Abstract
We evaluated mycophenolic acid (MPA) limited sampling strategies (LSSs) established using multiple linear regression (MLR) in children with nephrotic syndrome treated with mycophenolate mofetil (MMF). MLR-LSS is an easy-to-determine approach of therapeutic drug monitoring (TDM). We assessed the practicability of different LSSs for the estimation of MPA exposure as well as the optimal time points for MPA TDM. The literature search returned 29 studies dated 1998–2020. We applied 53 LSSs (n = 48 for MPA, n = 5 for free MPA [fMPA]) to predict the area under the time-concentration curve (AUCpred) in 24 children with nephrotic syndrome, for whom we previously determined MPA and fMPA concentrations, and compare the results with the determined AUC (AUCtotal). Nine equations met the requirements for bias and precision ±15%. The MPA AUC in children with nephrotic syndrome was predicted the best by four time-point LSSs developed for renal transplant recipients. Out of five LSSs evaluated for fMPA, none fulfilled the ±15% criteria for bias and precision probably due to very high percentage of bound MPA (99.64%). MPA LSS for children with nephrotic syndrome should include blood samples collected 1 h, 2 h and near the second MPA maximum concentration. MPA concentrations determined with the high performance liquid chromatography after multiplying by 1.175 may be used in LSSs based on MPA concentrations determined with the immunoassay technique. MPA LSS may facilitate TDM in the case of MMF, however, more studies on fMPA LSS are required for children with nephrotic syndrome.
1. Introduction
Mycophenolate mofetil (MMF) is an immunosuppressive drug administered in the prophylaxis against acute rejection after solid organ transplantation as well as in autoimmune diseases [1], nephrotic syndrome [2,3], and atopic dermatitis [4]. The MMF active moiety, mycophenolic acid (MPA), is characterized by complex and variable pharmacokinetics and high serum albumin binding (97–99%) [1,5]. MPA pharmacokinetics in renal transplant recipients are widely described in the literature [1,6,7,8,9,10], however, although the pharmacokinetics are assumed to be different, there are few studies concerning children with nephrotic syndrome treated with MMF [11,12,13,14]. In our previous study [11], we observed that the target values of the pharmacokinetic parameters, such as the concentration before the next dose (C0) and the area under the concentration—time curve from 0 to 12 h (AUCtotal), in children with nephrotic syndrome treated with MMF should be higher than those recommended after renal transplantation [1]. Similar observations were described by other authors [12,15].
MPA therapeutic drug monitoring (TDM) is frequently recommended, mainly to avoid underexposure [1,16]. TDM was shown to be favorable not only in renal transplant recipients [6], but also in patients with lupus nephritis [17] and steroid-dependent nephrotic syndrome [12,13]. One method of TDM is the limited sampling strategy (LSS), which allows us to predict AUCtotal on the basis of only few blood samples [6] instead of the time-consuming, expensive, and uncomfortable to patients method of collecting 8 to 15 blood samples over 12 h for a full pharmacokinetic profile [18]. LSS may be calculated using the Bayesian approach or multiple linear regression (MLR) analysis, which uses an equation derived from stepwise regression analysis based on concentrations measured at pre-defined times after dosing [16,19]. MLR is easier to use than Bayesian analysis, although one important limitation of the MLR approach is the reliance of the equations on the accuracy of the exact times of blood sample collection [7,16]. MLR LSSs have been proposed for MPA in many groups of patients [8,9,20,21]. Whereas many authors emphasize that each LSS should be applied to the same group of patients as it was established [22], Ting et al. [20] observed that the application of LSSs established for lung transplant recipients to the heart transplant population yielded satisfactory prediction results, Gellermann et al. [15] applied the LSSs established for children after renal transplantation and adult heart transplant recipients to evaluate AUC in children with nephrotic syndrome, and Katsuno et al. [17] used the LSS established for renal transplant recipients to predict AUC in patients with lupus nephritis. Additionally, Tong et al. [23] applied the LSS established with the high performance liquid chromatography (HPLC) method to evaluate the AUC for patients for whom the enzyme multiplied immunoassay technique (EMIT) was used for MPA determination, while Neuberger et al. [24] applied an MPA LSS established after the administration of another MPA formulation, enteric-coated mycophenolic sodium (EC-MPS), in MMF treated patients.
Due to the small number of studies on MPA pharmacokinetics in children with nephrotic syndrome, in this study we evaluated MLR-based LSSs found in the literature in children with nephrotic syndrome treated with MMF. The evaluation aimed to assess the practicability of different LSSs for the estimation of MPA exposure as well as to find the optimal time points for MPA TDM.
2. Results
2.1. MPA and fMPA Pharmacokinetics
The MPA and free MPA (fMPA) concentrations versus time in 24 children with nephrotic syndrome treated with MMF are presented in Figure 1. The results of MPA and fMPA maximum concentration (Cmax), time to reach Cmax (tmax), and AUCtotal values are presented in Table 1. MPA C0 was above 2.0 μg/mL and above 3.0 μg/mL in 67% (n = 16) and 42% (n = 10) of children, respectively. MPA Cmax was observed 1 h after MMF administration in 79% of children. Out of 24 children, 63% (n = 15) had MPA AUCtotal within the 30–60 μg∙h/mL range. For 21% (n = 5) of children, MPA AUCtotal was above 60 μg∙h/mL. Mean MPA binding to plasma protein was 99.65%, with only 0.35% of fMPA.
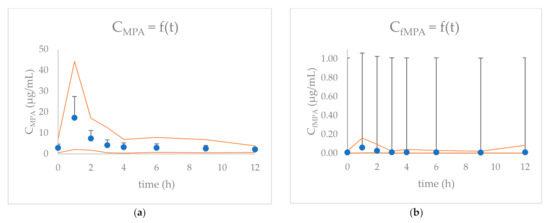
Figure 1.
The concentration (+SD) versus time graphs for: (a) MPA and (b) fMPA for 24 children included in the study. Orange curves indicate the maximum and minimum concentrations at each time-point.

Table 1.
Plasma concentrations and exposure of MPA and fMPA in children with nephrotic syndrome.
2.2. The Evaluation of MLR LSSs in Children with Nephrotic Syndrome
The search of the literature returned 29 studies meeting the requirements concerning MLR LSSs for MPA and fMPA, dated 1998–2020. We applied 48 MPA LSSs [8,9,14,21,22,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48] and five fMPA LSSs [35,36,42] found in the literature to calculate the predicted area under the (0–12 h) time–concentration curve (AUCpred) in children with nephrotic syndrome treated with MMF, and compared the results with AUCtotal. In the majority of studies, calcineurin inhibitors (CsA or tacrolimus (Tac)) were co-administered with MMF. In two studies, only MMF was administered and in one other study, only 8% of patients received CsA concomitantly. The majority of studies concerned patients after solid organ transplantation. We found seven studies including pediatric patients after renal transplantation (n = 4), with nephrotic syndrome (n = 2), and with lupus erythematosus (n = 1). In order to better describe the results, we divided the LSSs according to the methods of MPA determination and subdivided according to the indications for MMF treatment (Table 2 and Table 3). The LSSs for fMPA are presented separately (Table 4).

Table 2.
Predictive performance of MLR-based HPLC–MPA LSSs available in the literature for estimation of MPA AUCpred in children with nephrotic syndrome treated with MMF.

Table 3.
The predictive performance of MLR-based EMIT/PETINIA-MPA LSSs available in the literature for estimation of MPA AUCpred in children with nephrotic syndrome treated with MMF.

Table 4.
The predictive performance of MLR-based HPLC-fMPA LSSs available in the literature for the estimation of fMPA AUCpred in children with nephrotic syndrome treated with MMF.
The predictive performances for the estimation of MPA AUCpred using the 23 MPA MLR LSSs available in the literature in which MPA was determined based on HPLC method are presented in Table 2. Only two out of 23 equations (9%) met the requirements of ±15% for %MPE and 15% for %MAE. If the acceptable %MPE and %MAE were extended to ±20%, 13 equations (57%) would fulfill the criteria. For two of the 23 LSSs (9%), AUCpred was within ±15% of AUCtotal for more than 60% of children, concomitantly with r2 above 0.800. These LSSs included C1-C2-C4 and C1-C2-C6, both of which were established for Tac co-administration. High r2 was found in the Gota et al. [34] equation, concomitantly with low predictive performance. A number of 11 LSSs (48%) gave an AUCpred within ±15% of the AUCtotal for less than 50% of children.
The predictive performances of 25 MPA MLR LSSs in which MPA was determined based on EMIT or particle enhanced turbidimetric inhibition immunoassay (PETINIA) are presented in Table 3. Seven of 25 LSSs (28%) met the requirements of ±15% for %MPE and 15% for %MAE. If the acceptable %MPE and %MAE were extended to ±20%, ten equations (40%) would fulfill the criteria. For three of 25 LSSs (12%), the AUCpred was within ±15% of the AUCtotal for more than 60% of children, concomitantly with r2 above 0.800. These LSSs included C1-C2-C4-C6 (two LSSs) and C0-C1-C3-C6, all of which were established for Tac co-administration. In 13 of 25 LSSs (52%), the AUCpred was within ±15% of the AUCtotal in less than 50% of children.
We found five MLR LSSs for fMPA in three studies which we applied to calculate the fMPA AUCpred for children with nephrotic syndrome. The predictive performance of the fMPA MLR LSSs is presented in Table 4. In all three studies, MPA was determined with the HPLC method. None of the equations fulfilled the criteria for %MPE and %MAE. There was one four time point equation (C1-C2-C4-C6), which was established for patients after liver transplantation and co-treated with Tac, which met the requirements of ±20% for %MPE and %MAE, and demonstrated an r2 above 0.800.
2.3. Comparison of the Best Matched MLR LSSs
Nine LSSs with %MPE and %MAE ±15%, and r2 ≥ 0.799 were considered the best. These equations were established for adult renal transplant recipients (n = 3), adult liver transplant recipients (n = 2), and pediatric renal transplant recipients (n = 4). For these equations, the graphs describing the correlations between the AUCtotal and the AUCpred were drawn (Figure 2), and Bland–Altman (Figure 3) tests were performed. For the majority of equations, the Bland–Altman test showed only one or two values exceeding the fixed range of the mean ± 1.96 SD, which confirmed the agreement between the AUCtotal and the AUCpred.
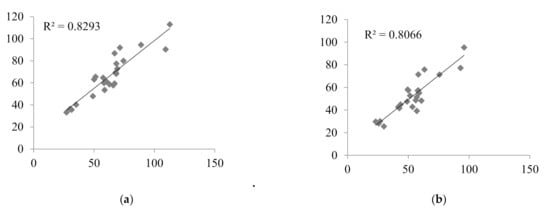
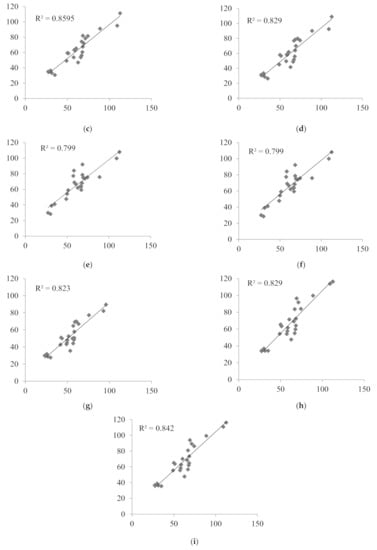
Figure 2.
Correlations between the MPA AUCtotal and the MPA AUCpred calculated for children with nephrotic syndrome using MLR LSS equations found in the literature that fulfilled the criteria for %MPE and %MAE ±15%; (a) AUCpred = 7.4 + 2.3 × C0 + 1.2 × C1 + 2.3 × C3 + 4.4 × C6 [30]; (b) AUCpred = 9.328 + 1.311 × C1 + 1.455 × C2 + 2.901 × C4 [43]; (c) AUCpred = 10.6 + 1.1 × C1 + 1.1 × C2 + 2.0 × C4 + 3.9 × C6 [30]; (d) AUCpred = 5.92 + 1.10 × C1 + 1.01 × C2 + 1.77 × C4 + 4.80 × C6 [28]; (e) AUCpred = 8.22 + 3.16 × C0 + 0.99 × C1 + 1.33 × C2 + 4.18 × C4 [32]; (f) AUCpred = 8.217 + 3.163 × C0 + 0.994 × C1 + 1.334 × C2 + 4.183 × C4 [8]; (g) AUCpred = 10.229 + 0.925 × C1 + 1.750 × C2 + 4.586 × C6 [29]; (h) AUCpred = 7.73 + 0.94 × C1 + 2.55 × C2 + 5.48 × C6 [32]; (i) AUCpred = 10.75 + 0.98 × C1 + 2.38 × C2 + 4.86 × C6 [33].
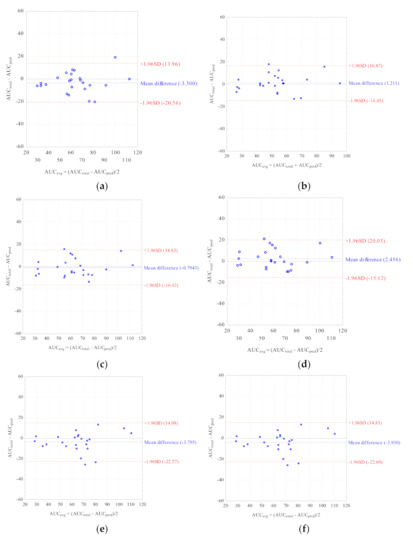
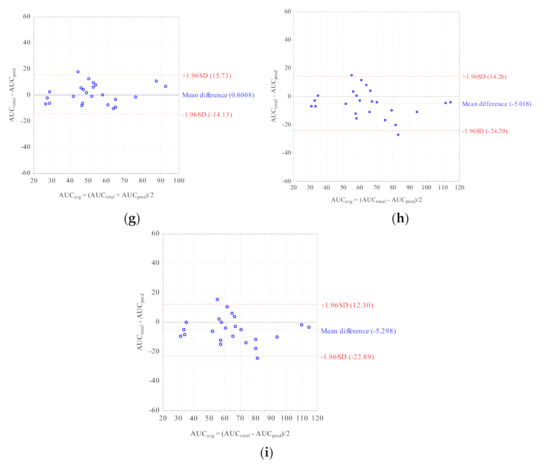
Figure 3.
Bland–Altman analyses testing agreement between the MPA AUCtotal and the MPA AUCpred calculated for children with nephrotic syndrome using the MLR LSS equations found in the literature that fulfilled the criteria for %MPE and %MAE ± 15%; (a) AUCpred = 7.4 + 2.3 × C0 + 1.2 × C1 + 2.3 × C3 + 4.4 × C6 [30]; (b) AUCpred = 9.328 + 1.311 × C1 + 1.455 × C2 + 2.901 × C4 [43]; (c) AUCpred = 10.6 + 1.1 × C1 + 1.1 × C2 + 2.0 × C4 + 3.9 × C6 [30]; (d) AUCpred = 5.92 + 1.10 × C1 + 1.01 × C2 + 1.77 × C4 + 4.80 × C6 [28]; (e) AUCpred = 8.22 + 3.16 × C0 + 0.99 × C1 + 1.33 × C2 + 4.18 × C4 [32]; (f) AUCpred = 8.217 + 3.163 × C0 + 0.994 × C1 + 1.334 × C2 + 4.183 × C4 [8]; (g) AUCpred = 10.229 + 0.925 × C1 + 1.750 × C2 + 4.586 × C6 [29]; (h) AUCpred = 7.73 + 0.94 × C1 + 2.55 × C2 + 5.48 × C6 [32]; (i) AUCpred = 10.75 + 0.98 × C1 + 2.38 × C2 + 4.86 × C6 [33].
3. Discussion
Estimating LSS is the approach of TDM applied for many drugs, e.g., MPA, levofloxacin, and etoposide [49,50,51]. We recently established and compared LSS for MPA in children with nephrotic syndrome using two different approaches [52]. In the present study, we used the MPA LSSs found in the literature in the attempt to assess their practicability for the estimation of MPA exposure and to find the optimal time points for MPA TDM in children with nephrotic syndrome. We verified the LSSs established for different indications, as in the literature we found studies in which LSS developed for one population was used to evaluate LSS in other population [20,24].
The novelty of our study is that we converted MPA concentrations determined with HPLC to evaluate the MPA LSSs established for EMIT or PETINIA. As MPA concentrations are 15–20% higher when established with EMIT or PETINIA due to MPA cross reaction with the MPA metabolite acyl-glucuronide [16,53], we multiplied the HPLC determined concentration by 1.175. Tong et al. [23] used MPA LSSs established for adult heart transplant recipients with the HPLC method to predict the AUC in children with nephrotic syndrome for whom MPA concentrations were determined with EMIT without any adjustment. Our results of predictive performance for both HPLC and EMIT/PETINIA did not differ significantly, and therefore we concluded that this approach may enable using LSSs established with EMIT or PETINIA to predict the MPA AUC based on HPLC-determined concentrations.
Nine MPA LSSs fulfilled the criteria of the best predictive performance. Because MMF is mainly administered as an acute rejection prophylaxis after renal transplantation and most of the studies concerned adults, five out of nine the best MLR LSSs were established for adults [28,29,30,43]. Four LSSs considered as the best were established for pediatric patients [8,32,33]. Among these four LSSs, although two equations were very similar, they were published in two different articles, and we therefore evaluated both of them [8,32]. Seven of nine LSSs included renal transplant recipients, both adult (n = 3) [30,43] and pediatric (n = 4) [8,32,33]. Two of nine the best LSSs included liver transplant recipients [28,29]. Surprisingly, the LSSs established for children with nephrotic syndrome [14,25] or lupus erythematosus [21] performed poorly as they did not fulfill the criteria: the values of r2 were below 0.800, and ≤50% of the AUCpred values were within ±15% of the AUCtotal. These poor results may be explained by one time point equation in the Hibino et al. study [14] and the relatively high intercept.
In our opinion, in the case of MPA, accurate and precise LSSs should consist of at least three time points. Among the best LSSs, four and five LSSs included four and three time points, respectively. The predictive performance for one and two time point LSSs were unsatisfactory. If the criteria were extended to ±20% for %MPE and %MAE, only one two-time-point equation would have fulfilled the criteria. However, the percentage of AUCpred within ±15% of AUCtotal was rather poor for this equation (50%). Moreover, equations with only one time-point performed poorly with respect to the percentage of the AUCpred within ±15% of the AUCtotal (≤33%). Interestingly, for one LSS, which included AUC1–4 instead of concentration at defined time points [34], r2 was >0.800, while the predictive performance and the percentage of the AUCpred within ±15% of the AUCtotal were unsatisfactory. Moreover, the LSSs which included logarithmic concentrations did not perform well [44].
The inclusion of particular time points may be of significant importance as they reflect MPA pharmacokinetics. In our study, eight of the nine (89%) best-matched equations included C1 and C2, and six equations included C6. Those three time points coincide with the MPA Cmax (1–2 h after dosing) and the second maximum concentration (Cmax2; 6–12 h after MMF administration) [10]. This evidence suggests that the MPA Cmax and Cmax2 influence its AUC the most, and the blood samples should be collected at least in three time points near Cmax and Cmax2 to precisely predict the AUC. According to the literature, for children with nephrotic syndrome C2 or time points up to 2 h after MMF administration should be included in the MPA LSS [14,25]. The inclusion of C6 makes using LSS cumbersome. However, according to the literature, better predictive performance was observed for LSSs which included time points in the latter half of the dosing interval [16]. Out of the nine best matched equations, only 3 (33%) included C0. This observation is in accordance with the literature data, as MPA C0 correlates poorly with AUCtotal [6].
We evaluated the MLR LSSs found in the literature regardless the drugs co-administered with MMF. Five of nine the best LSSs were established for MMF- and Tac-treated patients. According to the literature, Tac does not influence MPA clearance [3], and in patients with autoimmune disease MPA clearance is likely to be in close agreement with estimates from renal allograft recipients co-treated with Tac [54]. On the other hand, MPA concentrations are lower if co-administered with CsA [10]. CsA inhibits MPA enterohepatic recirculation, causing a decrease in MPA exposition, and therefore blood sampling does not require including time-points around the MPA Cmax2 when MMF is co-administered with CsA [16]. Among the LSSs applied in this study, only in three studies with MLR LSSs [21,25,46] did the patients not receive concomitant medications (in one study only 8% of patients received CsA [46]). Surprisingly, in our study, for these LSSs the predictive performance fell beyond ±15% range. The equation from the Prabha et al. [21] study would have fulfilled the extended criteria (±20%). One equation, which included C6, from the de Winter et al. study [46], was characterized by the r2 being >0.800, however, it did not fulfill even the extended criteria, therefore, we confirmed that choosing model equations based only on their r2 values may be misleading [55].
Out of five LSSs developed for fMPA [35,36,42], none fulfilled the criteria when used to evaluate the fMPA AUCpred in children with nephrotic syndrome. One equation would fulfill the criteria extended to ±20%, but the percentage of the AUCpred within ±15% of the AUCtotal for this formula was poor (38%). The obtained results may indicate differences in MPA protein binding in children with nephrotic syndrome. According to the literature, MPA is bound to plasma proteins in 97% to 99% [29,56]. In our previous study [11], similarly as in this study, the median fMPA fraction was 0.36%, which gives very high percentage of bound MPA (99.64%).
The limitation of our study is the fact that we were unable to apply the LSSs with time points 0.5, 0.75, or 1.5 h after MMF administration as blood sampling was not so frequent in the children included in the study.
4. Materials and Methods
4.1. Ethical Considerations
The study was approved by the Bioethical Committee at Poznan University of Medical Sciences and it is in accordance with the 1964 Declaration of Helsinki and its later amendments. Informed consent was obtained from the parents or guardians prior to initiating the study.
4.2. Children’s Characteristics
Our study included 24 children, aged 3–18 years, with nephrotic syndrome treated with MMF and steroids in the Department of Pediatric Nephrology and Hypertension, Poznan University of Medical Sciences, Poland. MMF was administered orally twice a day at the same dose. On the day of blood collection, 18 children were in remission whereas six children had trace proteinuria. MMF was given under fasting conditions, 30 min before breakfast. The exclusion criteria were cyclosporine (CsA) co-administration, MMF dosing at unequal morning and evening doses, administration of MMF shorter than 1 month and too low number of blood samples. Blood samples were collected into EDTA tubes before MMF administration (C0) and subsequently 1 h (C1), 2 h (C2), 3 h (C3), 4 h (C4), 6 h (C6), 9 h (C9), and 12 h (C12) after its administration. The samples were centrifuged to obtain plasma, then immediately frozen and kept at −20 °C until analysis. The demographic and biochemical characteristics of the children are presented in Table 5.

Table 5.
Demographic and biochemical characteristics of the study group.
4.3. Analytical Methods
MPA and fMPA concentrations were determined in the Department of Physical Pharmacy and Pharmacokinetics at Poznan University of Medical Sciences, Poland.
MPA plasma concentrations were determined using the HPLC method with ultraviolet detection. The analytical method for MPA determination was described elsewhere [11,57]. The calibration curve was linear, and within the range 0.25–40.0 μg/mL. The mean between-day coefficient of variation and average accuracy were 2.7% (range 0.5–6.1%) and 98.8% (range 93.8–103.0%), respectively [11].
Free MPA (fMPA) was determined using the HPLC method with fluorescence detection described previously [5,11]. The calibration curve was linear, and within the range of 0.0025–1.0 μg/mL. The mean between-day coefficient of variation and average accuracy were 6.5% (range 1.4–12.7%) and 99.9% (range 94.3–107.6%), respectively [11].
4.4. The Literature Data Search
We comprehensively searched the literature in December 2020 using the PUBMED database using the combination of ‘mycophenolic acid’ or ‘mycophenolate mofetil’ and the terms: ‘limited sampling strategy’, ‘limited sampling strategies’, ‘limited sampling’, ‘optimal sampling’, ‘sparse sampling’, and ‘minimal sampling’. We included English written studies determining LSS based on MLR calculations for adult and pediatric patients receiving MMF after solid organ transplantation or with autoimmune diseases, and identified those LSSs which covered the same blood sampling times as in our study. We included LSSs which were established based on HPLC and EMIT MPA determinations. We excluded articles describing LSS for EC-MPS as there is an evident difference in MPA pharmacokinetics for the two formulations MMF and EC-MPS (unpredictable absorption profile after EC-MPS administration) [58]. We also excluded studies using previously established LSSs, those with Bayesian estimators and with different than twice daily MMF dosing schedules.
4.5. Pharmacokinetic Calculations and Statistical Analyses
For children with nephrotic syndrome, firstly, we calculated the MPA AUCtotal using the linear trapezoidal rule. Secondly, based on the results of the literature data search, we calculated the AUCpred for these children using the MLR formulae found in the literature. We applied LSSs established using MPA concentrations determined with HPLC, EMIT, and PETINIA to evaluate LSS usefulness. Due to the 15–20% higher MPA concentrations established with EMIT [16] and the similar magnitude of the MPA overestimation found for PETINIA when compared with EMIT [53], we multiplied the MPA concentration determined in the children included in this study with the HPLC method by 1.175, and applied the re-calculated AUCtotal to the evaluation of the LSSs based on EMIT or PETINIA MPA determination. The multiplier of 1.175 was achieved by assuming that MPA concentrations established with EMIT are on average 17.5% higher than those determined with HPLC.
To assess the predictive performance of LSSs available in the literature, we calculated r2 as well as the bias and precision for AUCpred as the mean relative prediction error (%MPE) and the percentage of the mean absolute relative prediction error (%MAE), respectively, both with 95% confidence intervals. According to the literature, precision and bias ±15% were considered acceptable [22,59,60], although some authors defined the clinical acceptance as ±20% [18] or even as ±33% [61]. Although it does not translate into clinical practice, lower percentages of precision and bias result in more accurate calculations. We also calculated the percentage of the AUCpred within ±15% of the AUCtotal for each equation to analyze the agreement between the AUCpred and the AUCtotal. The equations used in the analysis were as follows [51,62]:
Statistical analyses were performed using STATISTICA 13.0 software (StatSoft, Inc., Tulsa, OK, USA). For the best matched MLR LSSs, the Bland–Altman method was used to assess the agreement between the AUCpred and the AUCtotal. To compare the HPLC and EMIT/PETINIA predictive performance results, the Mann–Whitney test was applied.
5. Conclusions
We concluded that the optimal MPA LSS for children with nephrotic syndrome should include C1, C2, and C6, as these time points coincide with MPA Cmax and Cmax2. MPA LSSs established using MPA concentrations determined with EMIT or PETINIA may be used in LSSs based on HPLC-determined MPA concentrations after multiplying the latter by 1.175. The MLR LSS which predicted MPA AUC the best in children with nephrotic syndrome was developed for MMF-treated renal transplant recipients. MPA binding with plasma protein is high in children with nephrotic syndrome, which suggests there are different fMPA pharmacokinetics in this group of patients than in renal, liver, and hematopoietic stem cell recipients treated with MMF. MPA LSSs may facilitate TDM in the case of MMF, however, more studies of fMPA LSS are required for children with nephrotic syndrome.
Author Contributions
Conceptualization, J.S.; methodology, J.S., M.R. and M.C.; validation, J.S. and M.R.; formal analysis, J.S.; investigation, J.S. and M.R.; resources, J.S., J.Z. and D.O.-N.; data curation, J.S.; writing—original draft preparation, J.S.; writing—review and editing, M.C., J.Z. and D.O.-N.; visualization, J.S.; supervision, M.C., J.Z. and D.O.-N.; project administration, J.S.; funding acquisition, J.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Poznan University of Medical Sciences, grant number 502-14-03306413-10156. The APC was funded by Poznan University of Medical Sciences.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of Poznan University of Medical Sciences (protocol code 542/16 and date of approval 5 May 2016).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors would like to acknowledge the nursing staff that participated in the samples collection.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Not applicable.
References
- Staatz, C.E.; Tett, S.E. Pharmacology and Toxicology of Mycophenolate in Organ Transplant Recipients: An Update. Arch. Toxicol. 2014, 88, 1351–1389. [Google Scholar] [CrossRef]
- Ostalska-Nowicka, D.; Malinska, A.; Silska, M.; Perek, B.; Zachwieja, J.; Nowicki, M. Mycophenolate Mofetil (MMF) Treatment Efficacy in Children with Primary and Secondary Glomerulonephritis. Arch. Med. Sci. 2011, 7, 1042–1048. [Google Scholar] [CrossRef]
- Kiang, T.K.; Ensom, M.H. Population Pharmacokinetics of Mycophenolic Acid: An Update. Clin. Pharm. 2018, 57, 547–558. [Google Scholar] [CrossRef]
- Dias-Polak, D.; Bergman, R.; Avitan-Hersh, E. Mycophenolate Mofetil Therapy in Adult Patients with Recalcitrant Atopic Dermatitis. J. Dermatol. Treat. 2019, 30, 49–51. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Gu, Z.; Chen, H.; Zhang, W.; Fen, X.; Cai, W.; Fan, Q. Establishment of High-Performance Liquid Chromatography and Enzyme Multiplied Immunoassay Technology Methods for Determination of Free Mycophenolic Acid and Its Application in Chinese Liver Transplant Recipients. Ther. Drug Monit. 2010, 32, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Filler, G.; Alvarez-Elías, A.C.; McIntyre, C.; Medeiros, M. The Compelling Case for Therapeutic Drug Monitoring of Mycophenolate Mofetil Therapy. Pediatr. Nephrol. 2017, 32, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Bruchet, N.K.; Ensom, M.H. Limited Sampling Strategies for Mycophenolic Acid in Solid Organ Transplantation: A Systematic Review. Expert Opin. Drug Metab. Toxicol. 2009, 5, 1079–1097. [Google Scholar] [CrossRef]
- Filler, G. Abbreviated Mycophenolic Acid AUC from CO, C1, C2, and C4 Is Preferable in Children after Renal Transplantation on Mycophenolate Mofetil and Tacrolimus Therapy. Transplant. Int. 2004, 17, 120–125. [Google Scholar] [CrossRef]
- David-Neto, E.; Araujo, L.M.; Sumita, N.M.; Mendes, M.E.; Castro, M.C.; Alves, C.F.; Kakehashi, E.; Romano, P.; Yagyu, E.M.; Queiroga, M.; et al. Mycophenolic Acid Pharmacokinetics in Stable Pediatric Renal Transplantation. Pediatr. Nephrol. 2003, 18, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Staatz, C.E.; Tett, S.E. Clinical Pharmacokinetics and Pharmacodynamics of Mycophenolate in Solid Organ Transplant Recipients. Clin. Pharm. 2007, 46, 13–58. [Google Scholar] [CrossRef]
- Sobiak, J.; Resztak, M.; Ostalska-Nowicka, D.; Zachwieja, J.; Gąsiorowska, K.; Piechanowska, W.; Chrzanowska, M. Monitoring of Mycophenolate Mofetil Metabolites in Children with Nephrotic Syndrome and the Proposed Novel Target Values of Pharmacokinetic Parameters. Eur. J. Pharm. Sci. 2015, 77, 189–196. [Google Scholar] [CrossRef]
- Hackl, Á.; Cseprekál, O.; Gessner, M.; Liebau, M.C.; Habbig, S.; Ehren, R.; Müller, C.; Taylan, C.; Dötsch, J.; Weber, L.T. Mycophenolate Mofetil Therapy in Children with Idiopathic Nephrotic Syndrome: Does Therapeutic Drug Monitoring Make a Difference? Ther. Drug Monit. 2016, 38, 274–279. [Google Scholar] [CrossRef]
- Tellier, S.; Dallocchio, A.; Guigonis, V.; Saint-Marcoux, F.; Llanas, B.; Ichay, L.; Bandin, F.; Godron, A.; Morin, D.; Brochard, K.; et al. Mycophenolic Acid Pharmacokinetics and Relapse in Children with Steroid–Dependent Idiopathic Nephrotic Syndrome. CJASN 2016, 11, 1777–1782. [Google Scholar] [CrossRef] [PubMed]
- Hibino, S.; Nagai, T.; Yamakawa, S.; Ito, H.; Tanaka, K.; Uemura, O. Pharmacokinetics of Mycophenolic Acid in Children with Clinically Stable Idiopathic Nephrotic Syndrome Receiving Cyclosporine. Clin. Exp. Nephrol. 2017, 21, 152–158. [Google Scholar] [CrossRef]
- Gellermann, J.; Weber, L.; Pape, L.; Tönshoff, B.; Hoyer, P.; Querfeld, U. Mycophenolate Mofetil versus Cyclosporin A in Children with Frequently Relapsing Nephrotic Syndrome. JASN 2013, 24, 1689–1697. [Google Scholar] [CrossRef] [PubMed]
- Abd Rahman, A.N.; Tett, S.E.; Staatz, C.E. How Accurate and Precise Are Limited Sampling Strategies in Estimating Exposure to Mycophenolic Acid in People with Autoimmune Disease? Clin. Pharm. 2014, 53, 227–245. [Google Scholar] [CrossRef]
- Katsuno, T.; Ozaki, T.; Ozeki, T.; Hachiya, A.; Kim, H.; Kato, N.; Ishimoto, T.; Kato, S.; Kosugi, T.; Tsuboi, N.; et al. Investigation on the Benefits of Mycophenolate Mofetil and Therapeutic Drug Monitoring in the Treatment of Japanese Patients with Lupus Nephritis. Clin. Exp. Nephrol. 2018, 22, 1341–1350. [Google Scholar] [CrossRef]
- Zicheng, Y.; Xianghui, W.; Peijun, Z.; Da, X.; Weixia, Z.; Hongzhuan, C. Evaluation of the Practicability of Limited Sampling Strategies for the Estimation of Mycophenolic Acid Exposure in Chinese Adult Renal Recipients. Ther. Drug Monit. 2007, 29, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Saint-Marcoux, F.; Guigonis, V.; Decramer, S.; Gandia, P.; Ranchin, B.; Parant, F.; Bessenay, L.; Libert, F.; Harambat, J.; Bouchet, S.; et al. Development of a Bayesian Estimator for the Therapeutic Drug Monitoring of Mycophenolate Mofetil in Children with Idiopathic Nephrotic Syndrome. Pharmacol. Res. 2011, 63, 423–431. [Google Scholar] [CrossRef]
- Ting, L.S.; Partovi, N.; Levy, R.D.; Ignaszewski, A.P.; Ensom, M.H. Performance of Limited Sampling Strategies for Predicting Mycophenolic Acid Area under the Curve in Thoracic Transplant Recipients. J. Heart Lung Transplant. 2008, 27, 325–328. [Google Scholar] [CrossRef]
- Prabha, R.; Mathew, B.; Jeyaseelan, V.; Kumar, T.; Agarwal, I.; Fleming, D. Development and Validation of Limited Sampling Strategy Equation for Mycophenolate Mofetil in Children with Systemic Lupus Erythematosus. Indian J. Nephrol. 2016, 26, 408–412. [Google Scholar] [CrossRef] [PubMed]
- Al-Khatib, M.; Shapiro, R.J.; Partovi, N.; Ting, L.S.; Levine, M.; Ensom, M.H. Limited Sampling Strategies for Predicting Area under the Concentration-Time Curve of Mycophenolic Acid in Islet Transplant Recipients. Ann. Pharm. 2010, 44, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Tong, K.; Mao, J.; Fu, H.; Shen, H.; Liu, A.; Shu, Q.; Du, L. The Value of Monitoring the Serum Concentration of Mycophenolate Mofetil in Children with Steroid-Dependent/Frequent Relapsing Nephrotic Syndrome. Nephron 2016, 132, 327–334. [Google Scholar] [CrossRef]
- Neuberger, M.; Sommerer, C.; Böhnisch, S.; Metzendorf, N.; Mehrabi, A.; Stremmel, W.; Gotthardt, D.; Zeier, M.; Weiss, K.H.; Rupp, C. Effect of Mycophenolic Acid on Inosine Monophosphate Dehydrogenase (IMPDH) Activity in Liver Transplant Patients. Clin. Res. Hepatol. Gastroenterol. 2020, 44, 543–550. [Google Scholar] [CrossRef]
- Benz, M.; Ehren, R.; Kleinert, D.; Müller, C.; Gellermann, J.; Fehrenbach, H.; Schmidt, H.; Weber, L. Generation and Validation of a Limited Sampling Strategy to Monitor Mycophenolic Acid Exposure in Children with Nephrotic Syndrome. Ther. Drug Monit. 2019, 41, 696–702. [Google Scholar] [CrossRef]
- Cai, W.; Cai, Q.; Xiong, N.; Qin, Y.; Lai, L.; Sun, X.; Hu, Y. Limited Sampling Strategy for Estimating Mycophenolic Acid Exposure on Day 7 Post-Transplant for Two Mycophenolate Mofetil Formulations Derived from 20 Chinese Renal Transplant Recipients. Transplant. Proc. 2018, 50, 1298–1304. [Google Scholar] [CrossRef]
- Chaabane, A.; Aouam, K.; Fredj, N.B.; Hammouda, M.; Chadly, Z.; May, M.E.; Boughattas, N.; Skhiri, H. Limited Sampling Strategy of Mycophenolic Acid in Adult Kidney Transplant Recipients: Influence of the Post-Transplant Period and the Pharmacokinetic Profile. J. Clin. Pharmacol. 2013, 53, 925–933. [Google Scholar] [CrossRef]
- Chen, H.; Gu, Z.; Chen, B.; Mao, H.; Zhang, W.; Fan, Q. Models for the Prediction of Mycophenolic Acid Area under the Curve Using a Limited-Sampling Strategy and an Enzyme Multiplied Immunoassay Technique in Chinese Patients Undergoing Liver Transplantation. Clin. Ther. 2008, 30, 2387–2401. [Google Scholar] [CrossRef]
- Chen, H.; Peng, C.; Yu, Z.; Shen, B.; Deng, X.; Qiu, W.; Fei, Y.; Shen, C.; Zhou, G.; Yang, W.; et al. Pharmacokinetics of Mycophenolic Acid and Determination of Area under the Curve by Abbreviated Sampling Strategy in Chinese Liver Transplant Recipients. Clin. Pharmacokinet. 2007, 46, 175–185. [Google Scholar] [CrossRef]
- Enokiya, T.; Nishikawa, K.; Muraki, Y.; Iwamoto, T.; Kanda, H.; Sugimura, Y.; Okuda, M. Usefulness of Limited Sampling Strategy for Mycophenolic Acid Area under the Curve Considering Postoperative Days in Living-Donor Renal Transplant Recipients with Concomitant Prolonged-Release Tacrolimus. J. Pharm. Health Care Sci. 2017, 3, 17. [Google Scholar] [CrossRef]
- Fatela-Cantillo, D.; Hinojosa-Pérez, R.; Peralvo-Rodríguez, M.I.; Serrano-Díaz Canedo, J.; Gómez-Bravo, M.A. Pharmacokinetic Evaluation of Mycophenolic Acid Profiles during the Period Immediately Following an Orthotopic Liver Transplant. Transplant. Proc. 2006, 38, 2482–2485. [Google Scholar] [CrossRef] [PubMed]
- Filler, G.; Feber, J.; Lepage, N.; Weiler, G.; Mai, I. Universal Approach to Pharmacokinetic Monitoring of Immunosuppressive Agents in Children. Pediatr. Transplant. 2002, 6, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Filler, G.; Mai, I. Limited Sampling Strategy for Mycophenolic Acid Area under the Curve. Ther. Drug Monit. 2000, 22, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Gota, V.; Purohit, V.; Gurjar, M.; Nayak, L.; Punatar, S.; Gokarn, A.; Bonda, A.; Bagal, B.; Vora, C.S.; Patil, A.; et al. A Limited Sampling Strategy for Therapeutic Drug Monitoring of Mycophenolate Mofetil for Prophylaxis of Acute Graft-Versus-Host Disease in Allogeneic Stem Cell Transplantation. Cell Transplant. 2020, 29, 0963689720912925. [Google Scholar] [CrossRef]
- Gu, Z.; Chen, B.; Song, Y.; Shen, B.; Zhu, Z.; Zhang, W.; Xie, J.; Deng, X.; Peng, C.; Fan, Q.; et al. Pharmacokinetics of Free Mycophenolic Acid and Limited Sampling Strategy for the Estimation of Area under the Curve in Liver Transplant Patients. Eur. J. Pharm. Sci. 2012, 47, 636–641. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Z.; Zhong, J.; Zhang, M.; Shi, X.; Yu, Y.; Lu, W. Total and Free Mycophenolic Acid and Its 7-O-Glucuronide Metabolite in Chinese Adult Renal Transplant Patients: Pharmacokinetics and Application of Limited Sampling Strategies. Eur. J. Clin. Pharm. 2007, 63, 27–37. [Google Scholar] [CrossRef]
- Johnson, A.G.; Rigby, R.J.; Taylor, P.J.; Jones, C.E.; Allen, J.; Franzen, K.; Falk, M.C.; Nicol, D. The Kinetics of Mycophenolic Acid and Its Glucuronide Metabolite in Adult Kidney Transplant Recipients. Clin. Pharmacol. Ther. 1999, 66, 492–500. [Google Scholar] [CrossRef]
- Karimani, A.; Abedi, H.; Nazemian, F.; Poortaji, A.; Pour, A.H. Estimation of Abbreviated Mycophenolic Acid Area under the Concentration-Time Curve during Stable Post-Transplant Period by Limited Sampling Strategy. Curr. Clin. Pharm. 2020. [Google Scholar] [CrossRef]
- Kuriata-Kordek, M.; Boratynska, M.; Falkiewicz, K.; Porazko, T.; Urbaniak, J.; Wozniak, M.; Patrzalek, D.; Szyber, P.; Klinger, M. The Influence of Calcineurin Inhibitors on Mycophenolic Acid Pharmacokinetics. Transplant. Proc. 2003, 35, 2369–2371. [Google Scholar] [CrossRef]
- Guellec, C.L.; Büchler, M.; Giraudeau, B.; Meur, Y.L.; Gakoué, J.E.; Lebranchu, Y.; Marquet, P.; Paintaud, G. Simultaneous Estimation of Cyclosporin and Mycophenolic Acid Areas under the Curve in Stable Renal Transplant Patients Using a Limited Sampling Strategy. Eur. J. Clin. Pharm. 2002, 57, 805–811. [Google Scholar] [CrossRef]
- Miura, M.; Satoh, S.; Niioka, T.; Kagaya, H.; Saito, M.; Hayakari, M.; Habuchi, T.; Suzuki, T. Limited Sampling Strategy for Simultaneous Estimation of the Area under the Concentration-Time Curve of Tacrolimus and Mycophenolic Acid in Adult Renal Transplant Recipients. Ther. Drug Monit. 2008, 30, 52–59. [Google Scholar] [CrossRef]
- Ng, J.; Rogosheske, J.; Barker, J.; Weisdorf, D.; Jacobson, P.A. A Limited Sampling Model for Estimation of Total and Unbound Mycophenolic Acid (MPA) Area under the Curve (AUC) in Hematopoietic Cell Transplantation (HCT). Ther. Drug Monit. 2006, 28, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Poulin, E.; Greanya, E.D.; Partovi, N.; Shapiro, R.J.; Al-Khatib, M.; Ensom, M.H. Development and Validation of Limited Sampling Strategies for Tacrolimus and Mycophenolate in Steroid-Free Renal Transplant Regimens. Ther. Drug Monit. 2011, 33, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Ting, L.S.; Partovi, N.; Levy, R.D.; Riggs, K.W.; Ensom, M.H. Limited Sampling Strategy for Predicting Area under the Concentration-Time Curve of Mycophenolic Acid in Adult Lung Transplant Recipients. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2006, 26, 1232–1240. [Google Scholar] [CrossRef] [PubMed]
- Wada, K.; Takada, M.; Kotake, T.; Ochi, H.; Morishita, H.; Komamura, K.; Oda, N.; Mano, A.; Hanatani, A.; Nakatani, T. Limited Sampling Strategy for Mycophenolic Acid in Japanese Heart Transplant Recipients Comparison of Cyclosporin and Tacrolimus Treatment. Circ. J. 2007, 7, 1022–1028. [Google Scholar] [CrossRef][Green Version]
- de Winter, B.C.; Neumann, I.; van Hest, R.M.; Gelder, T.; Mathot, R. Limited Sampling Strategies for Therapeutic Drug Monitoring of Mycophenolate Mofetil Therapy in Patients with Autoimmune Disease. Ther. Drug Monit. 2009, 31, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Fukuoka, N.; Kimura, S.; Watanabe, M.; Tani, K.; Tanaka, H.; Sofue, T.; Kosaka, S.; Inui, M.; Kakehi, Y.; et al. Limited Sampling Strategy for the Estimation of Mycophenolic Acid Area under the Concentration–Time Curve Treated in Japanese Living-Related Renal Transplant Recipients with Concomitant Extended-Release Tacrolimus. Biol. Pharm. Bull. 2013, 36, 1036–1039. [Google Scholar] [CrossRef]
- Yeung, S.; Tong, K.L.; Tsang, W.K.; Tang, H.L.; Fung, K.S.; Chan, H.W.; Chan, A.Y.; Chan, L. Determination of Mycophenolate Area under the Curve by Limited Sampling Strategy. Transplant. Proc. 2001, 33, 1052–1053. [Google Scholar] [CrossRef]
- Czyrski, A.; Kondys, K.; Szałek, E.; Karbownik, A.; Grześkowiak, E. The Pharmacokinetic Interaction between Levofloxacin and Sunitinib. Pharm. Rep. 2015, 67, 542–544. [Google Scholar] [CrossRef]
- Danielak, D.; Sobiak, J.; Wachowiak, J.; Glówka, F.; Chrzanowska, M. Development of a Limited Sampling Strategy for the Estimation of Exposure to High-Dose Etoposide after Intravenous Infusion in Pediatric Patients. Ther. Drug Monit. 2017, 39, 138–144. [Google Scholar] [CrossRef]
- Pawinski, T.; Luszczynska, P.; Durlik, M.; Majchrzak, J.; Baczkowska, T.; Chrzanowska, M.; Sobiak, J.; Glyda, M.; Kuriata-kordek, M.; Kamińska, D.; et al. Development and Validation of Limited Sampling Strategies for the Estimation of Mycophenolic Acid Area under the Curve in Adult Kidney and Liver Transplant Recipients Receiving Concomitant Enteric-Coated Mycophenolate Sodium and Tacrolimus. Ther. Drug Monit. 2013, 35, 760–769. [Google Scholar] [CrossRef]
- Sobiak, J.; Resztak, M.; Pawiński, T.; Żero, P.; Ostalska-Nowicka, D.; Zachwieja, J.; Chrzanowska, M. Limited Sampling Strategy to Predict Mycophenolic Acid Area under the Curve in Pediatric Patients with Nephrotic Syndrome: A Retrospective Cohort Study. Eur. J. Clin. Pharm. 2019, 75, 1249–1259. [Google Scholar] [CrossRef]
- Kunicki, P.K.; Pawinski, T.; Boczek, A.; Was, J.; Bodnar-Broniarczyk, M. A Comparison of the Immunochemical Methods, PETINIA and EMIT, with That of HPLC-UV for the Routine Monitoring of Mycophenolic Acid in Heart Transplant Patients. Ther. Drug Monit. 2015, 37, 311–318. [Google Scholar] [CrossRef]
- Abd Rahman, A.N.; Tett, S.E.; Staatz, C.E. Clinical Pharmacokinetics and Pharmacodynamics of Mycophenolate in Patients with Autoimmune Disease. Clin. Pharm. 2013, 52, 303–331. [Google Scholar] [CrossRef]
- Zhou, P.J.; Xu, D.; Yu, Z.C.; Wang, X.H.; Shao, K.; Zhao, J.P. Pharmacokinetics of Mycophenolic Acid and Estimation of Exposure Using Multiple Linear Regression Equations in Chinese Renal Allograft Recipients. Clin. Pharm. 2007, 46, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Downing, H.J.; Pirmohamed, M.; Beresford, M.W.; Smyth, R.L. Paediatric Use of Mycophenolate Mofetil. Br. J. Clin. Pharm. 2013, 75, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Elbarbry, F.A.; Shoker, A.S. Liquid Chromatographic Determination of Mycophenolic Acid and Its Metabolites in Human Kidney Transplant Plasma: Pharmacokinetic Application. J. Chromatogr. B 2007, 859, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Peng, B.; Li, L.; Wang, J.; Wang, X.; Qi, G.; Rong, R.; Wang, L.; Qiu, J.; Xu, M.; et al. Estimation of Mycophenolic Acid Area under the Curve with Limited-Sampling Strategy in Chinese Renal Transplant Recipients Receiving Enteric-Coated Mycophenolate Sodium. Ther. Drug Monit. 2017, 39, 29–36. [Google Scholar] [CrossRef]
- Gaies, E.; Ben Sassi, M.; El Jebari, H.; Jebabli, N.; Charfi, R.; Chokri, I.; Salouage, I.; Klouz, A.; Trabelsi, S. Limited Sampling Strategy for the Estimation of Mycophenolic Acid Area under the Curve in Tunisian Renal Transplant Patients. Nephrol. Ther. 2017, 13, 460–462. [Google Scholar] [CrossRef]
- Barraclough, K.; Isbel, N.; Franklin, M.; Lee, K.; Taylor, P.; Campbell, S.; Petchey, W.; Staatz, C. Evaluation of Limited Sampling Strategies for Mycophenolic Acid after Mycophenolate Mofetil Intake in Adult Kidney Transplant Recipients. Ther. Drug Monit. 2010, 32, 723–733. [Google Scholar] [CrossRef]
- Hao, C.; Erzheng, C.; Anwei, M.; Zhicheng, Y.; Baiyong, S.; Xiaxing, D.; Weixia, Z.; Chenghong, P.; Hongwei, L. Validation of Limited Sampling Strategy for the Estimation of Mycophenolic Acid Exposure in Chinese Adult Liver Transplant Recipients. Liver Transplant. 2007, 13, 1684–1693. [Google Scholar] [CrossRef] [PubMed]
- Miura, M.; Satoh, S.; Niioka, T.; Kagaya, H.; Saito, M.; Hayakari, M.; Habuchi, T.; Suzuki, T. Early Phase Limited Sampling Strategy Characterizing Tacrolimus and Mycophenolic Acid Pharmacokinetics Adapted to the Maintenance Phase of Renal Transplant Patients. Ther. Drug Monit. 2009, 31, 467–474. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).