Antimicrobial Peptide Dendrimers and Quorum-Sensing Inhibitors in Formulating Next-Generation Anti-Infection Cell Therapy Dressings for Burns
Abstract
:1. Introduction
2. Results and Discussion
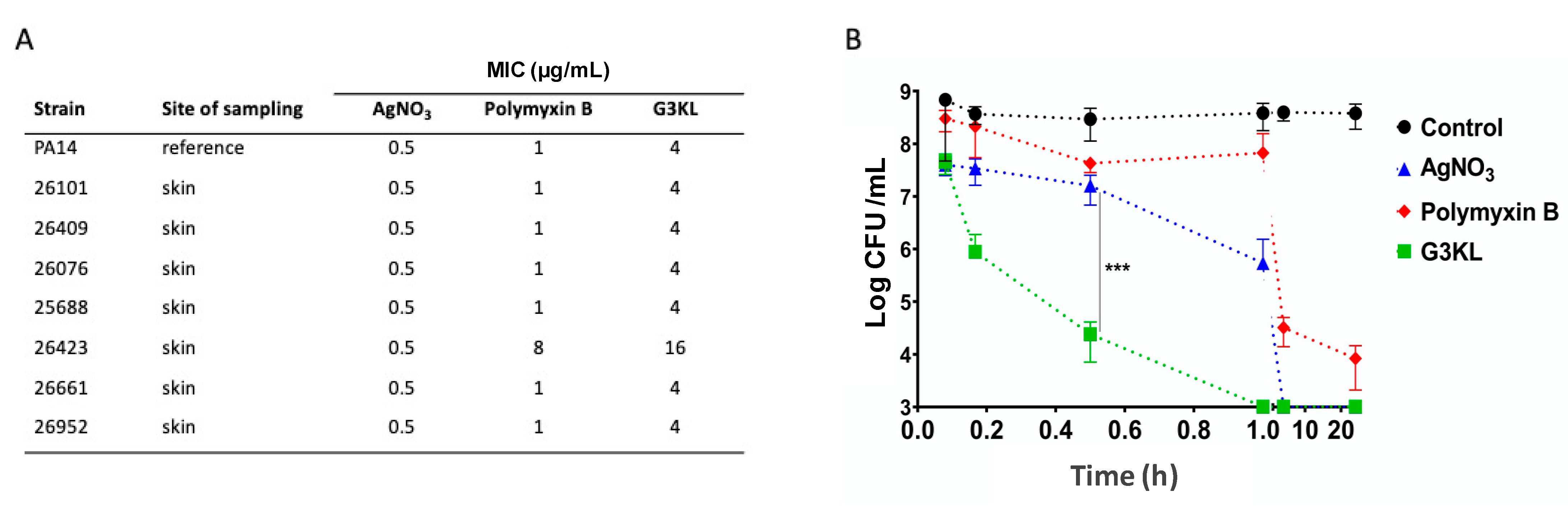
2.1. G3KL Has a Rapid Onset of Bactericidal Effect and Is Active against Clinical Isolates of P. aeruginosa from Burn Wounds
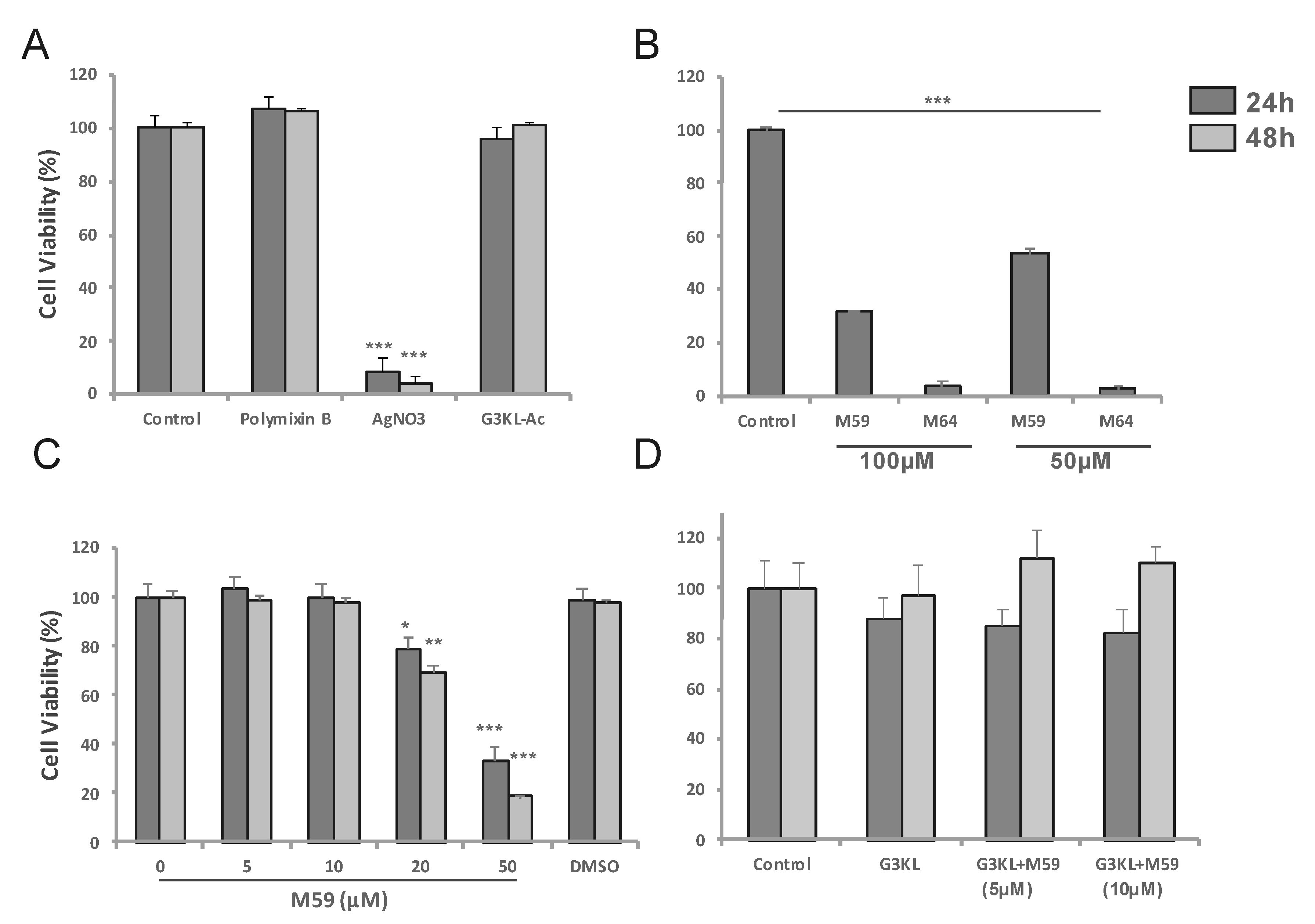
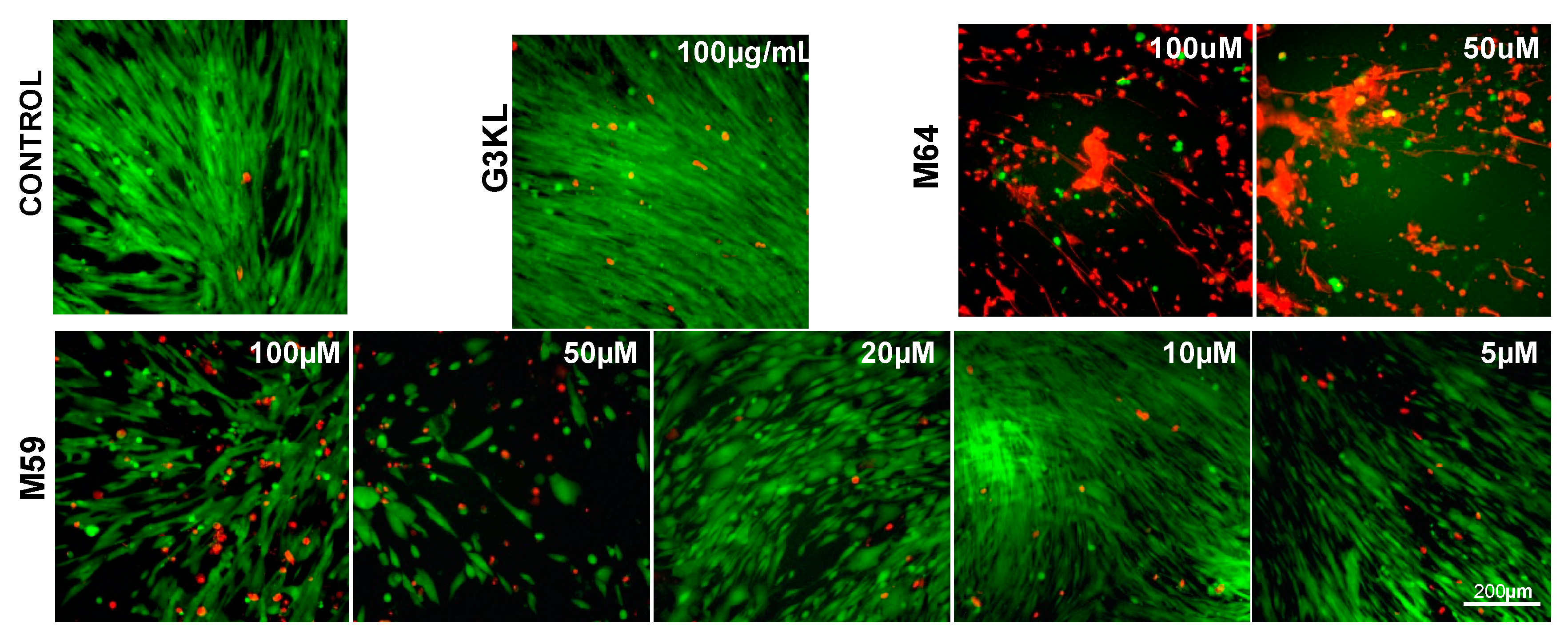
2.2. Identifying the Cytocompatible Concentrations of AMPDs and QS-Inhibitors
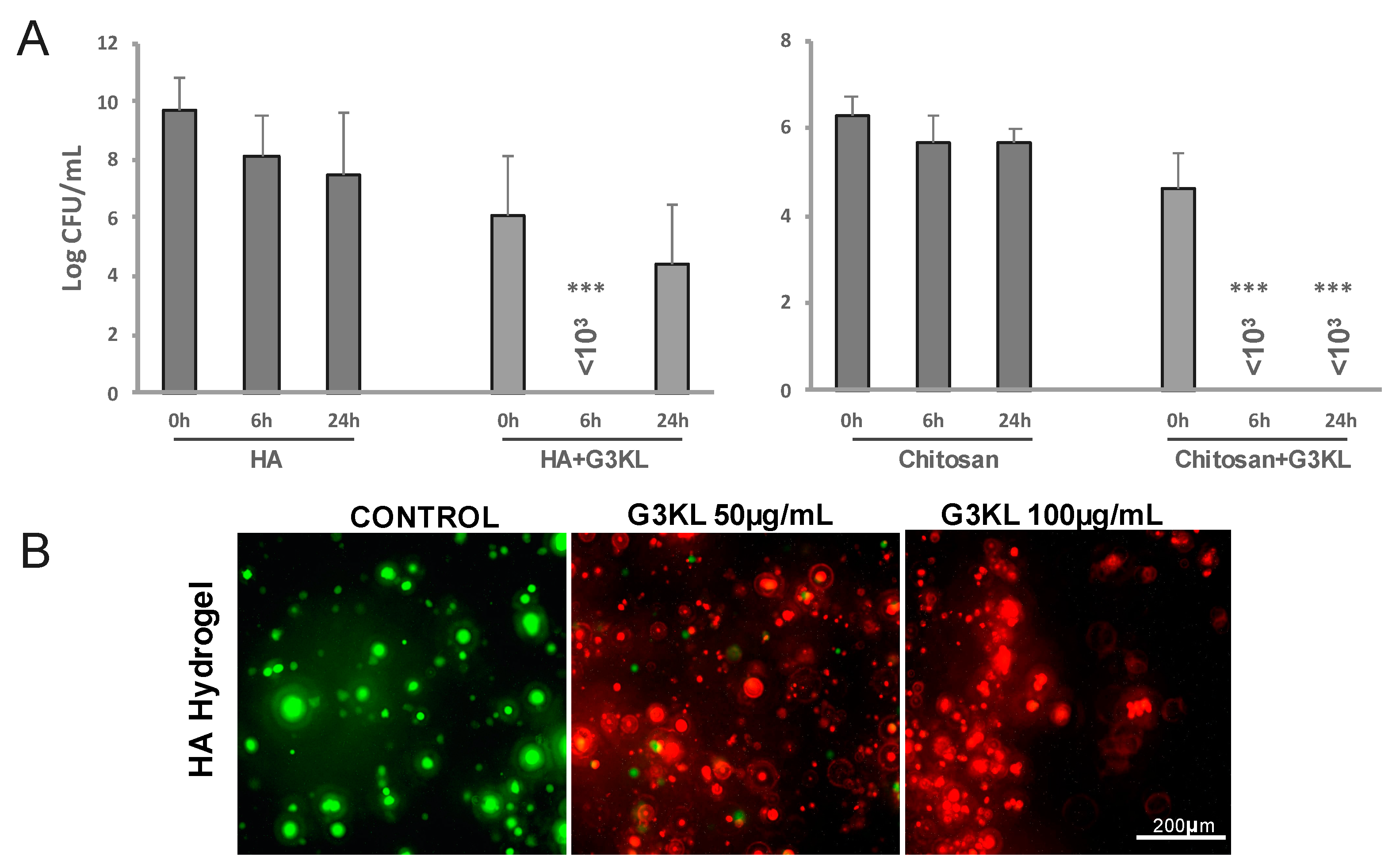
2.3. Identifying the Best Matrix for the Delivery of G3KL and Progenitor Skin Cells to Burn Wounds
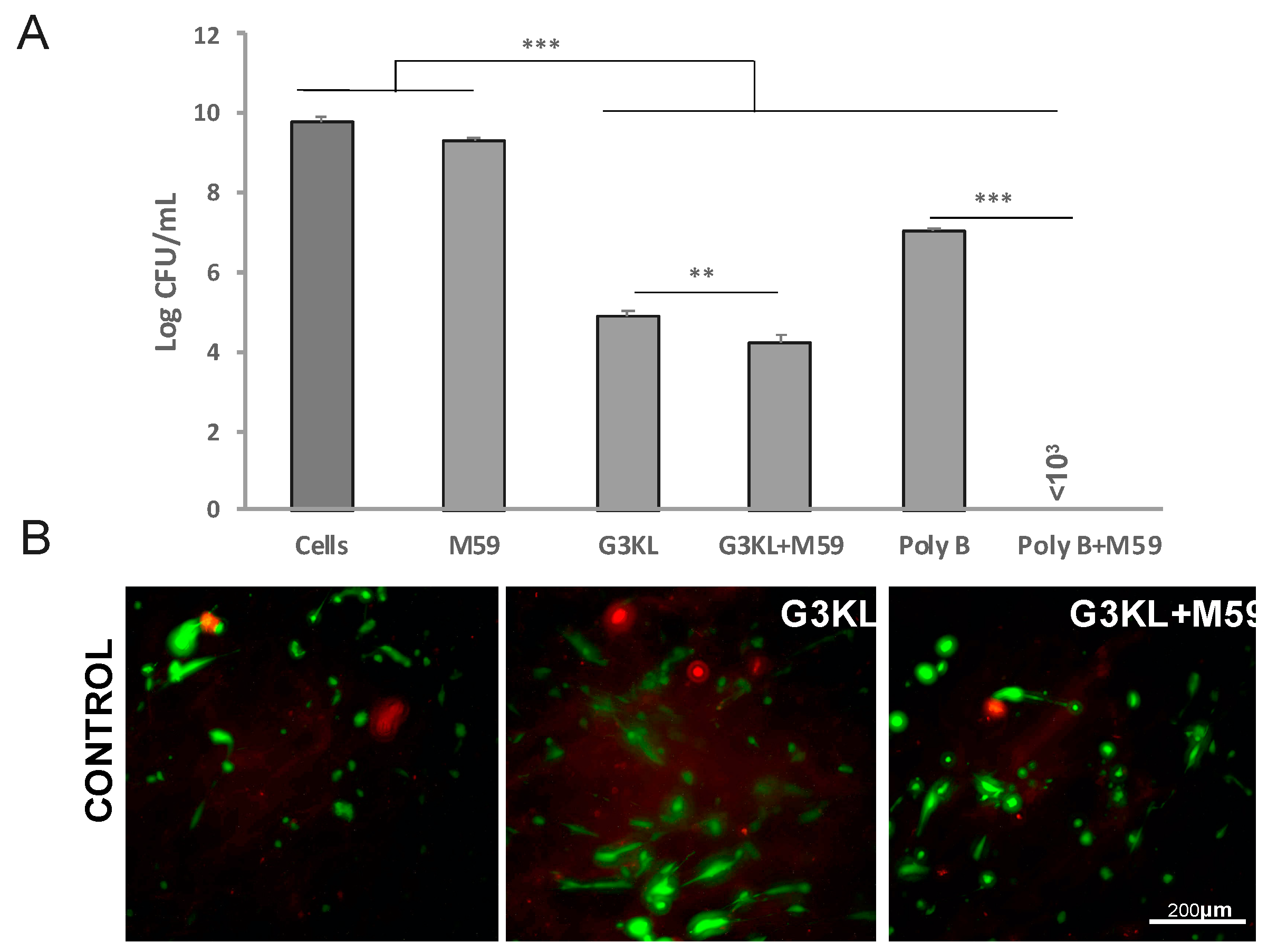
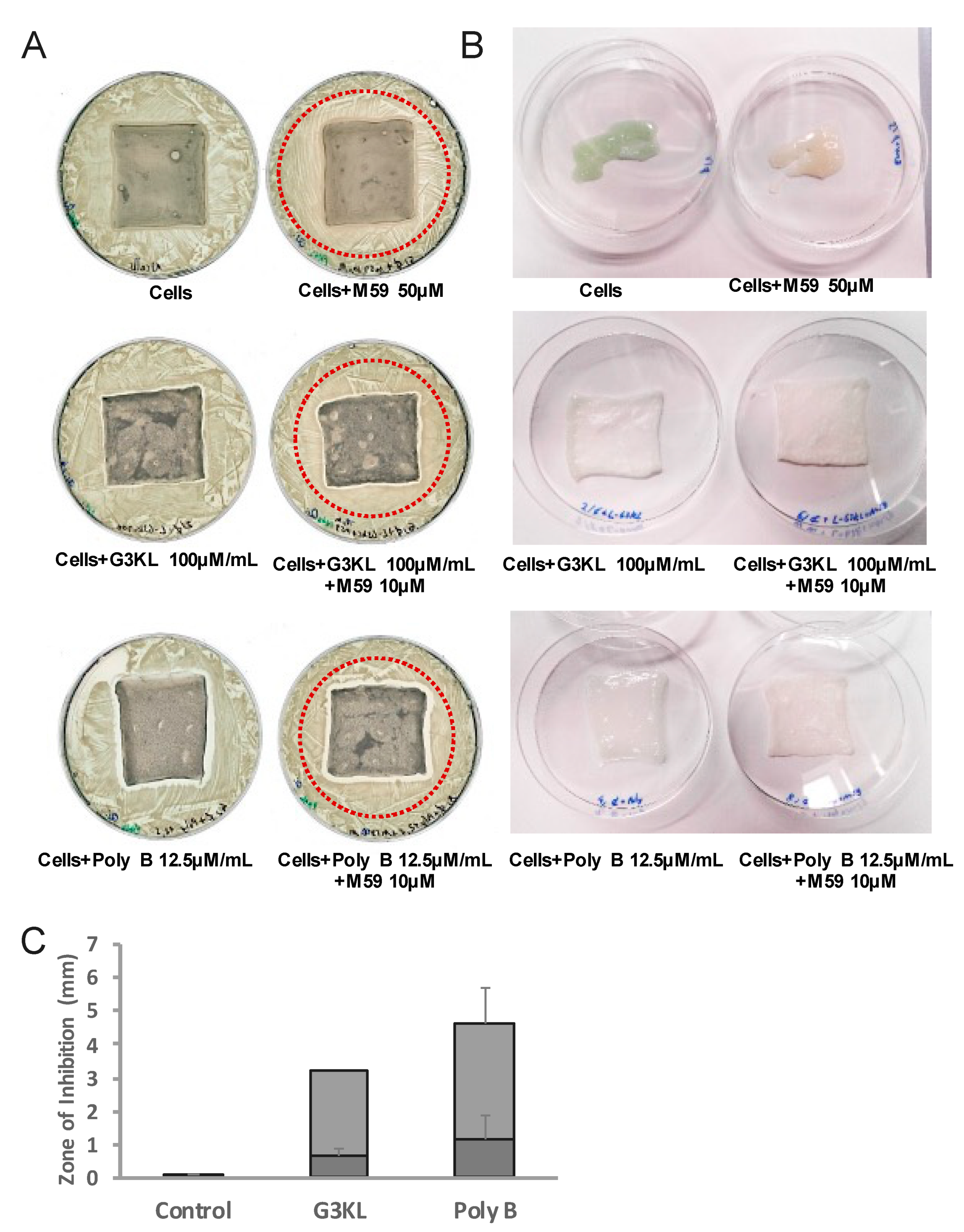
2.4. The Anti-Virulence Effect of QS-Inhibitor Compound M59 Is Retained When Embedded in the Collagen Matrix
2.5. Scaleing up and Final Assembly of the Components of Novel Anti-Infection Cell Therapy Dressings
3. Materials and Methods
3.1. Bacterial Strains, Clinical Grade Human Fibroblast Cells, and Chemicals
3.2. Cellular Toxicity Assays for Antibacterial Compounds
3.2.1. Live/Dead Assay
3.2.2. MTT Cell Titer Assay
3.3. Preparation of Cell-Embedded Dressings
3.4. Assessment of Antibacterial Activity
3.4.1. Minimal Inhibitory Concentrations (MIC) Assessment
3.4.2. Inhibition Zone Assay
3.4.3. Bacterial Killing Assay
3.4.4. Antibacterial Properties of Hydrogel Formulations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Church, D.; Elsayed, S.; Reid, O.; Winston, B.; Lindsay, R. Burn wound infections. Clin. Microbiol. Rev. 2006, 19, 403–434. [Google Scholar] [CrossRef] [Green Version]
- Lachiewicz, A.M.; Hauck, C.G.; Weber, D.J.; Cairns, B.A.; van Duin, D. Bacterial infections after burn injuries: Impact of multidrug resistance. Clin. Infect. Dis. 2017, 65, 2130–2136. [Google Scholar] [CrossRef] [PubMed]
- Erol, S.; Altoparlak, U.; Akcay, M.N.; Celebi, F.; Parlak, M. Changes of microbial flora and wound colonization in burned patients. Burns 2004, 30, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Clark, N.M.; Patterson, J.; Lynch, J.P., 3rd. Antimicrobial resistance among gram-negative organisms in the intensive care unit. Curr. Opin. Crit. Care 2003, 9, 413–423. [Google Scholar] [CrossRef]
- Herruzo-Cabrera, R.; Vizcaino-Alcaide, M.J.; Pinedo-Castillo, C.; Rey-Calero, J. Diagnosis of local infection of a burn by semiquantitative culture of the eschar surface. J. Burn Care Rehabil. 1992, 13, 639–641. [Google Scholar] [CrossRef] [PubMed]
- Lohana, P.; Hassan, S.; Watson, S.B. Integra in burns reconstruction: Our experience and report of an unusual immunological reaction. Ann. Burn. Fire Disasters 2014, 27, 17–21. [Google Scholar]
- Chalmers, R.L.; Smock, E.; Geh, J.L. Experience of Integra® in cancer reconstructive surgery. J. Plast. Reconstr. Aesthet. Surg. 2010, 63, 2081–2090. [Google Scholar] [CrossRef]
- Hicks, K.E.; Huynh, M.N.; Jeschke, M.; Malic, C. Dermal regenerative matrix use in burn patients: A systematic review. J. Plast. Reconstr. Aesthet. Surg. 2019, 72, 1741–1751. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, Q.; Li, Z.; Bai, X.; Wu, Y.; Liu, Y. Evaluating the effect of integra seeded with adipose tissue-derived stem cells or fibroblasts in wound healing. Curr. Drug Deliv. 2020, 17, 629–635. [Google Scholar] [CrossRef]
- Hohlfeld, J.; de Buys Roessingh, A.; Hirt-Burri, N.; Chaubert, P.; Gerber, S.; Scaletta, C.; Hohlfeld, P.; Applegate, L.A. Tissue engineered fetal skin constructs for paediatric burns. Lancet 2005, 366, 840–842. [Google Scholar] [CrossRef]
- Ramelet, A.A.; Hirt-Burri, N.; Raffoul, W.; Scaletta, C.; Pioletti, D.P.; Offord, E.; Mansourian, R.; Applegate, L.A. Chronic wound healing by fetal cell therapy may be explained by differential gene profiling observed in fetal versus old skin cells. Exp. Gerontol. 2009, 44, 208–218. [Google Scholar] [CrossRef] [Green Version]
- Laurent, A.; Scaletta, C.; Michetti, M.; Hirt-Burri, N.; Flahaut, M.; Raffoul, W.; de Buys Roessingh, A.S.; Applegate, L.A. Progenitor biological bandages: An authentic swiss tool for safe therapeutic management of burns, ulcers, and donor site grafts. Methods Mol. Biol. 2021, 2286, 49–65. [Google Scholar] [CrossRef]
- Singh, B.; Fleury, C.; Jalalvand, F.; Riesbeck, K. Human pathogens utilize host extracellular matrix proteins laminin and collagen for adhesion and invasion of the host. FEMS Microbiol. Rev. 2012, 36, 1122–1180. [Google Scholar] [CrossRef] [Green Version]
- Diacovich, L.; Gorvel, J.P. Bacterial manipulation of innate immunity to promote infection. Nat. Rev. Microbiol. 2010, 8, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Phillips, G.S.A.; Nizamoglu, M.; Wakure, A.; Barnes, D.; El-Muttardi, N.; Dziewulski, P. The use of dermal regeneration templates for primary burns surgery in a uk regional burns centre. Ann. Burn. Fire Disasters 2020, 33, 245–252. [Google Scholar]
- Altoparlak, U.; Erol, S.; Akcay, M.N.; Celebi, F.; Kadanali, A. The time-related changes of antimicrobial resistance patterns and predominant bacterial profiles of burn wounds and body flora of burned patients. Burns 2004, 30, 660–664. [Google Scholar] [CrossRef]
- Schmidtchen, A.; Holst, E.; Tapper, H.; Bjorck, L. Elastase-producing Pseudomonas aeruginosa degrade plasma proteins and extracellular products of human skin and fibroblasts, and inhibit fibroblast growth. Microb. Pathog. 2003, 34, 47–55. [Google Scholar] [CrossRef]
- Nagano, T.; Hao, J.L.; Nakamura, M.; Kumagai, N.; Abe, M.; Nakazawa, T.; Nishida, T. Stimulatory effect of pseudomonal elastase on collagen degradation by cultured keratocytes. Investig. Ophthalmol. Vis. Sci. 2001, 42, 1247–1253. [Google Scholar]
- Heck, L.W.; Morihara, K.; McRae, W.B.; Miller, E.J. Specific cleavage of human type III and IV collagens by Pseudomonas aeruginosa elastase. Infect. Immun. 1986, 51, 115–118. [Google Scholar] [CrossRef] [Green Version]
- Slutzkey, S.; Kozlovsky, A.; Artzi, Z.; Matalon, S. Collagen barrier membranes may accelerate bacterial growth in vitro: A potential clinical risk to regenerative procedures. Quintessence Int. 2015, 46, 43–50. [Google Scholar] [CrossRef]
- Fiorentini, C.; Bedini, A.; Mandel, V.D.; Bacca, E.; Menozzi, M.; Reggiani, C.; De Pace, B.; Meschiari, M.; Santoro, A.; Franceschini, E.; et al. Comparison of two perioperative antibiotic schedules in patients undergoing surgical reconstruction with dermal matrix after excision of skin cancer. Int. Wound J. 2020, 17, 937–943. [Google Scholar] [CrossRef]
- Weber, D.J.; van Duin, D.; DiBiase, L.M.; Hultman, C.S.; Jones, S.W.; Lachiewicz, A.M.; Sickbert-Bennett, E.E.; Brooks, R.H.; Cairns, B.A.; Rutala, W.A. Healthcare-associated infections among patients in a large burn intensive care unit: Incidence and pathogens, 2008–2012. Infect. Control. Hosp. Epidemiol. 2014, 35, 1304–1306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lachiewicz, A.M.; van Duin, D.; DiBiase, L.M.; Jones, S.W.; Carson, S.; Rutala, W.A.; Cairns, B.A.; Weber, D.J. Rates of hospital-associated respiratory infections and associated pathogens in a regional burn center, 2008–2012. Infect. Control. Hosp. Epidemiol. 2015, 36, 601–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keen, E.F., 3rd; Murray, C.K.; Robinson, B.J.; Hospenthal, D.R.; Co, E.M.; Aldous, W.K. Changes in the incidences of multidrug-resistant and extensively drug-resistant organisms isolated in a military medical center. Infect. Control. Hosp. Epidemiol. 2010, 31, 728–732. [Google Scholar] [CrossRef] [PubMed]
- Chato-Astrain, J.; Chato-Astrain, I.; Sanchez-Porras, D.; Garcia-Garcia, O.D.; Bermejo-Casares, F.; Vairo, C.; Villar-Vidal, M.; Gainza, G.; Villullas, S.; Oruezabal, R.I.; et al. Generation of a novel human dermal substitute functionalized with antibiotic-loaded nanostructured lipid carriers (NLCs) with antimicrobial properties for tissue engineering. J. Nanobiotechnol. 2020, 18, 174. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Naseri, M.; He, Y.; Xu, C.; Walsh, L.J.; Ziora, Z.M. Non-antibiotic antimicrobial agents to combat biofilm-forming bacteria. J. Glob. Antimicrob. Resist. 2020, 21, 445–451. [Google Scholar] [CrossRef]
- Cooper, R.; Kirketerp-Moller, K. Non-antibiotic antimicrobial interventions and antimicrobial stewardship in wound care. J. Wound Care 2018, 27, 355–377. [Google Scholar] [CrossRef]
- Sajjad, W.; Khan, T.; Ul-Islam, M.; Khan, R.; Hussain, Z.; Khalid, A.; Wahid, F. Development of modified montmorillonite-bacterial cellulose nanocomposites as a novel substitute for burn skin and tissue regeneration. Carbohydr. Polym. 2019, 206, 548–556. [Google Scholar] [CrossRef]
- Augustine, R.; Rehman, S.R.U.; Ahmed, R.; Zahid, A.A.; Sharifi, M.; Falahati, M.; Hasan, A. Electrospun chitosan membranes containing bioactive and therapeutic agents for enhanced wound healing. Int. J. Biol. Macromol. 2020, 156, 153–170. [Google Scholar] [CrossRef]
- Bankoti, K.; Rameshbabu, A.P.; Datta, S.; Goswami, P.; Roy, M.; Das, D.; Ghosh, S.K.; Das, A.K.; Mitra, A.; Pal, S.; et al. Dual functionalized injectable hybrid extracellular matrix hydrogel for burn wounds. Biomacromolecules 2021, 22, 514–533. [Google Scholar] [CrossRef]
- Sapru, S.; Ghosh, A.K.; Kundu, S.C. Non-immunogenic, porous and antibacterial chitosan and Antheraea mylitta silk sericin hydrogels as potential dermal substitute. Carbohydr. Polym. 2017, 167, 196–209. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, L.J.; White, R.J.; Chipman, J.K. Silver and nanoparticles of silver in wound dressings: A review of efficacy and safety. J. Wound Care. 2011, 20, 543–549. [Google Scholar] [CrossRef]
- You, C.; Li, Q.; Wang, X.; Wu, P.; Ho, J.K.; Jin, R.; Zhang, L.; Shao, H.; Han, C. Silver nanoparticle loaded collagen/chitosan scaffolds promote wound healing via regulating fibroblast migration and macrophage activation. Sci. Rep. 2017, 7, 10489. [Google Scholar] [CrossRef] [Green Version]
- Kalirajan, C.; Palanisamy, T. Bioengineered hybrid collagen scaffold tethered with silver-catechin nanocomposite modulates angiogenesis and TGF-beta toward scarless healing in chronic deep second degree infected burns. Adv. Healthc. Mater. 2020, 9, e2000247. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, X.; Tan, R.; She, Z.; Chen, Z.; Xia, Z. Preparation and characterization of a gallium-loaded antimicrobial artificial dermal scaffold. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 105, 110063. [Google Scholar] [CrossRef]
- Giuliani, A.; Pirri, G.; Bozzi, A.; Di Giulio, A.; Aschi, M.; Rinaldi, A.C. Antimicrobial peptides: Natural templates for synthetic membrane-active compounds. Cell. Mol. Life Sci. 2008, 65, 2450–2460. [Google Scholar] [CrossRef]
- Zasloff, M. Antimicrobial peptides of multicellular organisms. Nature 2002, 415, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Scorciapino, M.A.; Serra, I.; Manzo, G.; Rinaldi, A.C. Antimicrobial dendrimeric peptides: Structure, activity and new therapeutic applications. Int. J. Mol. Sci. 2017, 18, 542. [Google Scholar] [CrossRef] [Green Version]
- Giuliani, A.; Rinaldi, A.C. Beyond natural antimicrobial peptides: Multimeric peptides and other peptidomimetic approaches. Cell. Mol. Life Sci. 2011, 68, 2255–2266. [Google Scholar] [CrossRef]
- Aoki, W.; Ueda, M. Characterization of antimicrobial peptides toward the development of novel antibiotics. Pharmaceuticals 2013, 6, 1055–1081. [Google Scholar] [CrossRef] [Green Version]
- Roscia, G.; Falciani, C.; Bracci, L.; Pini, A. The development of antimicrobial peptides as new antibacterial drugs. Curr. Protein Pept. Sci. 2013, 14, 641–649. [Google Scholar] [CrossRef]
- Mardirossian, M.; Pompilio, A.; Crocetta, V.; De Nicola, S.; Guida, F.; Degasperi, M.; Gennaro, R.; Di Bonaventura, G.; Scocchi, M. In vitro and in vivo evaluation of BMAP-derived peptides for the treatment of cystic fibrosis-related pulmonary infections. Amino Acids 2016, 48, 2253–2260. [Google Scholar] [CrossRef]
- Kang, S.J.; Park, S.J.; Mishig-Ochir, T.; Lee, B.J. Antimicrobial peptides: Therapeutic potentials. Expert Rev. Anti Infect. Ther. 2014, 12, 1477–1486. [Google Scholar] [CrossRef] [PubMed]
- Pompilio, A.; Scocchi, M.; Pomponio, S.; Guida, F.; Di Primio, A.; Fiscarelli, E.; Gennaro, R.; Di Bonaventura, G. Antibacterial and anti-biofilm effects of cathelicidin peptides against pathogens isolated from cystic fibrosis patients. Peptides 2011, 32, 1807–1814. [Google Scholar] [CrossRef] [PubMed]
- Dickey, S.W.; Cheung, G.Y.C.; Otto, M. Different drugs for bad bugs: Antivirulence strategies in the age of antibiotic resistance. Nat. Rev. Drug Discov. 2017, 16, 457–471. [Google Scholar] [CrossRef] [PubMed]
- Maura, D.; Ballok, A.E.; Rahme, L.G. Considerations and caveats in anti-virulence drug development. Curr. Opin. Microbiol. 2016, 33, 41–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rutherford, S.T.; Bassler, B.L. Bacterial quorum sensing: Its role in virulence and possibilities for its control. Cold Spring Harb. Perspect. Med. 2012, 2, a012427. [Google Scholar] [CrossRef]
- Vega, N.M.; Allison, K.R.; Khalil, A.S.; Collins, J.J. Signaling-mediated bacterial persister formation. Nat. Chem. Biol. 2012, 8, 431–433. [Google Scholar] [CrossRef]
- Moker, N.; Dean, C.R.; Tao, J. Pseudomonas aeruginosa increases formation of multidrug-tolerant persister cells in response to quorum-sensing signaling molecules. J. Bacteriol. 2010, 192, 1946–1955. [Google Scholar] [CrossRef] [Green Version]
- Que, Y.A.; Hazan, R.; Strobel, B.; Maura, D.; He, J.; Kesarwani, M.; Panopoulos, P.; Tsurumi, A.; Giddey, M.; Wilhelmy, J.; et al. A quorum sensing small volatile molecule promotes antibiotic tolerance in bacteria. PLoS ONE 2013, 8, e80140. [Google Scholar] [CrossRef] [Green Version]
- Starkey, M.; Lepine, F.; Maura, D.; Bandyopadhaya, A.; Lesic, B.; He, J.; Kitao, T.; Righi, V.; Milot, S.; Tzika, A.; et al. Identification of anti-virulence compounds that disrupt quorum-sensing regulated acute and persistent pathogenicity. PLoS Pathog. 2014, 10, e1004321. [Google Scholar] [CrossRef] [PubMed]
- Cegelski, L.; Marshall, G.R.; Eldridge, G.R.; Hultgren, S.J. The biology and future prospects of antivirulence therapies. Nat. Rev. Microbiol. 2008, 6, 17–27. [Google Scholar] [CrossRef]
- De Buys Roessingh, A.S.; Hohlfeld, J.; Scaletta, C.; Hirt-Burri, N.; Gerber, S.; Hohlfeld, P.; Gebbers, J.O.; Applegate, L.A. Development, characterization, and use of a fetal skin cell bank for tissue engineering in wound healing. Cell Transplant. 2006, 15, 823–834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirt-Burri, N.; Scaletta, C.; Gerber, S.; Pioletti, D.P.; Applegate, L.A. Wound-healing gene family expression differences between fetal and foreskin cells used for bioengineered skin substitutes. Artif. Organs 2008, 32, 509–518. [Google Scholar] [CrossRef] [Green Version]
- Applegate, L.A.; Scaletta, C.; Hirt-Burri, N.; Raffoul, W.; Pioletti, D. Whole-cell bioprocessing of human fetal cells for tissue engineering of skin. Ski. Pharmacol. Physiol. 2009, 22, 63–73. [Google Scholar] [CrossRef] [Green Version]
- Laurent, A.; Lin, P.; Scaletta, C.; Hirt-Burri, N.; Michetti, M.; de Buys Roessingh, A.S.; Raffoul, W.; She, B.R.; Applegate, L.A. Bringing safe and standardized cell therapies to industrialized processing for burns and wounds. Front. Bioeng. Biotechnol. 2020, 8, 581. [Google Scholar] [CrossRef]
- Monnier, S.; Abdel-Sayed, P.; Roessingh, A.B.; Hirt-Burri, N.; Chemali, M.; Applegate, L.A.; Raffoul, W. Surgical management evolution between 2 massive burn cases at 17-year interval: Contribution of cell therapies in improving the surgical care. Cell Transplant. 2020, 29, 963689720973642. [Google Scholar] [CrossRef]
- Stach, M.; Siriwardena, T.N.; Kohler, T.; van Delden, C.; Darbre, T.; Reymond, J.L. Combining topology and sequence design for the discovery of potent antimicrobial peptide dendrimers against multidrug-resistant Pseudomonas aeruginosa. Angew. Chem. Int. Ed. Engl. 2014, 53, 12827–12831. [Google Scholar] [CrossRef] [PubMed]
- Pires, J.; Siriwardena, T.N.; Stach, M.; Tinguely, R.; Kasraian, S.; Luzzaro, F.; Leib, S.L.; Darbre, T.; Reymond, J.L.; Endimiani, A. In vitro activity of the novel antimicrobial peptide dendrimer G3KL against multidrug-resistant Acinetobacter baumannii and Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2015, 59, 7915–7918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siriwardena, T.N.; Capecchi, A.; Gan, B.H.; Jin, X.; He, R.; Wei, D.; Ma, L.; Kohler, T.; van Delden, C.; Javor, S.; et al. Optimizing Antimicrobial Peptide Dendrimers in Chemical Space. Angew. Chem. Int. Ed. Engl. 2018, 57, 8483–8487. [Google Scholar] [CrossRef] [PubMed]
- Gan, B.H.; Siriwardena, T.N.; Javor, S.; Darbre, T.; Reymond, J.L. Fluorescence imaging of bacterial killing by antimicrobial peptide dendrimer G3KL. ACS Infect. Dis. 2019, 5, 2164–2173. [Google Scholar] [CrossRef] [PubMed]
- Pompilio, A.; Geminiani, C.; Mantini, P.; Siriwardena, T.N.; Di Bonaventura, I.; Reymond, J.L.; Di Bonaventura, G. Peptide dendrimers as “lead compounds” for the treatment of chronic lung infections by Pseudomonas aeruginosa in cystic fibrosis patients: In vitro and in vivo studies. Infect. Drug Resist. 2018, 11, 1767–1782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siriwardena, T.N.; Stach, M.; He, R.; Gan, B.H.; Javor, S.; Heitz, M.; Ma, L.; Cai, X.; Chen, P.; Wei, D.; et al. Lipidated peptide dendrimers killing multidrug-resistant bacteria. J. Am. Chem. Soc. 2018, 140, 423–432. [Google Scholar] [CrossRef] [Green Version]
- Siriwardena, T.N.; Gan, B.H.; Kohler, T.; van Delden, C.; Javor, S.; Reymond, J.L. Stereorandomization as a method to probe peptide bioactivity. ACS Cent. Sci. 2021, 7, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Gan, B.H.; Cai, X.; Javor, S.; Kohler, T.; Reymond, J.L. Synergistic effect of propidium iodide and small molecule antibiotics with the antimicrobial peptide dendrimer G3KL against gram-negative bacteria. Molecules 2020, 25, 5643. [Google Scholar] [CrossRef]
- Ben Jeddou, F.; Falconnet, L.; Luscher, A.; Siriwardena, T.; Reymond, J.L.; van Delden, C.; Kohler, T. Adaptive and mutational responses to peptide dendrimer antimicrobials in Pseudomonas aeruginosa. Antimicrob. Agents hemother. 2020, 64, e02040-19. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Sayed, P.; Kaeppeli, A.; Siriwardena, T.; Darbre, T.; Perron, K.; Jafari, P.; Reymond, J.L.; Pioletti, D.P.; Applegate, L.A. Anti-microbial dendrimers against multidrug-resistant P. aeruginosa enhance the angiogenic effect of biological burn-wound bandages. Sci. Rep. 2016, 6, 22020. [Google Scholar] [CrossRef]
- Lewis, K. Riddle of biofilm resistance. Antimicrob. Agents Chemother. 2001, 45, 999–1007. [Google Scholar] [CrossRef] [Green Version]
- Hall, C.W.; Mah, T.F. Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol. Rev. 2017, 41, 276–301. [Google Scholar] [CrossRef]
- Cao, H.; Krishnan, G.; Goumnerov, B.; Tsongalis, J.; Tompkins, R.; Rahme, L.G. A quorum sensing-associated virulence gene of Pseudomonas aeruginosa encodes a LysR-like transcription regulator with a unique self-regulatory mechanism. Proc. Natl. Acad. Sci. USA 2001, 98, 14613–14618. [Google Scholar] [CrossRef] [Green Version]
- Deziel, E.; Gopalan, S.; Tampakaki, A.P.; Lepine, F.; Padfield, K.E.; Saucier, M.; Xiao, G.; Rahme, L.G. The contribution of MvfR to Pseudomonas aeruginosa pathogenesis and quorum sensing circuitry regulation: Multiple quorum sensing-regulated genes are modulated without affecting lasRI, rhlRI or the production of N-acyl-L-homoserine lactones. Mol. Microbiol. 2005, 55, 998–1014. [Google Scholar] [CrossRef]
- Gonzalez, M.R.; Fleuchot, B.; Lauciello, L.; Jafari, P.; Applegate, L.A.; Raffoul, W.; Que, Y.A.; Perron, K. Effect of human burn wound exudate on Pseudomonas aeruginosa virulence. mSphere 2016, 1, e00111-15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitao, T.; Lepine, F.; Babloudi, S.; Walte, F.; Steinbacher, S.; Maskos, K.; Blaesse, M.; Negri, M.; Pucci, M.; Zahler, B.; et al. Molecular insights into function and competitive inhibition of Pseudomonas aeruginosa multiple virulence factor regulator. mBio 2018, 9, e02158-17. [Google Scholar] [CrossRef] [Green Version]
- Maura, D.; Drees, S.L.; Bandyopadhaya, A.; Kitao, T.; Negri, M.; Starkey, M.; Lesic, B.; Milot, S.; Deziel, E.; Zahler, R.; et al. Polypharmacology approaches against the Pseudomonas aeruginosa MvfR regulon and their application in blocking virulence and antibiotic tolerance. ACS Chem. Biol. 2017, 12, 1435–1443. [Google Scholar] [CrossRef]
- Han, G.; Ceilley, R. Chronic wound healing: A review of current management and treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patrulea, V.; Borchard, G.; Jordan, O. An update on antimicrobial peptides (AMPs) and their delivery strategies for wound infections. Pharmaceutics 2020, 12, 840. [Google Scholar] [CrossRef] [PubMed]
- Longinotti, C. The use of hyaluronic acid based dressings to treat burns: A review. Burn. Trauma 2014, 2, 162–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hadisi, Z.; Farokhi, M.; Bakhsheshi-Rad, H.R.; Jahanshahi, M.; Hasanpour, S.; Pagan, E.; Dolatshahi-Pirouz, A.; Zhang, Y.S.; Kundu, S.C.; Akbari, M. Hyaluronic acid (HA)-based silk fibroin/Zinc oxide core-shell electrospun dressing for burn wound management. Macromol. Biosci. 2020, 20, e1900328. [Google Scholar] [CrossRef]
- Fahimirad, S.; Ajalloueian, F. Naturally-derived electrospun wound dressings for target delivery of bio-active agents. Int. J. Pharm. 2019, 566, 307–328. [Google Scholar] [CrossRef] [PubMed]
- Ueno, H.; Mori, T.; Fujinaga, T. Topical formulations and wound healing applications of chitosan. Adv. Drug Deliv. Rev. 2001, 52, 105–115. [Google Scholar] [CrossRef]
- Matica, M.A.; Aachmann, F.L.; Tondervik, A.; Sletta, H.; Ostafe, V. Chitosan as a wound dressing starting material: Antimicrobial properties and mode of action. Int. J. Mol. Sci. 2019, 20, 5889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, G.; Deziel, E.; He, J.; Lepine, F.; Lesic, B.; Castonguay, M.H.; Milot, S.; Tampakaki, A.P.; Stachel, S.E.; Rahme, L.G. MvfR, a key Pseudomonas aeruginosa pathogenicity LTTR-class regulatory protein, has dual ligands. Mol. Microbiol. 2006, 62, 1689–1699. [Google Scholar] [CrossRef] [PubMed]
- Van Delden, C.; Iglewski, B.H. Cell-to-cell signaling and Pseudomonas aeruginosa infections. Emerg. Infect. Dis. 1998, 4, 551–560. [Google Scholar] [CrossRef]
- Maura, D.; Rahme, L.G. Pharmacological inhibition of the Pseudomonas aeruginosa MvfR quorum-sensing system interferes with biofilm formation and potentiates antibiotic-mediated biofilm disruption. Antimicrob. Agents Chemother. 2017, 61, e01362-17. [Google Scholar] [CrossRef] [Green Version]
- Laurent, A.; Scaletta, C.; Michetti, M.; Hirt-Burri, N.; de Buys Roessingh, A.S.; Raffoul, W.; Applegate, L.A. GMP tiered cell banking of non-enzymatically isolated dermal progenitor fibroblasts for allogenic regenerative medicine. Methods Mol. Biol. 2021, 2286, 25–48. [Google Scholar] [CrossRef]
- Laurent, A.; Hirt-Burri, N.; Scaletta, C.; Michetti, M.; de Buys Roessingh, A.S.; Raffoul, W.; Applegate, L.A. Holistic approach of swiss fetal progenitor cell banking: Optimizing safe and sustainable substrates for regenerative medicine and biotechnology. Front. Bioeng. Biotechnol. 2020, 8, 557758. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 26th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2020. [Google Scholar]
- Dumville, J.C.; Lipsky, B.A.; Hoey, C.; Cruciani, M.; Fiscon, M.; Xia, J. Topical antimicrobial agents for treating foot ulcers in people with diabetes. Cochrane Database Syst. Rev. 2017, 6, CD011038. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jafari, P.; Luscher, A.; Siriwardena, T.; Michetti, M.; Que, Y.-A.; Rahme, L.G.; Reymond, J.-L.; Raffoul, W.; Van Delden, C.; Applegate, L.A.; et al. Antimicrobial Peptide Dendrimers and Quorum-Sensing Inhibitors in Formulating Next-Generation Anti-Infection Cell Therapy Dressings for Burns. Molecules 2021, 26, 3839. https://doi.org/10.3390/molecules26133839
Jafari P, Luscher A, Siriwardena T, Michetti M, Que Y-A, Rahme LG, Reymond J-L, Raffoul W, Van Delden C, Applegate LA, et al. Antimicrobial Peptide Dendrimers and Quorum-Sensing Inhibitors in Formulating Next-Generation Anti-Infection Cell Therapy Dressings for Burns. Molecules. 2021; 26(13):3839. https://doi.org/10.3390/molecules26133839
Chicago/Turabian StyleJafari, Paris, Alexandre Luscher, Thissa Siriwardena, Murielle Michetti, Yok-Ai Que, Laurence G. Rahme, Jean-Louis Reymond, Wassim Raffoul, Christian Van Delden, Lee Ann Applegate, and et al. 2021. "Antimicrobial Peptide Dendrimers and Quorum-Sensing Inhibitors in Formulating Next-Generation Anti-Infection Cell Therapy Dressings for Burns" Molecules 26, no. 13: 3839. https://doi.org/10.3390/molecules26133839
APA StyleJafari, P., Luscher, A., Siriwardena, T., Michetti, M., Que, Y.-A., Rahme, L. G., Reymond, J.-L., Raffoul, W., Van Delden, C., Applegate, L. A., & Köhler, T. (2021). Antimicrobial Peptide Dendrimers and Quorum-Sensing Inhibitors in Formulating Next-Generation Anti-Infection Cell Therapy Dressings for Burns. Molecules, 26(13), 3839. https://doi.org/10.3390/molecules26133839







