Rutin and Quercetin Decrease Cholesterol in HepG2 Cells but Not Plasma Cholesterol in Hamsters by Oral Administration
Abstract
:1. Introduction
2. Results
2.1. Effects on Cell Viability
2.2. Cholesterol Content in HepG2 Cells
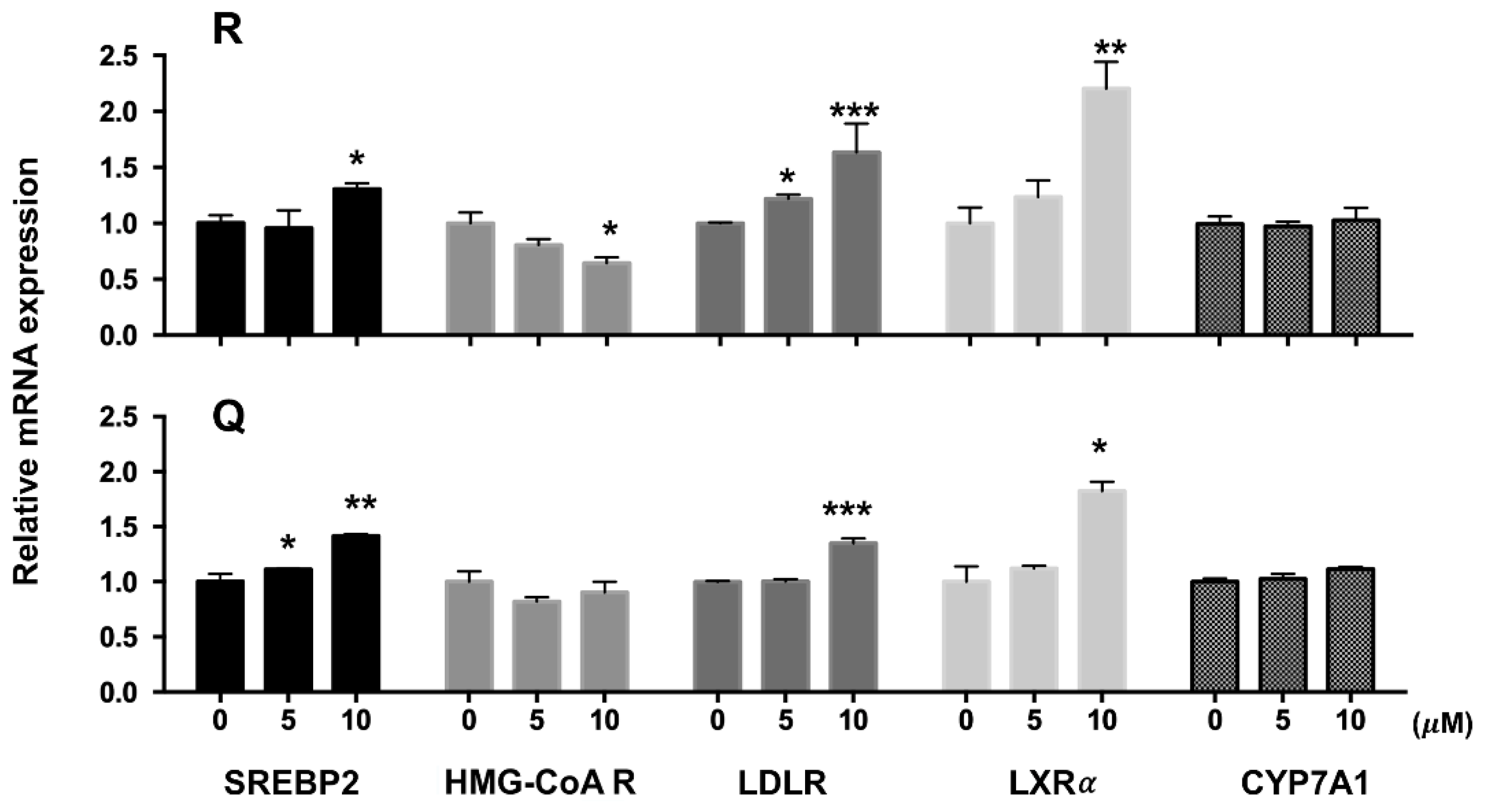
2.3. mRNA and Protein Abundances in HepG2 Cells
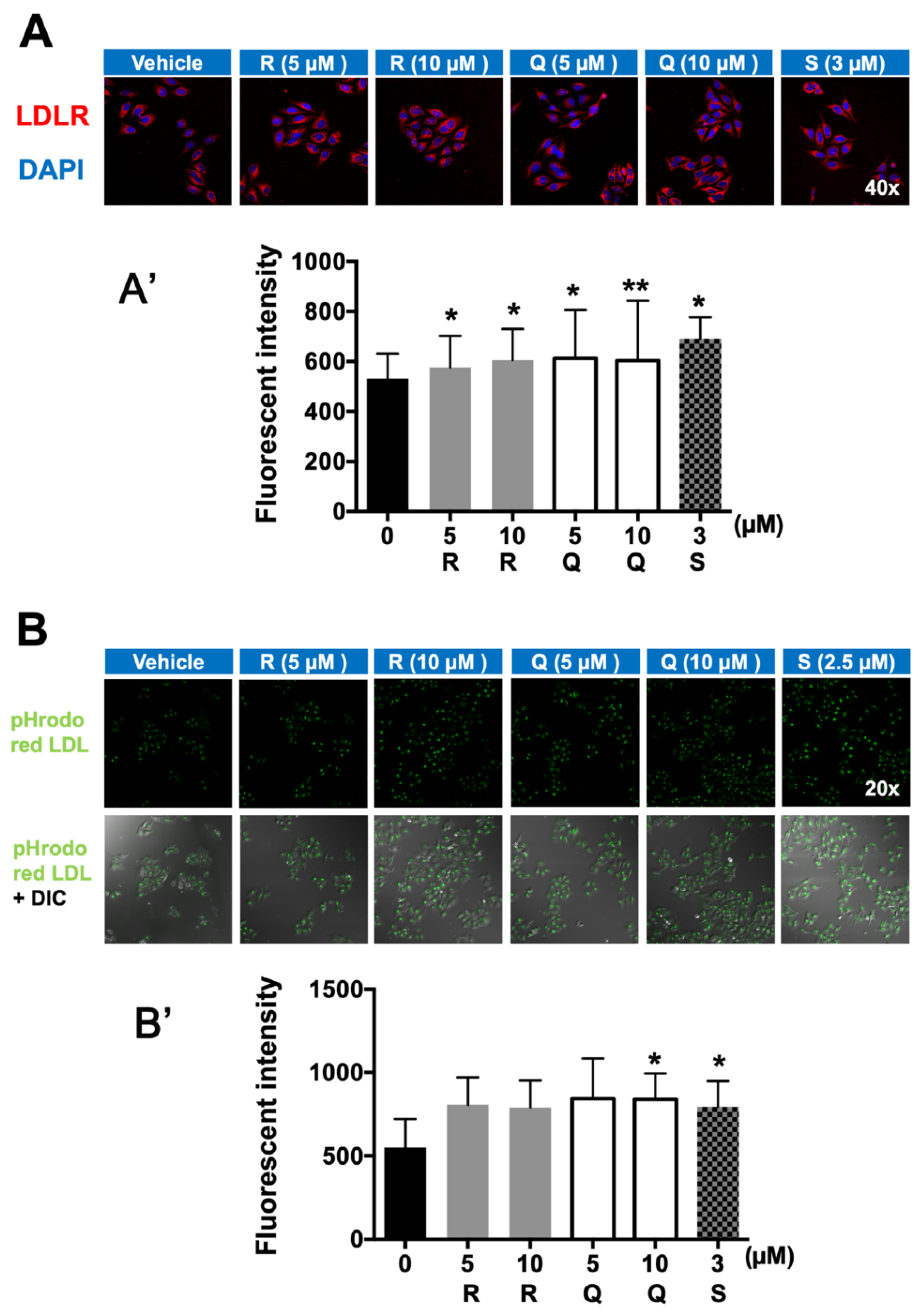
2.4. Fluorescence Immunostaining of LDLR and LDL-C Uptake
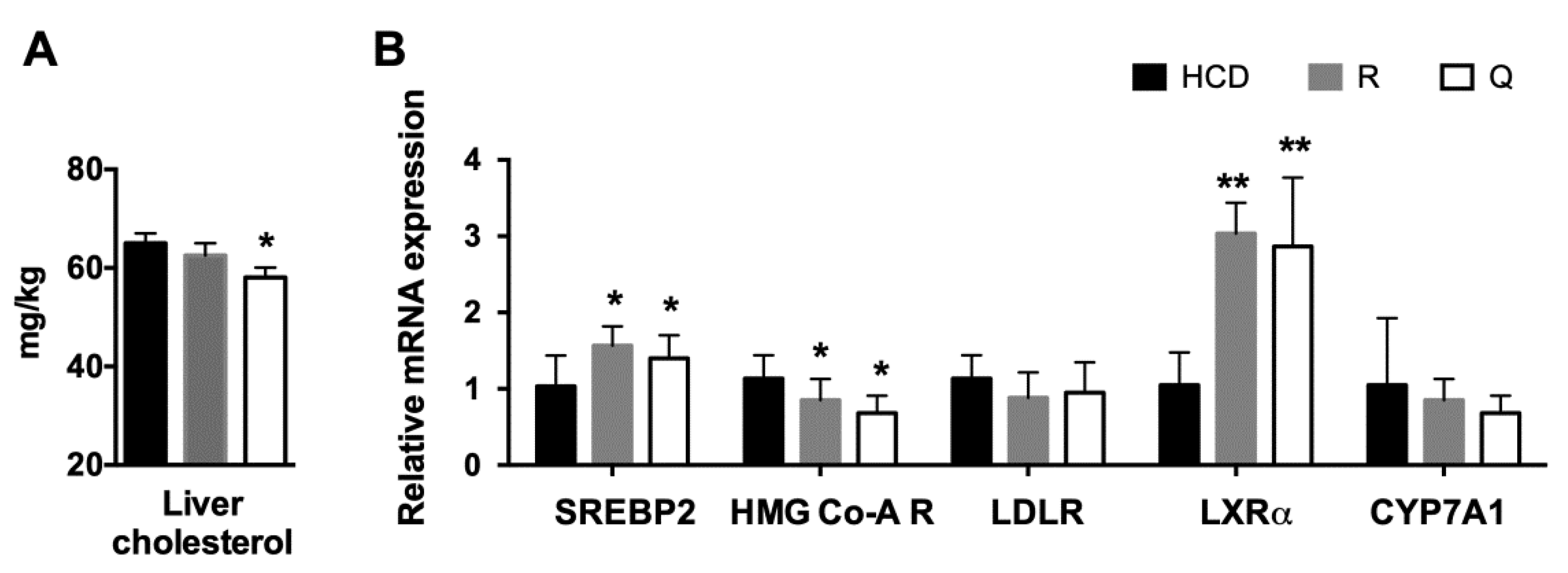
2.5. Plasma Profile and Liver Cholesterol
2.6. mRNA Abundance of Target Genes in the Liver
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cell Culture
4.3. MTT Assay
4.4. Cholesterol Determination in Cells
4.5. Real-Time PCR Analysis
4.6. Western Blotting
4.7. LDL Uptake Assay
4.8. Immunofluorescence Staining
4.9. Hamsters and Diets
4.10. Liver Cholesterol Determination
4.11. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Chen, Z.Y.; Ma, K.Y.; Liang, Y.; Peng, C.; Zuo, Y. Role and classification of cholesterol-lowering functional foods. J. Funct. Foods 2011, 3, 61–69. [Google Scholar] [CrossRef]
- Hoving, L.R.; Katiraei, S.; Heijink, M.; Pronk, A.; van der Wee-Pals, L.; Streefland, T.; Giera, M.; Willems van Dijk, K.; van Harmelen, V. Dietary Mannan Oligosaccharides Modulate Gut Microbiota, Increase Fecal Bile Acid Excretion, and Decrease Plasma Cholesterol and Atherosclerosis Development. Mol. Nutr. Food Res. 2018, 62, e1700942. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, T.; Harada, N.; Kuze, J.; Chiba, M.; Iwao, T.; Matsunaga, T. Human small intestinal epithelial cells differentiated from adult intestinal stem cells as a novel system for predicting oral drug absorption in humans. Drug Metab. Dispos. 2014, 42, 1947–1954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.Q.H. Regulation of Intestinal Cholesterol Absorption. Annu. Rev. Physiol. 2007, 69, 221–248. [Google Scholar] [CrossRef] [PubMed]
- Miserez, A.R.; Muller, P.Y.; Barella, L.; Barella, S.; Staehelin, H.B.; Leitersdorf, E.; Kark, J.D.; Friedlander, Y. Sterol-regulatory element-binding protein (SREBP)-2 contributes to polygenic hypercholesterolaemia. Atherosclerosis 2002, 164, 15–26. [Google Scholar] [CrossRef]
- Madison, B.B. SREBP2: A master regulator of sterol and fatty acid synthesis. J. Lip. Res. 2016, 57, 333–335. [Google Scholar] [CrossRef] [Green Version]
- Schaap, F.G.; Trauner, M.; Jansen, P.L. Bile acid receptors as targets for drug development. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 55–67. [Google Scholar] [CrossRef]
- Guo, S.; Li, L.; Yin, H. Cholesterol Homeostasis and Liver X Receptor (LXR) in Atherosclerosis. Cardiovasc. Hematol. Disord. Drug Targets 2018, 18, 27–33. [Google Scholar] [CrossRef]
- Gimenez-Bastida, J.A.; Zielinski, H. Buckwheat as a Functional Food and Its Effects on Health. J. Agric. Food Chem. 2015, 63, 7896–7913. [Google Scholar] [CrossRef]
- Liew, S.S.; Ho, W.Y.; Yeap, S.K.; Sharifudin, S.A.B. Phytochemical composition and in vitro antioxidant activities of Citrus sinensis peel extracts. PeerJ 2018, 6, e5331. [Google Scholar] [CrossRef] [Green Version]
- Al-Rejaie, S.S.; Aleisa, A.M.; Sayed-Ahmed, M.M.; Al-Shabanah, O.A.; Abuohashish, H.M.; Ahmed, M.M.; Al-Hosaini, K.A.; Hafez, M.M. Protective effect of rutin on the antioxidant genes expression in hypercholestrolemic male Westar rat. BMC Complement. Altern. Med. 2013, 13, 136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Umarani, V.; Muvvala, S.; Ramesh, A.; Lakshmi, B.V.; Sravanthi, N. Rutin potentially attenuates fluoride-induced oxidative stress-mediated cardiotoxicity, blood toxicity and dyslipidemia in rats. Toxicol. Mech. Methods 2015, 25, 143–149. [Google Scholar] [CrossRef]
- Sikder, K.; Kesh, S.B.; Das, N.; Manna, K.; Dey, S. The high antioxidative power of quercetin (aglycone flavonoid) and its glycone (rutin) avert high cholesterol diet induced hepatotoxicity and inflammation in Swiss albino mice. Food Funct. 2014, 5, 1294–1303. [Google Scholar] [CrossRef]
- Huang, R.; Shi, Z.; Chen, L.; Zhang, Y.; Li, J.; An, Y. Rutin alleviates diabetic cardiomyopathy and improves cardiac function in diabetic ApoEknockout mice. Eur. J. Pharmacol. 2017, 814, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.L.; Peng, C.C.; Chen, K.C.; Peng, R.Y. Rutin (quercetin rutinoside) induced protein-energy malnutrition in chronic kidney disease, but quercetin acted beneficially. J. Agric. Food Chem. 2013, 61, 7258–7267. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, R.; Li, Y.M.; Liang, N.; Zhao, Y.; Zhu, H.; He, Z.; Liu, J.; Hao, W.; Jiao, R.; et al. Cholesterol-Lowering Activity of Tartary Buckwheat Protein. J. Agric. Food Chem. 2017, 65, 1900–1906. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.Y.; Jiao, R.; Ma, K.Y. Cholesterol-lowering nutraceuticals and functional foods. J. Agric. Food Chem. 2008, 56, 8761–8773. [Google Scholar] [CrossRef] [PubMed]
- Edwards, C.A.; Havlik, J.; Cong, W.; Mullen, W.; Preston, T.; Morrison, D.J.; Combet, E. Polyphenols and health: Interactions between fibre, plant polyphenols and the gut microbiota. Nutr. Bull. 2017, 42, 356–360. [Google Scholar] [CrossRef]
- Braun, J.B.S.; Ruchel, J.B.; Adefegha, S.A.; Coelho, A.P.V.; Trelles, K.B.; Signor, C.; Rubin, M.A.; Oliveira, J.S.; Dornelles, G.L.; de Andrade, C.M.; et al. Neuroprotective effects of pretreatment with quercetin as assessed by acetylcholinesterase assay and behavioral testing in poloxamer-407 induced hyperlipidemic rats. Biomed. Pharma. Ther. 2017, 88, 1054–1063. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.B.S.; Ruchel, J.B.; Manzoni, A.G.; Abdalla, F.H.; Casalli, E.A.; Castilhos, L.G.; Passos, D.F.; Leal, D.B.R. Pretreatment with quercetin prevents changes in lymphocytes E-NTPDase/E-ADA activities and cytokines secretion in hyperlipidemic rats. Mol. Cell Biochem. 2018, 444, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; Jiang, T.; Zhao, G.J. Quercetin induces the selective uptake of HDL-cholesterol via promoting SR-BI expression and the activation of the PPARgamma/LXRalpha pathway. Food Funct. 2018, 9, 624–635. [Google Scholar] [CrossRef]
- Terao, J.; Kawai, Y.; Murota, K. Vegetable flavonoids and cardiovascular disease. Asia Pac. J. Clin. Nutr. 2008, 17 (Suppl. S1), 291–293. [Google Scholar] [PubMed]
- Kuipers, E.N.; Dam, A.D.V.; Held, N.M.; Mol, I.M.; Houtkooper, R.H.; Rensen, P.C.N.; Boon, M.R. Quercetin Lowers Plasma Triglycerides Accompanied by White Adipose Tissue Browning in Diet-Induced Obese Mice. Int. J. Mol. Sci. 2018, 19, 1786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamada, C.; da Silva, E.L.; Ohnishi-Kameyama, M.; Moon, J.H.; Terao, J. Attenuation of lipid peroxidation and hyperlipidemia by quercetin glucoside in the aorta of high cholesterol-fed rabbit. Free Radic. Res. 2005, 39, 185–194. [Google Scholar] [CrossRef]
- AlSharari, S.D.; Al-Rejaie, S.S.; Abuohashish, H.M.; Ahmed, M.M.; Hafez, M.M. Rutin Attenuates Hepatotoxicity in High-Cholesterol-Diet-Fed Rats. Oxid. Med. Cell Longev. 2016, 2016, 5436745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, C.L.; Wu, C.H.; Huang, S.L.; Yen, G.C. Phenolic compounds rutin and o-coumaric acid ameliorate obesity induced by high-fat diet in rats. J. Agric. Food Chem. 2009, 57, 425–431. [Google Scholar] [CrossRef]
- Liu, Q.; Pan, R.; Ding, L.; Zhang, F.; Hu, L.; Ding, B.; Zhu, L.; Xia, Y.; Dou, X. Rutin exhibits hepatoprotective effects in a mouse model of non-alcoholic fatty liver disease by reducing hepatic lipid levels and mitigating lipid-induced oxidative injuries. Int. Immunopharmacol. 2017, 49, 132–141. [Google Scholar] [CrossRef]
- Emini Veseli, B.; Perrotta, P.; de Meyer, G.R.A.; Roth, L.; van der Donckt, C.; Martinet, W.; de Meyer, G.R.Y. Animal models of atherosclerosis. Eur. J. Pharmacol. 2017, 816, 3–13. [Google Scholar] [CrossRef]
- Priyadharsini, R.P. Animal models to evaluate anti-atherosclerotic drugs. Fundam. Clin. Pharmacol. 2015, 29, 329–340. [Google Scholar] [CrossRef]
- Krumholz, H.M. Treatment of Cholesterol in 2017. JAMA 2017, 318, 417–418. [Google Scholar] [CrossRef]
- Marquart, T.J.; Allen, R.M.; Ory, D.S.; Baldan, A. miR-33 links SREBP-2 induction to repression of sterol transporters. Proc. Natl. Acad. Sci. USA 2010, 107, 12228–12232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Liu, J.; Hao, W.; Zhu, H.; Liang, N.; He, Z.; Ma, K.Y.; Chen, Z.Y. Structure-Specific Effects of Short-Chain Fatty Acids on Plasma Cholesterol Concentration in Male Syrian Hamsters. J. Agric. Food Chem. 2017, 65, 10984–10992. [Google Scholar] [CrossRef]
- Moon, J.; Lee, S.M.; Do, H.J.; Cho, Y.; Chung, J.H.; Shin, M.J. Quercetin up-regulates LDL receptor expression in HepG2 cells. Phytother. Res. 2012, 26, 1688–1694. [Google Scholar] [CrossRef] [PubMed]
- Cheurfa, M.; Abdallah, H.H.; Allem, R.; Noui, A.; Picot-Allain, C.M.N.; Mahomoodally, F. Hypocholesterolaemic and antioxidant properties of Olea europaea L. leaves from Chlef province, Algeria using in vitro, in vivo and in silico approaches. Food Chem. Toxicol. 2019, 123, 98–105. [Google Scholar] [CrossRef]
- Khamis, A.A.; Salama, A.F.; Kenawy, M.E.; Mohamed, T.M. Regulation of hepatic hydroxy methyl glutarate—CoA reductase for controlling hypercholesterolemia in rats. Biomed. Pharmacother. Ther. 2017, 95, 1242–1250. [Google Scholar] [CrossRef]
- Hong, C.; Tontonoz, P. Liver X receptors in lipid metabolism: Opportunities for drug discovery. Nat. Rev. Drug Discov. 2014, 13, 433–444. [Google Scholar] [CrossRef]
- Mariee, A.D.; Abd-Allah, G.M.; El-Beshbishy, H.A. Protective effect of dietary flavonoid quercetin against lipemic-oxidative hepatic injury in hypercholesterolemic rats. Pharm. Biol. 2012, 50, 1019–1025. [Google Scholar] [CrossRef] [PubMed]
- Nait Chabane, M.; al Ahmad, A.; Peluso, J.; Muller, C.D.; Ubeaud, G. Quercetin and naringenin transport across human intestinal Caco-2 cells. J. Pharm. Pharmacol. 2009, 61, 1473–1483. [Google Scholar] [CrossRef]
- Perez-Moral, N.; Saha, S.; Philo, M.; Hart, D.J.; Winterbone, M.S.; Hollands, W.J.; Spurr, M.; Bows, J.; van der Velpen, V.; Kroon, P.A.; et al. Comparative bio-accessibility, bioavailability and bioequivalence of quercetin, apigenin, glucoraphanin and carotenoids from freeze-dried vegetables incorporated into a baked snack versus minimally processed vegetables: Evidence from in vitro models and a human bioavailability study. J. Funct. Foods 2018, 48, 410–419. [Google Scholar]
- Chang, C.E.; Hsieh, C.M.; Huang, S.C.; Su, C.Y.; Sheu, M.T.; Ho, H.O. Lecithin-Stabilized Polymeric Micelles (LsbPMs) for Delivering Quercetin: Pharmacokinetic Studies and Therapeutic Effects of Quercetin Alone and in Combination with Doxorubicin. Sci. Rep. 2018, 8, 17640. [Google Scholar] [CrossRef]
- Erlund, I.; Kosonen, T.; Alfthan, G.; Maenpaa, J.; Perttunen, K.; Kenraali, J.; Parantainen, J.; Aro, A. Pharmacokinetics of quercetin from quercetin aglycone and rutin in healthy volunteers. Eur. J. Clin. Pharmacol. 2000, 56, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Gohlke, A.; Ingelmann, C.J.; Nurnberg, G.; Starke, A.; Wolffram, S.; Metges, C.C. Bioavailability of quercetin from its aglycone and its glucorhamnoside rutin in lactating dairy cows after intraduodenal administration. J. Dairy Sci. 2013, 96, 2303–2313. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Ali, A.; Ali, J.; Sahni, J.K.; Baboota, S. Rutin: Therapeutic potential and recent advances in drug delivery. Expert Opin. Investig. Drugs 2013, 22, 1063–1079. [Google Scholar] [CrossRef]
- Wein, S.; Beyer, B.; Zimmermann, B.F.; Blank, R.H.; Wolffram, S. Bioavailability of Quercetin from Onion Extracts after Intraruminal Application in Cows. J. Agric. Food Chem. 2018, 66, 10188–10192. [Google Scholar] [CrossRef]
- Magar, R.; Sohng, J. A Review on Structure, Modifications and Structure-Activity Relation of Quercetin and Its Derivatives. J. Microbiol. Biotechn. 2020, 30, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Riva, A.; Ronchi, M.; Petrangolini, G.; Bosisio, S.; Allegrini, P. Improved Oral Absorption of Quercetin from Quercetin Phytosome(R), a New Delivery System Based on Food Grade Lecithin. Eur. J. Drug Metab. Pharmacokinet. 2019, 44, 169–177. [Google Scholar] [CrossRef] [Green Version]
- Mel, M.; Gunathilake, K.; Fernando, C.A.N. Formulation of microencapsulated rutin and evaluation of bioactivity and stability upon in vitro digestive and dialysis conditions. Int. J. Biol. Macromol. 2020, 159, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Ravi, G.S.; Charyulu, R.N.; Dubey, A.; Prabhu, P.; Hebbar, S.; Mathias, A.C. Nano-lipid Complex of Rutin: Development, Characterisation and In Vivo Investigation of Hepatoprotective, Antioxidant Activity and Bioavailability Study in Rats. AAPS PharmSciTech 2018, 19, 3631–3649. [Google Scholar] [CrossRef]
- Li, X.; Guo, J.; Liang, N.; Jiang, X.; Song, Y.; Ou, S.; Hu, Y.; Jiao, R.; Bai, W. 6-Gingerol Regulates Hepatic Cholesterol Metabolism by Up-regulation of LDLR and Cholesterol Efflux-Related Genes in HepG2 Cells. Front. Pharmacol. 2018, 9, 159. [Google Scholar] [CrossRef] [Green Version]
- Liang, N.; Li, Y.; Chung, H.Y. Two natural eudesmane-type sesquiterpenes from Laggera alata inhibit angiogenesis and suppress breast cancer cell migration through VEGF- and Angiopoietin 2-mediated signaling pathways. Int. J. Oncol. 2017, 51, 213–222. [Google Scholar] [CrossRef]
- Wang, X.; Guan, L.; Zhao, Y.; Lei, L.; Liu, Y.; Ma, K.Y.; Wang, L.; Man, S.W.; Wang, J.; Huang, Y.; et al. Plasma cholesterol-lowering activity of dietary dihydrocholesterol in hypercholesterolemia hamsters. Atherosclerosis 2015, 242, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, M.; Wang, G.; Shao, F.; Chen, W.; Xia, C.; Wang, S.; Li, Y.; Zhou, G.; Liu, Z. EM23, A Natural Sesquiterpene Lactone from Elephantopus mollis, Induces Apoptosis in Human Myeloid Leukemia Cells through Thioredoxin- and Reactive Oxygen Species-Mediated Signaling Pathways. Front. Pharmacol. 2016, 7, 77. [Google Scholar] [CrossRef] [Green Version]
- He, Z.; Lei, L.; Kwek, E.; Zhao, Y.; Liu, J.; Hao, W.; Zhu, H.; Liang, N.; Ma, K.Y.; Ho, H.M.; et al. Ginger attenuates trimethylamine-N-oxide (TMAO)-exacerbated disturbance in cholesterol metabolism and vascular inflammation. J. Funct. Foods 2019, 52, 25–33. [Google Scholar] [CrossRef]


| Gene | For HepG2 Cells | For Hamster |
|---|---|---|
| GAPDH | Fw: CCCACTCCTCCACCTTTGAC | Fw: GAACATCATCCCTGCATCCA |
| Rv: TCTTCCTCTTGTGCTCTTGC | Rv: CCAGTGAGCTTCCCGTTCA | |
| SREBP2 | Fw: AACGGTCATTCACCCAGGTC | Fw: GGACTTGGTCATGGGAACAGATG |
| Rv: GGCTGAAGAATAGGAGTTGCC | Rv: TGTAATCAATGGCCTTCCTCAGAAC | |
| HMG-CoA R | Fw: TGATTGACCTTTCCAGAGCAAG | Fw: CGAAGGGTTTGCAGTGATAAAGGA |
| Rv: CTAAAATTGCCATTCCACGAGC | Rv: GCCATAGTCACATGAAGCTTCTGTA | |
| LDLR | Fw: ACGGCGTCTCTTCCTATGACA | Fw: GCCGGGACTGGTCAG ATG |
| Rv: CCCTTGGTATCCGCAACAGA | Rv: ACAGCCACCATTGTTGTCCA | |
| LXRα | Fw: TCTGGAGACATCTCGGAGGTA | Fw: GTTTGTCCTGAGCTTCGTCC |
| Rv: GGCCCTGGAGAACTCGAAG | Rv: CACCGCTGTGGCAAACATAG | |
| CYP7A1 | Fw: GCAATTTGGTGCCAATCCTCT | Fw: GGTAGTGTGCTGTTGTATATGGGTTA |
| Rv: GCACAACACCTTATGGTATGACA | Rv: ACAGCCCAGGTATGGAATCAAC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, N.; Li, Y.-M.; He, Z.; Hao, W.; Zhao, Y.; Liu, J.; Zhu, H.; Kwek, E.; Ma, K.-Y.; He, W.-S.; et al. Rutin and Quercetin Decrease Cholesterol in HepG2 Cells but Not Plasma Cholesterol in Hamsters by Oral Administration. Molecules 2021, 26, 3766. https://doi.org/10.3390/molecules26123766
Liang N, Li Y-M, He Z, Hao W, Zhao Y, Liu J, Zhu H, Kwek E, Ma K-Y, He W-S, et al. Rutin and Quercetin Decrease Cholesterol in HepG2 Cells but Not Plasma Cholesterol in Hamsters by Oral Administration. Molecules. 2021; 26(12):3766. https://doi.org/10.3390/molecules26123766
Chicago/Turabian StyleLiang, Ning, Yuk-Man Li, Zouyan He, Wangjun Hao, Yimin Zhao, Jianhui Liu, Hanyue Zhu, Erika Kwek, Ka-Ying Ma, Wen-Sen He, and et al. 2021. "Rutin and Quercetin Decrease Cholesterol in HepG2 Cells but Not Plasma Cholesterol in Hamsters by Oral Administration" Molecules 26, no. 12: 3766. https://doi.org/10.3390/molecules26123766
APA StyleLiang, N., Li, Y.-M., He, Z., Hao, W., Zhao, Y., Liu, J., Zhu, H., Kwek, E., Ma, K.-Y., He, W.-S., & Chen, Z.-Y. (2021). Rutin and Quercetin Decrease Cholesterol in HepG2 Cells but Not Plasma Cholesterol in Hamsters by Oral Administration. Molecules, 26(12), 3766. https://doi.org/10.3390/molecules26123766







