crystal Structure of the Neurotrophin-3 and P75NTR Symmetrical Complex. Nature 2008, 454, 789–793. [Google Scholar] [CrossRef]
- Marini, A.M.; Rabin, S.J.; Lipsky, R.H.; Mocchetti, I. Activity-Dependent Release of Brain-Derived Neurotrophic Factor Underlies the Neuroprotective Effect of N-Methyl-D-Aspartate. J. Biol. Chem. 1998, 273, 29394–29399. [Google Scholar] [CrossRef]
- Brandoli, C.; Sanna, A.; De Bernardi, M.A.; Follesa, P.; Brooker, G.; Mocchetti, I. Brain-Derived Neurotrophic Factor and Basic Fibroblast Growth Factor Downregulate NMDA Receptor Function in Cerebellar Granule Cells. J. Neurosci. Off. J. Soc. Neurosci. 1998, 18, 7953–7961. [Google Scholar] [CrossRef]
- Pattarawarapan, M.; Burgess, K. Molecular Basis of Neurotrophin-Receptor Interactions. J. Med. Chem. 2003, 46, 5277–5291. [Google Scholar] [CrossRef]
- Yeh, F.L.; Dong, M.; Yao, J.; Tepp, W.H.; Lin, G.; Johnson, E.A.; Chapman, E.R. SV2 Mediates Entry of Tetanus Neurotoxin into Central Neurons. PLoS Pathog. 2010, 6, e1001207. [Google Scholar] [CrossRef] [PubMed]
- Wiesmann, C.; de Vos, A.M. Nerve Growth Factor: Structure and Function: CMLS Cell. Mol. Life Sci. 2001, 58, 748–759. [Google Scholar] [CrossRef] [PubMed]
- Herrando-Grabulosa, M.; Casas, C.; Aguilera, J. The C-Terminal Domain of Tetanus Toxin Protects Motoneurons against Acute Excitotoxic Damage on Spinal Cord Organotypic Cultures. J. Neurochem. 2013, 124, 36–44. [Google Scholar] [CrossRef]
- Jacky, B.P.S.; Garay, P.E.; Dupuy, J.; Nelson, J.B.; Cai, B.; Molina, Y.; Wang, J.; Steward, L.E.; Broide, R.S.; Francis, J.; et al. Identification of Fibroblast Growth Factor Receptor 3 (FGFR3) as a Protein Receptor for Botulinum Neurotoxin Serotype A (BoNT/A). PLoS Pathog. 2013, 9, e1003369. [Google Scholar] [CrossRef]
- Roux, S.; Saint Cloment, C.; Curie, T.; Girard, E.; Mena, F.-J.M.; Barbier, J.; Osta, R.; Molgó, J.; Brûlet, P. Brain-Derived Neurotrophic Factor Facilitates In Vivo Internalization of Tetanus Neurotoxin C-Terminal Fragment Fusion Proteins in Mature Mouse Motor Nerve Terminals. Eur. J. Neurosci. 2006, 24, 1546–1554. [Google Scholar] [CrossRef]
- Boulanger, L.; Poo, M. Presynaptic Depolarization Facilitates Neurotrophin-Induced Synaptic Potentiation. Nat. Neurosci. 1999, 2, 346–351. [Google Scholar] [CrossRef]
- Shinoda, Y.; Ahmed, S.; Ramachandran, B.; Bharat, V.; Brockelt, D.; Altas, B.; Dean, C. BDNF Enhances Spontaneous and Activity-Dependent Neurotransmitter Release at Excitatory Terminals but Not at Inhibitory Terminals in Hippocampal Neurons. Front. Synaptic Neurosci. 2014, 6, 27. [Google Scholar] [CrossRef]
- Lalli, G.; Schiavo, G. Analysis of Retrograde Transport in Motor Neurons Reveals Common Endocytic Carriers for Tetanus Toxin and Neurotrophin Receptor P75NTR. J. Cell Biol. 2002, 156, 233–239. [Google Scholar] [CrossRef]
- Deinhardt, K.; Salinas, S.; Verastegui, C.; Watson, R.; Worth, D.; Hanrahan, S.; Bucci, C.; Schiavo, G. Rab5 and Rab7 Control Endocytic Sorting along the Axonal Retrograde Transport Pathway. Neuron 2006, 52, 293–305. [Google Scholar] [CrossRef]
- Terenzio, M.; Golding, M.; Russell, M.R.G.; Wicher, K.B.; Rosewell, I.; Spencer-Dene, B.; Ish-Horowicz, D.; Schiavo, G. Bicaudal- D 1 Regulates the Intracellular Sorting and Signalling of Neurotrophin Receptors. EMBO J. 2014, 33, 1582–1598. [Google Scholar] [CrossRef]
- Matteoli, M.; Verderio, C.; Rossetto, O.; Iezzi, N.; Coco, S.; Schiavo, G.; Montecucco, C. Synaptic Vesicle Endocytosis Mediates the Entry of Tetanus Neurotoxin into Hippocampal Neurons. Proc. Natl. Acad. Sci. USA 1996, 93, 13310–13315. [Google Scholar] [CrossRef] [PubMed]
- Terenzio, M.; Golding, M.; Schiavo, G. SiRNA Screen of ES Cell-Derived Motor Neurons Identifies Novel Regulators of Tetanus Toxin and Neurotrophin Receptor Trafficking. Front. Cell Neurosci. 2014, 8, 140. [Google Scholar] [CrossRef] [PubMed]
- Deinhardt, K.; Berninghausen, O.; Willison, H.J.; Hopkins, C.R.; Schiavo, G. Tetanus Toxin Is Internalized by a Sequential Clathrin-Dependent Mechanism Initiated within Lipid Microdomains and Independent of Epsin1. J. Cell Biol. 2006, 174, 459–471. [Google Scholar] [CrossRef]
- Bercsenyi, K.; Schmieg, N.; Bryson, J.B.; Wallace, M.; Caccin, P.; Golding, M.; Zanotti, G.; Greensmith, L.; Nischt, R.; Schiavo, G. Tetanus Toxin Entry. Nidogens Are Therapeutic Targets for the Prevention of Tetanus. Science 2014, 346, 1118–1123. [Google Scholar] [CrossRef]
- Macri, L.; Silverstein, D.; Clark, R.A.F. Growth Factor Binding to the Pericellular Matrix and Its Importance in Tissue Engineering. Adv. Drug Deliv. Rev. 2007, 59, 1366–1381. [Google Scholar] [CrossRef]
- Kanato, Y.; Ono, S.; Kitajima, K.; Sato, C. Complex Formation of a Brain-Derived Neurotrophic Factor and Glycosaminoglycans. Biosci. Biotechnol. Biochem. 2009, 73, 2735–2741. [Google Scholar] [CrossRef]
- Martino, M.M.; Briquez, P.S.; Güç, E.; Tortelli, F.; Kilarski, W.W.; Metzger, S.; Rice, J.J.; Kuhn, G.A.; Müller, R.; Swartz, M.A.; et al. Growth Factors Engineered for Super-Affinity to the Extracellular Matrix Enhance Tissue Healing. Science 2014, 343, 885–888. [Google Scholar] [CrossRef]
- O’Grady, P.; Thai, T.C.; Saito, H. The Laminin-Nidogen Complex Is a Ligand for a Specific Splice Isoform of the Transmembrane Protein Tyrosine Phosphatase LAR. J. Cell Biol. 1998, 141, 1675–1684. [Google Scholar] [CrossRef]
- Stryker, E.; Johnson, K.G. LAR, Liprin Alpha and the Regulation of Active Zone Morphogenesis. J. Cell. Sci. 2007, 120, 3723–3728. [Google Scholar] [CrossRef]
- Yang, T.; Yin, W.; Derevyanny, V.D.; Moore, L.A.; Longo, F.M. Identification of an Ectodomain within the LAR Protein Tyrosine Phosphatase Receptor That Binds Homophilically and Activates Signalling Pathways Promoting Neurite Outgrowth. Eur. J. Neurosci. 2005, 22, 2159–2170. [Google Scholar] [CrossRef]
- Yang, T.; Massa, S.M.; Longo, F.M. LAR Protein Tyrosine Phosphatase Receptor Associates with TrkB and Modulates Neurotrophic Signaling Pathways. J. Neurobiol. 2006, 66, 1420–1436. [Google Scholar] [CrossRef] [PubMed]
- Folador, E.L.; de Oliveira Junior, A.F.; Tiwari, S.; Jamal, S.B.; Ferreira, R.S.; Barh, D.; Ghosh, P.; Silva, A.; Azevedo, V. In Silico Protein-Protein Interactions: Avoiding Data and Method Biases Over Sensitivity and Specificity. Curr. Protein Pept. Sci. 2015, 16, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Kuenemann, M.A.; Sperandio, O.; Labbé, C.M.; Lagorce, D.; Miteva, M.A.; Villoutreix, B.O. In Silico Design of Low Molecular Weight Protein-Protein Interaction Inhibitors: Overall Concept and Recent Advances. Prog. Biophys. Mol. Biol. 2015, 119, 20–32. [Google Scholar] [CrossRef]
- Rognan, D. Rational Design of Protein-Protein Interaction Inhibitors. Med. Chem. Commun. 2015, 6, 51–60. [Google Scholar] [CrossRef]
- Guex, N.; Peitsch, M.C. SWISS-MODEL and the Swiss-Pdb Viewer: An Environment for Comparative Protein Modeling. Electrophoresis 1997, 18, 2714–2723. [Google Scholar] [CrossRef]
- Sillerud, L.; Larson, R. Design and Structure of Peptide and Peptidomimetic Antagonists of Protein- Protein Interaction. CPPS 2005, 6, 151–169. [Google Scholar] [CrossRef]
- Gallina, A.M.; Bork, P.; Bordo, D. Structural Analysis of Protein-Ligand Interactions: The Binding of Endogenous Compounds and of Synthetic Drugs: Structural Analysis of Protein-Ligand Interactions. J. Mol. Recognit. 2014, 27, 65–72. [Google Scholar] [CrossRef]
- Chan, W.C.; White, P.D. (Eds.) Fmoc Solid Phase Peptide Synthesis: A Practical Approach; The Practical Approach Series; Oxford University Press: New York, NY, USA, 2000; ISBN 978-0-19-963725-6. [Google Scholar]
- Pedretti, A.; Mazzolari, A.; Gervasoni, S.; Fumagalli, L.; Vistoli, G. The VEGA suite of programs: An versatile platform for cheminformatics and drug design projects. Bioinformatics 2020. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera?A Visualization System for Exploratory Research and Analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Schrödinger, LLC. The PyMOL Molecular Graphics System; Schrödinger, LLC.: New York, NY, USA, 2020. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Candalija, A.; Scior, T.; Rackwitz, H.-R.; Ruiz-Castelan, J.E.; Martinez-Laguna, Y.; Aguilera, J. Interaction between a Novel Oligopeptide Fragment of the Human Neurotrophin Receptor TrkB Ectodomain D5 and the C-Terminal Fragment of Tetanus Neurotoxin. Molecules 2021, 26, 3988. https://doi.org/10.3390/molecules26133988
Candalija A, Scior T, Rackwitz H-R, Ruiz-Castelan JE, Martinez-Laguna Y, Aguilera J. Interaction between a Novel Oligopeptide Fragment of the Human Neurotrophin Receptor TrkB Ectodomain D5 and the C-Terminal Fragment of Tetanus Neurotoxin. Molecules. 2021; 26(13):3988. https://doi.org/10.3390/molecules26133988
Chicago/Turabian StyleCandalija, Ana, Thomas Scior, Hans-Richard Rackwitz, Jordan E. Ruiz-Castelan, Ygnacio Martinez-Laguna, and José Aguilera. 2021. "Interaction between a Novel Oligopeptide Fragment of the Human Neurotrophin Receptor TrkB Ectodomain D5 and the C-Terminal Fragment of Tetanus Neurotoxin" Molecules 26, no. 13: 3988. https://doi.org/10.3390/molecules26133988
APA StyleCandalija, A., Scior, T., Rackwitz, H.-R., Ruiz-Castelan, J. E., Martinez-Laguna, Y., & Aguilera, J. (2021). Interaction between a Novel Oligopeptide Fragment of the Human Neurotrophin Receptor TrkB Ectodomain D5 and the C-Terminal Fragment of Tetanus Neurotoxin. Molecules, 26(13), 3988. https://doi.org/10.3390/molecules26133988





