How Climatic Seasons of the Amazon Biome Affect the Aromatic and Bioactive Profiles of Fermented and Dried Cocoa Beans?
Abstract
:1. Introduction
2. Results
2.1. Physicochemical Analysis in Fermented and Dried Cocoa Beans
2.2. Profile of Volatile Compounds in Fermented and Dried Cocoa Beans
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Material
4.3. Fermentation of Cocoa Beans and Artificial Drying
4.4. Methods
4.4.1. Physicochemical Analysis of Cocoa Beans
4.4.2. Determination of Total Phenolic Compounds in Fermented and Dried Cocoa Beans
4.4.3. Determination of Methylxanthines and Monomeric Compounds by HPLC-DAD
4.4.4. Extraction and Identification of Volatile Compounds in Fermented and Dried Cocoa Beans by GC-MS
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| ANOVA | Analysis of Variance |
| FID | Flame Ionization Detector |
| GC-MS | Gas chromatograph coupled to mass spectrometry |
| LOQ | Limit of quantification |
| mEq NaOH/100 g | milliequivalent sodium hydroxide per 100 g sample |
| mg ECE/g | milligram equivalent epicatechin per gram sample |
| PPO | Polyphenoloxidase |
| RI | Retention index |
References
- Ozturk, G.; Young, G.M. Food Evolution: The Impact of Society and Science on the Fermentation of Cocoa Beans. Compr. Rev. Food Sci. Food Saf. 2017, 16, 431–455. [Google Scholar] [CrossRef] [PubMed]
- Chagas Junior, G.; Espírito-Santo, J.C.A.; Ferreira, N.R.; Marques-da-Silva, S.H.; Oliveira, G.; Vasconcelos, S.; Almeida, S.F.O.; Silva, L.R.C.; Figueredo, H.M.; Lopes, A.S. Yeast isolation and identification during on-farm cocoa natural fermentation in a highly producer region in northern Brazil. Sci. Plena 2020, 16, 121502. [Google Scholar] [CrossRef]
- Chagas Junior, G.C.A.; Ferreira, N.R.; Lopes, A.S. The microbiota diversity identified during the cocoa fermentation and the benefits of the starter cultures use: An overview. Int. J. Food Sci. Technol. 2020, 1–9. [Google Scholar] [CrossRef]
- Martins, J.M.; Santos, J.H.F.; Silva, W.S.; Silva, V.B.; Arruda, J.A.P.; Nascimento, J.A.R.; Dortas, L.C.; Freitas, A.J.; Ramos, A.A. Melhoria da Qualidade do Cacau; CEPLAC/CENEX: Itabuna, Brazil, 2012. [Google Scholar]
- Bastos, V.S.; Uekane, T.M.; Bello, N.A.; de Rezende, C.M.; Flosi Paschoalin, V.M.; Del Aguila, E.M. Dynamics of volatile compounds in TSH 565 cocoa clone fermentation and their role on chocolate flavor in Southeast Brazil. J. Food Sci. Technol. 2019, 56, 2874–2887. [Google Scholar] [CrossRef]
- Serra, J.L.; Moura, F.G.; Pereira, G.V.d.M.; Soccol, C.R.; Rogez, H.; Darnet, S. Determination of the microbial community in Amazonian cocoa bean fermentation by Illumina-based metagenomic sequencing. LWT-Food Sci. Technol 2019, 106, 229–239. [Google Scholar] [CrossRef]
- Schwan, R.F.; Wheals, A.E. The microbiology of cocoa fermentation and its role in chocolate quality. Crit. Rev. Food Sci. Nutr. 2004, 44, 205–221. [Google Scholar] [CrossRef]
- Koné, M.K.; Guéhi, S.T.; Durand, N.; Ban-Koffi, L.; Berthiot, L.; Tachon, A.F.; Brou, K.; Boulanger, R.; Montet, D. Contribution of predominant yeasts to the occurrence of aroma compounds during cocoa bean fermentation. Food Res. Int. 2016, 89, 910–917. [Google Scholar] [CrossRef]
- Chagas Junior, G.C.A.; Ferreira, N.R.; Andrade, E.H.D.A.; Nascimento, L.D.D.; Siqueira, F.C.d.; Lopes, A.S. Profile of Volatile Compounds of On-Farm Fermented and Dried Cocoa Beans Inoculated with Saccharomyces cerevisiae KY794742 and Pichia kudriavzevii KY794725. Molecules 2021, 26, 344. [Google Scholar] [CrossRef]
- Visintin, S.; Ramos, L.; Batista, N.; Dolci, P.; Schwan, F.; Cocolin, L. Impact of Saccharomyces cerevisiae and Torulaspora delbrueckii starter cultures on cocoa beans fermentation. Int. J. Food Microbiol. 2017, 257, 31–40. [Google Scholar] [CrossRef]
- Moreira, I.M.V.; Vilela, L.F.; Miguel, M.G.C.P.; Santos, C.; Lima, N.; Schwan, R.F. Impact of a Microbial Cocktail Used as a Starter Culture on Cocoa Fermentation and Chocolate Flavor. Molecules 2017, 22, 766. [Google Scholar] [CrossRef] [Green Version]
- De Vuyst, L.; Leroy, F. Functional role of yeasts, lactic acid bacteria and acetic acid bacteria in cocoa fermentation processes. FEMS Microbiol. Rev. 2020, 44. [Google Scholar] [CrossRef]
- Efraim, P.; Alves, A.B.; Jardim, D.C.P. Revisão: Polifenóis em cacau e derivados: Teores, fatores de variação e efeitos na saúde. Brazilian J. Food Technol. 2011, 14, 181–201. [Google Scholar] [CrossRef]
- Chagas Junior, G.C.A.; Ferreira, N.R.; Gloria, M.B.A.; Martins, L.H.D.S.; Lopes, A.S. Chemical implications and time reduction of on-farm cocoa fermentation by Saccharomyces cerevisiae and Pichia kudriavzevii. Food Chem. 2021, 338, 127834. [Google Scholar] [CrossRef]
- Ceplac—Comissão Executiva do Plano da Lavoura Cacaueira—Pará Retoma Liderança na Produção Brasileira de Cacau, Com a União de Agricultores. Available online: https://g1.globo.com/economia/agronegocios/globo-rural/noticia/2019/11/03/lideranca-na-producao-brasileira-de-cacau-volta-para-casa-no-para-com-a-uniao-de-agricultores.ghtml (accessed on 10 November 2020).
- Fisch, G.; Marengo, J.A.; Nobre, C.A. Uma revisão geral sobre o clima da Amazônia. Acta Amaz. 1998, 28, 101. [Google Scholar] [CrossRef]
- Araujo, Q.R.; Fernandes, C.A.F.; Ribeiro, D.O.; Efraim, P.; Steinmacher, D.; Lieberei, R.; Bastide, P.; Araujo, T.G. Cocoa Quality Index—A proposal. Food Control 2014, 46, 49–54. [Google Scholar] [CrossRef]
- Afoakwa, E.O.; Quao, J.; Takrama, J.; Budu, A.S.; Saalia, F.K. Chemical composition and physical quality characteristics of Ghanaian cocoa beans as affected by pulp pre-conditioning and fermentation. J. Food Sci. Technol. 2013, 50, 1097–1105. [Google Scholar] [CrossRef] [Green Version]
- Afoakwa, E.O.; Paterson, A.; Fowler, M.; Ryan, A. Flavor formation and character in cocoa and chocolate: A critical review. Crit. Rev. Food Sci. Nutr. 2008, 48, 840–857. [Google Scholar] [CrossRef]
- Utrilla-Vázquez, M.; Rodríguez-Campos, J.; Avendaño-Arazate, C.H.; Gschaedler, A.; Lugo-Cervantes, E. Analysis of volatile compounds of five varieties of Maya cocoa during fermentation and drying processes by Venn diagram and PCA. Food Res. Int. 2020, 129, 108834. [Google Scholar] [CrossRef]
- Rodriguez-Campos, J.; Escalona-Buendía, H.B.; Contreras-Ramos, S.M.; Orozco-Avila, I.; Jaramillo-Flores, E.; Lugo-Cervantes, E. Effect of fermentation time and drying temperature on volatile compounds in cocoa. Food Chem. 2012, 132, 277–288. [Google Scholar] [CrossRef]
- Barišić, V.; Kopjar, M.; Jozinović, A.; Flanjak, I.; Ačkar, Đ.; Miličević, B.; Šubarić, D.; Jokić, S.; Babić, J. The chemistry behind chocolate production. Molecules 2019, 24, 3163. [Google Scholar] [CrossRef] [Green Version]
- Aprotosoaie, A.C.; Luca, S.V.; Miron, A. Flavor Chemistry of Cocoa and Cocoa Products-An Overview. Compr. Rev. Food Sci. Food Saf. 2016, 15, 73–91. [Google Scholar] [CrossRef]
- Tuenter, E.; Delbaere, C.; De Winne, A.; Bijttebier, S.; Custers, D.; Foubert, K.; Van Durme, J.; Messens, K.; Dewettinck, K.; Pieters, L. Non-volatile and volatile composition of West African bulk and Ecuadorian fine-flavor cocoa liquor and chocolate. Food Res. Int. 2020, 130, 108943. [Google Scholar] [CrossRef]
- Misnawi; Jinap, S.; Jamilah, B.; Nazamid, S. Effects of incubation and polyphenol oxidase enrichment on colour, fermentation index, procyanidins and astringency of unfermented and partly fermented cocoa beans. Int. J. Food Sci. Technol. 2003, 38, 285–295. [Google Scholar] [CrossRef]
- Hashim, P.; Selamat, J.; Muhammad, S.K.S.; Ali, A. Changes in free amino acid, peptide-N, sugar and pyrazine concentration during cocoa fermentation. J. Sci. Food Agric. 1998, 78, 535–542. [Google Scholar] [CrossRef]
- Jalil, A.M.M.; Ismail, A. Polyphenols in cocoa and cocoa products: Is there a link between antioxidant properties and health? Molecules 2008, 13, 2190–2219. [Google Scholar] [CrossRef] [Green Version]
- Wollgast, J.; Anklam, E. Polyphenols in chocolate: Is there a contribution to human health? Food Res. Int. 2000, 33, 449–459. [Google Scholar] [CrossRef]
- Lee, K.W.; Kim, Y.J.; Lee, H.J.; Lee, C.Y. Cocoa Has More Phenolic Phytochemicals and a Higher Antioxidant Capacity than Teas and Red Wine. J. Agric. Food Chem. 2003, 51, 7292–7295. [Google Scholar] [CrossRef]
- Bae, H.; Kim, S.H.; Kim, M.S.; Sicher, R.C.; Lary, D.; Strem, M.D.; Natarajan, S.; Bailey, B.A. The drought response of Theobroma cacao (cacao) and the regulation of genes involved in polyamine biosynthesis by drought and other stresses. Plant Physiol. Biochem. 2008, 46, 174–188. [Google Scholar] [CrossRef]
- Kongor, J.E.; Hinneh, M.; de Walle, D.V.; Afoakwa, E.O.; Boeckx, P.; Dewettinck, K. Factors influencing quality variation in cocoa (Theobroma cacao) bean flavour profile—A review. Food Res. Int. 2016, 82, 44–52. [Google Scholar] [CrossRef]
- Niether, W.; Smit, I.; Armengot, L.; Schneider, M.; Gerold, G.; Pawelzik, E. Environmental Growing Conditions in Five Production Systems Induce Stress Response and Affect Chemical Composition of Cocoa (Theobroma cacao L.) Beans. J. Agric. Food Chem. 2017, 65, 10165–10173. [Google Scholar] [CrossRef]
- Carrillo, L.C.; Londoño-Londoño, J.; Gil, A. Comparison of polyphenol, methylxanthines and antioxidant activity in Theobroma cacao beans from different cocoa-growing areas in Colombia. Food Res. Int. 2014, 60, 273–280. [Google Scholar] [CrossRef]
- Hansen, C.E.; Del Olmo, M.; Burri, C. Enzyme activities in cocoa beans during fermentation. J. Sci. Food Agric. 1998, 77, 273–281. [Google Scholar] [CrossRef]
- Biehl, B.; Brunner, E.; Passern, D.; Quesnel, V.C.; Adomako, D. Acidification, proteolysis and flavour potential in fermenting cocoa beans. J. Sci. Food Agric. 1985, 36, 583–598. [Google Scholar] [CrossRef]
- Matissek, R. Evaluation of xanthine derivatives in chocolate - nutritional and chemical aspects. Eur. Food Res. Technol. 1997, 205, 175–184. [Google Scholar] [CrossRef]
- Zheng, X.Q.; Koyama, Y.; Nagai, C.; Ashihara, H. Biosynthesis, accumulation and degradation of theobromine in developing Theobroma cacao fruits. J. Plant Physiol. 2004, 161, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Do Nascimento, L.D.; de Moraes, A.A.B.; da Costa, K.S.; Galúcio, J.M.P.; Taube, P.S.; Costa, C.M.L.; Cruz, J.N.; Andrade, E.H.d.A.; de Faria, L.J.G. Bioactive natural compounds and antioxidant activity of essential oils from spice plants: New findings and potential applications. Biomolecules 2020, 10, 988. [Google Scholar] [CrossRef]
- Guichard, E.; Christian, S.; Martine, M.; Bon, L.; Marie, A. Flavour: From Food to Perception; John Wiley & Sons Inc.: Chichester, UK, 2017; ISBN 9781118929414. [Google Scholar]
- Parker, J.K.; Elmore, J.S.; Methven, L. Flavour Development, Analysis and Perception in Food and Beverages; Elsevier: Amsterdam, The Netherlands, 2015; ISBN 978-1-78242-103-0. [Google Scholar]
- Oberparleiter, S.; Ziegleder, G. Amyl alcohols as compounds indicative of raw cocoa bean quality. Eur. Food Res. Technol. 1997, 204, 156–160. [Google Scholar] [CrossRef]
- Cevallos-Cevallos, J.M.; Gysel, L.; Maridueña-Zavala, M.G.; Molina-Miranda, M.J. Time-Related Changes in Volatile Compounds during Fermentation of Bulk and Fine-Flavor Cocoa (Theobroma cacao) Beans. J. Food Qual. 2018, 2018, 1758381. [Google Scholar] [CrossRef] [Green Version]
- Schmarr, H.G.; Engel, K.H. Analysis and stereodifferentiation of linalool in Theobroma cacao and cocoa products using enantioselective multidimensional gas chromatography. Eur. Food Res. Technol. 2012, 235, 827–834. [Google Scholar] [CrossRef]
- Hegmann, E.; Niether, W.; Phillips, W.; Rohsius, C.; Lieberei, R. Besides variety, also season and ripening stage have a major influence on fruit pulp aroma of cacao (Theobroma cacao L.). J. Appl. Bot. Food Qual. 2021, 93, 266–275. [Google Scholar] [CrossRef]
- Koné, K.M.; Assi-Clair, B.J.; Kouassi, A.D.D.; Yao, A.K.; Ban-Koffi, L.; Durand, N.; Lebrun, M.; Maraval, I.; Bonlanger, R.; Guehi, T.S. Pod storage time and spontaneous fermentation treatments and their impact on the generation of cocoa flavour precursor compounds. Int. J. Food Sci. Technol. 2020, 1–14. [Google Scholar] [CrossRef]
- Efraim, P.; Pezoa-García, N.H.; Jardim, D.C.P.; Nishikawa, A.; Haddad, R.; Eberlin, M.N. Influência da fermentação e secagem de amêndoas de cacau no teor de compostos fenólicos e na aceitação sensorial. Ciência e Tecnol. Aliment. 2010, 30, 142–150. [Google Scholar] [CrossRef] [Green Version]
- Horwitz, W.; Latimer, G.W. Official Methods of Analysis of AOAC International, 18th ed.; Association of Official Analytical Chemists: Rockville, MD, USA, 2006. [Google Scholar]
- Do Carmo Brito, B.d.N.; Campos Chisté, R.; da Silva Pena, R.; Abreu Gloria, M.B.; Santos Lopes, A. Bioactive amines and phenolic compounds in cocoa beans are affected by fermentation. Food Chem. 2017, 228, 484–490. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1998, 299, 152–178. [Google Scholar] [CrossRef]
- He, Q.; Lv, Y.; Zhou, L.; Shi, B. Simultaneous determination of caffeine and catechins in tea extracts by HPLC. J. Liq. Chromatogr. Relat. Technol. 2010, 33, 491–498. [Google Scholar] [CrossRef]
- Sandhya, M.V.S.; Yallappa, B.S.; Varadaraj, M.C.; Puranaik, J.; Rao, L.J.; Janardhan, P.; Murthy, P.S. Inoculum of the starter consortia and interactive metabolic process in enhancing quality of cocoa bean (Theobroma cacao) fermentation. LWT Food Sci. Technol. 2016, 65, 731–738. [Google Scholar] [CrossRef]
- Maia, O.G.S.; Andrade, L.H.A. Database of the amazon aromatic plants and their essential oils. Quim. Nova 2009, 32, 595–622. [Google Scholar] [CrossRef] [Green Version]
- Maia, J.G.S.; Andrade, E.H.A.; da Silva, M.H.L. Aroma volatiles of pequi fruit (Caryocar brasiliense Camb.). J. Food Compos. Anal. 2008, 21, 574–576. [Google Scholar] [CrossRef]
- De Sousa, E.M.; dos Anjos, T.O.; do Nascimento, L.D.; de Andrade, E.H.A.; Costa, C.M.L.; de Faria, L.J.G. Cinética de Secagem e Composição Química da Polpa do Fruto de Eugenia patrisii Vahl. (Myrtaceae). In Impactos das Tecnologias na Engenharia Química 2; Atena Editora: Ponta Grossa, Brazil, 2019; pp. 186–191. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential oil Components by Gas Chromatography/Mass Spectroscopy, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; ISBN 9781932633214. [Google Scholar]
- Mondello, L. Mass Spectra of Flavors and Fragrances of Natural and Synthetic Compounds; Wiley: Hoboken, NJ, USA, 2015. [Google Scholar]
- Stein, S.; Mirokhin, D.; Tchekhovskoi, D.; Mallard, G.; Mikaia, A.; Zaikin, V.; Sparkmanm, D. NIST 2008 User Guide. In The NIST Mass Spectral Search Program for the NIST/EPA/NIH Mass Spectra Library. Standard Reference Data Program of the National Institute of Standards and Technology; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2011. [Google Scholar]
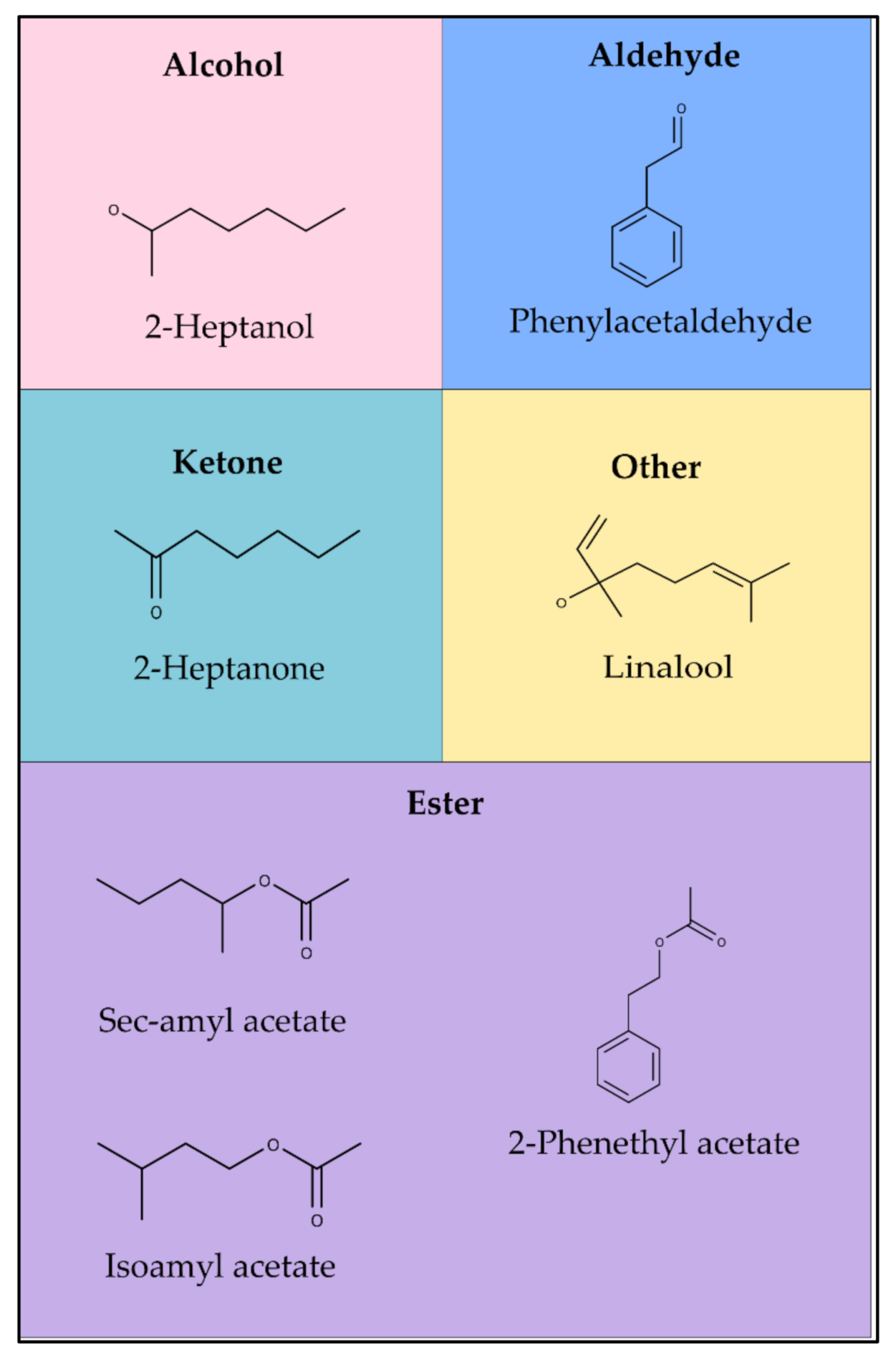
| Treatments/Means * | ||
|---|---|---|
| Parameters | Summer | Winter |
| pH | 4.09 ± 0.01 a | 4.07 ± 0.01 a |
| TTA (meq. NaOH/100 g) 1 | 18.72 ± 0.11 b | 20.01 ± 0.38 a |
| Total Phenolic Compounds (mg ECE/g) 2 | 25.10 ± 0.12 b | 30.89 ± 0.75a |
| Monomeric Compounds | ||
| Catechin (mg/g) | 0.37 ± 0.00 b | 0.41 ± 0.01 a |
| Epicatechin (mg/g) | 0.53 ± 0.01 b | 1.12 ± 0.01 a |
| Methylxanthines | ||
| Theobromine (mg/g) | 8.50 ± 0.18 a | 8.37 ± 1.54 a |
| Caffeine (mg/g) | 2.55 ± 0.05 a | 2.45 ± 0.25 a |
| Group | RI * | Compound | Summer Area % | Winter Area % | Attribute ** |
|---|---|---|---|---|---|
| Alcohols | 894 | 2-Heptanol | 3.52 | 6.78 | Sweet, citrus |
| 1106 | 2-Phenylethyl ethanol | 0.35 | 0.30 | Honey, floral, caramel | |
| Total Alcohols (%) | 3.87 | 7.08 | |||
| Aldehydes | 952 | Benzaldehyde | 6.34 | 2.44 | Bitter almonds, grass |
| 1036 | Phenylacetaldehyde | 36.73 | 25.45 | Honey | |
| Total Aldehydes (%) | 43.07 | 27.89 | |||
| Ketones | 889 | 2-Heptanone | 3.88 | 5.14 | Floral, fruity |
| 1059 | Acetophenone | 2.29 | 2.18 | Floral, almonds, sweet | |
| 1087 | 2-Nonanone | 2.40 | 4.16 | Floral | |
| Total Ketones (%) | 8.57 | 11.48 | |||
| Esters | 869 | Isoamyl acetate | 9.41 | 4.64 | Fruity |
| 871 | Sec-amyl acetate | 5.89 | 9.63 | Banana | |
| 1169 | Ethyl benzoate | 0.46 | 0.21 | Floral, fruity, chamomile | |
| 1196 | Ethyl octanoate | 0.40 | 0.30 | Floral, fruity | |
| 1243 | Ethyl phenylacetate | 1.50 | 0.81 | Sweet, waxy | |
| 1251 | 2-Phenethyl acetate | 15.38 | 4.68 | Roses | |
| 1433 | Isoamyl benzoate | 3.03 | 4.15 | Balsam, sweet | |
| 1594 | Ethyl dodecanoate | 0.06 | 0.23 | Floral, fruity | |
| Total Esters (%) | 36.13 | 24.65 | |||
| Others | 988 | Mycrene | 0.93 | 6.75 | Off-flavor |
| 1032 | Cis-beta-ocimene | 0.37 | 3.30 | Floral | |
| 1095 | Linalool | 3.99 | 11.14 | Floral | |
| 1128 | Allo-ocimeno | 0.23 | 2.47 | Floral | |
| 1200 | n-Dodecane | 0.22 | 0.24 | Off-flavor | |
| 1400 | n-Tetradecane | 0.26 | 0.39 | Off-flavor | |
| Total Others (%) | 6.00 | 24.29 | |||
| Total (all groups) (%) | 97.64 | 95.39 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaspar, D.P.; Chagas Junior, G.C.A.; de Aguiar Andrade, E.H.; Nascimento, L.D.d.; Chisté, R.C.; Ferreira, N.R.; Martins, L.H.d.S.; Lopes, A.S. How Climatic Seasons of the Amazon Biome Affect the Aromatic and Bioactive Profiles of Fermented and Dried Cocoa Beans? Molecules 2021, 26, 3759. https://doi.org/10.3390/molecules26133759
Gaspar DP, Chagas Junior GCA, de Aguiar Andrade EH, Nascimento LDd, Chisté RC, Ferreira NR, Martins LHdS, Lopes AS. How Climatic Seasons of the Amazon Biome Affect the Aromatic and Bioactive Profiles of Fermented and Dried Cocoa Beans? Molecules. 2021; 26(13):3759. https://doi.org/10.3390/molecules26133759
Chicago/Turabian StyleGaspar, Daniela Pinheiro, Gilson Celso Albuquerque Chagas Junior, Eloisa Helena de Aguiar Andrade, Lidiane Diniz do Nascimento, Renan Campos Chisté, Nelson Rosa Ferreira, Luiza Helena da Silva Martins, and Alessandra Santos Lopes. 2021. "How Climatic Seasons of the Amazon Biome Affect the Aromatic and Bioactive Profiles of Fermented and Dried Cocoa Beans?" Molecules 26, no. 13: 3759. https://doi.org/10.3390/molecules26133759
APA StyleGaspar, D. P., Chagas Junior, G. C. A., de Aguiar Andrade, E. H., Nascimento, L. D. d., Chisté, R. C., Ferreira, N. R., Martins, L. H. d. S., & Lopes, A. S. (2021). How Climatic Seasons of the Amazon Biome Affect the Aromatic and Bioactive Profiles of Fermented and Dried Cocoa Beans? Molecules, 26(13), 3759. https://doi.org/10.3390/molecules26133759










