Fermentative Production of Fructo-Oligosaccharides Using Aureobasidium pullulans: Effect of Dissolved Oxygen Concentration and Fermentation Mode
Abstract
:1. Introduction
2. Results and Discussion
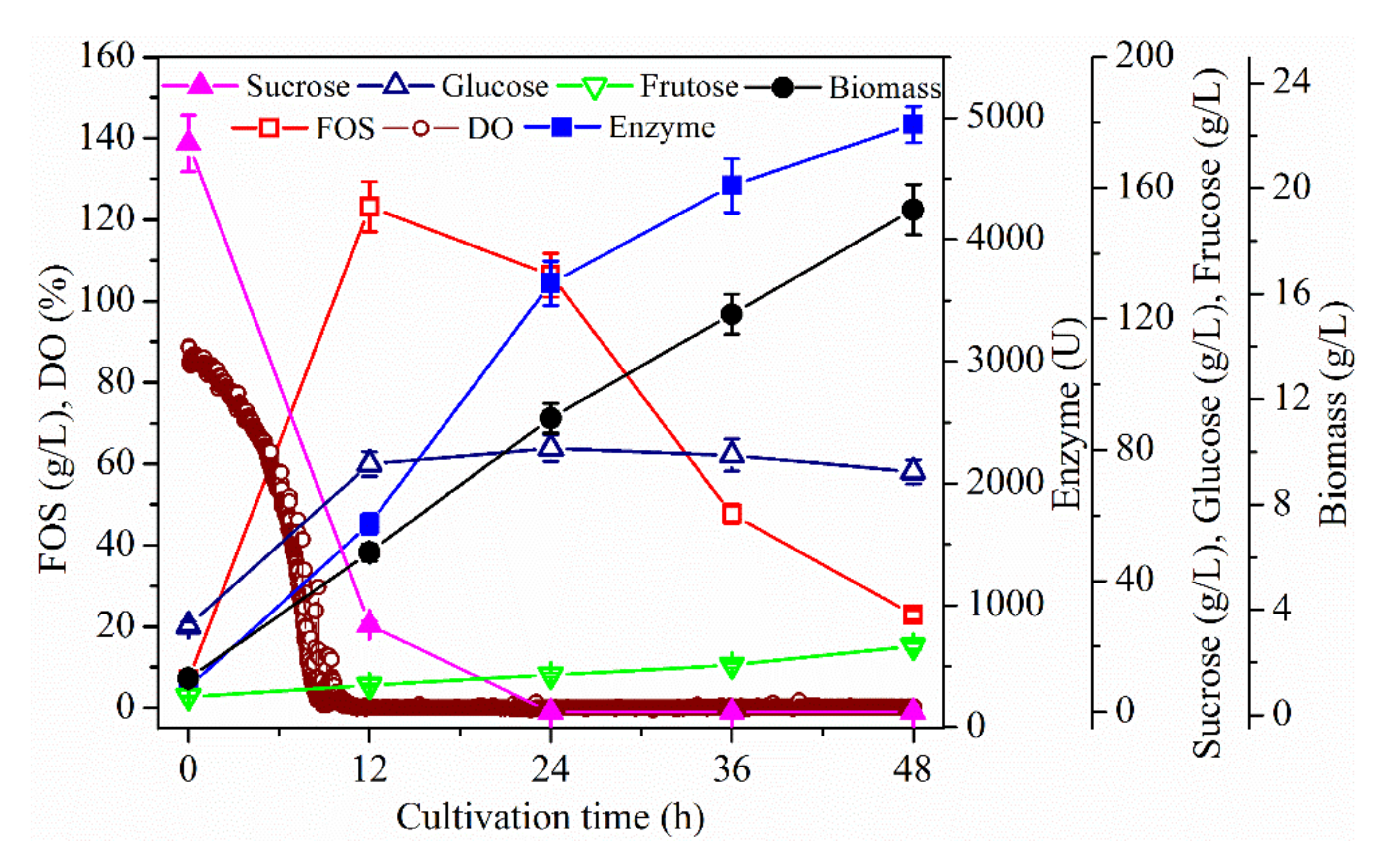
2.1. Effect of Dissolved Oxygen (DO) Concentration on Fructo-Oligosaccharides (FOS) Production
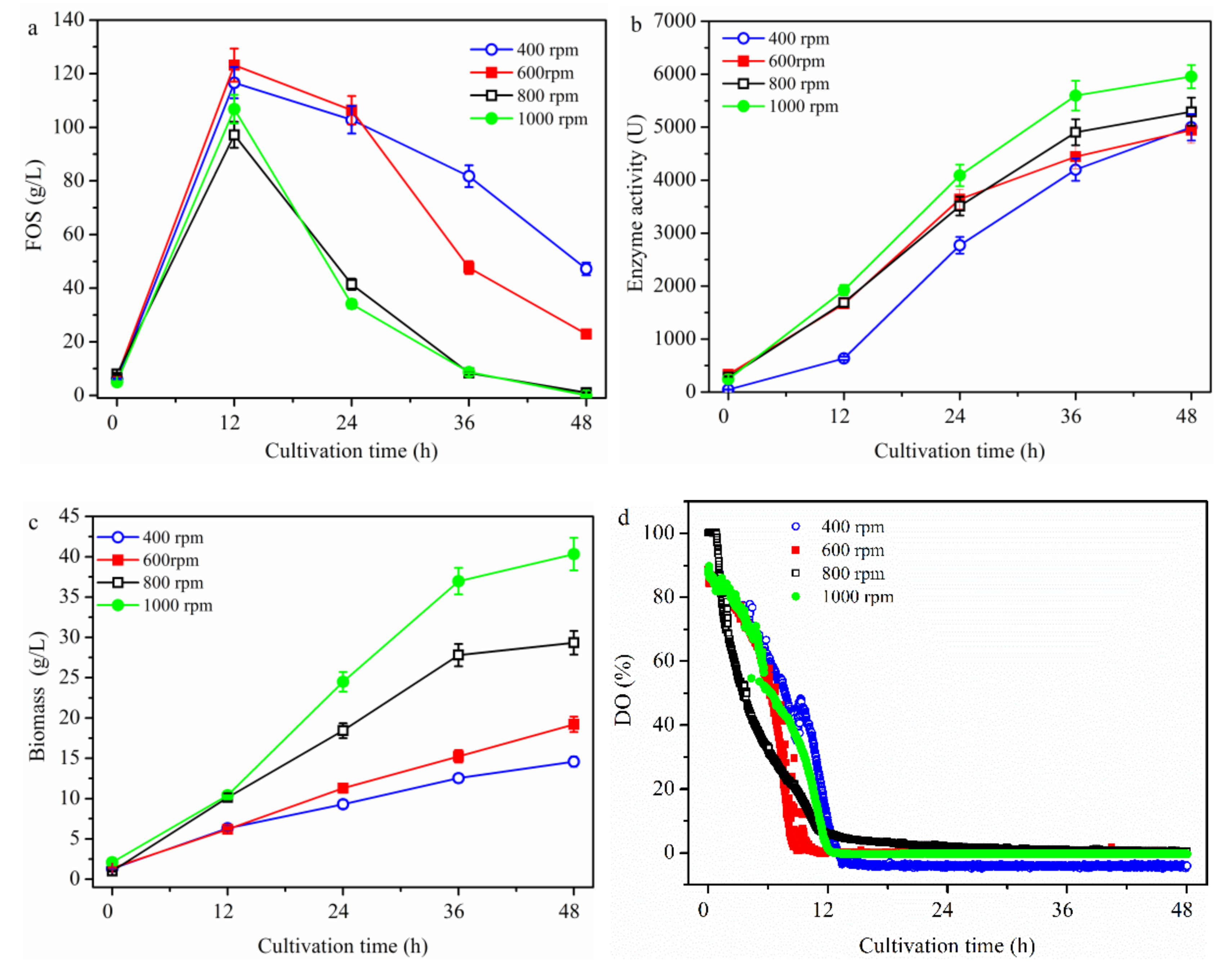
2.1.1. Effect of Stirring Speed on FOS Production
2.1.2. Effect of Aeration Rate on FOS Production
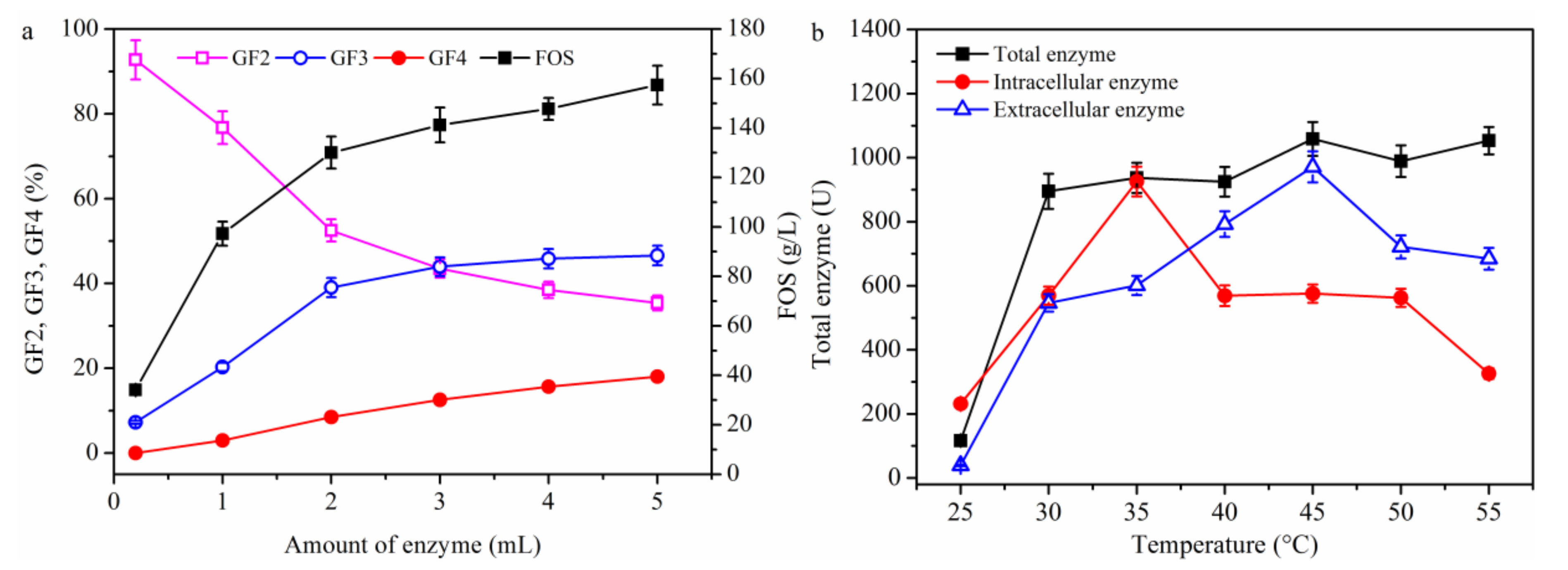
2.1.3. Effect of Bioreactor Partial Pressure on FOS Production
2.1.4. Effect of Temperature on FOS Production
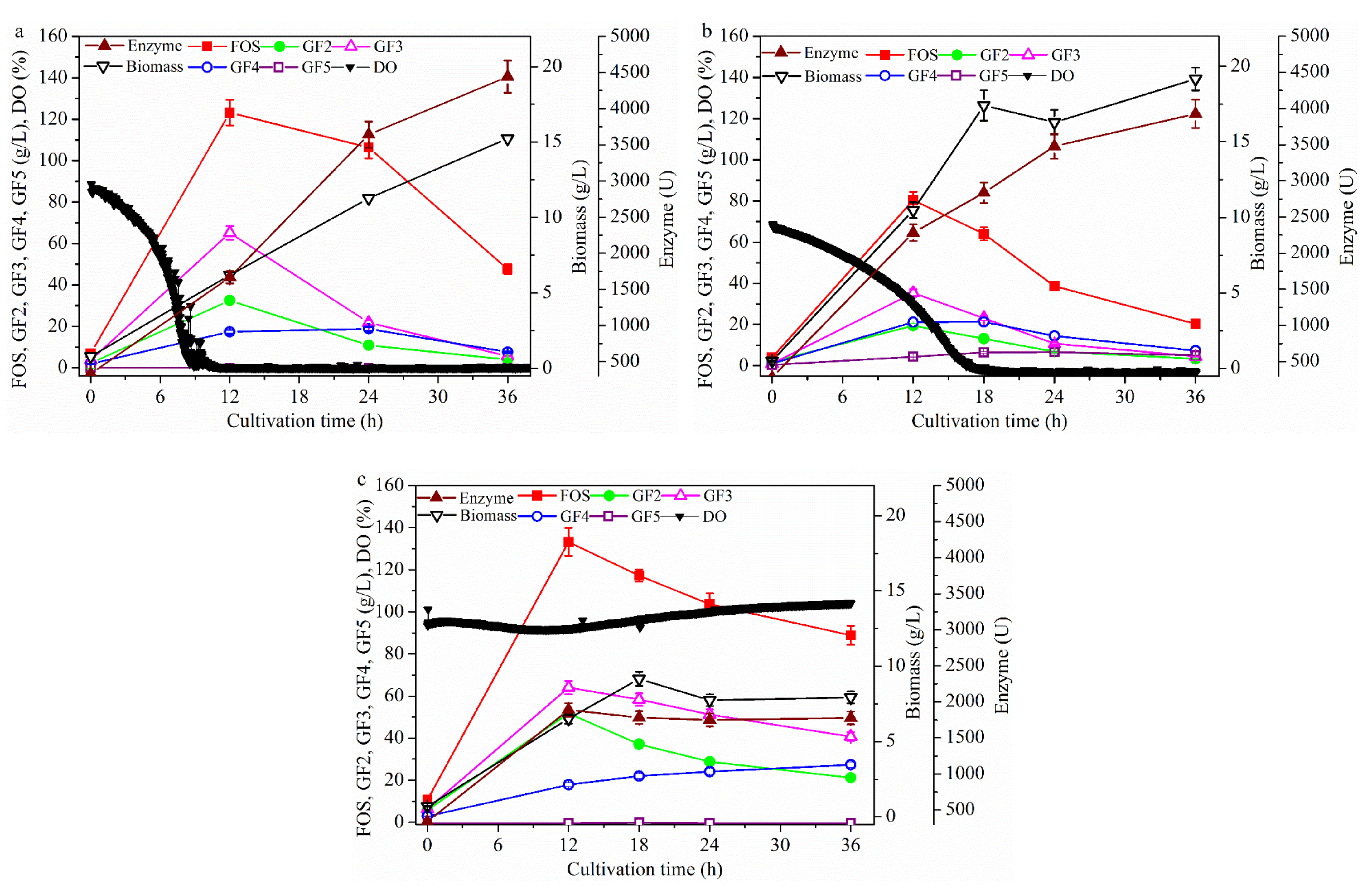
2.2. Effect of Fermentation Mode on FOS Production
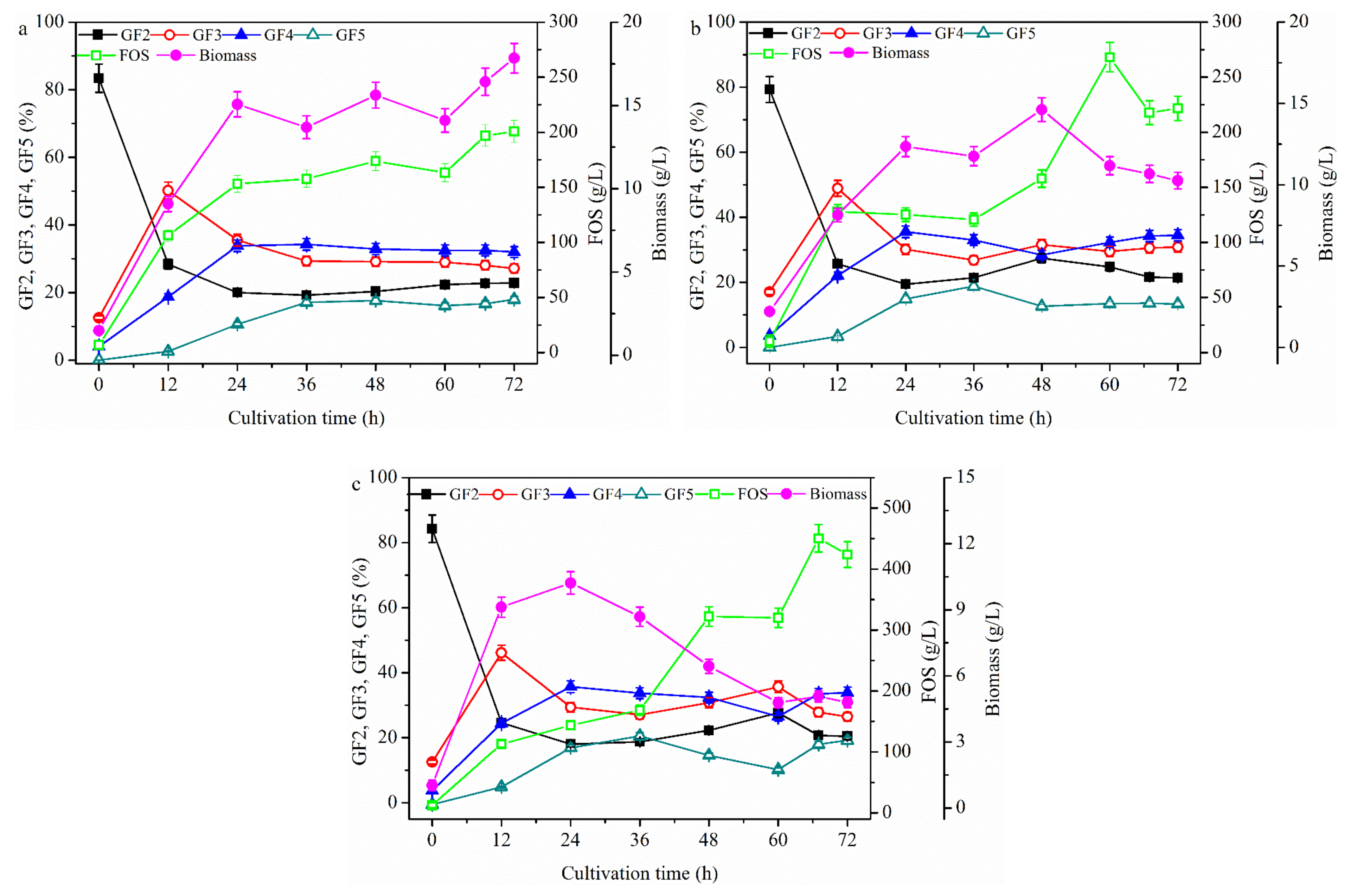
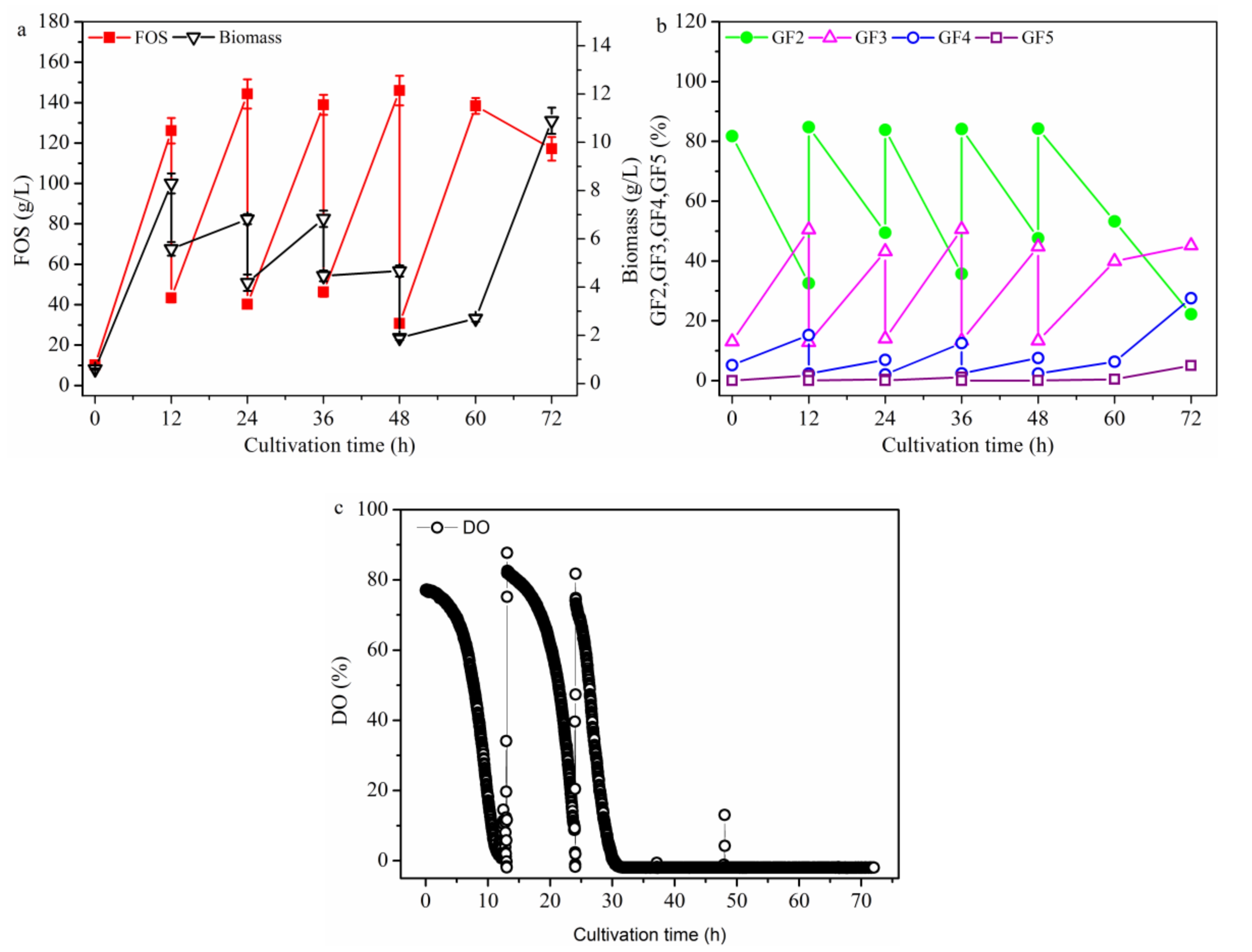
2.2.1. FOS Production in a Repeated-Batch Culture with 500 mL Broth Discharged Each Time
2.2.2. FOS Production in a Membrane Integrated Repeated Batch Culture
2.2.3. FOS Production in a Repeated-Culture with 1.6 L Broth Discharged Each Time
3. Materials and Methods
3.1. Design of Research Works
3.1.1. Effect of DO on FOS Production
3.1.2. Effect of Fermentation Mode on FOS Production
3.2. Microorganism
3.3. Culture Medium
3.4. Culture Method
3.5. Analytical Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FOS | fructo-oligosaccharides |
| DO | dissolved oxygen |
| GF4 | 1-fructofuranosyl nystose |
| GF5 | 1,1,1,1-kestohexose |
| GF2 | 1-kestose |
| GF3 | nystose |
| Yp/s | FOS yield |
| Yp/b | specific FOS production (FOS/biomass), |
| Ye/b | specific activity of fructosyltransferase (fructosyltransferase activity/biomass) |
References
- Bali, V.; Panesar, P.; Bera, M.B.; Panesar, R. Fructo-oligosaccharides: Production, Purification and Potential Applications. Crit. Rev. Food Sci. Nutr. 2015, 55, 1475–1490. [Google Scholar] [CrossRef]
- Dominguez, A.L.; Rodrigues, L.R.; Lima, N.M.; Teixeira, J.A. An overview of the recent developments on fructooligosaccharide production and applications. Food Bioprocess Tech. 2014, 7, 324–337. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Martínez, M.J.; Soto-Jover, S.; Antolinos, V.; Martínez-Hernández, G.B.; López-Gómez, A. Manufacturing of Short-Chain Fructooligosaccharides: From Laboratory to Industrial Scale. Food Eng. Rev. 2020, 12, 149–172. [Google Scholar] [CrossRef]
- Dominguez, A.; Nobre, C.; Rodrigues, L.R.; Peres, A.M.; Torres, D.; Rocha, I.; Lima, N.; Teixeira, J. New improved method for fructooligosaccharides production by Aureobasidium pullulans. Carbohydr. Poly. 2012, 89, 1174–1179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, P.; Dubey, K.K. Current perspectives and future strategies for fructooligosaccharides production through membrane bioreactor. In Applied Microbiology and Bioengineering; Shukla, P., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 185–202, Chapter 10. [Google Scholar]
- Castro, C.C.; Nobre, C.; Duprez, M.E.; De Weireld, G.; Hantson, A.L. Screening and selection of potential carriers to immobilize Aureobasidium pullulans cells for fructo-oligosaccharides production. Biochem. Eng. J. 2017, 118, 82–90. [Google Scholar] [CrossRef] [Green Version]
- Khatun, M.S.; Harrison, M.D.; Speight, R.; O’Hara, I.; Zhang, Z. Efficient production of fructo-oligosaccharides from sucrose and molasses by a novel Aureobasidium pullulan strain. Biochem. Eng. J. 2020, 163, 107747. [Google Scholar] [CrossRef]
- Fekih-Salem, R.; Schorsch, J.; Dewasme, L.; Castro, C.; Hantson, A.; Kinnaert, M.; Vande Wouwer, A. Data-driven modeling and optimal control of the production of Fructo-oligosaccharides by Aureobasidium Pullulans. J. Process Contr. 2019, 81, 136–149. [Google Scholar] [CrossRef]
- Zhang, S.; Jiang, H.; Xue, S.; Ge, N.; Chi, Z. Efficient conversion of cane molasses into FOSs by a glucose derepression mutant of Aureobasidium melanogenum with high β-fructofuranosidase activity. J. Agric. Food Chem. 2019, 67, 13665–13672. [Google Scholar] [CrossRef]
- Muñiz-Márquez, D.B.; Teixeira, J.A.; Mussatto, S.I.; Contreras-Esquivel, J.C.; Rodríguez-Herrera, R.; Aguilar, C.N. Fructo-oligosaccharides (FOS) production by fungal submerged culture using aguamiel as a low-cost by-product. LWT 2019, 102, 75–79. [Google Scholar] [CrossRef] [Green Version]
- Choukade, R.; Kango, N. Characterization of a mycelial fructosyltransferase from Aspergillus tamarii NKRC 1229 for efficient synthesis of fructooligosaccharides. Food Chem. 2019, 286, 434–440. [Google Scholar] [CrossRef]
- Magri, A.; Oliveira, M.; Baldo, C.; Tischer, C.; Sartori, D.; Mantovani, M.; Celligoi, M. Production of fructooligosaccharides by Bacillus subtilis natto CCT7712 and their antiproliferative potential. J. Appl. Microbiol. 2020, 128, 1414–1426. [Google Scholar] [CrossRef]
- Nobre, C.; do Nascimento, A.K.C.; Silva, S.P.; Coelho, E.; Coimbra, M.A.; Cavalcanti, M.T.H.; Teixeira, J.A.; Porto, A.L.F. Process development for the production of prebiotic fructo-oligosaccharides by Penicillium citreonigrum. Bioresour. Technol. 2019, 282, 464–474. [Google Scholar] [CrossRef] [Green Version]
- Bedzo, O.K.K.; Trollope, K.; Gottumukkala, L.D.; Coetzee, G.; Görgens, J.F. Amberlite IRA 900 versus calcium alginate in immobilization of a novel, engineered β-fructofuranosidase for short-chain fructooligosaccharide synthesis from sucrose. Biotechnol. Prog. 2019, e2797. [Google Scholar] [CrossRef]
- De Oliveira, R.L.; da Silva, M.F.; da Silva, S.P.; de Araújo, A.C.V.; Cavalcanti, J.V.F.L.; Converti, A.; Porto, T.S. Fructo-oligosaccharides production by an Aspergillus aculeatus commercial enzyme preparation with fructosyltransferase activity covalently immobilized on Fe3O4–chitosan-magnetic nanoparticles. Int. J. Biol. Macromol. 2020, 150, 922–929. [Google Scholar] [CrossRef]
- Ganaie, M.A.; Rawat, H.K.; Wani, O.A.; Gupta, U.S.; Kango, N. Immobilization of fructosyltransferase by chitosan and alginate for efficient production of fructooligosaccharides. Process Biochem. 2014, 49, 840–844. [Google Scholar] [CrossRef]
- Zeng, X.A.; Zhou, K.; Liu, D.; Brennan, C.S.; Brennan, M.; Zhou, J.; Yu, S. Preparation of fructooligosaccharides using Aspergillus niger 6640 whole-cell as catalyst for bio-transformation. LWT 2016, 65, 1072–1079. [Google Scholar] [CrossRef]
- Schorsch, J.; Castro, C.C.; Couto, L.D.; Nobre, C.; Kinnaert, M. Optimal control for fermentative production of fructo-oligosaccharides in fed-batch bioreactor. J. Process Control 2019, 78, 124–138. [Google Scholar] [CrossRef] [Green Version]
- Nobre, C.; Gonçalves, D.A.; Teixeira, J.A.; Rodrigues, L.R. One-step co-culture fermentation strategy to produce high-content fructo-oligosaccharides. Carbohydr. Polym. 2018, 201, 31–38. [Google Scholar] [CrossRef] [Green Version]
- Sangeetha, P.T.; Ramesh, M.N.; Prapulla, S.G. Maximization of fructooligosaccharide production by two stage continuous process and its scale up. J. Food. Eng. 2005, 68, 57–64. [Google Scholar] [CrossRef]
- Cao, W.; Qi, B.; Zhao, J.; Qiao, C.; Su, Y.; Wan, Y. Control strategy of pH, dissolved oxygen concentration and stirring speed for enhancing β-poly(malic acid) production by Aureobasidium pullulans ipe-1. J. Chem. Technol. Biotech. 2013, 88, 808–817. [Google Scholar] [CrossRef]
- Gibbs, P.; Seviour, R. Does the agitation rate and/or oxygen saturation influence exopolysaccharide production by Aureobasidium pullulans in batch culture? Appl. Microbiol. Biotechnol. 1996, 46, 503–510. [Google Scholar] [CrossRef]
- Gomez, R.; Schnabel, I.; Garrido, J. Pellet growth and citric acid yield of Aspergillus niger 110. Enzyme Microb. Technol. 1988, 10, 188–191. [Google Scholar]
- Mussatto, S.I.; Rodrigues, L.R.; Teixeira, J.A. β-Fructofuranosidase production by repeated batch fermentation with immobilized Aspergillus japonicus. J. Ind. Microbiol. Biotechnol. 2009, 36, 923–928. [Google Scholar] [CrossRef]
- Picazo, B.; Flores-Gallegos, A.C.; Muñiz-Márquez, D.B.; Flores-Maltos., A.; Michel-Michel, M.R.; de la Rosa, O.; Rodríguez-Jasso, R.M.; Rodríguez-Herrera, R.; Aguilar-González, C.N. Enzymes for fructooligosaccharides production. Achievements and opportunities. In Enzymes in Food Biotechnology; Kuddus, M., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 303–320, Chapter 18. [Google Scholar]
- Cao, W.; Luo, J.; Qi, B.; Zhao, J.; Qiao, C.; Ding, L.; Su, Y.; Wan, Y. β-poly(l-malic acid) production by fed-batch culture of Aureobasidium pullulans ipe-1 with mixed sugars. Eng. Life Sci. 2014, 14, 180–189. [Google Scholar] [CrossRef]
- Zhang, H.; Cai, J.; Dong, J.; Zhang, D.; Huang, L.; Xu, Z.; Cen, P. High-level production of poly (β-L-malic acid) with a new isolated Aureobasidium pullulans strain. Appl. Microbiol. Biotechnol. 2012, 92, 295–303. [Google Scholar] [CrossRef]
- Hayashi, S.; Nonokuchi, M.; Imada, K.; Ueno, H. Production of a fructosyl-transferring enzyme by Aureobasidium sp. ATCC 20524. J. Ind. Microbiol. 1990, 5, 395–399. [Google Scholar] [CrossRef]
- Shin, H.T.; Baig, S.Y.; Lee, S.W.; Suh, D.S.; Kwon, S.T.; Lim, Y.B.; Lee, J.H. Production of fructo-oligosaccharides from molasses by Aureobasidium pullulans cells. Bioresour. Technol. 2004, 93, 59–62. [Google Scholar] [CrossRef]
- Yun, J.W.; Lee, M.G.; Song, S.K. Batch production of high-content fructo-oligosaccharides from sucrose by the mixed-enzyme system of β-fructofuranosidase and glucose oxidase. J. Ferment. Bioeng. 1994, 77, 159–163. [Google Scholar] [CrossRef]
- Bolivar, J.M.; Mannsberger, A.; Thomsen, M.S.; Tekautz, G.; Nidetzky, B. Process intensification for O2-dependent enzymatic transformations in continuous single-phase pressurized flow. Biotechnol. Bioeng. 2019, 116, 503–514. [Google Scholar] [CrossRef] [Green Version]
- Ringborg, R.H.; Toftgaard Pedersen, A.; Woodley, J.M. Automated determination of oxygen-dependent enzyme kinetics in a tube-in-tube flow reactor. Chem. Cat. Chem. 2017, 9, 3273. [Google Scholar]
- Liu, S.; Steinbüchel, A. Investigation of poly (β-L-malic acid) production by strains of Aureobasidium pullulans. Appl. Microbiol. Biotechnol. 1996, 46, 273–278. [Google Scholar] [CrossRef]
- Fan, R.; Burghardt, J.P.; Prell, F.; Zorn, H.; Czermak, P. Production and purification of fructo-oligosaccharides using an enzyme membrane bioreactor and subsequent fermentation with probiotic Bacillus coagulans. Sep. Purif. Technol. 2020, 251, 117291. [Google Scholar] [CrossRef]
- Burghardt, J.P.; Coletta, L.A.; van der Bolt, R.; Ebrahimi, M.; Gerlach, D.; Czermak, P. Development and characterization of an enzyme membrane reactor for fructo-oligosaccharide production. Membranes 2019, 9, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, W.F.; Luo, J.Q.; Zhao, J.; Qiao, C.S.; Ding, L.H.; Qi, B.K.; Su, Y.; Wan, Y.H. Intensification of β-poly(L-malic acid) production by Aureobasidium pullulans ipe-1 in the late exponential growth phase. J. Ind. Microbiol. Biotechnol. 2012, 39, 1073–1080. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, X.; Li, C.; Cao, W.; Cao, W.; Shen, F.; Wan, Y. Fermentative Production of Fructo-Oligosaccharides Using Aureobasidium pullulans: Effect of Dissolved Oxygen Concentration and Fermentation Mode. Molecules 2021, 26, 3867. https://doi.org/10.3390/molecules26133867
Liang X, Li C, Cao W, Cao W, Shen F, Wan Y. Fermentative Production of Fructo-Oligosaccharides Using Aureobasidium pullulans: Effect of Dissolved Oxygen Concentration and Fermentation Mode. Molecules. 2021; 26(13):3867. https://doi.org/10.3390/molecules26133867
Chicago/Turabian StyleLiang, Xinquan, Chenglin Li, Weifeng Cao, Weilei Cao, Fei Shen, and Yinhua Wan. 2021. "Fermentative Production of Fructo-Oligosaccharides Using Aureobasidium pullulans: Effect of Dissolved Oxygen Concentration and Fermentation Mode" Molecules 26, no. 13: 3867. https://doi.org/10.3390/molecules26133867
APA StyleLiang, X., Li, C., Cao, W., Cao, W., Shen, F., & Wan, Y. (2021). Fermentative Production of Fructo-Oligosaccharides Using Aureobasidium pullulans: Effect of Dissolved Oxygen Concentration and Fermentation Mode. Molecules, 26(13), 3867. https://doi.org/10.3390/molecules26133867





