Measurement of Antioxidant Capacity of Meat and Meat Products: Methods and Applications
Abstract
:1. Introduction
2. Determination of Antioxidant Capacity in Meat and Meat Products
2.1. Extraction of Antioxidant Compounds from Meat and Meat Products
2.2. Antioxidant Capacity Assays Frequently Employed in Meat and Meat Products
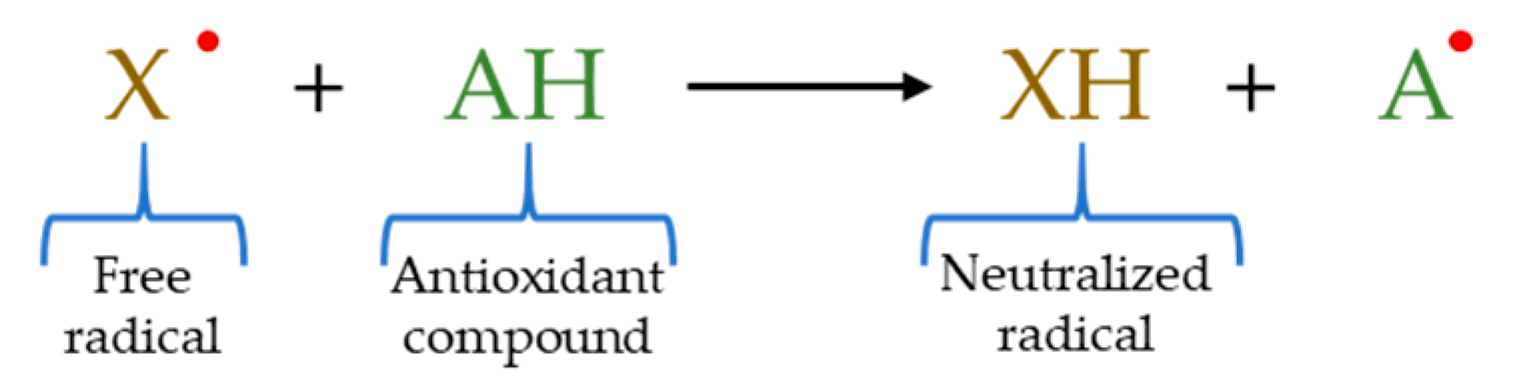
2.3. HAT–Based Methods
2.3.1. Oxygen Radical Absorbance Capacity (ORAC) Assay
2.3.2. Hydroxyl Radical Averting Capacity (HORAC) Assay
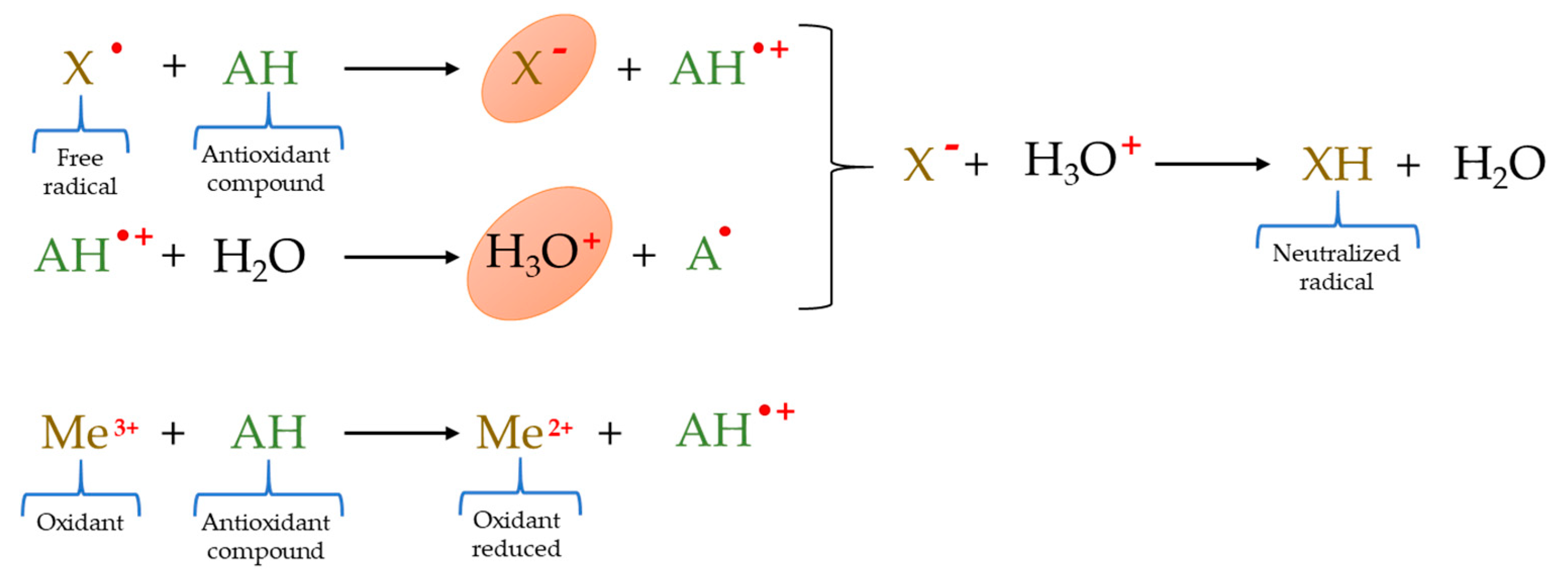
2.4. ET–Based Methods
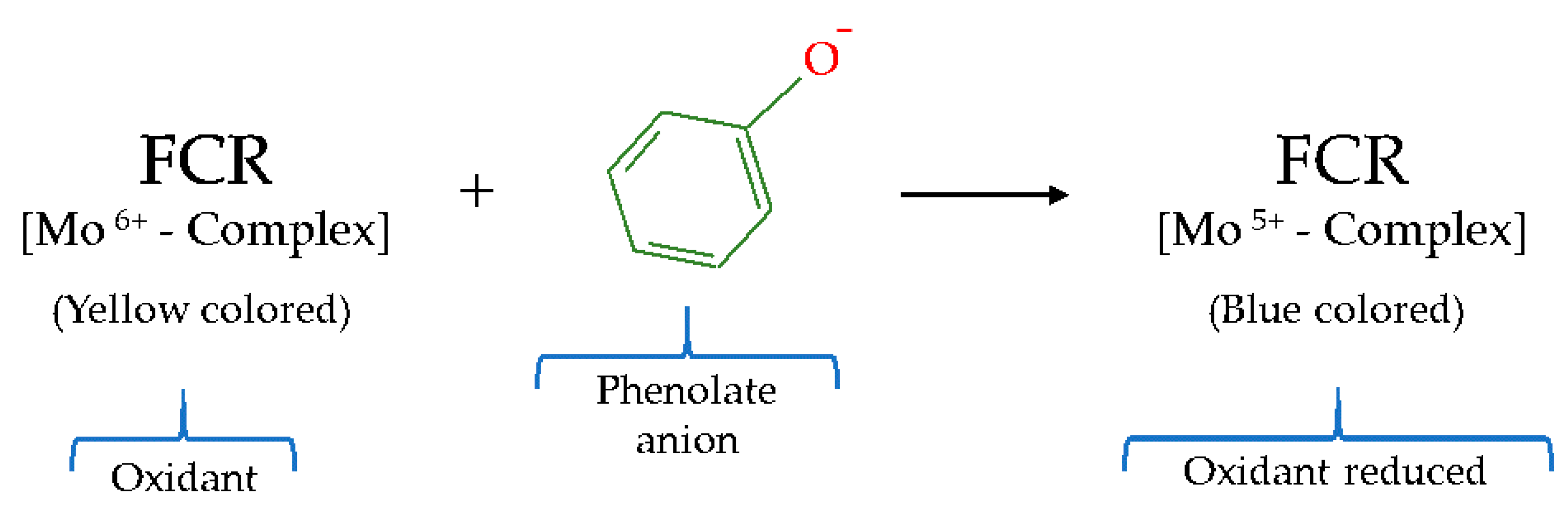
2.4.1. Total Phenol Content (TPC) by Folin–Ciocalteu Assay
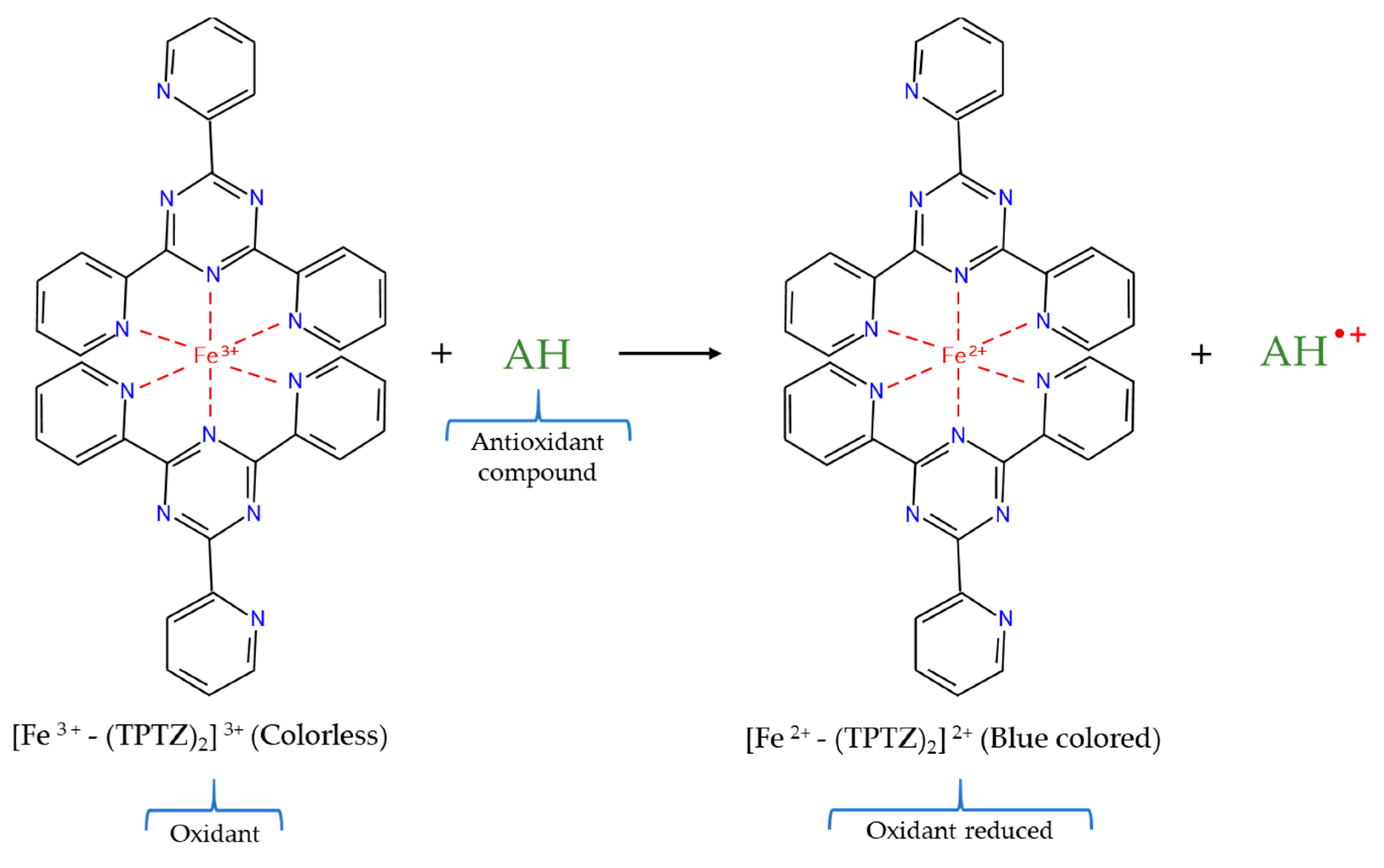
2.4.2. Ferric Ion Reducing Antioxidant Power (FRAP) Assay
2.5. Mixed Mode (HAT– and ET–Based) Methods
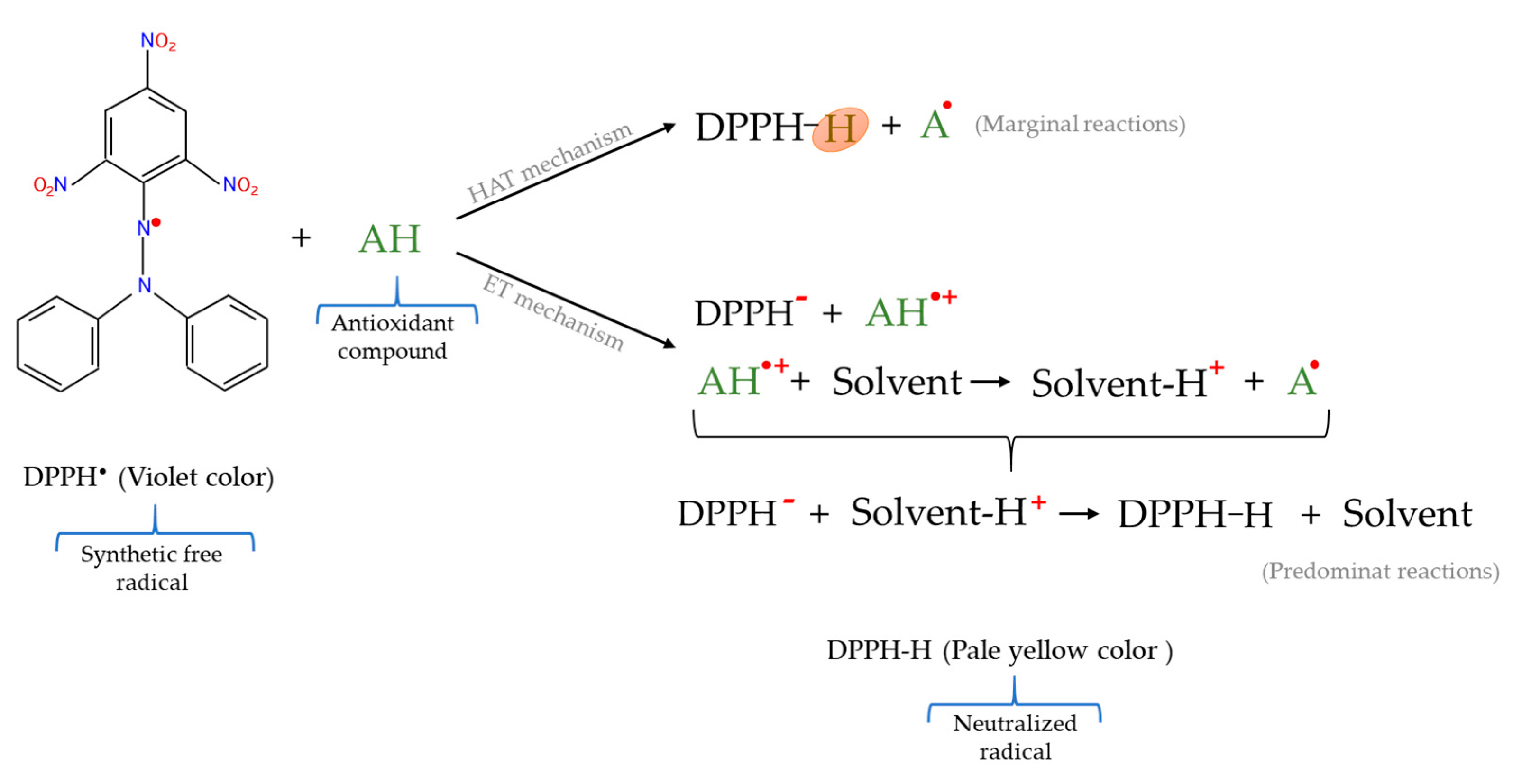
2.5.1. 2,2–Diphenyl–1–picrylhydrazyl Radical (DPPH•) Scavenging Assay
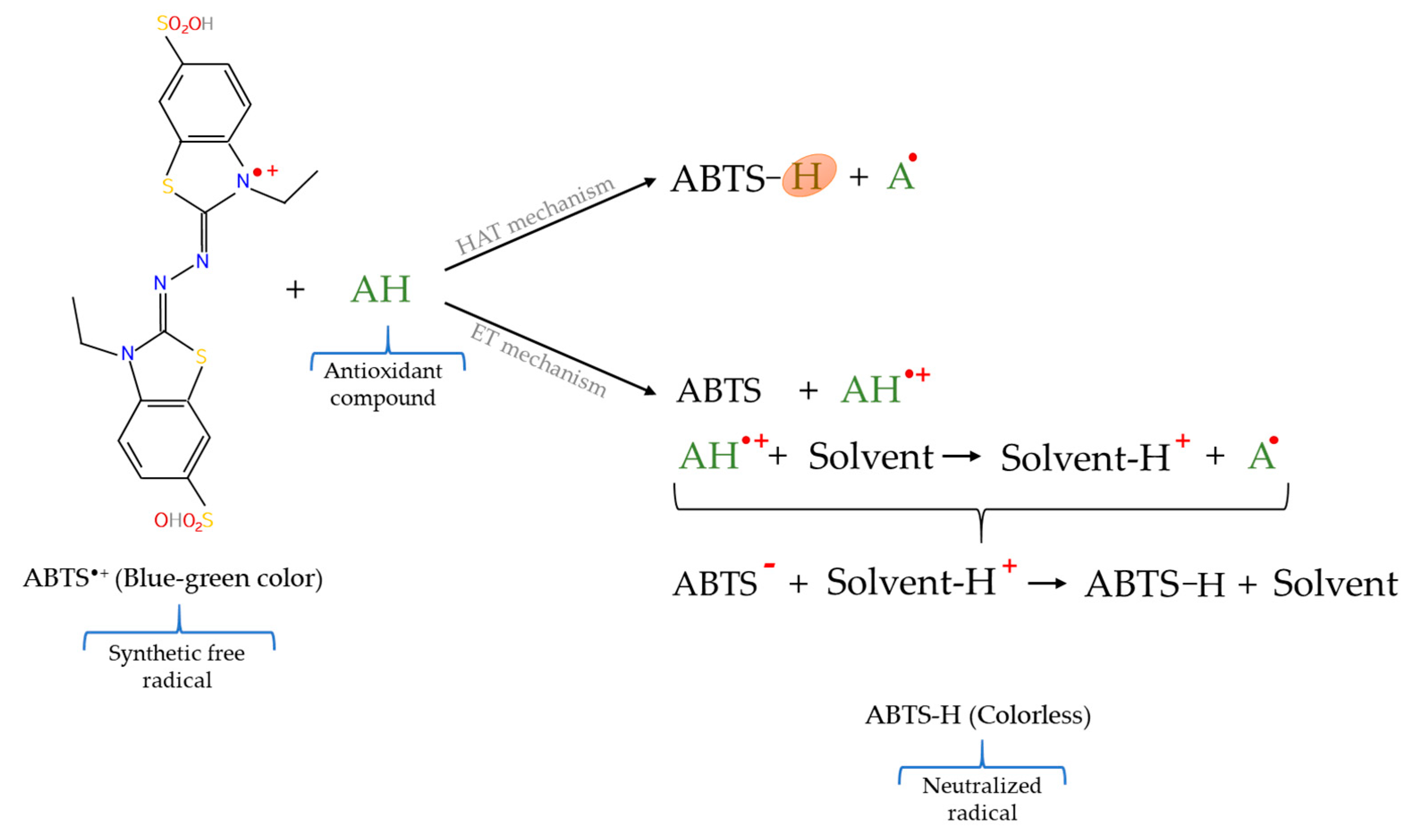
2.5.2. 2,2′–Azinobis–(3–ethylbenzothiazoline–6–sulphonic acid) Radical Cation (ABTS•+) Scavenging Assay
3. Applications of Antioxidant Assays in Meat and Meat Products
3.1. Study of the Influence of Animal Diet on the Antioxidant Capacity of Meat
3.2. Study of the Influence of Animal Breed on the Antioxidant Capacity of Meat
3.3. Study of the Functional Ingredients Addition on the Antioxidant Capacity of Meat Products
3.4. Characterization of the Antioxidant Capacity of Proteins Obtained from Meat
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Domínguez, R.; Munekata, P.E.; Pateiro, M.; López-Fernández, O.; Lorenzo, J.M. Immobilization of oils using hydrogels as strategy to replace animal fats and improve the healthiness of meat products. Curr. Opin. Food Sci. 2021, 37, 135–144. [Google Scholar] [CrossRef]
- Mukumbo, F.E.; Descalzo, A.M.; Collignan, A.; Hoffman, L.C.; Servent, A.; Muchenje, V.; Arnaud, E. Effect of Moringa oleifera leaf powder on drying kinetics, physic-chemical properties, ferric reducing antioxidant power, α-tocopherol, β-carotene, and lipid oxidation of dry pork sausages during processing and storage. J. Food Process. Preserv. 2019, e14300. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A comprehensive review on lipid oxidation in meat and meat products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, J.S.; Santos, M.J.M.C.; Silva, L.K.R.; Pereira, L.C.L.; Santos, I.A.; da Silva Lannes, S.C.; da Silva, M.V. Natural antioxidants used in meat products: A brief review. Meat Sci. 2019, 148, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Min, B.; Nam, K.C.; Cordray, J.; Ahn, D.U. Endogenous factors affecting oxidative stability of beef loin, pork loin, and chicken breast and thigh meats. J. Food Sci. 2008, 73, C439–C446. [Google Scholar] [CrossRef]
- Kim, Y.H.; Nam, K.C.; Ahn, D.U. Volatile profiles, lipid oxidation and sensory characteristics of irradiated meat from different animal species. Meat Sci. 2002, 61, 257–265. [Google Scholar] [CrossRef]
- Králová, M. The effect of lipid oxidation on the quality of meat and meat products. Maso Int. J. Food Sci. Technol. 2015, 2, 125–132. [Google Scholar]
- Chaijan, M.; Panpipat, W. Mechanism of oxidation in foods of animal origin. In Natural Antioxidants: Applications in Foods of Animal Origin; Apple Academic Press: New York, NY, USA, 2017; pp. 1–37. ISBN 9781771884600. [Google Scholar]
- Domínguez, R.; Purriños, L.; Pérez-Santaescolástica, C.; Pateiro, M.; Barba, F.J.; Tomasevic, I.; Campagnol, P.C.B.; Lorenzo, J.M. Characterization of volatile compounds of dry-cured meat products using HS-SPME-GC/MS technique. Food Anal. Methods 2019, 12, 1263–1284. [Google Scholar] [CrossRef]
- Huang, X.; Ahn, D.U. Lipid oxidation and its implications to meat quality and human health. Food Sci. Biotechnol. 2019, 28, 1275–1285. [Google Scholar] [CrossRef]
- Wu, H.; Yin, J.; Zhang, J.; Richards, M.P. Factors affecting lipid oxidation due to pig and turkey hemolysate. J. Agric. Food Chem. 2017, 65, 8011–8017. [Google Scholar] [CrossRef]
- Mottram, D.S. Flavour formation in meat and meat products: A review. Food Chem. 1998, 62, 415–424. [Google Scholar] [CrossRef]
- Purriños, L.; Bermúdez, R.; Franco, D.; Carballo, J.; Lorenzo, J.M. Development of volatile compounds during the manufacture of dry-cured “Lacón,” a Spanish traditional meat product. J. Food Sci. 2011, 76, C89–C97. [Google Scholar] [CrossRef]
- de Carvalho, F.A.L.; Munekata, P.E.S.; Lopes de Oliveira, A.; Pateiro, M.; Domínguez, R.; Trindade, M.A.; Lorenzo, J.M. Turmeric (Curcuma longa L.) extract on oxidative stability, physicochemical and sensory properties of fresh lamb sausage with fat replacement by tiger nut (Cyperus esculentus L.) oil. Food Res. Int. 2020, 136, 109487. [Google Scholar] [CrossRef] [PubMed]
- Barden, L.; Decker, E.A. Lipid oxidation in low-moisture food: A review. Crit. Rev. Food Sci. Nutr. 2016, 56, 2467–2482. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Pateiro, M.; Domínguez, R.; Barba, F.J.; Putnik, P.; Kovačević, D.B.; Shpigelman, A.; Granato, D.; Franco, D. Berries extracts as natural antioxidants in meat products: A review. Food Res. Int. 2018, 106, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.L.F.; Abreu, V.K.G. Lipid peroxidation in meat and meat products. In Lipid Peroxidation Research; Mansour, M.A., Ed.; IntechOpen: London, UK, 2020; pp. 29–42. ISBN 978-1-83968-548-4. [Google Scholar]
- Broncano, J.M.; Petrón, M.J.; Parra, V.; Timón, M.L. Effect of different cooking methods on lipid oxidation and formation of free cholesterol oxidation products (COPs) in Latissimus dorsi muscle of Iberian pigs. Meat Sci. 2009, 83, 431–437. [Google Scholar] [CrossRef]
- Domínguez, R.; Gullón, P.; Pateiro, M.; Munekata, P.E.S.; Zhang, W.; Lorenzo, J.M. Tomato as potential source of natural additives for meat industry. A review. Antioxidants 2020, 9, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Csala, M.; Kardon, T.; Legeza, B.; Lizák, B.; Mandl, J.; Margittai, É.; Puskás, F.; Száraz, P.; Szelényi, P.; Bánhegyi, G. On the role of 4-hydroxynonenal in health and disease. Biochim. Biophys. Acta Mol. Basis Dis. 2015, 1852, 826–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Q.; Wang, Q.; Zhu, J.; Xiao, Q.; Zhang, L. Reactive oxygen species: Key regulators in vascular health and diseases. Br. J. Pharmacol. 2018, 175, 1279–1292. [Google Scholar] [CrossRef] [PubMed]
- Min, B.; Ahn, D.U. Mechanism of lipid peroxidation in meat and meat products—A review. Food Sci. Biotechnol. 2005, 14, 152–163. [Google Scholar]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [Green Version]
- Estévez, M.; Ollilainen, V.; Heinonen, M. Analysis of protein oxidation markers α-aminoadipic and γ-glutamic semialdehydes in food proteins using liquid chromatography (LC)–electrospray ionization (ESI)–multistage tandem mass spectrometry (MS). J. Agric. Food Chem. 2009, 57, 3901–3910. [Google Scholar] [CrossRef]
- Mancini, R.A.; Hunt, M.C. Current research in meat color. Meat Sci. 2005, 71, 100–121. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Vargas, F.C.; Strozzi, I.; Pateiro, M.; Furtado, M.M.; Sant’Ana, A.S.; Rocchetti, G.; Barba, F.J.; Dominguez, R.; Lucini, L.; et al. Influence of pitanga leaf extracts on lipid and protein oxidation of pork burger during shelf-life. Food Res. Int. 2018, 114, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Jang, A.; Liu, X.D.; Shin, M.H.; Lee, B.D.; Lee, S.K.; Lee, J.H.; Jo, C. Antioxidative potential of raw breast meat from broiler chicks fed a dietary medicinal herb extract mix. Poult. Sci. 2008, 87, 2382–2389. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, R.; Munekata, P.E.S.; Pateiro, M.; Maggiolino, A.; Bohrer, B.; Lorenzo, J.M. Red beetroot. A potential source of natural additives for the meat industry. Appl. Sci. 2020, 10, 8340. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Rocchetti, G.; Pateiro, M.; Lucini, L.; Domínguez, R.; Lorenzo, J.M. Addition of plant extracts to meat and meat products to extend shelf-life and health-promoting attributes: An overview. Curr. Opin. Food Sci. 2020, 31, 81–87. [Google Scholar] [CrossRef]
- Neri-Numa, I.A.; Arruda, H.S.; Geraldi, M.V.; Maróstica Júnior, M.R.; Pastore, G.M. Natural prebiotic carbohydrates, carotenoids and flavonoids as ingredients in food systems. Curr. Opin. Food Sci. 2020, 33, 98–107. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Gullón, B.; Pateiro, M.; Tomasevic, I.; Domínguez, R.; Lorenzo, J.M. Natural antioxidants from seeds and their application in meat products. Antioxidants 2020, 9, 815. [Google Scholar] [CrossRef] [PubMed]
- Pateiro, M.; Barba, F.J.; Domínguez, R.; Sant’Ana, A.S.; Mousavi Khaneghah, A.; Gavahian, M.; Gómez, B.; Lorenzo, J.M. Essential oils as natural additives to prevent oxidation reactions in meat and meat products: A review. Food Res. Int. 2018, 113, 156–166. [Google Scholar] [CrossRef]
- Christieans, S.; Picgirard, L.; Parafita, E.; Lebert, A.; Gregori, T. Impact of reducing nitrate/nitrite levels on the behavior of Salmonella Typhimurium and Listeria monocytogenes in French dry fermented sausages. Meat Sci. 2018, 137, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Pateiro, M.; Gómez-Salazar, J.A.; Jaime-Patlán, M.; Sosa-Morales, M.E.; Lorenzo, J.M. Plant extracts obtained with green solvents as natural antioxidants in fresh meat products. Antioxidants 2021, 10, 181. [Google Scholar] [CrossRef]
- Echegaray, N.; Munekata, P.E.S.; Centeno, J.A.; Domínguez, R.; Pateiro, M.; Carba-Llo, J.; Lorenzo, J.M. Total phenol content and antioxidant activity of different celta pig carcass locations as affected by the finishing diet (chestnuts or commercial feed). Antioxidants 2021, 10, 5. [Google Scholar] [CrossRef] [PubMed]
- González, E.; Tejeda, J.F.; Motilva, M.J.; Romero, M.P. Phenolic compounds in subcutaneous adipose tissue from Iberian pigs. Options Méditerranéennes, Ser. A 2003, 76, 115–118. [Google Scholar]
- Ortuño, J.; Serrano, R.; Jordán, M.J.; Bañón, S. Relationship between antioxidant status and oxidative stability in lamb meat reinforced with dietary rosemary diterpenes. Food Chem. 2016, 190, 1056–1063. [Google Scholar] [CrossRef]
- Simonetti, A.; Perna, A.; Gambacorta, E. Comparison of antioxidant compounds in pig meat from Italian autochthonous pig Suino Nero Lucano and a modern crossbred pig before and after cooking. Food Chem. 2019, 292, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Lengkidworraphiphat, P.; Wongpoomchai, R.; Taya, S.; Jaturasitha, S. Effect of genotypes on macronutrients and antioxidant capacity of chicken breast meat. Asian-Australas. J. Anim. Sci. 2020, 33, 1817–1823. [Google Scholar] [CrossRef] [PubMed]
- Antonini, E.; Torri, L.; Piochi, M.; Cabrino, G.; Meli, M.A.; De Bellis, R. Nutritional, antioxidant and sensory properties of functional beef burgers formulated with chia seeds and goji puree, before and after in vitro digestion. Meat Sci. 2020, 161, 108021. [Google Scholar] [CrossRef]
- Mancini, S.; Preziuso, G.; Dal Bosco, A.; Roscini, V.; Parisi, G.; Paci, G. Modifications of fatty acids profile, lipid peroxidation and antioxidant capacity in raw and cooked rabbit burgers added with ginger. Meat Sci. 2017, 133, 151–158. [Google Scholar] [CrossRef]
- de Carvalho, F.A.L.; Lorenzo, J.M.; Pateiro, M.; Bermúdez, R.; Purriños, L.; Trindade, M.A. Effect of guarana (Paullinia cupana) seed and pitanga (Eugenia uniflora L.) leaf extracts on lamb burgers with fat replacement by chia oil emulsion during shelf life storage at 2 °C. Food Res. Int. 2019, 125, 108554. [Google Scholar] [CrossRef]
- Pateiro, M.; Vargas, F.C.; Chincha, A.A.I.A.; Sant’Ana, A.S.; Strozzi, I.; Rocchetti, G.; Barba, F.J.; Domínguez, R.; Lucini, L.; do Amaral Sobral, P.J.; et al. Guarana seed extracts as a useful strategy to extend the shelf life of pork patties: UHPLC-ESI/QTOF phenolic profile and impact on microbial inactivation, lipid and protein oxidation and antioxidant capacity. Food Res. Int. 2018, 114, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, M.M.; dos Santos, M.; Ribeiro, W.O.; de Azambuja Ferreira, N.C.; Picone, C.S.F.; Domínguez, R.; Lorenzo, J.M.; Pollonio, M.A.R. Radish powder and oregano essential oil as nitrite substitutes in fermented cooked sausages. Food Res. Int. 2021, 140, 109855. [Google Scholar] [CrossRef] [PubMed]
- Floegel, A.; Kim, D.O.; Chung, S.J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Saura-Calixto, F. Literature data may underestimate the actual antioxidant capacity of cereals. J. Agric. Food Chem. 2005, 53, 5036–5040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.; Duckett, S.K.; Neel, J.P.S.; Fontenot, J.P.; Clapham, W.M. Influence of finishing systems on hydrophilic and lipophilic oxygen radical absorbance capacity (ORAC) in beef. Meat Sci. 2008, 80, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Capanoglu, E.; Kamiloglu, S.; Ozkan, G.; Apak, R. Evaluation of antioxidant activity/capacity measurement methods for food products. In Measurement of Antioxidant Activity and Capacity: Recent Trends and Applications; Apak, R., Capanoglu, E., Shahidi, F., Eds.; Wiley: Oxford, UK, 2018; ISBN 9781119135388. [Google Scholar]
- Böhm, V.; Müller, L. Methods to measure the antioxidant capacity of meat products. In Handbook of Processed Meats and Poultry Analysis; Nollet, L.M., Toldra, F., Eds.; CRC Press: Boca Raton, FL, USA, 2009; pp. 246–289. ISBN 978-1-4200-4531-4. [Google Scholar]
- Echegaray, N.; Munekata, P.E.S.; Gullón, P.; Dzuvor, C.K.O.; Gullón, B.; Kubi, F.; Lorenzo, J.M. Recent advances in food products fortification with anthocyanins. Crit. Rev. Food Sci. Nutr. 2020, 1–15. [Google Scholar] [CrossRef]
- López-Fernández, O.; Domínguez, R.; Pateiro, M.; Munekata, P.E.S.; Rocchetti, G.; Lorenzo, J.M. Determination of polyphenols using liquid chromatography–tandem mass spectrometry technique (LC–MS/MS): A review. Antioxidants 2020, 9, 479. [Google Scholar] [CrossRef]
- Perna, A.; Simonetti, A.; Grassi, G.; Gambacorta, E. Effect of a cauliflower (Brassica oleraceae var. Botrytis) leaf powder-enriched diet on performance, carcass and meat characteristics of growing rabbit. Meat Sci. 2018, 149, 134–140. [Google Scholar] [CrossRef]
- Mancini, S.; Preziuso, G.; Dal Bosco, A.; Roscini, V.; Szendro, Z.; Fratini, F.; Paci, G. Effect of turmeric powder (Curcuma longa L.) and ascorbic acid on physical characteristics and oxidative status of fresh and stored rabbit burgers. Meat Sci. 2015, 110, 93–100. [Google Scholar] [CrossRef]
- Serpen, A.; Gökmen, V.; Fogliano, V. Total antioxidant capacities of raw and cooked meats. Meat Sci. 2012, 90, 60–65. [Google Scholar] [CrossRef]
- Gökmen, V.; Serpen, A.; Fogliano, V. Direct measurement of the total antioxidant capacity of foods: The “QUENCHER” approach. Trends Food Sci. Technol. 2009, 20, 278–288. [Google Scholar] [CrossRef]
- Carrillo, C.; Barrio, Á.; del Mar Cavia, M.; Alonso-Torre, S. Global antioxidant response of meat. J. Sci. Food Agric. 2017, 97, 2358–2365. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Prior, R.L. Comparison of different analytical methods for assessing total antioxidant capacity of human serum. Clin. Chem. 1998, 44, 1309–1315. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [Green Version]
- Alam, M.N.; Bristi, N.J.; Rafiquzzaman, M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J. 2013, 21, 143–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amaral, A.B.; Da Solva, M.V.; Lannes, S.C.D.S. Lipid oxidation in meat: Mechanisms and protective factors—A review. Food Sci. Technol. 2018, 38, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Prior, R.L.; Wu, X.L.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. Rev. Pneumol. Clin. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- DeLange, R.J.; Glazer, A.N. Phycoerythrin fluorescence-based assay for peroxy radicals: A screen for biologically relevant protective agents. Anal. Biochem. 1989, 177, 300–306. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef] [PubMed]
- Karadag, A.; Ozcelik, B.; Saner, S. Review of methods to determine antioxidant capacities. Food Anal. Methods 2009, 2, 41–60. [Google Scholar] [CrossRef]
- Ou, B.; Huang, D.; Hampsch-Woodill, M.; Flanagan, J.A.; Deemer, E.K. Analysis of antioxidant activities of common vegetables employing oxygen radical absorbance capacity (ORAC) and ferric reducing antioxidant power (FRAP) assays: A comparative study. J. Agric. Food Chem. 2002, 50, 3122–3128. [Google Scholar] [CrossRef]
- Dorta, E.; Fuentes-Lemus, E.; Speisky, H.; Lissi, E.; López-Alarcón, C. Evaluation of the antioxidant capacity of food samples: A chemical examination of the oxygen radical absorbance capacity assay. In Measurement of Antioxidant Activity and Capacity: Recent Trends and Applications; Apak, R., Capanoglu, E., Shahidi, F., Eds.; Wiley: Oxford, UK, 2018; pp. 39–55. ISBN 9781119135388. [Google Scholar]
- Magalhães, L.M.; Segundo, M.A.; Reis, S.; Lima, J.L.F.C. Methodological aspects about in vitro evaluation of antioxidant properties. Anal. Chim. Acta 2008, 613, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Deemer, E.K. Development and validation of oxygen radical absorbance capacity assay for lipophilic antioxidants using randomly methylated β-cyclodextrin as the solubility enhancer. J. Agric. Food Chem. 2002, 50, 1815–1821. [Google Scholar] [CrossRef] [PubMed]
- Naguib, Y.M.A. Antioxidant activities of astaxanthin and related carotenoids. J. Agric. Food Chem. 2000, 48, 1150–1154. [Google Scholar] [CrossRef]
- Badarinath, A.V.; Rao, K.M.; Madhu, C.; Chetty, S.; Ramkanth, S.; Rajan, T.V.S.; Gnanaprakash, K. A Review on in-vitro antioxidant methods: Comparisions, correlations and considerations. Int. J. PharmTech Res. 2010, 2, 1276–1285. [Google Scholar]
- Ou, B.; Hampsch-Woodill, M.; Flanagan, J.; Deemer, E.K.; Prior, R.L.; Huang, D. Novel fluorometric assay for hydroxyl radical prevention capacity using fluorescein as the probe. J. Agric. Food Chem. 2002, 50, 2772–2777. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Nat. Med. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- MacDonald-Wicks, L.K.; Wood, L.G.; Garg, M.L. Methodology for the determination of biological antioxidant capacity in vitro: A review. J. Sci. Food Agric. 2006, 86, 2046–2056. [Google Scholar] [CrossRef]
- Folin, O. Tyrosine and tryptophan determinations in proteins. J. Biol. Chem. 1927, 73, 649–672. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Lamuela-Raventós, R.M. Folin-Ciocalteu method for the measurement of total phenolic content and antioxidant capacity. In Measurement of Antioxidant Activity and Capacity: Recent Trends and Applications; Apak, R., Capanoglu, E., Shahidi, F., Eds.; Wiley: Oxford, UK, 2018; pp. 107–115. ISBN 9781119135388. [Google Scholar]
- Katsube, T.; Tabata, H.; Ohta, Y.; Yamasaki, Y.; Anuurad, E.; Shiwaku, K.; Yamane, Y. Screening for antioxidant activity in edible plant products: Comparison of low-density lipoprotein oxidation assay, DPPH radical scavenging assay, and Folin-Ciocalteu assay. J. Agric. Food Chem. 2004, 52, 2391–2396. [Google Scholar] [CrossRef] [PubMed]
- Vinson, J.A.; Su, X.; Zubik, L.; Bose, P. Phenol antioxidant quantity and quality in foods: Fruits. J. Agric. Food Chem. 2001, 49, 5315–5321. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Zhong, Y. Measurement of antioxidant activity. J. Funct. Foods 2015, 18, 757–781. [Google Scholar] [CrossRef]
- Peterson, G.L. Review of the Folin phenol protein quantitation method of Lowry et al. Anal. Biochem. 1979, 18, 201–220. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gülçin, I.; Elmastaş, M.; Aboul-Enein, H.Y. Antioxidant activity of clove oil—A powerful antioxidant source. Arab. J. Chem. 2012, 5, 489–499. [Google Scholar] [CrossRef] [Green Version]
- Pisoschi, A.M.; Negulescu, G.P. Methods for total antioxidant activity determination: A review. Biochem. Anal. Biochem. 2012, 1, 106. [Google Scholar] [CrossRef] [Green Version]
- Antolovich, M.; Prenzler, P.D.; Patsalides, E.; McDonald, S.; Robards, K. Methods for testing antioxidant activity. Analyst 2002, 127, 183–198. [Google Scholar] [CrossRef]
- Nilsson, J.; Pillai, D.; Önning, G.; Persson, C.; Nilsson, Å.; Åkesson, B. Comparison of the 2,2′-azinobis-3-ethylbenzotiazoline-6-sulfonic acid (ABTS) and ferric reducing antioxidant power (FRAP) methods to asses the total antioxidant capacity in extracts of fruit and vegetables. Mol. Nutr. Food Res. 2005, 49, 239–246. [Google Scholar] [CrossRef]
- Prior, R.L.; Cao, G. In vivo total antioxidant capacity: Comparison of different analytical methods. Free Radic. Biol. Med. 1999, 27, 1173–1181. [Google Scholar] [CrossRef]
- Somogyi, A.; Rosta, K.; Pusztai, P.; Tulassay, Z.; Nagy, G. Antioxidant measurements. Physiol. Meas. 2007, 28, R41. [Google Scholar] [CrossRef] [PubMed]
- Apak, R.; Özyürek, M.; Güçlü, K.; Çapanoʇlu, E. Antioxidant activity/capacity measurement. 1. Classification, physicochemical principles, mechanisms, and electron transfer (ET)-based assays. J. Agric. Food Chem. 2016, 64, 997–1027. [Google Scholar] [CrossRef] [PubMed]
- Pulido, R.; Bravo, L.; Saura-Calixto, F. Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J. Agric. Food Chem. 2000, 48, 3396–3402. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Lindmark-Månsson, H.; Gorton, L.; Åkesson, B. Antioxidant capacity of bovine milk as assayed by spectrophotometric and amperometric methods. Int. Dairy J. 2003, 13, 927–935. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Devaki, M. The ferric reducing/antioxidant power (FRAP) assay for non-enzymatic antioxidant capacity: Concepts, procedures, limitations and applications. In Measurement of Antioxidant Activity and Capacity: Recent Trends and Applications; Apak, R., Capanoglu, E., Shahidi, F., Eds.; Wiley: Oxford, UK, 2018; pp. 77–106. ISBN 9781119135388. [Google Scholar]
- Foti, M.; Daquino, C.; Geraci, C. Esters with the DPPH• radical in alcoholic solutions. J. Org. Chem 2004, 2309–2314. [Google Scholar] [CrossRef] [PubMed]
- Nenadis, N.; Tsimidou, M.Z. DPPH (2,2-di(4-tert-octylphenyl)-1- picrylhydrazyl) radical scavenging mixed-mode colorimetric assay(s). In Measurement of Antioxidant Activity and Capacity: Recent Trends and Application; Apak, R., Capanoglu, E., Shahidi, F., Eds.; Wiley: Hoboken, NJ, USA, 2018; pp. 141–164. ISBN 978-1-119-13535-7. [Google Scholar]
- Bedlovičová, Z.; Strapáč, I.; Baláž, M.; Salayová, A. A brief overview on antioxidant activity determination of silver nanoparticles. Molecules 2020, 25, 3191. [Google Scholar] [CrossRef] [PubMed]
- Blois, M. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Mishra, K.; Ojha, H.; Chaudhury, N.K. Estimation of antiradical properties of antioxidants using DPPH-Assay: A critical review and results. Food Chem. 2012, 130, 1036–1043. [Google Scholar] [CrossRef]
- Bondet, V.; Brand-Williams, W.; Berset, C. Kinetics and mechanisms of antioxidant activity using the DPPH• free radical method. LWT Food Sci. Technol. 1997, 30, 609–615. [Google Scholar] [CrossRef]
- Kedare, S.B.; Singh, R.P. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef] [Green Version]
- Roginsky, V.; Lissi, E.A. Review of methods to determine chain-breaking antioxidant activity in food. Food Chem. 2005, 92, 235–254. [Google Scholar] [CrossRef]
- Arnao, M.B. Some methodological problems in the determination of antioxidant activity using chromogen radicals: A practical case. Trends Food Sci. Technol. 2000, 11, 419–421. [Google Scholar] [CrossRef]
- Miller, N.J.; Rice-Evans, C.; Davies, M.J.; Gopinathan, V.; Milner, A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin. Sci. 1993, 84, 407–412. [Google Scholar] [CrossRef] [Green Version]
- Miller, N.J.; Sampson, J.; Candeias, L.P.; Bramley, P.M.; Rice-Evans, C.A. Antioxidant activities of carotenes and xanthophylls. FEBS Lett. 1996, 384, 240–242. [Google Scholar] [CrossRef] [Green Version]
- Van Den Berg, R.; Haenen, G.R.M.M.; Van Den Berg, H.; Bast, A. Applicability of an improved Trolox equivalent antioxidant capacity (TEAC) assay for evaluation of antioxidant capacity measurements of mixtures. Food Chem. 1999, 66, 511–517. [Google Scholar] [CrossRef]
- Cano, A.; Arnao, M.B. An end-point method for estimation of the total antioxidant activity in plant material. Phytochem. Anal. 1998, 9, 196–202. [Google Scholar] [CrossRef]
- Alonso, Á.M.; Domínguez, C.; Guillén, D.A.; Barroso, C.G. Determination of antioxidant power of red and white wines by a new electrochemical method and its correlation with polyphenolic content. J. Agric. Food Chem. 2002, 50, 3112–3115. [Google Scholar] [CrossRef] [PubMed]
- Cano, A.; Arnao, M.B. ABTS/TEAC (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)/Trolox®-Equivalent Antioxidant Capacity) radical scavenging mixed-mode assay. In Measurement of Antioxidant Activity and Capacity: Recent Trends and Applications; Apak, R., Capanoglu, E., Shahidi, F., Eds.; Wiley: Hoboken, NJ, USA, 2018; pp. 117–139. ISBN 978-1-119-13535-7. [Google Scholar]
- Lemańska, K.; Szymusiak, H.; Tyrakowska, B.; Zieliński, R.; Soffers, A.E.M.F.; Rietjens, I.M.C.M. The influence of pH on antioxidant properties and the mechanism of antioxidant action of hydroxyflavones. Free Radic. Biol. Med. 2001, 31, 869–881. [Google Scholar] [CrossRef]
- Awika, J.M.; Rooney, L.W.; Wu, X.; Prior, R.L.; Cisneros-Zevallos, L. Screening methods to measure antioxidant activity of sorghum (Sorghum bicolor) and sorghum products. J. Agric. Food Chem. 2003, 51, 6657–6662. [Google Scholar] [CrossRef] [PubMed]
- Borrajo, P.; Pateiro, M.; Gagaoua, M.; Franco, D.; Zhang, W.; Lorenzo, J.M. Evaluation of the antioxidant and antimicrobial activities of porcine liver protein hydrolysates obtained using alcalase, bromelain, and papain. Appl. Sci. 2020, 10, 2290. [Google Scholar] [CrossRef] [Green Version]
- Duthie, G.; Campbell, F.; Bestwick, C.; Stephen, S.; Russell, W. Antioxidant effectiveness of vegetable powders on the lipid and protein oxidative stability of cooked turkey meat patties: Implications for health. Nutrients 2013, 5, 1241–1252. [Google Scholar] [CrossRef] [Green Version]
- Nishimura, K.; Suzuki, M.; Saeki, H. Glucose-conjugated chicken myofibrillar proteins derived from random-centroid optimization present potent hydroxyl radical scavenging activity. Biosci. Biotechnol. Biochem. 2019, 83, 2307–2317. [Google Scholar] [CrossRef]
- Tejerina, D.; García-Torres, S.; Cabeza De Vaca, M.; Vázquez, F.M.; Cava, R. Effect of production system on physical-chemical, antioxidant and fatty acids composition of Longissimus dorsi and Serratus ventralis muscles from Iberian pig. Food Chem. 2012, 133, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Arshad, M.S.; Anjum, F.M.; Khan, M.I.; Shahid, M.; Akhtar, S.; Sohaib, M. Wheat germ oil enrichment in broiler feed with α-lipoic acid to enhance the antioxidant potential and lipid stability of meat. Lipids Health Dis. 2013, 12, 164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kopec, W.; Wiliczkiewicz, A.; Jamroz, D.; Biazik, E.; Pudlo, A.; Hikawczuk, T.; Skiba, T.; Korzeniowska, M. Antioxidant status of turkey breast meat and blood after feeding a diet enriched with histidine. Poult. Sci. 2016, 95, 53–61. [Google Scholar] [CrossRef]
- Choi, S.-U.; Choi, I.-H.; Chung, T.-H. Investigation of breast meat traits of broilers fed different amounts of Hermetia illucens and Protaetia brevitarsis seulensis powder. Entomol. Res. 2021. [Google Scholar] [CrossRef]
- Bermúdez, R.; Franco, I.; Franco, D.; Carballo, J.; Lorenzo, J.M. Influence of inclusion of chestnut in the finishing diet on fatty acid profile of dry-cured ham from Celta pig breed. Meat Sci. 2012, 92, 394–399. [Google Scholar] [CrossRef]
- De Brito, G.F.; Ponnampalam, E.N.; Hopkins, D.L. The effect of extensive feeding systems on growth rate, carcass traits, and meat quality of finishing lambs. Compr. Rev. Food Sci. Food Saf. 2017, 16, 23–38. [Google Scholar] [CrossRef]
- Simitzis, P.E.; Charismiadou, M.A.; Goliomytis, M.; Charalambous, A.; Ntetska, I.; Giamouri, E.; Deligeorgis, S.G. Antioxidant status, meat oxidative stability and quality characteristics of lambs fed with hesperidin, naringin or α-tocopheryl acetate supplemented diets. J. Sci. Food Agric. 2019, 99, 343–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, K.P.; Fan, Y.X.; Ma, T.W.; Wang, Z.; TanTai, W.J.; Nie, H.T.; Guo, Y.X.; Yu, X.Q.; Sun, L.W.; Wang, F. Carcass traits, meat quality, antioxidant status and antioxidant gene expression in muscle and liver of Hu lambs fed perilla seed. J. Anim. Physiol. Anim. Nutr. 2018, 102, e828–e837. [Google Scholar] [CrossRef]
- Goliomytis, M.; Kartsonas, N.; Charismiadou, M.A.; Symeon, G.K.; Simitzis, P.E.; Deligeorgis, S.G. The influence of naringin or hesperidin dietary supplementation on broiler meat quality and oxidative stability. PLoS ONE 2015, 10, e0141652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cadavez, V.A.P.; Popova, T.; Bermúdez, R.; Osoro, K.; Purriños, L.; Bodas, R.; Lorenzo, J.M.; Gonzales-Barron, U. Compositional attributes and fatty acid profile of lamb meat from Iberian local breeds. Small Rumin. Res. 2020, 106244. [Google Scholar] [CrossRef]
- Echegaray, N.; Pateiro, M.; Gonzales-Barron, U.; Lorenzo, M. Influence of feeding system on Longissimus thoracis et lumborum volatile compounds of an Iberian local lamb breed. Small Rumin. Res. 2021, 201, 1064017. [Google Scholar] [CrossRef]
- Cividini, A.; Levart, A.; Žgur, S.; Kompan, D. Fatty acid composition of lamb meat from the autochthonous Jezersko-Solčava breed reared in different production systems. Meat Sci. 2014, 97, 480–485. [Google Scholar] [CrossRef]
- Domínguez, R.; Bohrer, B.; Munekata, P.E.S.; Pateiro, M.; Lorenzo, J.M. Recent discoveries in the field of lipid bio-based ingredients for meat processing. Molecules 2021, 26, 190. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, R.; Barba, F.J.; Gómez, B.; Putnik, P.; Bursać Kovačević, D.; Pateiro, M.; Santos, E.M.; Lorenzo, J.M. Active packaging films with natural antioxidants to be used in meat industry: A review. Food Res. Int. 2018, 113, 93–101. [Google Scholar] [CrossRef]
- Barros, J.C.; Munekata, P.E.S.; De Carvalho, F.A.L.; Pateiro, M.; Barba, F.J.; Domínguez, R.; Trindade, M.A.; Lorenzo, J.M. Use of tiger nut (Cyperus esculentus L.) oil emulsion as animal fat replacement in beef burgers. Foods 2020, 9, 44. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Xing, L.; Fu, Q.; Zhou, G.H.; Zhang, W.G. A review of antioxidant peptides derived from meat muscle and by-Products. Antioxidants 2016, 5, 32. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.J.; Kim, K.H.; Kim, Y.S.; Kim, E.K.; Hwang, J.W.; Lim, B.O.; Moon, S.H.; Jeon, B.T.; Jeon, Y.J.; Ahn, C.B.; et al. Biological activity from the gelatin hydrolysates of duck skin by-Products. Process Biochem. 2012, 47, 1150–1154. [Google Scholar] [CrossRef]
| Reaction Mechanisms | Assay | Oxidizing Agent | Probe | Detection | Monitored Changes |
|---|---|---|---|---|---|
| HAT | ORAC | ROO• | Fluorescein | Fluorometry | Fluorescence → Non–fluorescence product |
| HORAC | OH• | Fluorescein | Fluorometry | ||
| ET | Folin–Ciocalteu | Mo6+ | FCR | Spectrophotometric | Yellow color → Blue color |
| FRAP | Fe3+ | TPTZ | Spectrophotometric | Colorless → Blue color | |
| HAT + ET | DPPH• | DPPH• radical | DPPH• radical | Spectrophotometric | Violet color → Pale yellow color |
| ABTS•+ | ABTS•+ radical cation | ABTS•+ radical cation | Spectrophotometric | Blue–green color → Colorless |
| Method | Advantages | Disadvantages |
|---|---|---|
| ORAC | Versatile technique Useful in complex matrices Representative free radical (ROO•) | Specialized equipment necessity (fluorometer) Long reaction times Temperature sensitive |
| HORAC | Representative free radical (OH•) | Specialized equipment necessity (fluorometer) Long reaction times |
| TPC by Folin–Ciocalteu | Simple method Reproducible technique Robust assay | Detection of possible interferences Temperature sensitive pH sensitive |
| FRAP | Simple method Quick test No specialized equipment | Substances with lower redox potential than Fe3+/Fe2+ act as interferences Not quantify antioxidants with –SH groups Not representative conditions of biological systems pH conditions that favor protein precipitation Possible interferences in the measurement of absorbance |
| DPPH• | Simple method Quick test Reactive DPPH• not need previous generation No specialized equipment | Steric impediment of reactions between large molecules and DPPH• Substances with an absorption like DPPH• act as interferences Not appropriate for hydrophilic antioxidants Not suitable for emulsions Causes protein precipitation Not a biological radical |
| ABTS•+ | Simple method Quick test Permits working in a wide pH range Useful for hydro– and lipophilic antioxidants No specialized equipment | Requires previous generation of radical Not a biological radical |
| Assay | Meat Matrix | Purpose of the Antioxidant Capacity Determination | References |
|---|---|---|---|
| ORAC | Angus–crossbred steers meat | Study of the effect of grazing forage species | [47] |
| Cooked beef burgers | Research of the addition of chia seeds and/or goji puree | [40] | |
| Liver protein hydrolysates | Select suitable hydrolysis conditions | [109] | |
| HORAC | Cooked turkey patties | Study of the addition of different vegetable powders | [110] |
| Chicken myofibrillar proteins | Ensure the obtaining of functional proteins | [111] | |
| TPC by Folin–Ciocalteu | Iberian pig meat | Study of the influence of the acorns and grass in the pig diet | [112] |
| Celta pig meat and liver | Investigation of the effect of chestnut in the pig diet | [35] | |
| Pig meat | Study of the influence of a local pig breed | [38] | |
| Cooked turkey patties | Study of the addition of different vegetable powders | [110] | |
| Cooked beef burgers | Research of the addition of chia seeds and/or goji puree | [40] | |
| Cobb chicken meat | Contemplation of the effect of a dietary herbal extract | [27] | |
| Broiler chicken meat | Study of dietary supplementation with natural antioxidants | [113] | |
| Rabbit meat | Study of the inclusion of a diet enriched in cauliflower powder | [52] | |
| FRAP | Celta pig meat and liver | Investigation of the effect of chestnut in the pig diet | [35] |
| Rabbit meat | Study of the inclusion of a diet enriched in cauliflower powder | [52] | |
| Lamb meat | Study of the effect of dietary rosemary extract | [37] | |
| Broiler chicken meat | Study of dietary supplementation with natural antioxidants | [113] | |
| Turkey breast meat | Investigation of dietary supplementation with histidine | [114] | |
| Chicken meat | Contemplation of the influence of the chicken genotype | [39] | |
| Rabbit meat hamburgers | Investigation of the effect of turmeric powder addition | [53] | |
| Rabbit meat hamburgers | Research of the addition of ginger powder | [41] | |
| Liver protein hydrolysates | Select suitable hydrolysis conditions | [109] | |
| DPPH• | Celta pig meat and liver | Investigation of the effect of chestnut in the pig diet | [35] |
| Lamb meat | Study of the effect of dietary rosemary extract | [37] | |
| Cobb chicken meat | Contemplation of the effect of a dietary herbal extract | [27] | |
| Broiler chicken meat | Study of dietary supplementation with natural antioxidants | [113] | |
| Arbor Acres chicken meat | Research of the employ of insects as protein sources in chicken diet | [115] | |
| Turkey breast meat | Investigation of dietary supplementation with histidine | [114] | |
| Chicken meat | Contemplation of the influence of the chicken genotype | [39] | |
| Cooked beef burgers | Research of the addition of chia seeds and/or goji puree | [40] | |
| Rabbit meat hamburgers | Investigation of the effect of turmeric powder addition | [53] | |
| Rabbit meat hamburgers | Research of the addition of ginger powder | [41] | |
| Reformulated lamb hamburgers | Study of guarana seed and pitanga leaf extracts addition | [42] | |
| Reformulated lamb sausages | Investigation of the addition of turmeric extract | [14] | |
| Liver protein hydrolysates | Select suitable hydrolysis conditions | [109] | |
| ABTS•+ | Iberian pig meat | Study of the influence of the acorns and grass in the pig diet | [112] |
| Celta pig meat and liver | Investigation of the effect of chestnut in the pig diet | [35] | |
| Lamb meat | Study of the effect of dietary rosemary extract | [37] | |
| Cobb chicken meat | Contemplation of the effect of a dietary herbal extract | [27] | |
| Turkey breast meat | Investigation of dietary supplementation with histidine | [114] | |
| Rabbit meat | Study of the inclusion of a diet enriched in cauliflower powder | [52] | |
| Chicken meat | Contemplation of the influence of the chicken genotype | [39] | |
| Cooked beef burgers | Research of the addition of chia seeds and/or goji puree | [40] | |
| Rabbit meat hamburgers | Investigation of the effect of turmeric powder addition | [53] | |
| Rabbit meat hamburgers | Research of the addition of ginger powder | [41] | |
| Liver protein hydrolysates | Select the suitable hydrolysis conditions | [109] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Echegaray, N.; Pateiro, M.; Munekata, P.E.S.; Lorenzo, J.M.; Chabani, Z.; Farag, M.A.; Domínguez, R. Measurement of Antioxidant Capacity of Meat and Meat Products: Methods and Applications. Molecules 2021, 26, 3880. https://doi.org/10.3390/molecules26133880
Echegaray N, Pateiro M, Munekata PES, Lorenzo JM, Chabani Z, Farag MA, Domínguez R. Measurement of Antioxidant Capacity of Meat and Meat Products: Methods and Applications. Molecules. 2021; 26(13):3880. https://doi.org/10.3390/molecules26133880
Chicago/Turabian StyleEchegaray, Noemí, Mirian Pateiro, Paulo E. S. Munekata, José M. Lorenzo, Zakariya Chabani, Mohamed A. Farag, and Rubén Domínguez. 2021. "Measurement of Antioxidant Capacity of Meat and Meat Products: Methods and Applications" Molecules 26, no. 13: 3880. https://doi.org/10.3390/molecules26133880
APA StyleEchegaray, N., Pateiro, M., Munekata, P. E. S., Lorenzo, J. M., Chabani, Z., Farag, M. A., & Domínguez, R. (2021). Measurement of Antioxidant Capacity of Meat and Meat Products: Methods and Applications. Molecules, 26(13), 3880. https://doi.org/10.3390/molecules26133880












