2.1. Nutritional Characteristics of Honeys
Honey moisture content depends on the environmental conditions and the beekeeper manipulation in the harvest period [
14]. Honey with high water content is more likely to ferment, making the preservation and storage more difficult. The results of water content in the tested honey samples are shown in
Table 1. This parameter in conventional honeys ranges from 14.8% to 19.8%, while in organic honeys from 16.1% to 19.9%, which is in the acceptable range according to the EC Directive 2001/110 [
15]. The lowest content of water was found in honeydew honeys (average value of 15.4%), while the highest (average value of 19.1%)—in dandelion honeys. Linden honey was characterized by similar values of this quality factor, regardless of the production method. The difference found in this case was at the level of approx. 2.5%. On the other hand, the greatest disproportion was noticed in the case of forest honeys and it amounted to approx. 12%.
The results of potential acidity measured by titration with sodium hydroxide solution are presented in
Table 1. The highest values of the parameter were found for conventional buckwheat and forest honeys (47 and 43 mEq/kg, respectively). The lowest acidity was determined in conventional linden honey (32 mEq/kg). The largest difference (approx. 19%) between organic and conventional production methods was noticed for the linden honeys. Honey pH is affected by the conditions during processing and storage, which also influences texture, stability and shelf-life. The low pH value and the high acidity correspond to the longer honey shelf life because these conditions do not favor microbial growing. On the other hand, high acidic values may indicate the presence of undesirable fermentation, especially in honey with higher water content [
16]. The results of the determination of active acidity (pH measurement) are shown in
Table 1. High values of this indicator were measured for both dandelion honeys, conventional linden honey and organic honeydew honey (pH in the range of 4.8–4.9). The rest of honeys were more acidic, and the pH value was in the range of 3.6–4.3. The lowest pH value among the analyzed honey samples was determined for conventional buckwheat honey. It was found that the greatest difference between the pH of organic and conventional production methods concerned linden honeys. In this case, organic honey was characterized by a pH value lower by approx. 12% than conventional honey. On the other hand, the method of honey production and origin had no effect on the active acidity of forest and dandelion honeys.
The nutritional quality of food is based on vitamin C analysis as one of the most frequently used indicators of quality [
9]. It may strengthen honey’s antibacterial activity [
17]. The processing and storage of honey, as well as its botanical origin influence vitamin content and its antioxidant capacity [
18]. This vitamin has been found in almost all types of honey [
19]. Honey is not an important source of vitamin C, as is described in the present study as well as other literature. In our study (
Table 1), honeys with high amounts of vitamin C were: honeydew (3.86–4.17 mg/kg), forest (2.56–2.77 mg/kg), buckwheat (2.51–2.73 mg/kg), lime (2.17–4.47 mg/kg) and dandelion (3.75–4.11 mg/kg). Ciulu et al. [
20,
21] used RP-HPLC method to simultaneously determine five water-soluble vitamins (B2, B3, B5, B9 and C) in 25 Sardinian honey samples of 10 different botanical origins, in addition to three monofloral honeys (acacia and linden) from northern Italy. The amount of water-soluble vitamin including vitamin C found in honey was also quite low: the overall concentration of all analytes did not exceed 40 mg/kg. The content of vitamin C was low (over 5 mg/kg) and invariant with respect to the origin of honey. The correlation of analyzed vitamins to the botanical origin of the samples may be a useful tool to determine the origin of honeys.
However, honey may contain undesirable compounds like furan derivatives (it may lead to toxicity), which create during a heat treatment facilitating the filtration process, reduce viscosity, delay the crystallization and prevent fermentation during honey processing [
22]. The Maillard reaction is a chemical reaction between amino acids and reducing sugars and occurs during cooking conditions, as well as in food storage at low temperature [
23,
24,
25]. Hydroxymethyl-furfural (5-hydroxy-2-furaldehyde, HMF) is one of many important Maillard reaction products and the evaluation of HMF level is a well-known procedure to investigate the quality of honey (an indicator of quality different food products). Due to its adverse effects on human health, like cytotoxic, mutagenic, genotoxic and carcinogenic consequences, the HMF level is limited for some foods such as molasses and honey [
22,
26,
27,
28,
29,
30]. HMF is absent in fresh honeys immediately stored by bees and tends to increase during processing and/or aging of the product; therefore, the HMF content is widely recognized as a parameter of honey sample freshness [
31,
32]. In our study (
Table 1), the levels of HMF were distributed over a wide range of concentrations (6.05–54.25 mg/kg) in honey samples. The HMF content was the lowest in honeydew honeys (10.85 mg/kg in conventional and 6.05 mg/kg in organic). These results are in agreement with the results published by other authors [
33,
34,
35,
36,
37,
38,
39,
40]. The exceeded amount of HMF was detected in the conventional and organic buckwheat honey samples (48.42 and 54.25 mg/kg, respectively). These values were higher than requirements (<40 mg/kg) established by Polish and international standards for honey [
41,
42,
43]. It can be assumed that these honeys were probably adulterated by invert syrup or they were exposed to excessive heating.
Fructose, glucose and sucrose, as the most important sugars analyzed in the honey samples, are related to those present in the nectar foraged by bees to make honey in such a way that identification of source is possible [
44]. Mean sugar content with the corresponding standard deviation, sum of reducing sugars and fructose/glucose ratio (F/G) are shown in
Table 2. The two reducing sugars fructose and glucose were detected in all honey samples. Sucrose, however, was not detected in 6 samples. Fructose and glucose represented the largest portion of honey (in case of organic honeys average 72.06 g/100 g and 70.37 g/100 g in the case of conventional honeys). Sucrose formed the lowest portion (in case of organic honeys average 1.29 g/100 g and 0.94 g/100 g in case of conventional honeys). The proportion of fructose to glucose fluctuates considerably but there is always more fructose than glucose in fresh nectar obtained from flower [
45,
46,
47]. The results of presented study agree with these findings. Reducing sugars, mainly fructose and glucose, stand for the largest portion of honey composition, while sucrose content was the lowest in all honey samples. These results are in conformity with the standard requirements [
48]. This proves adequate honey treatment, good maturity, energy value and high viscosity [
49,
50,
51,
52].
Fructose levels not were significantly different in the honey samples. Its level varied from 37.01 to 42.75 g/100 g.
The lowest fructose content was in organic dandelion honey, while the same honey variety from conventional method was characterized by the highest amount of this sugar. Fructose levels were significantly higher in all samples compared with glucose. Most of the physical and nutritional characteristics of honey depends on the content of this sugar [
53]. Mendes et al. [
54] found fructose to be the largest portion of 50 Portuguese honey samples evaluated. Golob and Plestenjak [
47] found that the differences in fructose and glucose mass fraction among different types of honey are significant. The maximum value of fructose (42.75 g/100 g) in other study was similar to maximum values 40.1 and 40.6 g/100 g in two honey samples from Spain evaluated by Mateo and Bosch-Reig [
55]. Fructose range was different, indicating the variety of floral sources from which the honey samples originated.
The second important sugar in honey after fructose is glucose. Its levels were significantly different in the honey samples. The organic honeydew honey contained the lowest amount of glucose (27.17 g/100 g), while the organic linden honey has the highest amount (36.47 g/100 g). The glucose mean value (31.80 g/100 g for conventional honeys and 31.27 g/100 g for organic honeys) of the present study is similar with the results reported by Golob and Plestenjak [
47] and Gomez Barez et al. [
56]. These researchers have shown the glucose levels for Slovene (29.4 g/100 g) and Spanish (29.2 g/100 g) honeys.
The sucrose content in the studied honeys was ranging from 0 g/100 g in most samples to 4.57 g/100 g in organic linden honey. Sucrose content was the lowest among the 3 tested sugars, which were determined in honey samples as a result of enzyme invertase action, which breaks down the disaccharide molecule of sucrose in the nectar into the monosaccharides, glucose and fructose during the process of ripening of honey [
49,
57,
58]. However, sucrose, which is not highly soluble in honey’s water, can provide information about adulteration and botanical origin of the honey [
35,
36,
53]. In the present study, the mean sucrose was 1.21 g/100 g in organic honeys and 0.94 g/100 g in conventional honeys—similar to that found by Perez-Arquillue et al. [
59] and Golob and Plestenjak [
47] in Spanish and Slovakian honeys, respectively. In turn, it is lower than the 3.3 g/100 g and 3.0 g/100 g sucrose reported by Abu-Tarboush et al. [
60] and Yilmaz and Yavuz [
61] in Saudi and Turkish honeys, respectively. Perez-Arquillue et al. [
59] reported sucrose content below 1 g/100 g in several Spanish honey samples of different botanical origins. Absence of sucrose in six samples may be due to these honeys being obtained from nectars, which contain just fructose and glucose as a result of total conversion of sucrose into these monosaccharides before their secretion nectars [
62,
63]. Only fructose and glucose in some of their analyzed honey samples from Indonesia and Nigeria were detected by White et al. [
64] and Agwu et al. [
46]. The high level of sucrose in honeys may be a result of unripe honey as it was collected from uncapped honeycomb [
46,
49,
63].
An indication of the ability of honey to crystallize is fructose to glucose ratio (F/G), which is used to typify honey samples from different origins, it is the aforementioned indicator, the tendency of crystallization. The organic honeydew honey with low F/G ratio (0.73) crystallized after its collection and storage at room temperature. F/G ratio in organic honey samples ranged between 0.73–0.95 and in conventional honey samples ranged between 0.74–0.83. The highest F/G ratio was observed in organic linden honey (0.95). A high or low ratio signifies liquid or crystallized honey, respectively [
60]. Similar results were found with Litchi honeys analyzed by Suryanarayana et al. [
65]. The viscosity of honey can be examined by its texture. The wide range of F/G ratio in present study may be indicative of the variety of floral sources from which the honey samples originated. Mateo and Bosch-Reig [
55] obtained a similar range of F/G ratio for Spanish honey samples (0.99–1.40 g/100 g). While Perez-Arquillue et al. [
59] reported narrower range (1.06–1.13 g/100 g) in rosemary honeys, which displayed a remarkable low variation among unfloral samples.
2.2. Organoleptic Characteristics of Honeys
The results of the organoleptic evaluation are presented in
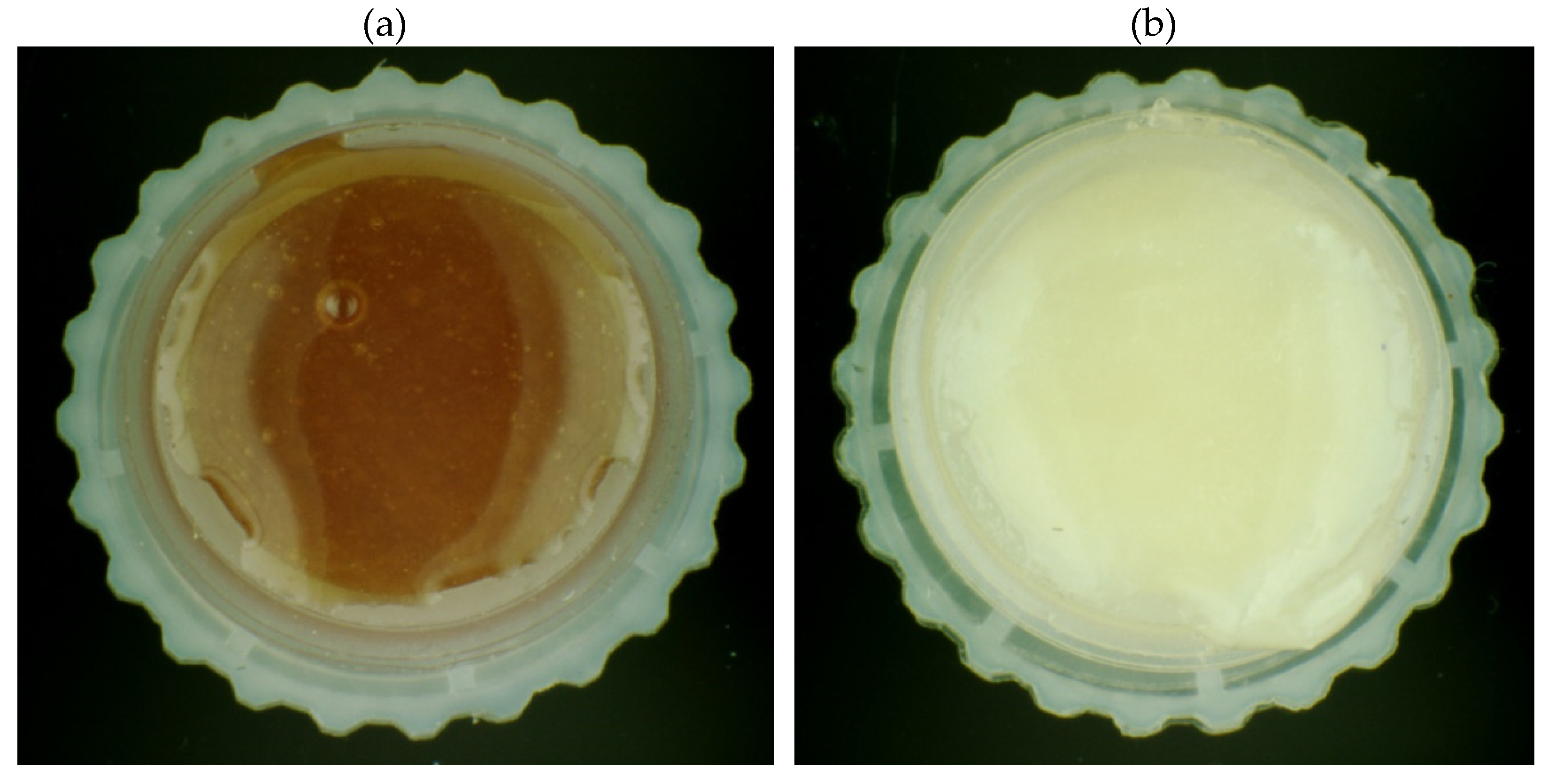
Table 3. On their basis, it was found that the color of organic and conventional honeys does not differ significantly in the case of the linden, forest and honeydew honeys. On the other hand, color differences were observed within buckwheat and dandelion honeys. Differences in color of tested honey samples show selected photos (
Figure 1). The taste of all nectar honeys was characterized as spicy with varying degrees of intensity. Honeydew and forest honeys were described as mild, with notes of resin and pine needles in the scent. Nectar honeys, on the other hand, were characterized by a subtle scent of flowers from which the main pollen was obtained. The consistency of the honey, depending on the individual characteristics of the variety, showed a fine-crystalline structure for linden, dandelion and forest honeys produced conventionally. The consistency of the honeydew honey was medium-grained crystallization, while the organic forest honey contained coarse-grained crystals. In buckwheat honeys, the crystal size was not specified because they were in the form of a strained honey.
The results of the organoleptic evaluation carried out in the study indicated a certain differentiation of the tested honey samples, both in terms of variety (honeydew, forest, buckwheat, linden, dandelion) and the method of its production (organic, conventional). The final color of honey is created by the content of pollen and pollutants from the environment. It confirms the possibility of unconscious creation of honey features uncharacteristic for the variety. Different organoleptic characteristics such as consistency, taste and aroma of the same varieties from organic and conventional honeys may result from the degree of crystallization, type of fruit, harvest time or environmental conditions. Moreover, it is indicated that the length of honey storage has an impact on the content of essential oils, therefore buckwheat, honeydew, dandelion and linden honeys may differ in aromas within the same variety.
Popov-Raljić et al. [
66] also presents significant differences in the organoleptic characteristics in the case of monofloral (acacia) and multifloral (meadow) honeys from Serbia. They reported that variation in honey properties is a consequence of different types of honey, different geographical and botanical origin, chemical composition, weather conditions, beekeeping and other factors. Silvano et al. [
67] analyzed the physicochemical parameters and the sensory properties in honeys from different regions of Buenos Aires province. They suggest that could be possible to classify honeys according to the geographic origin based on the physicochemical parameters, while the sensory properties were not good predictors.
2.3. Content of Water- and Ethanol-Soluble Phenolics in Honeys
The total contents of water-soluble phenolics in the tested honeys are presented in
Table 4. They were from 6.84 mg/100 g for conventional linden honey to 206.25 mg/100 g for conventional buckwheat honey. In turn, ethanol-soluble phenolics constituted from 2.92 mg/100 g to 216.78 mg/100 g for the same honeys, respectively. The lowest content of phenolics among the tested organic honeys was found in dandelion honey (water and ethanol extracted compounds up to 17 mg/100 g). No relationship was observed between the origin of the honey and the total content of phenolics. In total, 11 organic and 11 conventional honey samples from Poland were investigated by Halagarda et al. [
9]. Authors determined the phenolic profiles of several varieties of Polish honey and their correlation with various factors influencing the quality of honey. They also verified the impact of production method (conventional/organic) and the pollen content on these profiles.
The total content of flavonoids in the tested honeys was clearly lower (
Table 4). The water-soluble flavonoids ranged from 2.44 mg/100 g for conventional buckwheat honey to 13.61 mg/100 g for organic honeydew honey. On the other hand, when extracting these compounds with ethanol, their content was 1.6–3.0 times lower. The lowest content of flavonoids among the tested organic honeys was found in linden honey, while the lowest content of flavonoids among conventional honeys was found in buckwheat honey. No relationship was observed between the origin of the honey and the content of flavonoids. However, a relationship was demonstrated between the solvent used and the total content of flavonoids in honey. Higher values were found in the case of extracting flavonoids with water. Pauliuc et al. [
68] described the physicochemical characteristics of honey (raspberry, mint, rape, sunflower, thyme and multifloral) produced in Romania and reported that the flavonoid content was influenced not by botanical origin but year.
Many studies have shown that honey has valuable activity against respiratory pathogens [
69], including viruses that cause several viral diseases [
70,
71,
72,
73]. In addition, honey also possesses anti-inflammatory properties and is recognized as an immune booster, which complements it as an effective means of reducing the severity of viral diseases [
74,
75,
76]. Most of the medicinal and especially nutritional properties of honey have been linked to the antioxidant phenolic compounds contained in it [
77]. The diverse chemical structures including phenolic acids and polyphenols (e.g., flavonoids) are characterized of phenolic compounds in honey. The most abundant phenolic acids are gallic acid, chlorogenic acid, syringic acid, vanillic acid,
p-coumaric acid,
p-hydroxybenzoic acid and caffeic acid, while the most abundant flavonoids in honey are apigenin, chrysin, quercetin, luteolin, kaempferol, galangin, genistein, pinocembrin and pinobanksin [
77,
78]. A study of 10 monofloral and multifloral honeys showed that the antioxidant activities, based on their phenolic content, of some monofloral honeys were higher compared to multifloral honeys, whereas other monofloral honeys showed lower antioxidant activities [
79,
80].
2.4. Antioxidant Activity of Water- and Ethanol-Soluble Compounds in Honeys
The results of the determination of the antioxidant activity of the tested honeys against DPPH radicals are presented in
Table 5. In the case of water-soluble compounds, the DPPH radical scavenging capacity was within the range of 0.64 mM TE/100 g for organic dandelion honey to 1.58 mM TE/100 g for conventional buckwheat, while in the case of ethanol-soluble compounds, this parameter ranged from 0.60 to 1.06 mM TE/100 g for the same honeys, respectively. It was found that linden honey was characterized by the lowest antioxidant activity among conventional honeys, while buckwheat honey was characterized by the highest antioxidant activity among organic honeys. The strongest antioxidant activity for buckwheat honey was also confirmed by Dżugan et al. [
69]. The difference between conventional and organic honeys ranged from 8% (honeydew honeys) to 30% (forest honeys). In general, water-soluble compounds of honeys showed stronger antioxidant properties in the test with the DPPH radical. Moreover, in most cases, conventional honeys showed a higher DPPH radical scavenging activity. The only exception was linden honey, in the case of which the tendency was opposite.
The results of the ORAC test are presented in
Table 5. It was shown that with the use of ethanol for the extraction of honey antioxidants, the oxygen radical absorption capacity was in the ranged from 0.86 mM TE/100 g for organic dandelion honey to 3.72 mM TE/100 g for forest honey from the same production method. However, in the case of the use of water, the values of this parameter were less differentiated and ranged from 1.98 mM TE/100 g for organic honeydew honey to 3.40 mM TE/100 g for conventional linden honey. Among organic honeys, buckwheat and honeydew honeys showed the highest oxygen radical absorption capacity.
The results of the research showed a strong correlation between the content of total phenolics and the antioxidant activity determined in the test with DPPH radical. The correlation coefficient for all tested honeys was 0.83; for honeys from conventional method production r = 0.81, while for organic honeys r = 0.93. There was a moderate correlation between the antioxidant activity determined in the ORAC test and the total phenolics content in organic honeys, where r = 0.57. However, the content of flavonoids in honeys does not affect their antioxidant activity.
The correlations between antioxidant activity and total concentration of phenolics were confirmed for strawberry tree honeys from Italy [
81] and floral origin honeys from Romania [
82]. The antioxidant activities based on the free radical scavenging, reducing power, and bleaching inhibition were investigated for the three commonly used honeys in Malaysia (tualang, gelam and acacia honey) by Chua et al. [
83]. The authors showed that antioxidant capacity of the honey samples correlated with the content of total phenolics and flavonoids. The total flavonoid content of honeys strongly correlated with the three antioxidative tests, while the total phenolics content not correlated with the DPPH test. In turn, no significant correlation between phenolic contents and antioxidant activity of raw honeys from Algeria was found in work of Ahmed et al. [
7].
The differences in antioxidant activity of water-soluble compounds were relatively more visible among honeys analyzed using the DPPH test, while in the ORAC test—ethanol-soluble compounds. The differences may be a result of the distinct mechanisms of these tests. Hydrogen atom transfer (HAT) mechanism is studied in ORAC test and single electron transfer (SET) mechanism is typical for DPPH test [
84]. Our study shows that the HAT mechanism generally predominated for honey compounds regardless of the method of their extraction used.