First-in-Class Phosphorus Dendritic Framework, a Wide Surface Functional Group Palette Bringing Noteworthy Anti-Cancer and Anti-Tuberculosis Activities: What Lessons to Learn?
Abstract
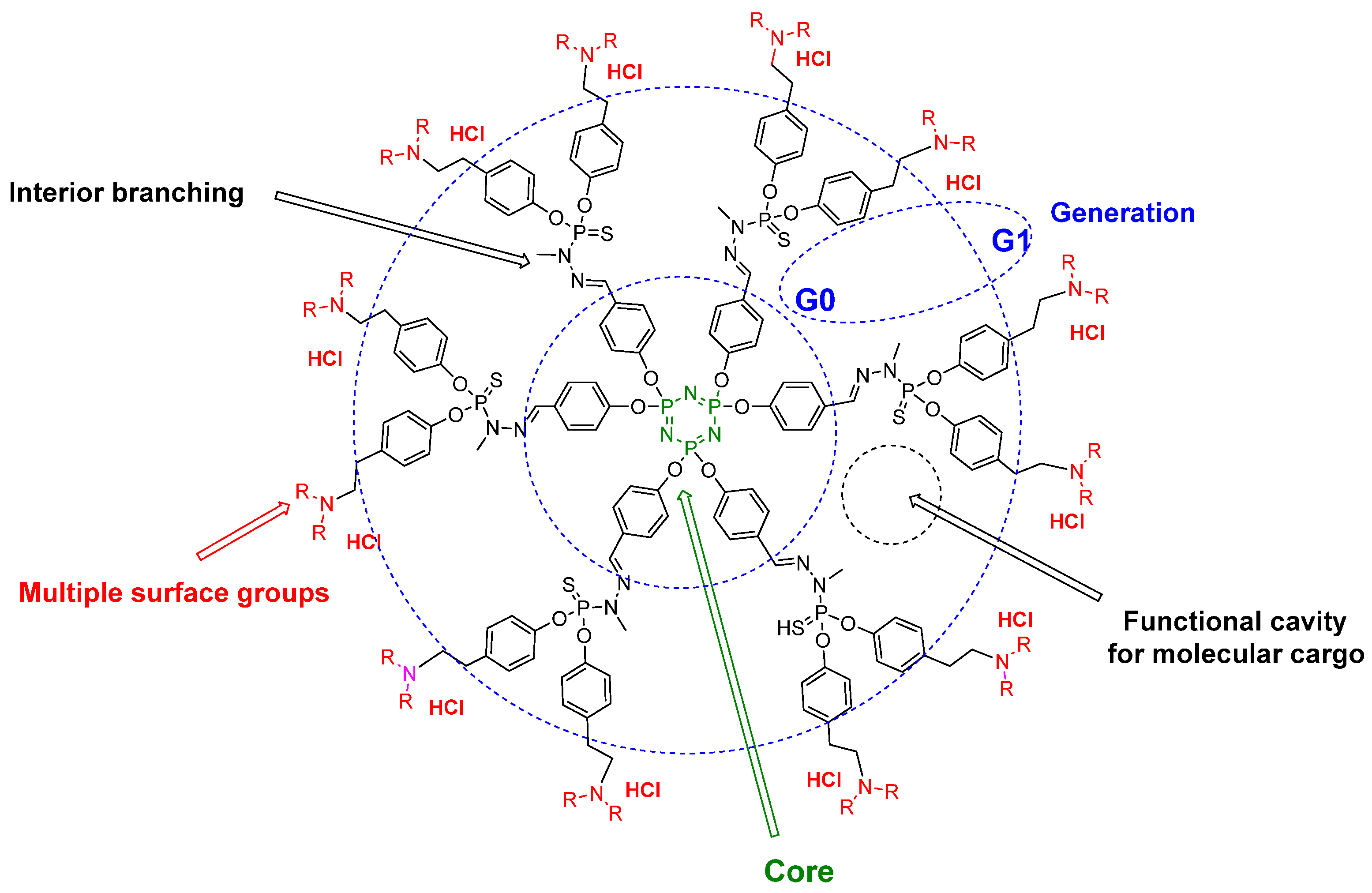
1. Introduction
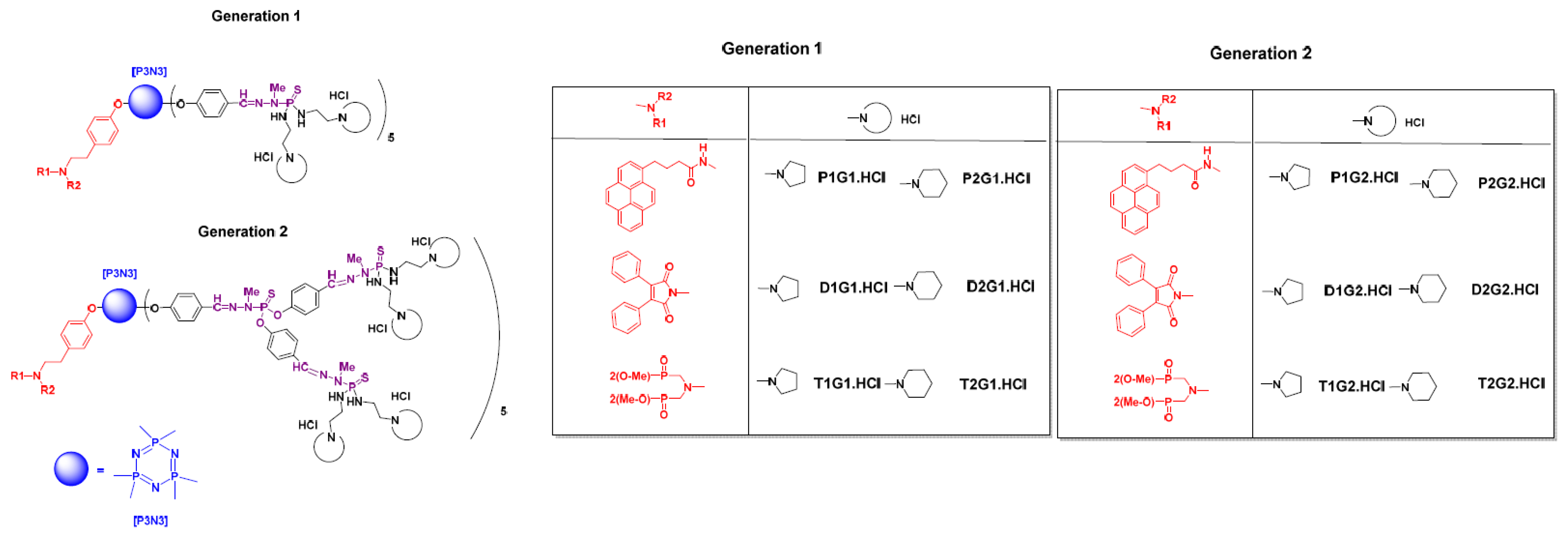
2. Neutral Phosphorus Dendrimers as Anti-Cancer Agents
3. Polycationic Phosphorus Dendrimers
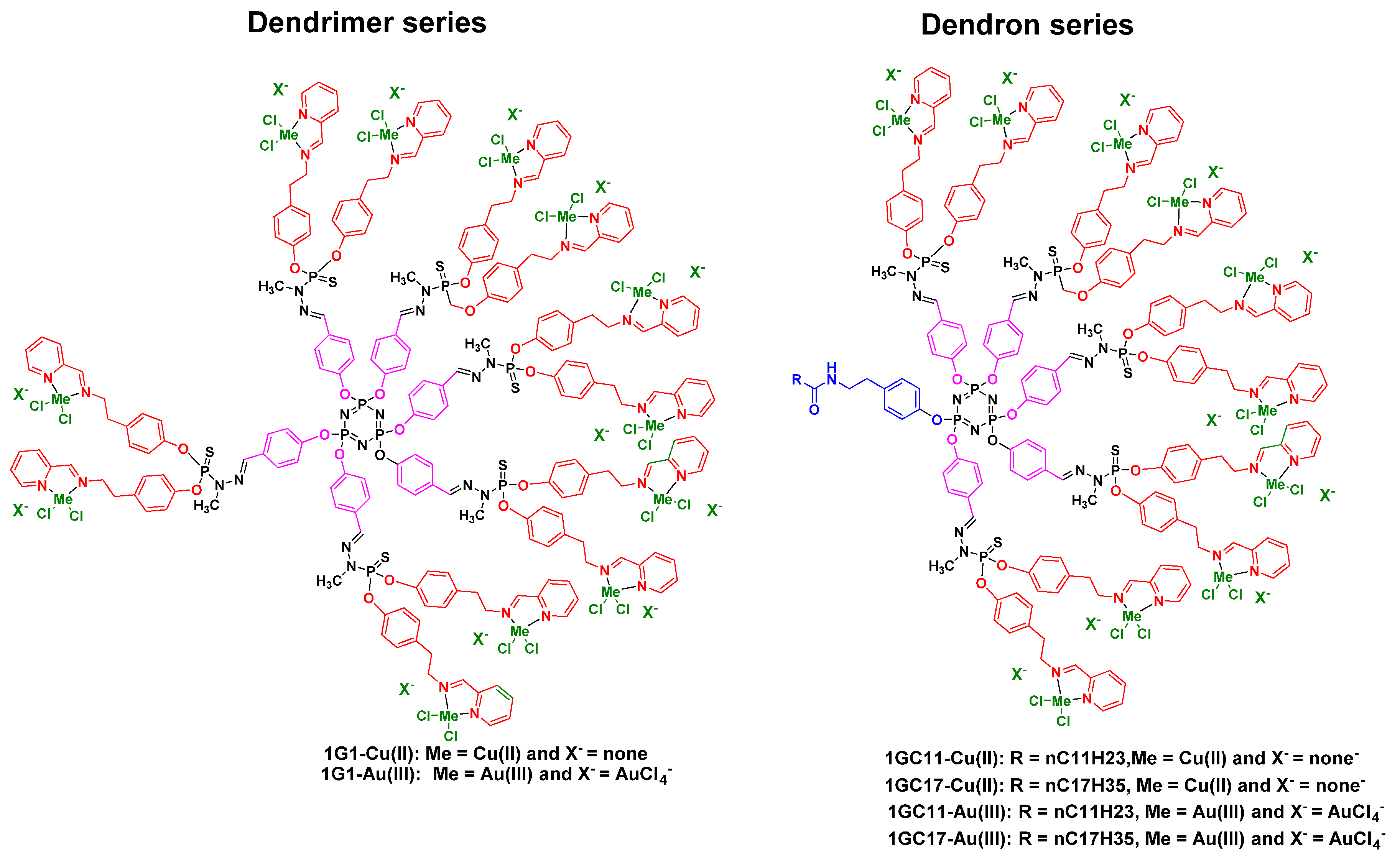
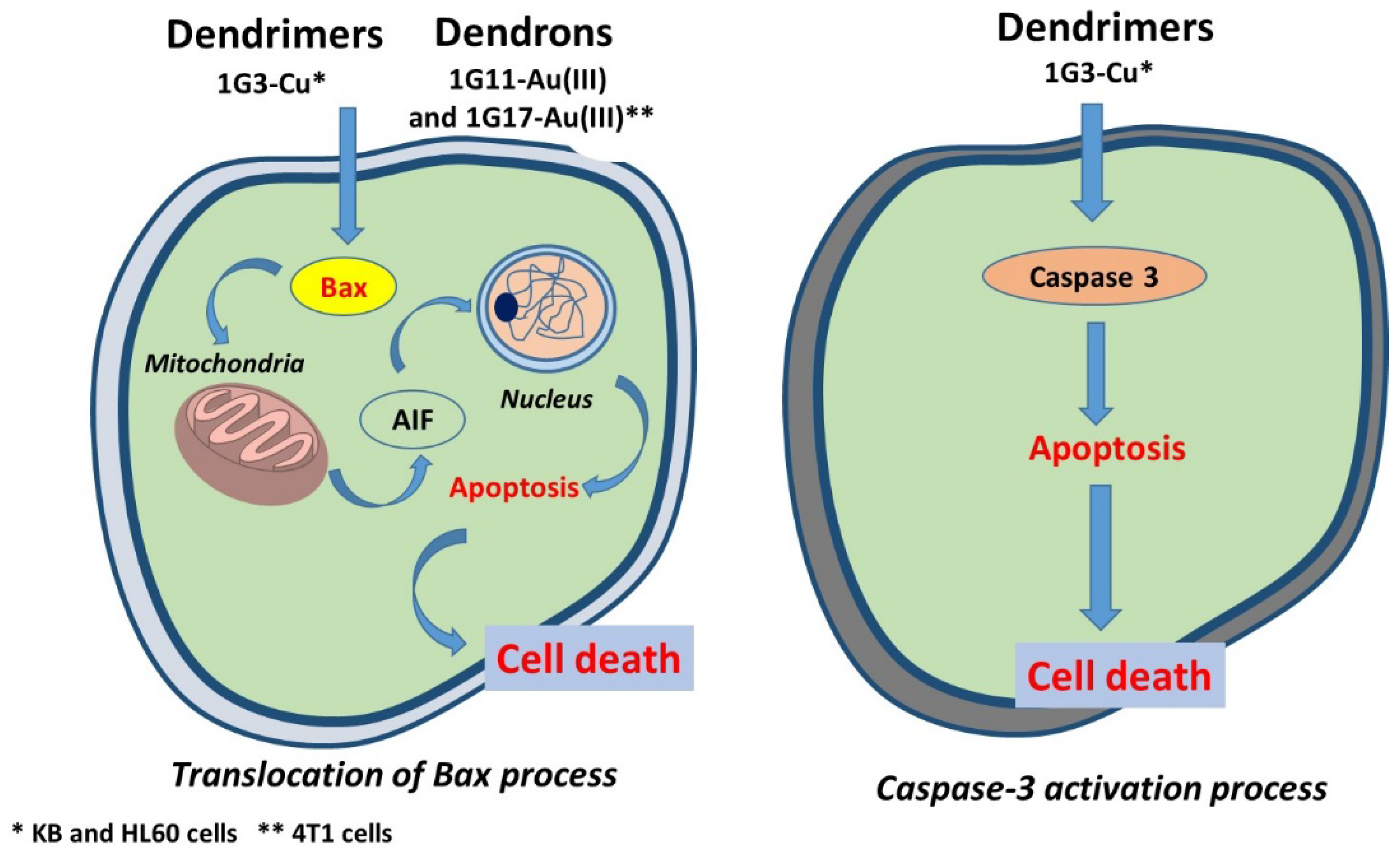
3.1. As Anti-Cancer Agents
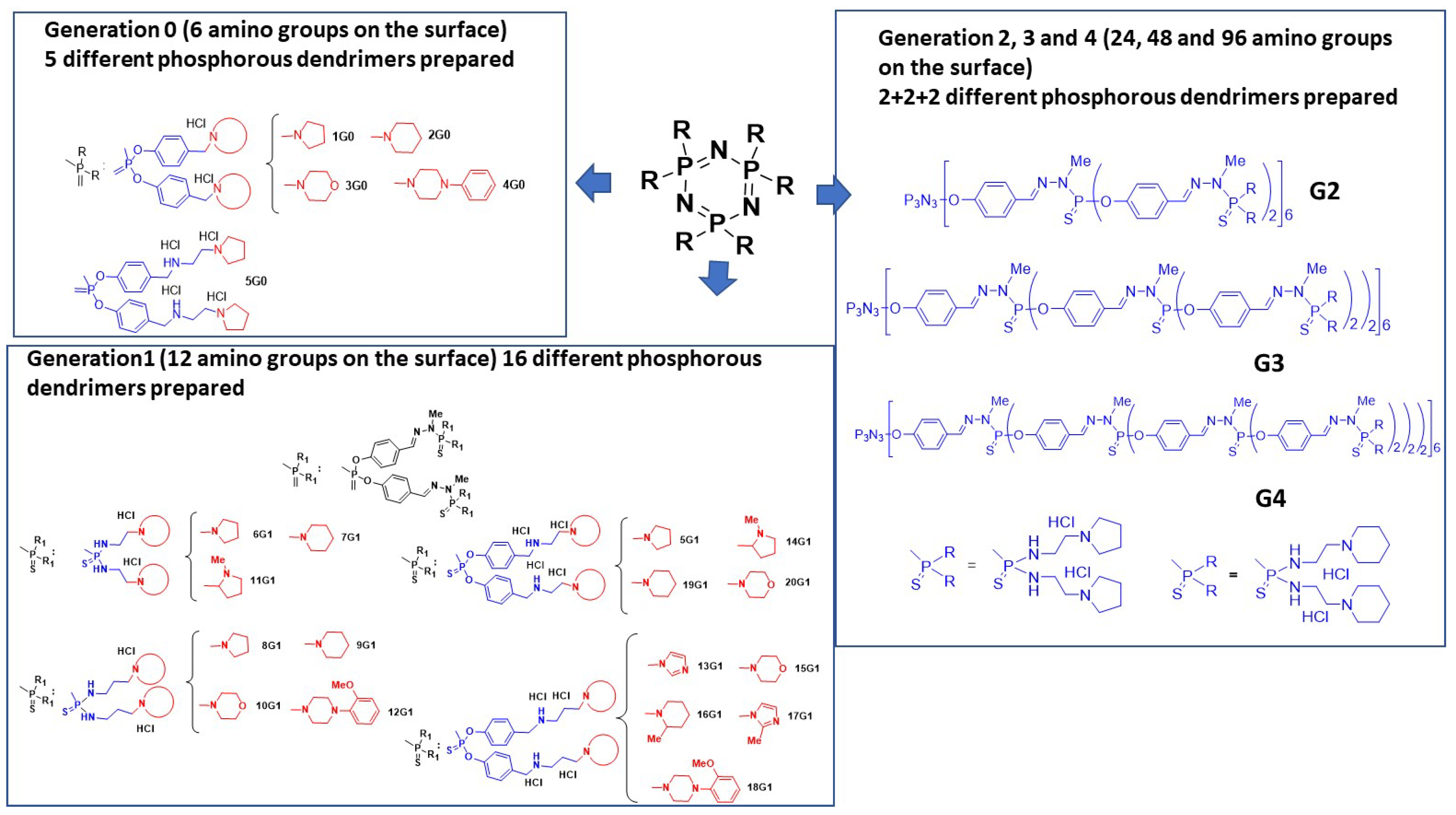
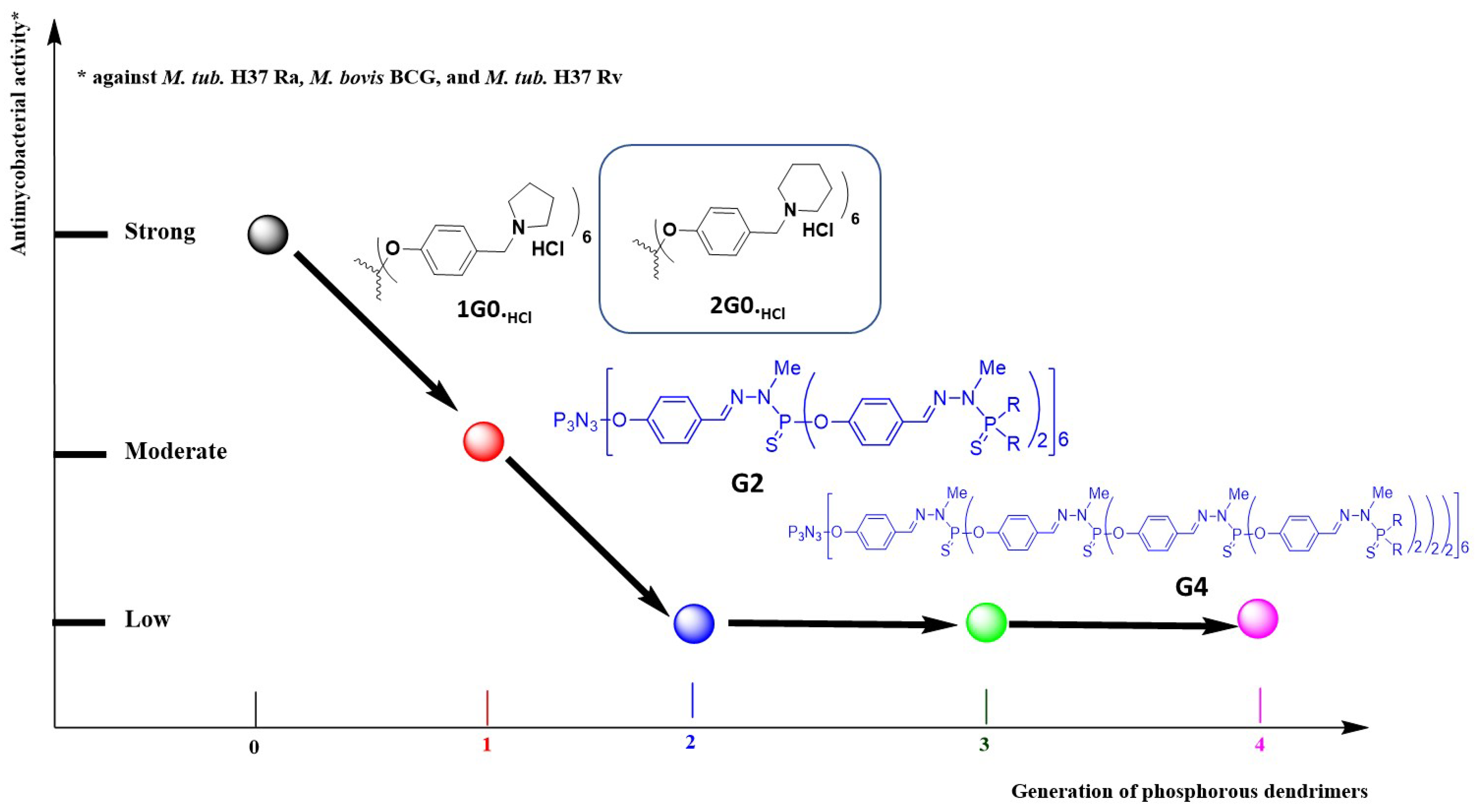
3.2. As Anti-Tuberculosis Agents
4. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Shahi, S.R.; Kulkarni, M.S.; Karva, G.S.; Giram, P.S.; Gugulkar, R.R. Dendrimers. Int. J. Pharm. Sci. Rev. Res. 2015, 33, 187–198. [Google Scholar]
- Mignani, S.; El Kazzouli, S.; Bousmina, M.M.; Majoral, J.-P. Dendrimer space concept for innovative nanomedicine: A futuristic vision for medicinal chemistry. Prog. Polym. Sci. 2013, 38, 993–1008. [Google Scholar] [CrossRef]
- Caminade, A.M.; Turrin, C.O.; Majoral, J.P. Phosphorous Dendrimers in Biology and Nanomedicine: Syntheses, Characterization, and Properties; Jenny Stanford Publishing: Boca Raton, FL, USA, 2018. [Google Scholar]
- Caminade, A.M.; Ouali, A.; Laurent, R.; Turrin, C.-O.; Majoral, J.-P. Coordination chemistry with phosphorus dendrimers. Applications as catalysts, for materials, and in biology. Coord. Chem. Rev. 2016, 308, 478–497. [Google Scholar] [CrossRef]
- Waltera, M.V.; Malkoch, M. Simplifying the synthesis of dendrimers: Accelerated approaches. Chem. Soc. Rev. 2012, 41, 4593–4609. [Google Scholar] [CrossRef] [PubMed]
- Maraval, V.; Caminade, A.M.; Majoral, J.-P.; Blais, J.C. Dendrimer design: How to circ54umvent the dilemma of a reduction of steps or an increase of fuction multiplicity? Angew. Chem. Int. Ed. Engl. 2003, 42, 1822–1826. [Google Scholar] [CrossRef] [PubMed]
- Larre, C.; Caminade, A.M.; Majoral, J.-P. Chemoselective polyalkylations of phosphorus-containing dendrimers. Angew. Chem. Int. Ed. Engl. 1997, 36, 596–599. [Google Scholar] [CrossRef]
- Sebastian, R.M.; Griffe, L.; Turrin, C.-O.; Donnadieu, B.; Caminade, A.M.; Majoral, J.-P. Synthesis and core and surface reactivity of phosphorus-based dendrons. Eur. J. Inorg. Chem. 2004, 12, 2459–2466. [Google Scholar] [CrossRef]
- Rodriguez, L.I.; Zablocka, M.; Caminade, A.M.; Seco, M.; Rossell, O.; Majoral, J.-P. Phosphorus dendrimers and dendrons functionalized with the cage ligand tris(1,2-dimethylhydrazino)diphosphate. Heteroat. Chem. 2010, 21, 290–297. [Google Scholar] [CrossRef]
- Katir, K.; El Brahmi, N.; El Kadib, A.; Mignani, S.; Caminade, A.M.; Bousmina, M.; Majoral, J.-P. Synthesis of onion-peel nanodendritic structures with sequential functional phosphorus diversity. Chem. Eur. J. 2015, 21, 6400–6408. [Google Scholar] [CrossRef]
- Caminade, A.M.; Laurent, R.; Delavaux-Nicot, B.; Majoral, J.-P. ‘Janus’ dendrimers: Syntheses and properties. New J. Chem. 2012, 36, 217–226. [Google Scholar] [CrossRef]
- Noriega-Luna, B.; Godínez, L.A.; Rodríguez, F.J.; Rodríguez, A.; de Larrea, G.Z.-L.; Sosa-Ferreyra, C.F.; Mercado-Curiel, R.F.; Manríquez, J.; Bustos, E. Applications of Dendrimers in Drug Delivery Agents, Diagnosis, Therapy, and Detection. J. Nanomater. 2014. [Google Scholar] [CrossRef]
- Ihre, H.R.; de Jesús, O.L.P.; Szoka, F.C.; Fréchet, J.M.J. Polyester dendritic systems for drug delivery applications: Design, synthesis, and characterization. Bioconjug. Chem. 2020, 13, 443–452. [Google Scholar] [CrossRef]
- Kim, Y.; Park, E.J.; Na, D.H. Recent progress in dendrimer-based nanomedicine development. Arch. Pharm. Res. 2018, 41, 571–580. [Google Scholar] [CrossRef]
- Mignani, S.; Majoral, J.-P. Dendrimers as macromolecular tools to tackle from colon to brain tumor types: A concise overview. New J. Chem. 2013, 37, 3337–3357. [Google Scholar] [CrossRef]
- Sanz del Olmo, N.; Carloni, R.; Bajo, A.M.; Ortega, P.; Gómez, A.F.R.; Ottaviani, M.F.; García-Gallego, S.; Cangiotti, M.; de la Mata, F.J. Insight into the antitumor activity of carbosilane Cu(II)–metallodendrimers through their interaction with biological membrane models. Nanoscale 2019, 11, 13330–13342. [Google Scholar] [CrossRef]
- Carloni, R.; del Olmo, N.S.; Ortega, P.; Fattori, A.; Gómez, R.; Ottaviani, M.F.; García-Gallego, S.; Cangiotti, M.; de la Mata, F.J. Exploring the Interactions of Ruthenium (II) Carbosilane Metallodendrimers and Precursors with Model Cell Membranes through a Dual Spin-Label Spin-Probe Technique Using EPR. Biomolecules 2019, 9, 540. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.C.; MacKay, J.A.; Fréchet, J.M.; Szoka, F.C. Designing dendrimers for biological applications. Nat. Biotechnol. 2005, 23, 1517–1526. [Google Scholar] [CrossRef]
- Khandare, J.; Calderon, M.; Dagia, N.M.; Haag, R. Multifunctional dendritic polymers in nanomedicine: Opportunities and challenges. Chem. Soc. Rev. 2012, 41, 2824–2848. [Google Scholar] [CrossRef]
- Mignani, S.; Huber, S.; Tomás, H.; Rodrigues, J.; Majoral, J.-P. Compound high-quality criteria: A new vision to guide the development of drugs, current situation. Drug Discov. Today 2016, 21, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Mignani, S.; Rodrigues, J.; Roy, R.; Shi, X.; Cena, V.; El Kazzouli, S.; Majoral, J.-P. Exploration of biomedical dendrimer space based on in-vivo physicochemical parameters: Key factor analysis (Part 2). Drug Discov. Today 2019, 24, 1184–1192. [Google Scholar] [CrossRef]
- Mignani, S.; Shi, X.; Rodrigues, J.; Roy, R.; Muñoz-Fernández, A.; Ceña, V.; Majoral, J.-P. Dendrimers toward Translational Nanotherapeutics: Concise Key Step Analysis. Bioconjugate Chem. 2020, 31, 2060–2071. [Google Scholar] [CrossRef]
- Mignani, S.; Shi, X.; Rodrigues, J.; Tomas, H.; Karpus, A.; Majoral, J.-P. First-in-class and best-in-class dendrimer nanoplatforms from concept to clinic: Lessons learned moving forward. Eur. J. Med. Chem. 2021, 219, 113456. [Google Scholar] [CrossRef] [PubMed]
- Maciejewski, M. Concepts of trapping topologically by shell molecules. Macromol. Sci. Chem. A 1982, 17, 689–703. [Google Scholar] [CrossRef]
- StarPharma. Available online: https://starpharma.com/news/328. (accessed on 16 June 2021).
- Tyssen, D.; Henderson, S.A.; Johnson, A.; Sterjovski, J.; Moore, K.; La, J.; Zanin, M.; Sonza, S.; Karellas, P.; Giannis, M.P.; et al. Structure Activity Relationship of Dendrimer Microbicides with Dual Action Antiviral Activity. PLoS ONE 2010, 5, e12309. [Google Scholar] [CrossRef] [PubMed]
- Hayder, M.; Poupot, M.; Baron, M.; Nigou, D.; Turrin, C.O.; Caminade, A.M.; Majoral, J.P.; Eisenberg, R.A.; Fournié, J.J.; Cantagrel, A.; et al. A phosphorus-based dendrimer targets inflammation and osteoclastogenesis in experimental arthritis. Sci. Transl. Med. 2011, 3, 81ra35. [Google Scholar] [CrossRef]
- Bohr, A.; Tsapis, N.; Andreana, I.; Chamarat, A.; Foged, C.; Delomenie, C.; Noiray, M.; El Brahmi, N.; Majoral, J.-P.; Mignani, S.; et al. Anti-Inflammatory Effect of Anti-TNF-α SiRNA Cationic Phosphorus Dendrimer Nanocomplexes Administered Intranasally in a Murine Acute Lung Injury Model. Biomacromolecules 2017, 18, 2379–2388. [Google Scholar] [CrossRef]
- Mignani, S.; Srivastava, K.K.; Tripathi, R.P.; Soam, D.; Chopra, S.; Dasgupta, A.; Mishra, D.P.; Tripathi, V.D.; Majoral, J.-P.; Caminade, A.M. Functional neutral phosphorus dendrimers and polycationic phosphorus dendrimers for the treatment of Mycobacterium Tuberculosis. Indian Patent Application IN 202011002577, 21 January 2020. [Google Scholar]
- Milowska, K.; Grochowina, J.; Katir, N.; El Kadib, A.; Majoral, J.-P.; Bryszewska, M.; Gabryelak, T. Viologen-phosphorus dendrimers inhibit α-synuclein fibrillation. Mol. Pharm. 2013, 10, 1131–1137. [Google Scholar] [CrossRef]
- Solassol, J.; Crozet, C.; Perrier, V.; Leclaire, J.; Beranger, F.; Caminade, A.M.; Meunier, B.; Dormont, D.; Majoral, J.-P.; Lehmann, S. Cationic phosphorus-containing dendrimers reduce prion replication both in cell culture and in mice infected with scrapie. J. Gen. Virol. 2004, 85, 1791–1799. [Google Scholar] [CrossRef]
- Mignani, S.; El Brahmi, N.; Cresteil, T.; Majoral, J.-P. First-in-class combination therapy of a copper(II) metallo-phosphorus dendrimer with cytotoxic agents. Oncology 2018, 94, 324–328. [Google Scholar] [CrossRef]
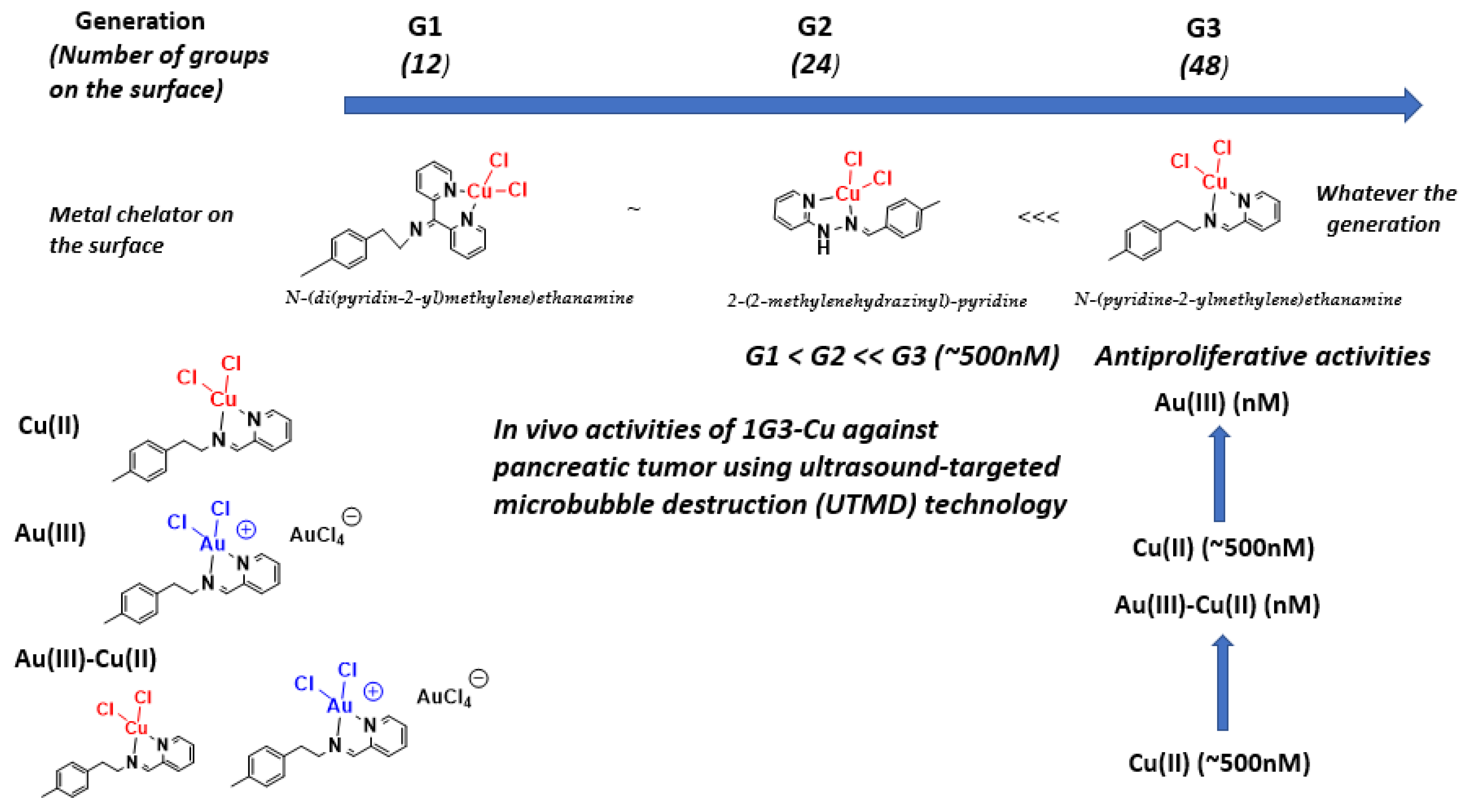
- Mignani, S.M.; El Brahmi, N.; El Kazzouli, S.; Laurent, R.; Ladeira, S.; Caminade, A.M.; Pedziwiatr-Werbicka, E.; Szewczyk, E.M.; Bryszewska, M.; Bousmina, M.M.; et al. Original multivalent gold(III) and dual gold(III)–copper(II) conjugated phosphorus dendrimers as potent antitumoral and antimicrobial agents. Mol. Pharm. 2017, 14, 4087–4097. [Google Scholar] [CrossRef] [PubMed]
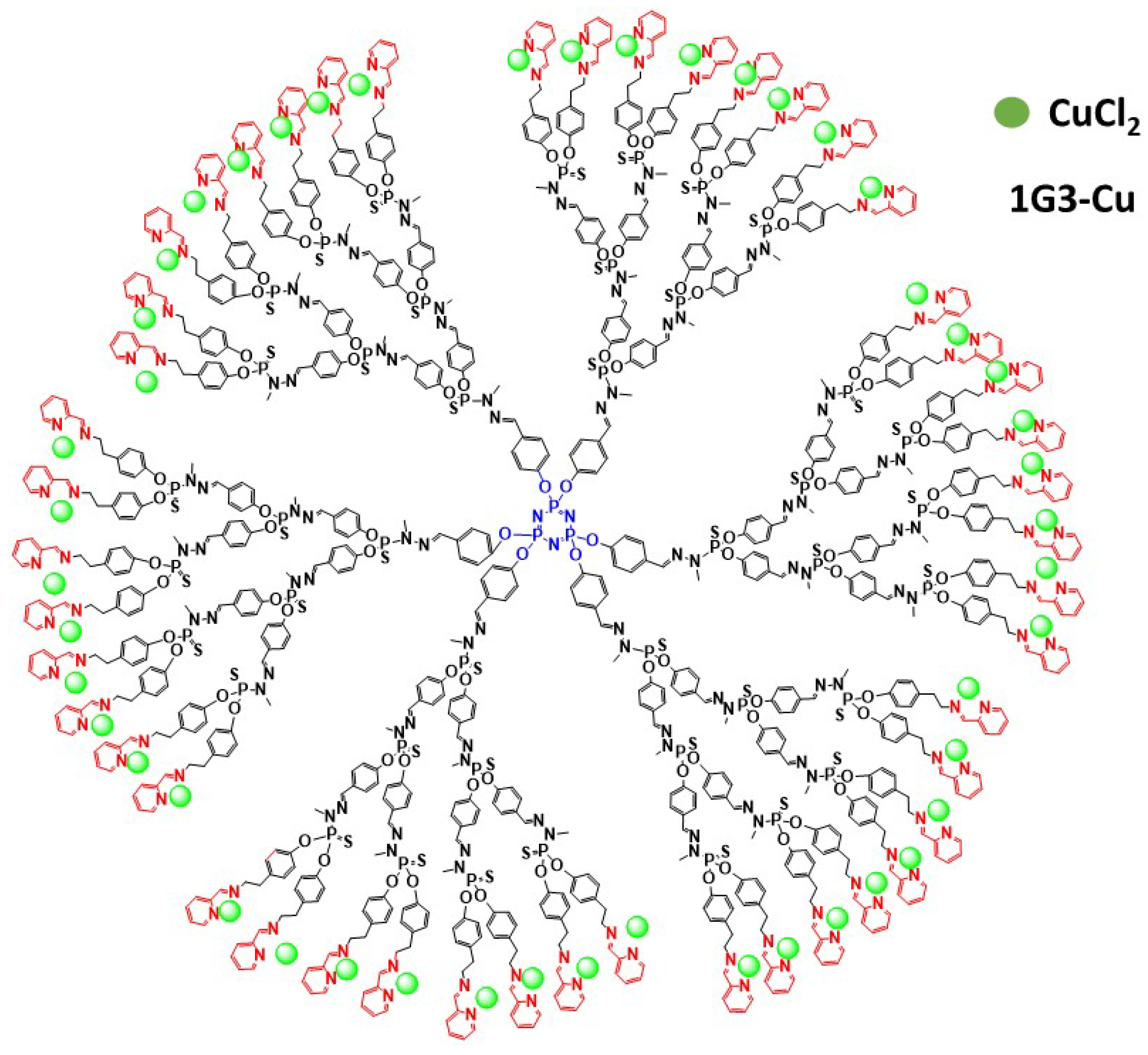
- Mignani, S.; Shi, X.; Steinmetz, A.; Majoral, J.-P. Multivalent Copper(II)-Conjugated Phosphorus Dendrimers with Noteworthy In Vitro and In Vivo Antitumor Activities: A Concise Overview. Mol. Pharm. 2020. [Google Scholar] [CrossRef]
- Mignani, S.M.; El Brahmi, N.; Eloy, L.; Poupon, J.; Nicolas, V.; Steinmetz, A.; El Kazzouli, S.; Bousmina, M.M.; Blanchard-Desce, M.; Caminade, A.M.; et al. Anticancer copper(II) phosphorus dendrimers are potent proapoptotic Bax activators. Eur. J. Med. Chem. 2017, 132, 142e156. [Google Scholar] [CrossRef] [PubMed]
- Mignani, S.; Rodrigues, J.; Tomas, H.; Caminade, A.-M.; Laurent, R.; Shi, X.; Majoral, J.-P. Recent therapeutic applications of the theranostic principle with dendrimers in oncology. Sci. China Mater. 2018, 61, 1367–1386. [Google Scholar] [CrossRef]
- Chen, L.; Fan, Y.; Qiu, J.; Laurent, R.; Bignon, J.; Mignani, S.; Caminade, A.M.; Shi, X.; Majoral, J.-P. Potent Anticancer Efficacy of First-In-Class CuII and AuIII Metaled Phosphorus Dendrons with Distinct Cell Death Pathways. Chem. A Eur. J. 2020, 26. [Google Scholar] [CrossRef]
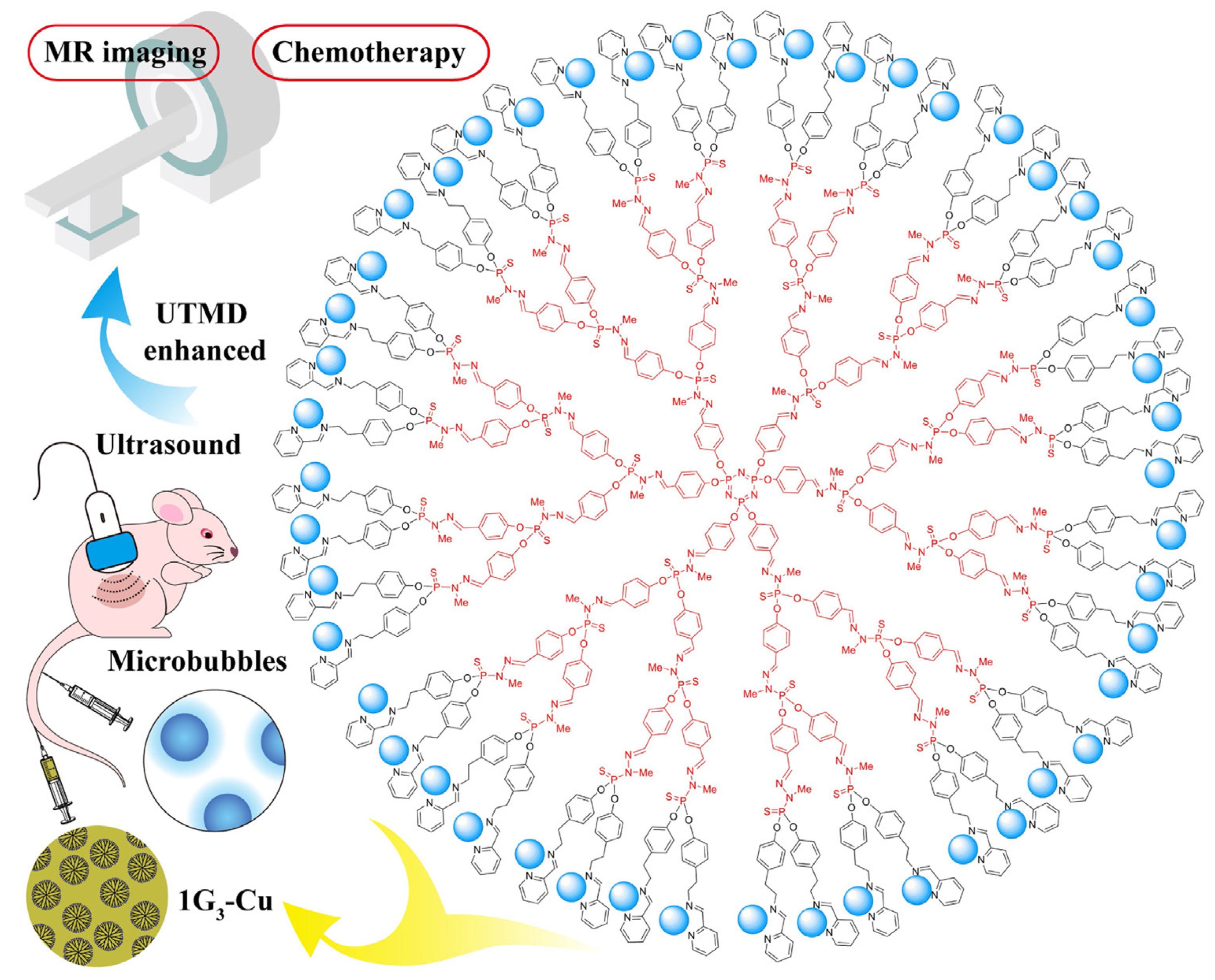
- Fan, Y.; Lin, L.; Yinc, F.; Zhua, Y.; Shena, M.; Wang, H.; Dub, L.; Mignani, S.; Majoral, J.-P.; Shi, X. Phosphorus dendrimer-based copper(II) complexes enableultrasound-enhanced tumor theranostics. Nano Today 2020, 33, 100899. [Google Scholar] [CrossRef]
- Qiu, J.; Liang, C.; Zhan, M.; Laurent, R.; Bignon, J.; Mignani, S.; Shi, X.; Caminade, A.-M.; Majoral, J.-P. Facile Synthesis of Amphiphilic Fluorescent Phosphorus Dendron-Based Micelles as Antiproliferative Agents: First Investigations. Bioconjugate Chem. 2021, 32, 339–349. [Google Scholar] [CrossRef]
- Vasan, N.; Baselga, J.; Hyman, D.M. A view on drug resistance in cancer. Nature 2019, 575, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Fan, Y.; Gao, F.; Jin, L.; Li, D.; Sun, W.; Li, F.; Qin, P.; Shi, Q.; Shi, X.; et al. UTMD-Promoted Co-Delivery of Gemcitabine and miR-21 Inhibitor by Dendrimer-Entrapped Gold Nanoparticles for Pancreatic Cancer Therapy. Theranostics 2018, 8, 1923–1939. [Google Scholar] [CrossRef] [PubMed]
- Caminade, A.M.; Hameau, A.; Majoral, J.P. Multicharged and/or water-soluble fluorescent dendrimers: Properties and uses. Chem. Eur. J. 2009, 15, 9270–9285. [Google Scholar] [CrossRef]
- Qiu, J.R.; Hameau, A.; Shi, X.Y.; Mignani, S.; Majoral, J.P.; Caminade, A.M. Fluorescent phosphorus dendrimers: Towards material and biological applications. ChemPlusChem 2019, 84, 1070–1080. [Google Scholar] [CrossRef] [PubMed]
- Ang, C.W.; Jarrad, A.M.; Cooper, M.A.; Blaskovich, M.A.T. Nitroimidazoles: Molecular Fireworks That Combat a Broad Spectrum of Infectious Diseases. J. Med. Chem. 2017, 60, 7636–7657. [Google Scholar] [CrossRef] [PubMed]
- Zumla, A.; Nahid, P.; Cole, S.T. Advances in the development of new tuberculosis drugs and treatment regimens. Nat. Rev. Drug Discov. 2013, 12, 388–404. [Google Scholar] [CrossRef]
- Costa-Gouveia, J.; Aınsa, J.A.; Brodin, P.; Lucıa, A. How can nanoparticles contribute to antituberculosis therapy? Drug Discov. Today 2017, 22, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Webescence-Citations. Available online: https://citations.webescence.com/citations/Henri-Matisse (accessed on 16 June 2021).
- Mignani, S.; Shi, X.; Ceña, V.; Shcharbin, D.; Bryszewska, M.; Majoral, J.-P. In vivo therapeutic applications of phosphorus dendrimers: State of the art. Drug Discov. Today 2021. [Google Scholar] [CrossRef] [PubMed]
- Ottl, J.; Leder, L.; Schaefer, J.V.; Dumelin, C.E. Encoded library technologies as integrated lead finding platforms for drug discovery. Molecules 2019, 24, 1629. [Google Scholar] [CrossRef]

| Materials | Cell Lines | ||||||
|---|---|---|---|---|---|---|---|
| 4T1 | MCF-7 | HL-60 | HCT-116 | NIH-3T3 * | MCR5 * | ||
| Dendrons | 1GC11-Cu(II) | 0.585+/−0.11 | 1.489+/−0.27 | 1.93+/−1.37 | 3.37+/−0.87 | 1.491+/−0.22 | 2.46+/−1.20 |
| 1GC17-Cu(II) | 1.025+/−0.09 | 2.755+/−0.286 | 2.65+/−0.65 | 3.03+/−0.254 | 4.075+/−0.65 | 2.66+/−0.64 | |
| 1GC11-Au(III) | 0.164+/−0.04 | 0.286+/−0.07 | 1.33+/−0.25 | 2.09+/−0.23 | 0.468+/−0.12 | 2.71+/−0.71 | |
| 1GC17-Au(III) | 0.339+/−0.06 | 0.802+/−0.15 | 1.34+/−0.30 | 4.44+/−0.87 | 0.132+/−0.06 | 3.18+/−0.27 | |
| Dendrimers | 1G1-Cu(II) | 1.00+/−0.20 | 3.26+/−0.71 | ||||
| 1G1-Au(III) | 0.65+/−0.04 | 2.90+/−0.87 | |||||
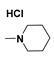
| Generation | Cyclic Amine | Pyrene Series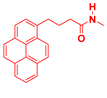 | Maleimide Series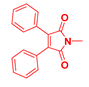 | Azabisdimethyl Phosphonate Series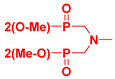 |
|---|---|---|---|---|
| 1 | 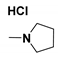 | 3.56 | 76.9 | 153.7 |
 | 2.96 | 73.3 | 109 | |
| 2 | 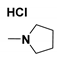 | 1.42 | 35.3 | 60 |
 | 2.31 | 26.3 | 45.5 |
| Cell Lines | IC50s | Cell Lines | IC50s |
|---|---|---|---|
| L929 * | 8.75+/−0.51 | HL60 | 4.1+/−0.15 |
| A549 | 0.36+/−0.06 | HCT116 | 0.86+/−0.02 |
| MCF7 | 2.89+/−0.17 | K562 | 1.1+/−0.06 |
| PC3 | 0.56+/−0.03 | K562R | 2.47+/−0.07 |
| U87-MG | 0.27+/−0.03 | MDA-MB-231 | 0.31+/−0.01 |
| Compound | M. tuberculosis H37Rv (µg/mL) | MTB Strain Resistant to | |||
|---|---|---|---|---|---|
| INH (µg/mL) | RIF (µg/mL) | ETB (µg/mL) | STR (µg/mL) | ||
| 2G0.HCl | 3.12 | 6.25 | 6.25 | 6.25 | 6.25 |
| RIF | 0.04 | 0.04 | >64 | 0.19 | 0.04 |
| INH | 0.04 | >64 | 0.04 | 0.04 | 0.04 |
| ETB | 3.12 | NT | NT | >64 | 0.78 |
| STR | 1.56 | NT | NT | 12.5 | >64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mignani, S.; Bignon, J.; Shi, X.; Majoral, J.-P. First-in-Class Phosphorus Dendritic Framework, a Wide Surface Functional Group Palette Bringing Noteworthy Anti-Cancer and Anti-Tuberculosis Activities: What Lessons to Learn? Molecules 2021, 26, 3708. https://doi.org/10.3390/molecules26123708
Mignani S, Bignon J, Shi X, Majoral J-P. First-in-Class Phosphorus Dendritic Framework, a Wide Surface Functional Group Palette Bringing Noteworthy Anti-Cancer and Anti-Tuberculosis Activities: What Lessons to Learn? Molecules. 2021; 26(12):3708. https://doi.org/10.3390/molecules26123708
Chicago/Turabian StyleMignani, Serge, Jérôme Bignon, Xiangyang Shi, and Jean-Pierre Majoral. 2021. "First-in-Class Phosphorus Dendritic Framework, a Wide Surface Functional Group Palette Bringing Noteworthy Anti-Cancer and Anti-Tuberculosis Activities: What Lessons to Learn?" Molecules 26, no. 12: 3708. https://doi.org/10.3390/molecules26123708
APA StyleMignani, S., Bignon, J., Shi, X., & Majoral, J.-P. (2021). First-in-Class Phosphorus Dendritic Framework, a Wide Surface Functional Group Palette Bringing Noteworthy Anti-Cancer and Anti-Tuberculosis Activities: What Lessons to Learn? Molecules, 26(12), 3708. https://doi.org/10.3390/molecules26123708








