Methanolic Extract of Piper sarmentosum Attenuates Obesity and Hyperlipidemia in Fructose-Induced Metabolic Syndrome Rats
Abstract
1. Introduction
2. Results
2.1. Physiological Parameters
2.2. Metabolic Parameters
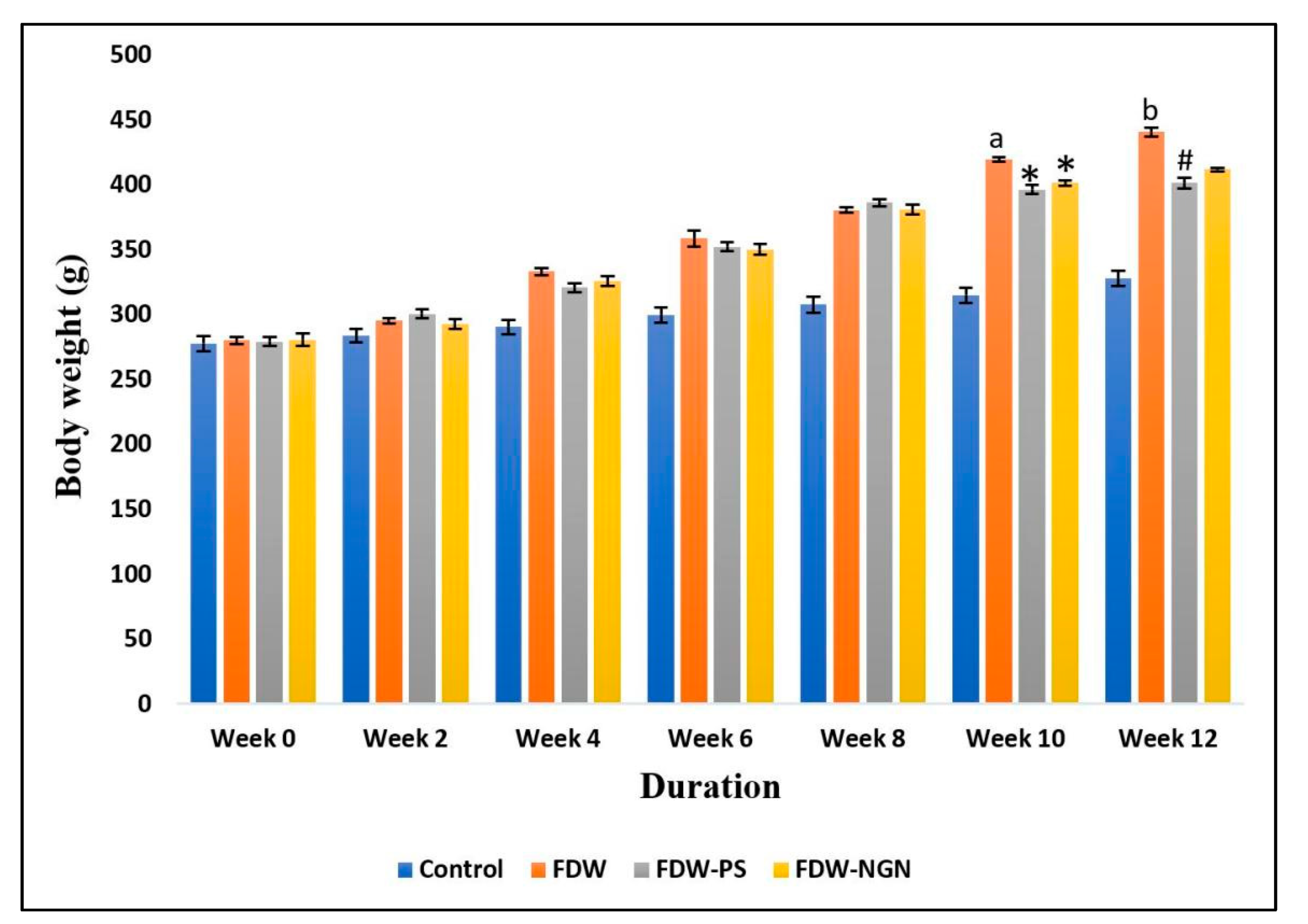
2.2.1. Body Weight
2.2.2. Fat Mass and Percentage
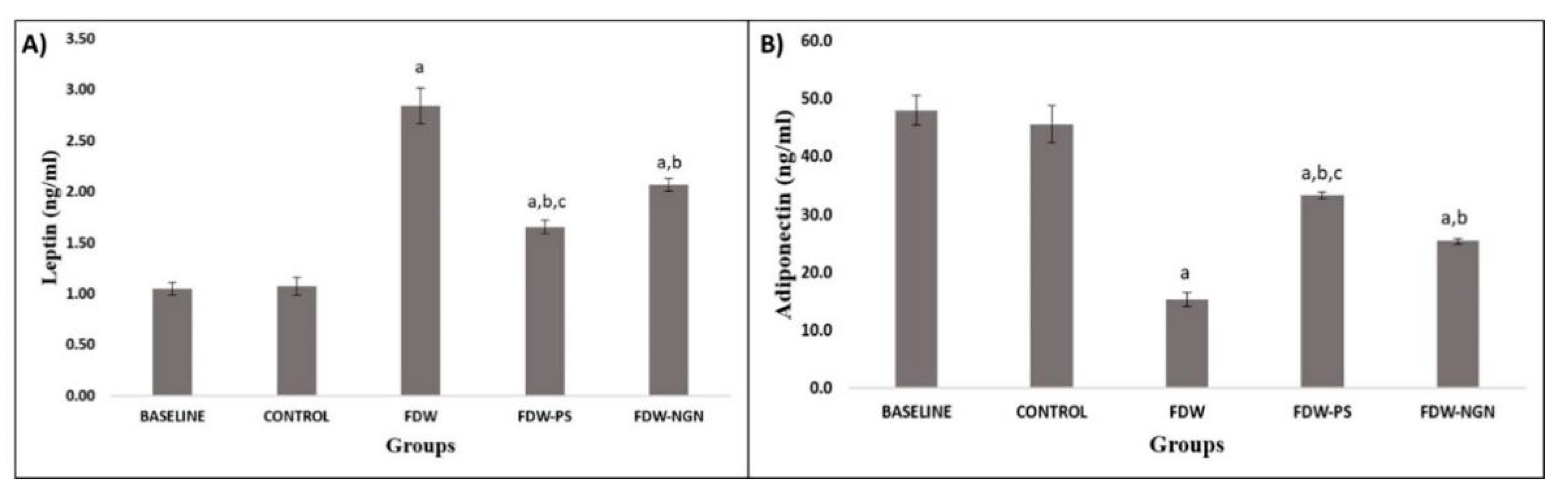
2.2.3. Adipocytokines
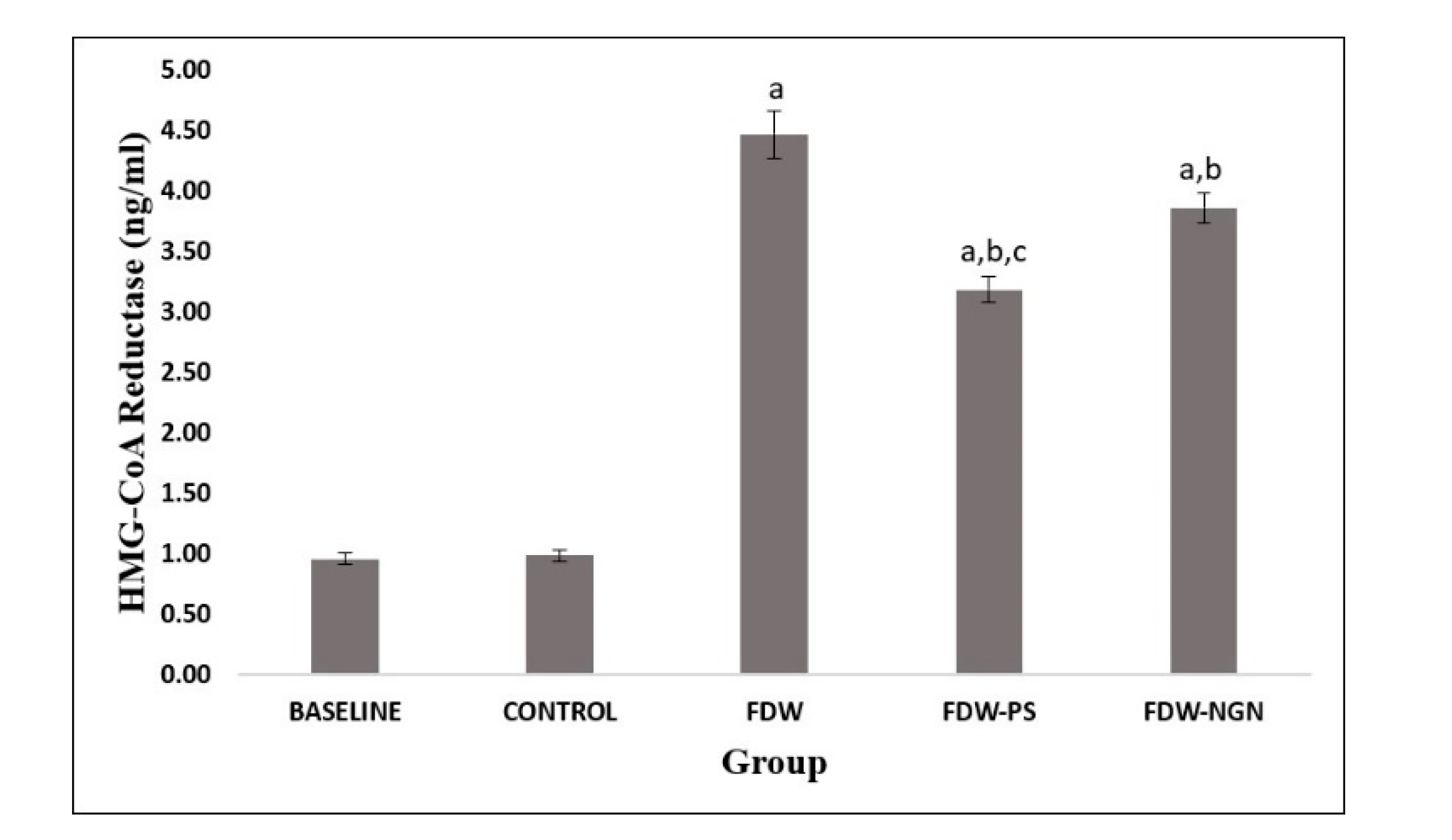
2.2.4. HMG-CoA Reductase Activity
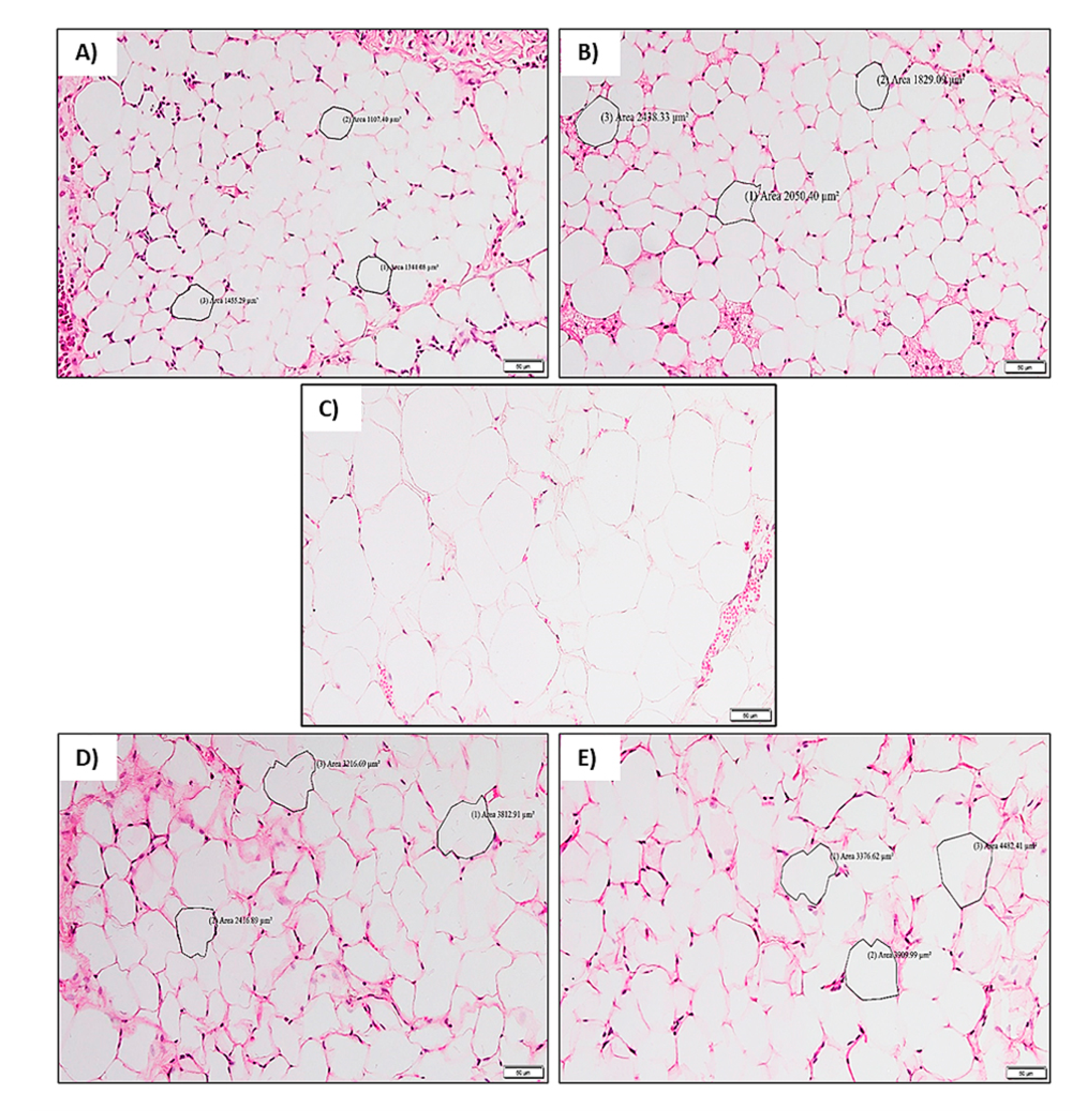
2.2.5. Histological Analysis
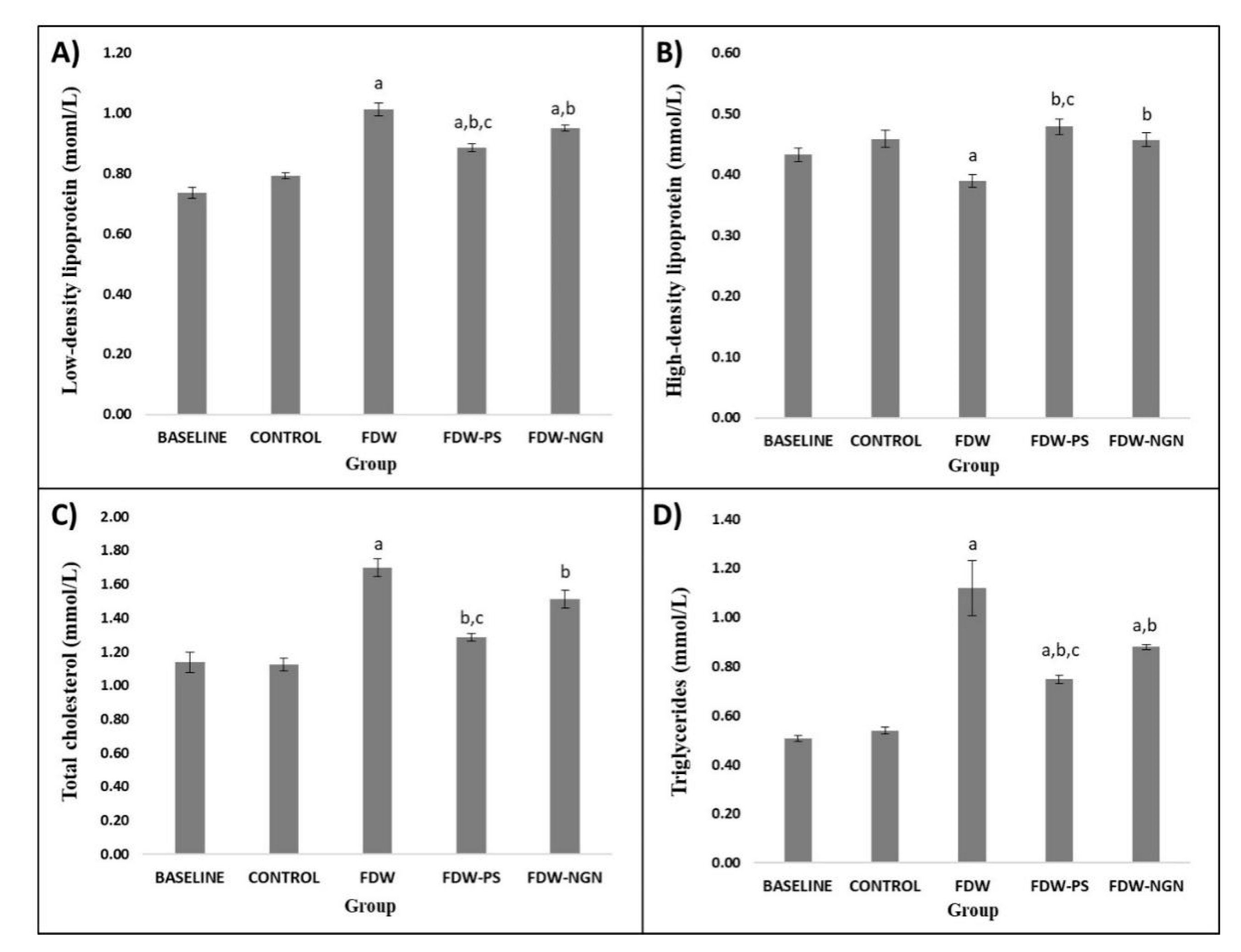
2.2.6. Lipid Profiles
3. Discussion
4. Materials and Methods
4.1. Preparation of Piper Sarmentosum Roxb. [Piperaceae] (PS) Methanol Extract
4.2. Animal Preparation
4.3. Administration of Fructose Drinking Water
4.4. Dosage of Treatment
4.5. Physiological Measurements
4.6. Metabolic Parameters
4.6.1. Body Weight
4.6.2. Fat Mass and Percentage
4.6.3. Adipocytokines
4.6.4. HMG-CoA Reductase Activity
4.6.5. Histological Analysis
4.6.6. Lipid Profiles
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Obesity and Overweight. Available online: https://www.who.int/dietphysicalactivity/media/en/gsfs_obesity.pdf (accessed on 20 January 2020).
- Hruby, A.; Hu, B.F. The Epidemiology of Obesity: A Big Picture. Pharmacoeconomics 2015, 33, 673–689. [Google Scholar] [CrossRef] [PubMed]
- Global Health Observatory (GHO) data: Overweight and Obesity. Available online: https://www.who.int/gho/ncd/riskfactors/overweight_text/en/ (accessed on 20 January 2020).
- Klop, B.; Elte, J.W.F.; Cabezas, M.C. Dyslipidemia in Obesity: Mechanisms and Potential Targets. Nutrients 2013, 5, 1218–1240. [Google Scholar] [CrossRef]
- Karr, S. Epidemiology and management of hyperlipidemia. Am. J. Manag. Care 2017, 23, S139–S148. [Google Scholar]
- Global Health Observatory (GHO) data: Raised Cholesterol. Available online: https://www.who.int/gho/ncd/risk_factors/cholesterol_text/en/ (accessed on 20 January 2020).
- Marvasti, T.B.; Adeli, K.H. Pharmacological management of metabolic syndrome and its lipid complications. DARU J. Pharm. Sci. 2010, 18, 146–154. [Google Scholar]
- Mallare, J.T.; Karabell, A.H.; Velasquez-Mieyer, P.; Stender, S.R.S.; Christensen, M.L. Current and Future Treatment of Metabolic Syndrome and Type 2 Diabetes in Children and Adolescents. Diabetes Spectr. 2005, 18, 220–228. [Google Scholar] [CrossRef]
- Zetia (ezetimibe) Tablets. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/021445s019lbl.pdf (accessed on 20 January 2020).
- Nature’s Medicinal Bounty: Don’t Throw It Away. World Health Forum Vol 14. Available online: http://apps.who.int/iris/bitstream/handle/10665/51722/WHF_1993_14_p390-395.pdf;jsessionid=D788660CAC98E76C77089E330D2C8DA8?sequence=1 (accessed on 20 January 2020).
- Oryan, A. Plant-derived compounds in treatment of leishmaniasis. Iran. J. Vet. Res. 2015, 16, 1–19. [Google Scholar]
- Rahman, S.F.S.; Sijam, K.; Omar, D. A Mini Review of Ethnobotany, Phytochemistry and Pharmacology. J. Anal. Pharm. Res. 2016, 2, 1–3. [Google Scholar]
- Chanwitheesuk, A.; Teerawutgulrag, A.; Rakariyatham, N. Screening of antioxidant activity and antioxidant compounds of some edible plants of Thailand. Food Chem. 2005, 92, 491–497. [Google Scholar] [CrossRef]
- Subramaniam, V.; Adenan, M.I.; Ahmad, A.R.; Sahdan, R. Natural Antioxidants: Piper sarmentosum (Kadok) and Morinda elliptica (Mengkudu). Malays. J. Nutr. 2003, 9, 41–51. [Google Scholar]
- Niamsa, N.; Chantrapromma, K. Chemical constituents isolated from Piper sarmentosum Roxb. J. Sci. Technol. 1983, 5, 151–152. [Google Scholar]
- Hussain, K.; Hashmi, F.K.; Latif, A.; Ismail, Z.; Sadikun, A. A review of the literature and latest advances in research of Piper sarmentosum. J. Pharm. Bio. 2012, 50, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.R.; Ramli, E.S.M.; Nasir, N.A.A.; Ismail, N.M.; Fahami, N.A.M. Preventive Effect of Naringin on Metabolic Syndrome and Its Mechanism of Action: A Systematic Review. Evid. Based Complement. Alternat. Med. 2019, 9752826. [Google Scholar] [CrossRef]
- Herman, M.A.; Samuel, V.T. The sweet path to metabolic demise: Fructose and lipid synthesis. Trends Endrocrinol. Metab. 2016, 27, 719–730. [Google Scholar] [CrossRef] [PubMed]
- Ado, M.A.; Abas, F.; Mohammed, A.S.; Ghazali, H.M. Anti-and Pro-Lipase Activity of Selected Medicinal, Herbal and Aquatic Plants, and Structure Elucidation of an Anti-Lipase Compound. Molecules 2013, 18, 14651–14669. [Google Scholar] [CrossRef] [PubMed]
- Sahib, N.G.; Hamid, A.A.; Saari, N.; Abas, F.; Pak Dek, S.; Rahim, M. Anti-Pancreatic Lipase and Antioxidant Activity of Selected Tropical Herbs. Int. J. Food Prop. 2012, 15, 569–578. [Google Scholar] [CrossRef]
- Lee, J.H.; Cho, S.; Paik, H.D.; Choi, C.W.; Nam, K.T.; Hwang, S.G.; Kim, S.K. Investigation on antibacterial and antioxidant activities, phenolic and flavonoid contents of some Thai edible plants as an alternative for antibiotics. Asian Australas J. Anim. Sci. 2014, 27, 1461–1468. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Buchholz, T.; Melzig, M.F. Polyphenolic compounds as pancreatic lipase inhibitors. Planta Med. 2015, 81, 771–783. [Google Scholar] [CrossRef]
- Zubiría, M.G.; Alzamendi, A.; Moreno, G.; Rey, M.A.; Spinedi, E.; Giovambattista, A. Long-term fructose intake increases adipogenic potential: Evidence of direct effects of fructose on adipocyte precursor cells. Nutrients 2016, 8, 198. [Google Scholar] [CrossRef] [PubMed]
- Kazemipoor, M.; Radzi, C.W.J.W.M.; Cordell, G.A.; Yaze, I. Potential of traditional medicinal plants for treating obesity: A review. In Proceedings of the International Conference on Nutrition and Food Sciences, Singapore, 23–24 July 2012; IACSIT Press: Singapore, 2012; pp. 164–169. [Google Scholar]
- Pessin, J.E.; Kwon, H. Adipokines mediate inflammation and insulin resistance. Front. Endocrinol. 2013, 4, 71. [Google Scholar] [CrossRef]
- Yadav, H.; Lee, J.H.; Lloyd, J.; Walter, P.; Rane, S.G. Beneficial metabolic effects of a probiotic via butyrate-induced GLP-1 hormone secretion. J. Biol. Chem. 2013, 288, 25088–25097. [Google Scholar] [CrossRef] [PubMed]
- Staiger, K.; Stefan, N.; Staiger, H.; Brendel, M.D.; Brandhorst, D.; Bretzel, R.G.; Häring, H.U. Adiponectin is functionally active in human islets but does not affect insulin secretory function or β-cell lipoapoptosis. J. Clin. Endocrinol. Metab. 2005, 90, 6707–6713. [Google Scholar] [CrossRef]
- Ibars, M.; Aragones, G.; Ardid-Ruiz, A.; Gilbert-Ramos, A.; Arola-Arnal, A.; Suarez, M.; Bladé, C. Seasonal consumption of polyphenol-rich fruits affects the hypothalamic leptin signaling system in a photoperiod-dependent mode. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef]
- Hasimun, P.; Sulaeman, A.; Mulyani, Y.; Nur Islami, W.; Lubis, F.A.T. Antihyperlipidemic Activity and HMG CoA Reductase Inhibiton of Ethanolic Extract of Zingiber Cassumunar Roxb in Fructose-Induced Hyperlipidemic Wistar Rats. J. Pharm. Sci. Res. 2019, 11, 1897–1901. [Google Scholar]
- Thirunavukkarasu, V.; Nandhini, A.T.A.; Anuradha, C.V. Effect of α-Lipoic Acid on Lipid Profile in Rats Fed a High-Fructose Diet. Exp. Diab. Res. 2004, 5, 195–200. [Google Scholar] [CrossRef]
- Ulug, E.; Nergiz-Unal, R. Does High Fructose Intake increase De Novo Cholesterol Synthesis and Cholesterol Levels in Blood and Liver. Clin. Nutr. 2018, 37, 46–47. [Google Scholar] [CrossRef]
- Ali, A.A.; Suhaimi, F.H.; Saad, Q.H.M.; Fahami, N.A.M. Lipid lowering effects of Piper sarmentosum Extract in Ovariectomy-induced obese rats. Int. J. Appl. Res. Nat. Prod. 2017, 10, 1–6. [Google Scholar]
- Baskaran, G.; Salvamani, S.; Azlan, A.; Ahmad, S.A.; Yeap, S.K.; Shukor, M.Y. Hypocholesterolemic and antiatherosclerotic potential of Basella alba leaf extract in hypercholesterolemia-induced rabbits. Evid. Based Complement Alternat Med. 2015, 751714. [Google Scholar] [CrossRef]
- Atiax, E.; Ahmad, F.; Sirat, H.M.; Arbain, D. Antibacterial activity and cytotoxicity screening of sumatran kaduk (Piper sarmentosum Roxb.). Iran. J. Pharmacol. Ther. 2010, 10, 1–5. [Google Scholar]
- Nguyen, T.T. The cholesterol-lowering action of plant stanol esters. J. Nutr. 1999, 129, 2109–2112. [Google Scholar] [CrossRef]
- Trautwein, E.A.; Duchateau, G.S.; Lin, Y.; Mel'nikov, S.M.; Molhuizen, H.O.; Ntanios, F.Y. Proposed mechanisms of cholesterol-lowering action of plant sterols. Eur. J. Lipid Sci. Tech. 2003, 105, 171–185. [Google Scholar] [CrossRef]
- Bactiar, C.F.; Nur Azlina, M.F. LC-MS Analysis of Phytocomponents in the Methanol Extract of Piper Sarmentosum Leaves. Pharmacogn. J. 2019, 11, 1071–1076. [Google Scholar] [CrossRef]
- Azlina, A.A.; Farihah, H.S.; Qodriyah, H.M.S.; Nur Azlina, M.F. Effects of Piper sarmentosum water extract on 11-β hydroxysteroid dehydrogenase type 1 bioactivity in ovariectomy-induced obese rats. IJP Int. J. Pharmacol. 2009, 5, 362–369. [Google Scholar] [CrossRef]
- Alam, M.; Kauter, K.; Brown, L. Naringin improves diet-induced cardiovascular dysfunction and obesity in high carbohydrate, high fat diet-fed rats. Nutrients 2013, 5, 637–650. [Google Scholar] [CrossRef]
- Chanet, A.; Wizinska, P.; Polakof, S.; Mazur, A.; Bennetau-Pelissero, C.; Morand, C.; Milenkovic, D. Naringin at a nutritional dose modulates expression of genes related to lipid metabolism and inflammation in liver of mice fed a high-fat diet. Nutr. Aging 2012, 1, 113–123. [Google Scholar] [CrossRef]
- Sharma, A.K.; Bharti, S.; Ojha, S.; Bhatia, J.; Kumar, N.; Ray, R.; Arya, D.S. Up-regulation of PPARγ, heat shock protein-27 and-72 by naringin attenuates insulin resistance, β-cell dysfunction, hepatic steatosis and kidney damage in a rat model of type 2 diabetes. Br. J. Nutr. 2011, 106, 1713–1723. [Google Scholar] [CrossRef] [PubMed]

| Variables | CTRL | FDW | FDW-PS | FDW-NGN |
|---|---|---|---|---|
| Food intake (g/day) | 15.23 ± 0.54 | 20.91 ± 0.67 a | 18.15 ± 0.51 a,b | 17.55 ± 0.53 a,b |
| Fluid intake (ml/day) | 44.87 ± 1.12 | 51.75 ± 1.03 a | 49.75 ± 1.03 a,b | 50.5 ± 1.18 a,b |
| Calorie intake (Kcal/day) | 42.66 ± 1.53 | 100.3 ± 1.94 a,b | 90.6 ± 1.19 a,b | 88.9 ± 1.92 a,b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, S.R.; Mohd Ramli, E.S.; Abdul Nasir, N.A.; Mohd Ismail, N.; Mohd Fahami, N.A. Methanolic Extract of Piper sarmentosum Attenuates Obesity and Hyperlipidemia in Fructose-Induced Metabolic Syndrome Rats. Molecules 2021, 26, 3985. https://doi.org/10.3390/molecules26133985
Kumar SR, Mohd Ramli ES, Abdul Nasir NA, Mohd Ismail N, Mohd Fahami NA. Methanolic Extract of Piper sarmentosum Attenuates Obesity and Hyperlipidemia in Fructose-Induced Metabolic Syndrome Rats. Molecules. 2021; 26(13):3985. https://doi.org/10.3390/molecules26133985
Chicago/Turabian StyleKumar, Sivanesan Raja, Elvy Suhana Mohd Ramli, Nurul Alimah Abdul Nasir, Nafeeza Mohd Ismail, and Nur Azlina Mohd Fahami. 2021. "Methanolic Extract of Piper sarmentosum Attenuates Obesity and Hyperlipidemia in Fructose-Induced Metabolic Syndrome Rats" Molecules 26, no. 13: 3985. https://doi.org/10.3390/molecules26133985
APA StyleKumar, S. R., Mohd Ramli, E. S., Abdul Nasir, N. A., Mohd Ismail, N., & Mohd Fahami, N. A. (2021). Methanolic Extract of Piper sarmentosum Attenuates Obesity and Hyperlipidemia in Fructose-Induced Metabolic Syndrome Rats. Molecules, 26(13), 3985. https://doi.org/10.3390/molecules26133985






