Abstract
The natural products pulchrol and pulchral, isolated from the roots of the Mexican plant Bourreria pulchra, have previously been shown to possess antiparasitic activity towards Trypanosoma cruzi, Leishmania braziliensis and L. amazonensis, which are protozoa responsible for Chagas disease and leishmaniasis. These infections have been classified as neglected diseases, and still require the development of safer and more efficient alternatives to their current treatments. Recent SARs studies, based on the pulchrol scaffold, showed which effects exchanges of its substituents have on the antileishmanial and antitrypanosomal activity. Many of the analogues prepared were shown to be more potent than pulchrol and the current drugs used to treat leishmaniasis and Chagas disease (miltefosine and benznidazole, respectively), in vitro. Moreover, indications of some of the possible interactions that may take place in the binding sites were also identified. In this study, 12 analogues with modifications at two or three different positions in two of the three rings were prepared by synthetic and semi-synthetic procedures. The molecules were assayed in vitro towards T. cruzi epimastigotes, L. braziliensis promastigotes, and L. amazonensis promastigotes. Some compounds had higher antiparasitic activity than the parental compound pulchrol, and in some cases even benznidazole and miltefosine. The best combinations in this subset are with carbonyl functionalities in the A-ring and isopropyl groups in the C-ring, as well as with alkyl substituents in both the A- and C-rings combined with a hydroxyl group in position 1 (C-ring). The latter corresponds to cannabinol, which indeed was shown to be potent towards all the parasites.
1. Introduction
Natural products have been one of the main sources for bioactive compounds used to treat a wide range of diseases [1]. The chemical diversity, potential selectivity, and the availability of traditional knowledge about the use of natural materials, played an important role in the development of modern drugs. Some examples are morphine isolated from opium [2]; taxol isolated from Taxus brevifolia [3]; important antiparasitic drugs, such as quinine, isolated from Cinchona officinalis [4]; and artemisinin extracted from Artemisia annua [5].
Natural products are often isolated in limited quantities and can be assayed in just a few biological systems. In order to extend the bioactivity scope, synthetic routes can be designed to obtain sufficient quantities of the product, and can also be used to prepare new derivatives and analogues, which may possess superior biological properties [1,6]. For diseases in which the mechanism of action and the drug target are not fully understood, as is the case for leishmaniasis and Chagas disease [7,8,9], derivatives can be prepared and assayed towards cells or whole organisms to measure their activity [10,11,12,13], leading to the development of structure–activity relationships studies (SARs). These can be used to design more potent and less toxic analogues, assuming that just one target is involved [11,14].
The vegetal specie, Bourreria pulchra, which is native in the Yucatan province in Mexico, is traditionally used to treat cutaneous diseases, fevers, and infections [15]. The isolation of the main chemical compounds from its roots yielded pulchrol (1a, see Figure 1), which was shown to possess antiparasitic activity against Leishmania braziliensis, L. amazonensis and L. mexicana promastigotes, as well as against Trypanosoma cruzi epimastigotes [16].
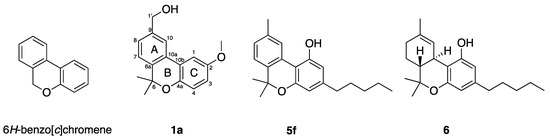
Figure 1.
The 6H-benzo[c]chromene scaffold studied here (left), and structures of pulchrol (1a), cannabinol (5f) and Δ9-tetrahydrocannabinol (6).
Leishmania and Trypanosoma parasites are part of the family Trypanosomatidae, and are responsible for leishmaniasis and Chagas disease, respectively. Both are considered neglected diseases, but still affect millions of people in developing countries, mainly placed in the tropical and subtropical regions of the world [7,17,18,19,20,21]. Leishmaniasis can appear as cutaneous, mucocutaneous or visceral leishmaniasis, and around 700,000 to 1 million new cases are diagnosed every year. The treatments for leishmaniasis (mainly amphotericin, miltefosine and pentavalent antimonials) may give several toxic side effects and may require hospitalization [22,23]. Likewise, Chagas disease is able to produce damage in the hearth tissue and eventually cause death. Twelve million people are affected by this disease and the existing treatments are far from ideal (nifurtimox or benznidazole) [24,25,26,27,28]. Currently, there are few validated drug targets for leishmaniasis and Chagas disease, and too little is understood of the complex life cycle of these pathogens [9].
Pulchrol (1a) is one example of the many natural products that are based on a 6H-benzo[c]chromene scaffold. This type of compound has been shown to possess different kinds of biological activities [29,30,31,32,33,34]. The most studied benzo[c]chromenes are probably the cannabinoids, isolated mainly from the plant Cannabis sativa, and known for their affinity to the cannabinoid receptors CB1 and CB2. The natural product cannabinol (5f, see Figure 1), which possess the same skeleton as pulchrol, has been shown to be selective for CB2, a receptor expressed on immune cells, macrophages, and in other peripheral organs, while Δ9-tetrahydrocannabinol (THC, 6, see Figure 1), also isolated from C. sativa, showed greater affinity for CB1, which is the receptor associated with the psychotropic activity of C. sativa [35]. SARs including cannabinol (5f) have been studied previously, and indicated that hydroxyl groups at positions 1 and 1′, together with bulky alkyl substituents at position 3, are important for the affinity to CB1 [36]. In addition, shorter and bulkier alkyl substituents at C-3 improved the affinity for the CB2 receptor [37,38]. Cannabinoids have also shown immunomodulatory properties in the treatment of psoriasis [39], antinociceptive properties related to their capacity to induce vasorelaxation and release neuropeptides [40,41], and antineoplastic activity on Lewis lung tumors [33].
The potential shown by pulchrol (1a) as an antiparasitic agent against T. cruzi and the Leishmania species led to the development of a synthetic route [42,43] that yields sufficient amounts of pulchrol to perform additional biological assays. This could also be adapted for the preparation of a series of analogues with individual transformations of the benzyl alcohol moiety in the A-ring [44], variations at positions 1, 2 and 3 in the C-ring, and modifications at the only available position in the B-ring (C-6) [45]. In this investigation, we prepared analogues with combinations of different functionalities in the A-, B-, and C-rings (see Figure 2), inspired by some of the SARs observed in the previous studies [44,45]. Cannabinol (5f) and its 3-methyl analogue 5e (see Figure 2) were also prepared and assayed. Our main objective was to study the effect that several substituents coexisting at different positions in the benzo[c]chromene skeleton may have in the activity towards the parasites under study, and retrieve more information about the chemical surroundings of the active site in which pulchrol and its analogues may be interacting.
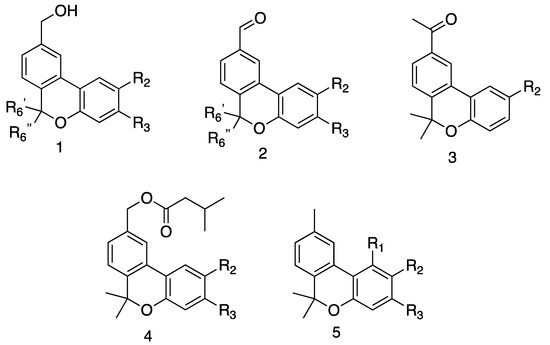
Figure 2.
Prepared derivatives. 1a R2 = OMe, R3 = H, R6′ = Me, R6″ = Me; 1b R2 = isopropyl, R3 = H, R6′ = Me, R6″ = Me; 1c R2 = H, R3 = isopropyl, R6′ = Me, R6″ = Me; 1d R2 = OMe, R3 = H, R6′ = Me, R6″ = H; 1e R2 = OMe, R3 = H, R6′ = H, R6″ = Me; 2a R2 = OMe, R3 = H, R6′ = Me, R6″ = Me; 2b R2 = isopropyl, R3 = H, R6′ = Me, R6″ = Me; 2c R2 = H, R3 = isopropyl, R6′ = Me, R6″ = Me; 2d R2 = OMe, R3 = H, R6′ = Me, R6″ = H; 2e R2 = OMe, R3 = H, R6′ = H, R6″ = Me; 3a R2 = OMe; 3b R2 = isopropyl; 4a R2 = OMe, R3 = H; 4b R2 = isopropyl, R3 = H; 4c R2 = H, R3 = isopropyl; 5a R1 = H, R2 = OMe, R3 = H; 5b R1 = H, R2 = isopropyl, R3 = H; 5c R1 = H, R2 = H, R3 = isopropyl; 5d R1 = H, R2 = OH, R3 = H; 5e R1 = OH, R2 = H, R3 = Me; 5f R1 = OH, R2 = H, R3 = n-pentyl. See Experimental part for synthetic details.
2. Results
2.1. Preparation
In this study, analogues of pulchrol were prepared, containing combinations of two or three modifications in the A-, B, and C-rings (see Figure 2). The synthetic routes used to prepare the analogues were partially based on the already reported procedure that was used to prepare pulchrol from biaryl intermediates [42,43]. The molecules in the 1-series (see Figure 2) were previously reported [44,45], and were used as the starting material to prepare the compounds from the 2-, 3-, 4- and 5-series, containing a 1′-aldehyde, 1′-methyl ketone, 3-methylbutanoic acid ester, and 9-methyl functionalities, respectively (see Figure 2). Compound 5f (cannabinol) and its analogue 5e were obtained through iodine-mediated deconstructive annulation in a one-pot synthesis, using citral and resorcinol analogues as the starting material [46]. In total, 12 molecules were prepared and assayed in vitro against T. cruzi epimastigotes, as well as L. braziliensis and L. amazonensis promastigotes (see Table 1). Their antiparasitic activities were compared with those previously reported for the analogues in the 1-series in addition to compounds 2a (pulchral), 3a, 4a, and 5a [44]. Figure 2 summarizes the structure types of the analogues prepared, while the biological activities are given in Table 1.

Table 1.
Antileishmanial and antitrypanosomal activity pulchrol derivatives, compared to the positive controls benznidazole and miltefosine. See experimental for details about the assays.

Table 2.
Proton chemical shifts (in ppm) for the compounds prepared in this study, measured at 400 MHz. The assignments were made with 2D NMR spectroscopy, COSY, HMQC and HMBC experiments.

Table 3.
13C NMR chemical shifts (in ppm) for compounds of series 1 to 5 determined at 100 MHz in CDCl3. The assignments were made with 2D NMR spectroscopy, COSY, HMQC and HMBC experiments.
2.2. Selected Functionalities
The natural products pulchrol (1a) and pulchral (2a) have been studied in the past, and both of them have been shown to be active against Trypanosoma and Leishmania parasites [44]. Pulchrol (1a) has been reported to be moderately active against L. braziliensis and L. amazonensis promastigotes (IC50 59.2 μM and IC50 77.7 μM, respectively), while it has been shown to possess potent toxicity towards T. cruzi epimastigotes (IC50 18.5 μM), comparable to that of the drug benznidazole (19.2 μM) that currently is used as treatment for Chagas disease. Meanwhile, pulchral (2a) has been shown to be active against all of the three parasites (T. cruzi, IC50 24.2 μM; L. braziliensis, IC50 24.2 μM; L. amazonensis, IC50 29.8 μM) [44,45].
In previous investigations, we studied the effect that individual transformations in the A-, B-, and C-rings have on the activity towards T. cruzi, L. braziliensis and L. amazonensis [44,45]. Initially, we reported the effect of the modifications at position 1′ in the A-ring, and concluded preliminarily that the benzyl alcohol functionality was important for pulchrol’s activity towards all of the three parasites, possibly acting as a hydrogen bond acceptor. It was also observed that the 1′-carbonyl analogues were equipotent towards T. cruzi compared to pulchrol, while they were more potent towards L. braziliensis and L. amazonensis. On the other hand, the 9-methyl analogue was reported to possess considerably less activity than pulchrol against all the parasites. Finally, the ester analogues were shown to be, in general, more potent than pulchrol, with longer and branched alkyl substituents showing considerable improvements [44]. The effects that modifications in the B- and C-rings have on the antiparasitic activity were also investigated, and were mainly focused on the effects that variations in the lipophilicity may have in the antiparasitic activity. The 6-monoalkyl (methyl and ethyl) derivatives, also as pure enantiomers, were less potent towards the parasites compared to pulchrol, and a preference for 6,6-dialkyl analogues was established. Alkyl substituents in the C-ring were shown to be beneficial for the antiparasitic activity; most notably, longer and branched alkyl substituents at positions 2 and 3 increased the potency considerably [45].
3. Discussion
In this study, we analyse the effect that two or three transformations in the different rings of the pulchrol scaffold may have on the antiparasitic activity. Most of the analogues have transformations in the A- and C-rings, whereas just a pair have the A- and B-rings modified. The 1′-aldehyde, the 3-methyl butanoic acid ester, and the 9-methyl functionalities in the A-ring were combined with isopropyl substituents at C-2 and C-3 in the C-ring (2b and 2c; 4b and 4c; and 5b and 5c, respectively) to see how an increase in lipophilicity would affect their antiparasitic activity. An analogue with the 1′-methyl ketone functionality in the A-ring and an isopropyl substituent at position 2 was also prepared (3b) to see if any differences would arise with respect to the corresponding 1′-aldehyde analogue (2b). The 9-methyl functionality was also combined with a hydroxyl substituent at C-2 in the C-ring (5d), and the natural compound cannabinol (5f) and its analogue 5e were prepared, both of them containing a 9-methyl substituent in the A-ring, a hydroxyl group at C-1, and an alkyl group at C-3 in the C-ring; cannabinol (5f) with an n-pentyl substituent and 5e with a methyl substituent at C-3. Finally, the 1′-aldehyde functionality was combined with the 6-monomethyl substituent in the B-ring, as a pair of enantiomers (2d and 2e), to evaluate whether the carbonyl group helps to improve their activity or hamper it in comparison with the already reported benzyl alcohol enantiomers 1d and 1e [45].
3.1. Antiparasitic Activities towards Trypanosoma Cruzi Epimastigotes
It has previously been reported that the transformation of the benzyl alcohol functionality in pulchrol (1a, IC50 = 18.5 μM) to a 1′-carbonyl group (2a, IC50 = 24.2 μM and 3a, IC50 = 21.3 μM) was not beneficial for the antiparasitic activity towards T. cruzi [44], while the replacement of the methoxy functionality (in 1a) for an isopropyl group in positions 2 and 3 in the C-ring was shown to improve the IC50 values for this parasite (1b, IC50 = 12.4 μM and 1c, IC50 = 14.2 μM, respectively) [45]. In order to evaluate the effect that combinations involving the functionalities mentioned before may have in the antitrypanosomal activity, analogues with an aldehyde functionality in the A-ring and with an isopropyl substituent at positions 2 or 3 in the C-ring were prepared and found to be considerably more active (2b, IC50 = 10.7 μM; and 2c, IC50 = 7.1 μM) compared to the previously reported corresponding benzyl alcohols 1b and 1c [45]. This could be caused by a change in orientation in a hypothetical binding site, by the combined functionalities, possibly enhancing the hydrophobic interactions of the isopropyl substituents in 2b and 2c. The methyl ketone 3b, substituted with an isopropyl group at C-2, was actually the most potent compound towards T. cruzi (IC50 = 3.4 μM) of all the analogues prepared in this study, showing six-fold higher activity than the positive control benznidazole (IC50 = 19.2 μM), indicating possible interactions between the methyl substituent in the ketone and the active site.
Contrary to the improvements shown by the analogues substituted with an isopropyl group in the 2- and 3-series, 2- and 3-isopropyl analogues of the 4-series (combined with a 3-methylbutanoic acid ester) were less potent towards T. cruzi (4b, IC50 = 10.9 μM; 4c, IC50 = 13.6 μM) compared to the corresponding 2-methoxy ester 4a (IC50 = 4.2 μM), which has previously been reported to possess better activity than the positive control benznidazole [44].
Among the analogues of the 5-series, which contain a 9-methyl substituent in the A-ring, analogue 5a (with a methoxy group at C-2, IC50 = 51.1 μM) has previously been shown to possess less potency than pulchrol (1a) [44]. Similarly, in this study analogue 5d, with a hydroxyl functionality at C-2, showed comparable activity (IC50 = 54.8 μM) to 5a towards T. cruzi. It is possible that both analogues, 5a and 5d, interact with the binding site in a rotated position, in which the methoxy group in 5a and the hydroxy group in 5d act as hydrogen bond acceptors where pulchrol’s benzyl alcohol usually interacts. On the other hand, more lipophilic analogues, 5b and 5c, with isopropyl substituents at positions 2 and 3 (IC50 = 23.7 μM and IC50 = 50.3 μM, respectively) were found to be less toxic than pulchrol (1a, IC50 = 18.5 μM). However, cannabinol (5f) and its analogue 5e, which also has an alkyl substituent at C-3 and, in addition, a hydroxyl functionality at C-1, were shown to be the most potent (5f, IC50 = 7.4 μM; 5e, IC50 = 5.9 μM) compounds from the 5-series towards T. cruzi. Analogue 5e, with a methyl substituent at C-3 instead of the n-pentyl group in cannabinol (5f), is slightly more active than 5f and possesses one of the highest activities towards T. cruzi among all the derivatives prepared in this project, possibly due to interactions between the hydroxyl group at C-1 and the target protein.
Finally, the aldehyde enantiomers 2d and 2e, substituted with a single methyl group at position 6 in the B-ring, possess considerably lower antiparasitic activity compared to pulchrol (1a, IC50 = 18.5 μM). They are also less potent compared to their synthetic precursors, the benzyl alcohols 1d and 1e [45], indicating that a possible change in orientation may disrupt hydrophobic interactions around position 6 in the B-ring.
3.2. Antiparasitic Activities towards Leishmania Braziliensis Promastigotes
The opposite to what has been reported for T. cruzi, the transformation of pulchrol’s benzyl alcohol to a 1′-carbonyl functionality (2a and 3a) has been shown to considerably increase the antiparasitic activity towards L. braziliensis (2a, IC50 = 24.2 μM; and 3a, IC50 = 28.3 μM) [44]. Likewise, isopropyl substituents in the C-ring (1b and 1c) also increase the potency (1b, IC50 = 18.1 μM; and 3a, IC50 = 19.1 μM) compared to pulchrol (1a, IC50 = 59.2 μM) [45]. In this study, the aldehydes 2b and 2c (with isopropyl substituents at C-2 and C-3, respectively) were prepared and found to be more toxic towards L. braziliensis (2b, IC50 = 12.1 μM; and 2c, IC50 = 17.8 μM) than their benzyl alcohol precursors (1b and 1c). As for T. cruzi, the 2-isopropyl ketone 3b was the most potent compound (IC50 = 8.8 μM) in this investigation. Furthermore, the 1′-carbonyl analogues 2b and 3b are also more potent than the positive control miltefosine (IC50 = 13.0 μM). Possibly, the increase in potency shown by the carbonyl analogues combined with alkyl substituents in the C-ring, may be due to better hydrophobic interactions with a target protein, produced by a change in the orientation of the alkyl groups.
Similar results to those obtained for T. cruzi were observed for the analogues from the 4-series; the 2- and 3-isopropyl esters 4b and 4c were less active towards L. braziliensis (4b, IC50 = 272.9 μM; and 4c, IC50 = 63.3 μM) than the previously reported ester 4a (with a methoxy substituent at C-2, IC50 = 13.1 μM) [44]. The opposite to T. cruzi, analogue 4b was inactive, indicating a possible limitation in the volume of the lipophilic pocket where the 3-alkyl substituents interact.
The transformation of the benzyl alcohol moiety in pulchrol (1a) to a 9-methyl substituent, as in 5a, has previously been determined to be unfavourable for the activity (1a, IC50 = 59.2 μM; and 5a, IC50 = 69.6 μM) [44]. In this investigation, the 9-methyl/2-isopropyl analogue 5b and the 9-methyl/2-hydroxy analogue 5d are more potent towards L. braziliensis (5b, IC50 = 49.2 μM; and 5d, IC50 = 30.4 μM), opposite to T. cruzi. On the other hand, the 9-methyl/3-isopropyl analogue 5c was shown to be essentially inactive (IC50 = 312 μM), suggesting again that there is a limit in the volume around positions 2 and 3. Similar to the results obtained for T. cruzi, 5e and cannabinol (5f) were found to possess higher activity (IC50 = 15.7 μM and 10.3 μM, respectively) than 5a and pulchrol (1a). However, the longer chain at C-3 in cannabinol (5f) seems to be better for the activity against L. braziliensis compared to 5e, with a methyl group at C-3, while the opposite is true for T. cruzi.
As with T. cruzi, the aldehyde enantiomers 2d and 2e were found to be less potent (IC50 = 70.8 μM and 118 μM, respectively) compared to pulchrol (1a), although they are more potent than their corresponding benzyl alcohol enantiomers 1e and 1f (IC50 = 156 μM and 129 μM, respectively) [45].
3.3. Antiparasitic Activities towards Leishmania Amazonensis Promastigotes
For L. amazonensis, as with L. braziliensis, pulchral (2a), and the analogues 1b and 1c (substituted with an isopropyl group at C-2 and C-3 in the C-ring, respectively) have been reported to be more potent than pulchrol [44,45]. Similar to T. cruzi and L. braziliensis, the combination of the 9-aldehyde functionality with isopropyl substituents at positions 2 and 3 (2b and 2c, respectively) is beneficial for the antiparasitic activity, and the highest activities were shown by the aldehyde 2b (IC50 = 11.4 μM), and the methyl ketone 3b (IC50 = 9.5 μM), which are equipotent with the positive control miltefosine (IC50 = 10.8 μM). All the ester analogues (4a to 4b) were more toxic than pulchrol towards L. amazonensis, and as with the other parasites, the previously reported 4a (with a methoxy substituent on C-2) was still the most potent (IC50 = 14.5 μM) among the esters.
The analogues possessing a 9-methyl substituent were similarly as potent towards L. amazonensis as towards L. braziliensis. Most of the 9-methyl analogues (5b, 5d, 5e and 5f) are more potent than pulchrol (1a, IC50 = 77.7 μM), while the 9-methyl/3-isopropyl analogue 5c is much less active (IC50 = 236.5 μM) towards L. braziliensis. The most potent compounds in the 5-series are cannabinol (5f, IC50 = 14.2 μM) and its analogue 5e (IC50 = 21.2 μM). Finally, the enantiomers 2d (IC50 = 44.0 μM) and 2e (IC50 = 80.6 μM) were still less potent than the corresponding 6,6-dimethyl aldehyde 2a (pulchral, IC50 = 29.8 μM) [44], similar to what was observed for L. braziliensis and T. cruzi.
4. Materials and Methods
4.1. General
1H NMR spectra (400 MHz) and 13C NMR spectra (100 MHz) (See Supplementary Materials) were recorded with a Bruker Avance II (Bruker Biospin AG, Industriestrasse 26, 8117 Fällanden, Switzerland) in CDCl3. The individual 1D signals were assigned using 2D NMR experiments (COSY, HSQC, HMBC). The chemical shifts are given in ppm with the solvent signal as reference (7.27 ppm for 1H and 77.0 for 13C). Infrared spectra were recorded with a Bruker Alpha-P FT/IR instrument (Bruker Biospin AG, Industriestrasse 26, 8117 Fällanden, Switzerland) with a Diamond ATR sensor as films, and the intensities are given as vw (very weak), w (weak), m (medium), s (strong) and vs (very strong). High-resolution mass spectra (HRMS) were recorded with Waters XEVO-G2 QTOF equipment (Waters Corp, Milford, Worcester County, MA, USA), with electrospray ionization (ESI). Synthetic reactions were monitored by TLC using alumina plates coated with silica gel and visualized using either UV light and/or spraying/heating with vanillin/H2SO4. Flash chromatography was performed with silica gel (35–70 μm, 60 Å). THF was distilled from sodium, acetonitrile was distilled from CaH2 and other reaction solvents were dried with Al2O3. Commercially available compounds were obtained from Aldrich.
4.2. Synthetic Procedures
Methyl 4-(hydroxymethyl)-2-iodobenzoate (intermediate in the synthesis of 1a–1e): BH3-THF (1 M, 47.1 mL, 47.1 mmol) was slowly added to a stirred solution of 1-methyl-2-iodoterephthalate (4.8 g, 15.7 mmol) in dry THF (250 mL) at 0 °C. After 30 h, saturated aqueous NaHCO3/H2O was added, and the aqueous phase was extracted with ethyl acetate (3 × 250 mL) before drying (Na2SO4) and removal of solvent under reduced pressure. Purification by column chromatography (SiO2, 4:6 heptane/ethyl acetate) gave 4.06 g (89%) of the pure product as yellow crystals, identical to that previously reported [42].
Methyl 4-(((tert-butyldiphenylsilyl)oxy)methyl)-2-iodobenzoate (intermediate in the synthesis of 1a–1e): TBDPSCl (4.3 mL, 16.7 mmol) was added to a stirred solution of methyl 4-(hydroxymethyl)-2-iodobenzoate (4.06 g, 13.9 mmol) in pyridine (80 mL) at rt. After 24 h, saturated aqueous NH4Cl/H2O was added and the aqueous phase was extracted with diethyl ether (3 × 200 mL), then the organic phase was washed with brine (2 × 500 mL) before drying (Na2SO4) and removal of solvent under reduced pressure. Purification by column chromatography (SiO2, 20:2 heptane/ethyl acetate) gave 4.2 g (57%) of the pure product as white crystals, identical to that previously reported [42].
General procedure for Suzuki coupling (intermediates in the synthesis of 1a–1e): corresponding boronic acid (1.5 equiv), K2CO3 (5 equiv) and Tetrakis(triphenylphosphine)palladium(0) (0.17 equiv) were added to a stirred solution of methyl 4-(((tert-butyldiphenylsilyl)oxy)methyl)-2-iodobenzoate (1 equiv) dissolved in 4:1 DME/water (15 mL), the mixture (contained in a microtube) was degasified under vaccuum/N2 at −78 °C five times. The microwave reaction conditions were 100 °C, high pressure, and 10 s of pre-stirring. After 30 to 60 min in the microwave reactor, the mixture was filtered through a plug of celite and washed with ethyl acetate (250 mL) before drying (Na2SO4) and removal of solvent under reduced pressure. Purification by column chromatography (SiO2, 20:3 heptane/ethyl acetate) gave the pure products.
Methyl 5-(((tert-butyldiphenylsilyl)oxy)methyl)-2′,5′-dimethoxy-[1,1′-biphenyl]-2-carboxylate (intermediate in the synthesis of 1a): the pure product was obtained as an orange wax (yield 94%) identical to that previously reported [42].
Methyl 5-(((tert-butyldiphenylsilyl)oxy)methyl)-5′-isopropyl-2′-methoxy-[1,1′-biphenyl]-2-carboxylate (intermediate in the synthesis of 1b): the pure product was obtained as colorless wax (yield 97%) identical to that previously reported [45].
Methyl 5-(((tert-butyldiphenylsilyl)oxy)methyl)-2′-methoxy-4′-isopropyl-[1,1′-biphenyl]-2-carboxylate (intermediate in the synthesis of 1c): the pure product was obtained as a yellowish wax (yield 38%) identical to that previously reported [45].
General procedure for organo-lithic addition (intermediate in the synthesis of 1a–1e): Corresponding organo-lithic reagent (4 equiv) was added to a stirred solution of the Suzuki coupling product (1 equiv) in dry THF (70 mL), at 0 or −78 °C, depending on the organo-lithic reagent. After 12 h, saturated aqueous NH4Cl/H2O was added, and the aqueous phase was extracted with ethyl acetate (3 × 100 mL) before drying (Na2SO4) and removal of solvent under reduced pressure. Purification by column chromatography (SiO2, 20:4 heptane/ethyl acetate) gave the pure product.
2-(5-(((tert-butyldiphenylsilyl)oxy)methyl)-2′,5′-dimethoxy-[1,1′-biphenyl]-2-yl)propan-2-ol (intermediate in the synthesis of 1a): the pure product was obtained as a yellowish wax (yield 65.2%) identical to that previously reported [42].
2-(5-(((tert-butyldiphenylsilyl)oxy)methyl)-5′-isopropyl-2′-methoxy-[1,1′-biphenyl]-2-yl)propan-2-ol (intermediate in the synthesis of 1b): the pure product was obtained as a transparent wax (yield 78.5%) identical to that previously reported [45].
2-(5-(((tert-butyldiphenylsilyl)oxy)methyl)-2′-methoxy-4′-isopropyl-[1,1′-biphenyl]-2-yl)propan-2-ol (intermediate in the synthesis of 1c): the pure product was obtained as a transparent wax (yield 64%) identical to that previously reported [45].
General procedure to prepare compounds 1a–1c: HI (55%, 10 equiv) was added to a stirred solution of the corresponding starting material in acetonitrile (25 mL), at rt. After 30 min, saturated aqueous Na2S2O3 (25 mL) was added, and the aqueous layer was extracted with ethyl acetate (3 × 50 mL), before drying (Na2SO4) and removal of solvent under reduced pressure. TBAF (1 M, 1.1 equiv) was added to the crude product in THF (150 mL). After 3 h, aqueous saturated NaHCO3 (50 mL) was added, and the aqueous layer was extracted with ethyl acetate (3 × 50 mL), before drying (Na2SO4) and removal of solvent under reduced pressure. Purification by column chromatography (SiO2, 1:1 heptane/ethyl acetate) gave the pure product.
(2-methoxy-6,6-dimethyl-6H-benzo[c]chromen-9-yl)methanol (1a): the pure product was obtained as a yellowish wax (yield 77%) identical to that previously reported [42].
(2-isopropyl-6,6-dimethyl-6H-benzo[c]chromen-9-yl)methanol (1b): the pure product was obtained as a transparent wax (yield 88%) identical to that previously reported [45].
(3-isopropyl-6,6-dimethyl-6H-benzo[c]chromen-9-yl)methanol (1c): the pure product was obtained as a transparent wax (yield 85%) identical to that previously reported [45].
5-(((tert-butyldiphenylsilyl)oxy)methyl)-2′,5′-dimethoxy-[1,1′-biphenyl]-2-carbaldehyde (intermediate in the synthesis of 1d and 1e): Morpholine (0.2 mL, 2.2 mmol) was added to a solution of DIBALH (1 M, 1.1 mL, 1.1 mmol) in dry THF (30 mL) at 0 °C. After 3 h, methyl 5-(((tert-butyldiphenylsilyl)oxy)methyl)-2′,5′-dimethoxy-[1,1′-biphenyl]-2-carboxylate (600 mg, 1.1 mmol) in dry THF (20 mL) was added, 10 min later, DIBALH (1 M, 1.1 mL, 1.1 mmol) was added again at 0 °C. After 4 h, aqueous HCL (1 N, 20 mL) was added, and the aqueous phase was extracted with diethyl ether (3 × 50 mL) before drying (Na2SO4) and removal of solvent under reduced pressure. Purification by column chromatography (SiO2, 20:4 heptane/ethyl acetate) gave the pure product as a yellowish wax (89.1 mg, 16%) identical to that previously reported [45].
General procedure to prepare compounds 1d and 1e: MeLi (3 M, 2 equiv) was added to 5-(((tert-butyldiphenylsilyl)oxy)methyl)-2′,5′-dimethoxy-[1,1′-biphenyl]-2-carbaldehyde (1 equiv) in dry THF (5 mL) at 0 °C. After 6 h, saturated aqueous NH4Cl/H2O was added, and the aqueous phase was extracted with ethyl acetate (3 × 20 mL) before drying (Na2SO4) and removal of solvent under reduced pressure. PBr3 (0.34 equiv) was added to the crude product (1 equiv) in dichloromethane (10 mL) at rt. After 2 h, LiI (3 equiv) was added at rt. After 12 h, saturated aqueous Na2S2O3/H2O was added, and the aqueous phase was extracted with ethyl acetate (3 × 20 mL) before drying (Na2SO4) and removal of solvent under reduced pressure. TBAF (2 equiv) was added to the crude product in THF (25 mL) at rt. After 5 h, saturated aqueous NaHCO3/H2O was added, and the aqueous phase was extracted with ethyl acetate (3 × 25 mL) before drying (Na2SO4) and removal of solvent under reduced pressure. Purification by column chromatography (SiO2, 1:1 heptane/ethyl acetate) and the enantiomers were separated using a semipreparative HPLC (Chiralpack B column, 96:4 hexane/isopropanol).
(2-methoxy-6-methyl-6H-benzo[c]chromen-9-yl)methanol (1d): the pure product was obtained as a yellowish wax (yield 3%), αD20-17.8 identical to that previously reported [45].
(2-methoxy-6-methyl-6H-benzo[c]chromen-9-yl)methanol (1e): the pure product was obtained as a yellowish wax (yield 3%), αD20 +23.8° identical to that previously reported [45].
General procedure to obtain compounds 2b–2e: Dess–Martin periodinane 15% (2 equiv) was added to a stirred solution of corresponding starting material (1 equiv) in CH2Cl2 at rt. After five hours, saturated aqueous Na2S2O3/H2O was added and the aqueous phase was extracted three times with CH2Cl2 before drying (Na2SO4) and removal of solvent under reduced pressure. Purification by column chromatography (SiO2, 20:1 CH2Cl2/methanol).
2-isopropyl-6,6-dimethyl-6H-benzo[c]chromene-9-carbaldehyde (2b): The pure product was obtained as a transparent wax (85.7 mg, yield 88%). 1H NMR and 13C NMR data in Table 2 and Table 3, respectively. HRMS-ESI+ (m/z): [M + Na]+ calcd for C19H20O2, 303.1361; found 303.1355.
3-isopropyl-6,6-dimethyl-6H-benzo[c]chromene-9-carbaldehyde (2c): the pure product was obtained as a transparent wax (15.3 mg, yield 77%). 1H NMR and 13C NMR data in Table 2 and Table 3, respectively. HRMS-ESI+ (m/z): [M + Na]+ calcd for C19H20O2, 303.1361; found 303.1357.
2-methoxy-6-methyl-6H-benzo[c]chromene-9-carbaldehyde (2d): the pure product was obtained as a yellowish wax (5.4 mg, yield 96%). 1H NMR and 13C NMR data in Table 2 and Table 3, respectively. HRMS-ESI+ (m/z): [M + Na]+ calcd for C16H14O5, 309.0738; found 309.0739 (+2O due to oxidation).
2-methoxy-6-methyl-6H-benzo[c]chromene-9-carbaldehyde (2e): the pure product was obtained as a yellowish wax (6.7 mg, yield 95%). 1H NMR and 13C NMR data in Table 2 and Table 3, respectively. HRMS-ESI+ (m/z): [M + Na]+ calcd for C16H14O5, 309.0739; found 309.0739 (+2O due to oxidation).
1-(2-isopropyl-6,6-dimethyl-6H-benzo[c]chromen-9-yl)ethan-1-one (3b): MeMgI (3 M, 0.24 mL, 0.7 mmol) was added to 2b (65 mg, 0.23 mol) in dry ethyl ether (5 mL), at 0 °C. After 20 h, saturated aqueous NH4Cl/H2O was added, and the aqueous phase was extracted with diethyl ether (3 × 5 mL). The organic product was washed with brine once before drying (Na2SO4) and removal of solvent under reduced pressure. Celite (250 mg) and PCC (63 mg, 0.25 mmol) were added to the crude product in dry CH2Cl2 (5 mL) under nitrogen, at rt. After 12 h diethyl ether (10 mL) was added and the mixture was filtered over a plug of silica gel and washed with ethyl acetate (50 mL) before removal of solvent under reduce pressure. Purification by column chromatography (SiO2, 20:4 hept/ethyl acetate) gave 30.0 mg, 44% as a transparent wax.%). 1H NMR and 13C NMR data in Table 2 and Table 3, respectively. HRMS-ESI+ (m/z): [M + Na]+ calcd for C20H22O2, 317.1514; found 317.1517.
General procedure to obtain compounds 4b–4c: Isovaleric anhydride (1.5 equiv), DMAP (1.2 equiv), and Et3N (1.5 equiv) were added to a stirred solution of appropriate starting material (1 equiv) in CH2Cl2 (25 mL) at rt. After three hours, saturated aqueous NH4Cl/H2O was added and the aqueous phase was extracted with CH2Cl2 (3 × 25 mL) before drying (Na2SO4) and removal of solvent under reduced pressure. Purification by column chromatography (SiO2, 20:4 heptane/ethyl acetate) gave the desired products.
(2-isopropyl-6,6-dimethyl-6H-benzo[c]chromen-9-yl)methyl 3-methylbutanoate (4b): The pure product was obtained as a transparent wax (64.9, yield 53%). 1H NMR and 13C NMR data in Table 2 and Table 3, respectively. HRMS-ESI+ (m/z): [M + Na]+ calcd for C24H30O3, 389.2093; found 389.2099.
(3-isopropyl-6,6-dimethyl-6H-benzo[c]chromen-9-yl)methyl 3-methylbutanoate (4c): The pure product was obtained as a transparent wax (15.2, yield 59%). 1H NMR and 13C NMR data in Table 2 and Table 3, respectively. HRMS-ESI+ (m/z): [M + Na]+ calcd for C24H30O3, 389.2093; found 389.2091.
General procedure to obtain compounds 5a–5c: Et3SiH (5 equiv) and PdCl2 (2 equiv) were added to a stirred solution of corresponding starting material (1 equiv) in EtOH. After 3h, ethyl acetate was added and the mixture was filtered through a plug of celite and washed with ethyl acetate before drying (Na2SO4) and removal of solvent under reduced pressure. Purification by column chromatography (SiO2, 20:1 heptane/ethyl acetate).
2-Methoxy-6,6,9-trimethyl-6H-benzo[c]chromene (5a): the pure product was obtained as a yellowish wax (yield 92%) identical to that previously reported [44].
2-isopropyl-6,6,9-trimethyl-6H-benzo[c]chromene (5b): the pure product was obtained as a transparent wax (105 mg, yield 89%). 1H NMR and 13C NMR data in Table 2 and Table 3, respectively. HRMS-ESI+ (m/z): [M + Na]+ calcd for C19H22O, 289.1568; found 289.1563.
3-isopropyl-6,6,9-trimethyl-6H-benzo[c]chromene (5c): the pure product was obtained as a transparent wax (16 mg, yield 85%). 1H NMR and 13C NMR data in Table 2 and Table 3, respectively. HRMS-ESI+ (m/z): [M + Na]+ calcd for C19H22O, 289.1568; found 289.1565.
6,6,9-trimethyl-6H-benzo[c]chromen-2-ol (5d): Sodium ethanethiolate (132 mg, 1.57 mmol2) was added to a stirred solution of 5a (0.157 mmol) in DMF (4 mL), and reacted in a microwave reactor at 160 °C. After one hour, saturated aqueous NH4Cl/H2O was added and the aqueous phase was extracted with ethyl acetate (3 × 100 mL) before drying (Na2SO4) and removal of solvent under reduced pressure. Purification by column chromatography (SiO2, 20:4 heptane/ethyl acetate) gave 27 mg (72%) of the pure product as an orange wax.%). 1H NMR and 13C NMR data in Table 2 and Table 3, respectively. HRMS-ESI+ (m/z): [M + H]+ calcd for C16H17O2, 241.1229; found 241.1231.
General procedure to obtain compounds 5eand 5f: Citral (1 equiv) and n-butylamine (1 equiv) were added to the corresponding resorcinol analogue (1 equiv) in toluene at 110 °C. After 12 h, DOWEX 50WX8 (200 mg) was added to the stirred mixture at rt. After 10 min the mixture was filtered over a plug of celite. Iodine (2 equiv) was added to the filtrate at 110 °C. After 3 h, saturated aqueous Na2S2O3/H2O was added and the aqueous phase was extracted three times with EtOAc before drying (Na2SO4) and removal of solvent under reduced pressure. Purification by column chromatography (SiO2, 100:5 heptane/ethyl acetate).
4.3. Biological Assays
4.3.1. Evaluations against Leishmania Parasites
Promastigotes of Leishmania (Leishmania): L. amazonensis, clone 1, NHOM-BR-76-LTB-012 (Lma, donated by the Paul Sabatier Université, France) and Leishmania (Viannia): L. braziliensis M2904 C192 RJA (M2904, donated by Dr. Jorge Arévalo from Universidad Peruana Cayetano Heredia, Peru) [47]. All strains were cultured in Schneider’s insect medium (pH 6.2), supplemented with 10% FBS and incubated at 26 °C. Medium changes were made every 72 h to maintain a viable parasitic population. Leishmanicidal activity was determined according to Williams with some modifications [48]. Samples were dissolved in DMSO (maximum final concentration 1%) at 10 mg/mL. Promastigotes in logarithmic phase of growth, at the concentration 3 × 106 parasites/mL, were distributed (100 μL/well) in 96-well flat bottom microtiter plates. Samples with different concentrations (3.1–100 μg/mL) were added (100 μL). Miltefosine (3.1–100 μg/mL) was used as control drug [49]. Assays were performed in triplicates. The microwell plates were incubated for 72 h at 26 °C. After incubation, a solution of XTT (1 mg/mL, [2,3-bis[2-methoxy-4-nitro-5-sulfophenyl]-2H-tetrazolium-5-carboxanilide]) in PBS (pH 7.0 at 37 °C) with PMS (0.06 mg/mL phenazine methosulfate) was added (50 μL/well), and incubated for 3 h at 26 °C. All assays were carried out as triplicates. The optical density of each well was measured with a Synergy HT microplate reader at 200–450 nm. The IC50 values were calculated using the Gen5 program (BioTek).
4.3.2. Evaluations against Trypanosoma cruzi
Cultures of Trypanosoma cruzi (epimastigotes, donated by the Parasitology Department of INLASA, Tc-INLASA) were maintained in medium LIT (liver infusion tryptose) (pH 7.2), supplemented with 10% FBS and incubated at 26 °C. The LIT medium was prepared with NaCl, 40 g/L; KCl, 4 g/L; Na2HPO4, 80 g/L; tryptose, 50 g/L; 10% liver infusion broth, 50 mL/L; 40% glucose solution, 10 mL/L; penicillin, 200 units/mL; streptomycin, 200 units/mL; and neomycin, 200 units/mL. Medium changes were made every 72 h to maintain a viable parasitic population. Trypanocidal activity was determined according to Muelas-Serrano with some modifications [50]. Samples were dissolved in DMSO (maximum final concentration 1%) at 10 mg/mL. Epimastigotes in logarithmic phase of growth, at a concentration of 3 × 106 parasites/mL, were distributed (100 μL/well) in 96-well flat bottom microtiter plates. Samples at different concentrations (3.1–100 μg/mL) were added (100 μL). Benznidazol (3.1–100 μg/mL) was used as the control drug. Assays were performed in triplicates. The microwell plates were incubated for 72 h at 26 °C. After incubation, a solution of XTT (1 mg/mL) in PBS (pH 7.0 at 37 °C) with PMS (0.06 mg/mL) was added (50 μL/well) and incubated for 4 h at 26 °C. All assays were carried out as triplicates. The optical density of each well was measured with a Synergy HT microplate reader at 200–450 nm. The IC50 values were calculated using the Gen5 program (BioTek).
5. Conclusions
Twelve compounds with chemical transformations of the benzyl alcohol functionality in the A-ring, and with modifications either in the C-ring or the B-ring, were prepared and assayed towards T. cruzi, L. braziliensis and L. amazonensis. Although Trypanosoma and Leishmania species belong to the same order (Trypanosomatida), they apparently possess different molecular targets in their different life stages, and the ways they infect humans are not fully understood. Moreover, the active site or active sites on which pulchrol acts on each of the parasites are unknown; therefore, the bioactivity results reported in this work just provide some suggestions of SARs and the surrounding near a possible binding site. The effects of the antiparasitic activity that lipophilic groups in the C-ring may have was studied by testing analogues substituted with isopropyl groups at position 2 and 3, combined with different transformations of pulchrol’s benzyl alcohol functionality. The combination of a 1′-carbonyl group in the A-ring was found to be beneficial for the activity towards all of the three parasites; however, the 3-isopropyl analogues showed slightly better leishmanicidal activity than the 2-isopropyl analogues. The latter are more potent towards T. cruzi, suggesting that differences in the binding sites of the protein targets may exist. The most interesting antiparasitic activity was showed by the analogue containing a 1′-methyl ketone functionality in the A-ring and the isopropyl group at position 2. Moreover, all the carbonyl/isopropyl analogues were more potent than the analogues containing just the carbonyl functionality in the A-ring or just the isopropyl group in the C-ring, indicating that the combination may improve the orientation in the binding site.
Supplementary Materials
The following are available online: 1D and 2D NMR spectra of all isolated compounds.
Author Contributions
P.T is a PhD student and has planned and carried out the synthesis of the reported compounds, and written the manuscript; E.S. has carried out the biological assays, supervised by A.G.; M.D. has supervised the work of P.T. at UMSS; S.M. has supervised the work of P.T. in Lund; while O.S. has supervised the work of P.T. in Lund and compiled the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Swedish International Development Agency (SIDA, grant No 2017-258), and a Ph.D. scholarships for P.T. from SIDA is gratefully acknowledge.
Institutional Review Board Statement
Not applicable, as the study did not involve humans or animals.
Informed Consent Statement
Not applicable, as the study did not involve humans or animals.
Data Availability Statement
All data are available in this publication and in the Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest. The sponsors had no role in the design, execution, interpretation, or writing of the study.
Sample Availability
Samples of the compounds are not available from the authors, but all information about how to prepare them are given in the paper.
References
- Li, G.; Lou, H.-X. Strategies to diversify natural products for drug discovery. Med. Res. Rev. 2018, 38, 1255–1294. [Google Scholar] [CrossRef]
- Sertürner, F.W. Säure im opium. Trommsdorffs J. Pharm. 1805, 13, 234. [Google Scholar]
- Wani, M.C.; Taylor, H.L.; Wall, M.E.; Coggon, P.; McPhail, A.T. Plant antitumor agents. VI. Isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J. Am. Chem. Soc. 1971, 93, 2325–2327. [Google Scholar] [CrossRef] [PubMed]
- Achan, J.; Talisuna, A.O.; Erhart, A.; Yeka, A.; Tibenderana, J.K.; Baliraine, F.N.; Rosenthal, P.J.; D’Alessandro, U. Quinine, an old anti-malarial drug in a modern world: Role in the treatment of malaria. Malar. J. 2011, 10, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qinghaosu Antimalarial Coordinating Research Group. Antimalaria studies on qinghaosu. Chin. Med. J. 1979, 92, 811–816. [Google Scholar]
- Rodrigues, T.; Reker, D.; Schneider, P.; Schneider, G. Counting on natural products for drug design. Nat. Chem. 2016, 8, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Molyneux, D.H.; Savioli, L.; Engels, D. Neglected tropical diseases: Progress towards addressing the chronic pandemic. Lancet 2017, 389, 312–325. [Google Scholar] [CrossRef]
- Gilbert, I.H. Drug discovery for neglected diseases: Molecular target-based and phenotypic approaches. J. Med. Chem. 2013, 56, 7719–7726. [Google Scholar] [CrossRef]
- Weng, H.-B.; Chen, H.-X.; Wang, M.-W. Innovation in neglected tropical disease drug discovery and development. Infect. Dis. Poverty 2018, 7, 1–9. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.-M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [Green Version]
- Moffat, J.G.; Vincent, F.; Lee, J.A.; Eder, J.; Prunotto, M. Opportunities and challenges in phenotypic drug discovery: An industry perspective. Nat. Rev. Drug Discov. 2017, 16, 531–543. [Google Scholar] [CrossRef]
- Zheng, W.; Thorne, N.; McKew, J.C. Phenotypic screens as a renewed approach for drug discovery. Drug Discov. Today 2013, 18, 1067–1073. [Google Scholar] [CrossRef] [Green Version]
- Al-Ali, H. The evolution of drug discovery: From phenotypes to targets, and back. Med. Chem. Commun. 2016, 7, 788–798. [Google Scholar] [CrossRef]
- Haasen, D.; Schopfer, U.; Antczak, C.; Guy, C.; Fuchs, F.; Selzer, P. How Phenotypic Screening Influenced Drug Discovery: Lessons from Five Years of Practice. Assay Drug Dev. Technol. 2017, 15, 239–246. [Google Scholar] [CrossRef]
- Campos-Ríos, M.G. Revisión del Género Bourreria P. Browne (Boraginaceae) en México. Polibotánica 2005, 19, 39–103. [Google Scholar]
- Erosa-Rejón, G.J.; Yam-Puc, A.; Chan-Bacab, M.J.; Giménez-Turbax, A.; Salamanca, E.; Peña-Rodríguez, L.M.; Sterner, O. Benzochromenes from the roots of Bourreria pulchra. Phytochem. Lett. 2010, 3, 9–12. [Google Scholar] [CrossRef]
- World Health Organization Home Page. Chagas Disease (American Tripanosomiasis). Available online: https://www.who.int/chagas/disease/en/ (accessed on 19 December 2019).
- World Health Organization Home Page. WHO Report on Global Surveillance of Epidemic-Prone Infectious Diseases—Leishmaniasis. Available online: https://www.who.int/csr/resources/publications/CSR_ISR_2000_1leish/en/ (accessed on 19 December 2019).
- World Health Organization Home Page. Available online: https://www.who.int/neglected_diseases/diseases/en/ (accessed on 14 July 2020).
- Jacobson, J.; Bush, S. Neglected Tropical Diseases, Neglected Communities, and Conflict: How Do We Leave No One Behind? Trends Parasitol. 2018, 34, 175–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization Home Page. Second WHO Report on Neglected Tropical Diseases. Available online: https://www.who.int/neglected_diseases/9789241564540/en/ (accessed on 14 July 2020).
- World Health Organization Home Page. Available online: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis (accessed on 14 July 2020).
- Croft, S.L.; Sundar, S.; Fairlamb, A.H. Drug resistance in leishmaniasis. Clin. Microbiol. Rev. 2006, 19, 111–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Field, M.C.; Horn, D.; Fairlamb, A.H.; Ferguson, M.A.J.; Gray, D.W.; Read, K.D.; De Rycker, M.; Torrie, L.S.; Wyatt, P.G.; Wyllie, S.; et al. Anti-trypanosomatid drug discovery: An ongoing challenge and a continuing need. Nat. Rev. Microbiol. 2017, 15, 217–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Molina, J.A.; Molina, I. Chagas disease. Lancet 2018, 391, 82–94. [Google Scholar] [CrossRef]
- Pinazo, M.; Munoz, J.; Posada, E.; Lopez-Chejade, P.; Gallego, M.; Ayala, E.; del Cacho, E.; Soy, D.; Gascon, J. Tolerance of Benznidazole in treatment of Chagas’ disease in adults. Antimicrob. Agents Chemother. 2020, 54, 4896–4899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatelain, E. Chagas disease research and development: Is there light at the end of the tunnel? Comput. Struct. Biotechnol. J. 2016, 15, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Tarleton, R.L. Chagas Disease: A Solvable Problem, Ignored. Trends Mol. Med. 2016, 22, 835–838. [Google Scholar] [CrossRef] [Green Version]
- Gulcan, H.O.; Unlu, S.; Esiringu, İ.; Ercetin, T.; Sahin, Y.; Oz, D.; Sahin, M.F. Design, synthesis and biological evaluation of novel 6H-benzo[c]chromen-6-one, and 7,8,9,10-tetrahydro-benzo[c]chromen-6-one derivatives as potential cholinesterase inhibitors. Biorgan. Med. Chem. 2014, 22, 5141–5154. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Cama, L.D.; Birzin, E.T.; Warrier, S.; Locco, L.; Mosley, R.; Hammond, M.L.; Rohrer, S.P. 6H-Benzo[c]chromen-6-one derivatives as selective ERβ agonists. Biorgan. Med. Chem. Lett. 2006, 16, 1468–1472. [Google Scholar] [CrossRef]
- Appendino, G.; Gibbons, S.; Giana, A.; Pagani, A.; Grassi, G.; Stavri, M.; Smith, E.; Rahman, M.M. Antibacterial Cannabinoids from Cannabis sativa: A Structure-Activity Study. J. Nat. Prod. 2008, 71, 1427–1430. [Google Scholar] [CrossRef]
- Kaplan, B.L.F.; Rockwell, C.E.; Kaminski, N.E. Evidence for Cannabinoid Receptor-Dependent and -Independent Mechanisms of Action in Leukocytes. J. Pharmacol. Exp. Ther. 2003, 306, 1077–1085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munson, A.E.; Harris, L.S.; Friedman, M.A.; Dewey, W.L.; Carchman, R.A. Antineoplastic Activity of Cannabinoids. J. Natl. Cancer Inst. 1975, 55, 597–602. [Google Scholar] [CrossRef]
- Khanolkar, A.D.; Lu, D.; Ibrahim, M.; Duclos, J.R.I.; Thakur, G.A.; Malan, J.T.P.; Porreca, F.; Veerappan, V.; Tian, X.; George, C.; et al. Cannabilactones: A Novel Class of CB2 Selective Agonists with Peripheral Analgesic Activity. J. Med. Chem. 2007, 50, 6493–6500. [Google Scholar] [CrossRef] [PubMed]
- Munro, S.; Thomas, K.L.; Abu-Shaar, M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 1993, 365, 61–65. [Google Scholar] [CrossRef]
- Thakur, G.A.; Bajaj, S.; Paronis, C.; Peng, Y.; Bowman, A.L.; Barak, L.S.; Caron, M.G.; Parrish, D.; Deschamps, J.R.; Makriyannis, A. Novel Adamantyl Cannabinoids as CB1 Receptor Probes. J. Med. Chem. 2013, 56, 3904–3921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Husni, A.S.; McCurdy, C.R.; Radwan, M.M.; Ahmed, S.A.; Slade, D.; Ross, S.A.; ElSohly, M.A.; Cutler, S.J. Evaluation of Phytocannabinoids from High Potency Cannabis sativa using In Vitro Bioassays to Determine Structure-Activity Relationships for Cannabinoid Receptor 1 and Cannabinoid Receptor 2. Med. Chem. Res. 2014, 23, 4295–4300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahadevan, A.; Siegel, C.; Martin, B.R.; Abood, M.E.; Beletskaya, I.; Razdan, R.K. Novel Cannabinol Probes for CB1 and CB2 Cannabinoid Receptors. J. Med. Chem. 2000, 43, 3778–3785. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, J.D.; Williamson, E.M. Cannabinoids inhibit human keratinocyte proliferation through a non-CB1/CB2 mechanism and have a potential therapeutic value in the treatment of psoriasis. J. Dermatol. Sci. 2007, 45, 87–92. [Google Scholar] [CrossRef]
- Zygmunt, P.M.; Andersson, D.A.; Hogestatt, E.D. Delta 9-tetrahydrocannabinol and cannabinol activate capsaicin-sensitive sensory nerves via a CB1 and CB2 cannabinoid receptor-independent mechanism. J. Neurosci. 2002, 22, 4720–4727. [Google Scholar] [CrossRef] [Green Version]
- Pertwee, R.G. Novel Pharmacological Targets for Cannabinoids. Curr. Neuropharmacol. 2004, 2, 9–29. [Google Scholar] [CrossRef]
- Killander, D.; Sterner, O. Synthesis of the Bioactive Benzochromenes Pulchrol and Pulchral, Metabolites of Bourreria pulchra. Eur. J. Org. Chem. 2014, 2014, 1594–1596. [Google Scholar] [CrossRef]
- Killander, D.; Sterner, O. Reagent-Controlled Cyclization—Deprotection Reaction to Yield either Fluorenes or Benzochromenes. Eur. J. Org. Chem. 2014, 2014, 6507–6512. [Google Scholar] [CrossRef]
- Terrazas, P.; Salamanca, E.; Dávila, M.; Manner, S.; Giménez, A.; Sterner, O. SAR:s for the Antiparasitic Plant Metabolite Pulchrol. 1. The Benzyl Alcohol Functionality. Molecules 2020, 25, 3058. [Google Scholar] [CrossRef]
- Terrazas, P.; Salamanca, E.; Dávila, M.; Manner, S.; Giménez, A.; Sterner, O. SAR:s for the Antiparasitic Plant Metabolite Pulchrol. 2. B- and C-ring substituents. Molecules 2020, 25, 4510. [Google Scholar] [CrossRef]
- Caprioglio, D.; Mattoteia, D.; Minassi, A.; Pollastro, F.; Lopatriello, A.; Muňoz, E.; Taglialatela-Scafati, O.; Appendino, G. One-Pot Total Synthesis of Cannabinol via Iodine-Mediated Deconstructive Annulation. Org. Lett. 2019, 21, 6122–6125. [Google Scholar] [CrossRef]
- Bilbao-Ramos, P.; Dea-Ayuela, M.A.; Cardenas-Alegría, O. Leishmaniasis in the major endemic region of Plurinational State of Bolivia: Species identification, phylogeography and drug susceptibility implications. Acta Trop. 2017, 176, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.; Espinosa, O.A.; Montenegro, H. Hydrosoluble formazan XTT: Its application to natural products drug discovery for Leishmania. J. Microbiol. Methods 2003, 55, 813–816. [Google Scholar] [CrossRef] [PubMed]
- Campos-Buzzi, F.; Fracasso, M.; Clase, B.K.; Ticona, J.C.; Gimenez, A.; Cechinel-Filho, V. Evaluation of antinociceptive effects of Galipea longiflora alkaloid extract and major alkaloid 2-fenilquinoline. Methods Find. Exp. Clin. Pharmacol. 2010, 32, 707–711. [Google Scholar] [CrossRef] [PubMed]
- Muelas-Serrano, S.; Nogal-Ruiz, J.; Gomez-Barrio, A. Setting of a colorimetric method to determine the viability of Trypanosoma cruzi epimastigotes. Parasitol. Res. 2000, 86, 999–1002. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).