Efficient Generation of Microdroplets Using Tail Breakup Induced with Multi-Branch Channels
Abstract
1. Introduction
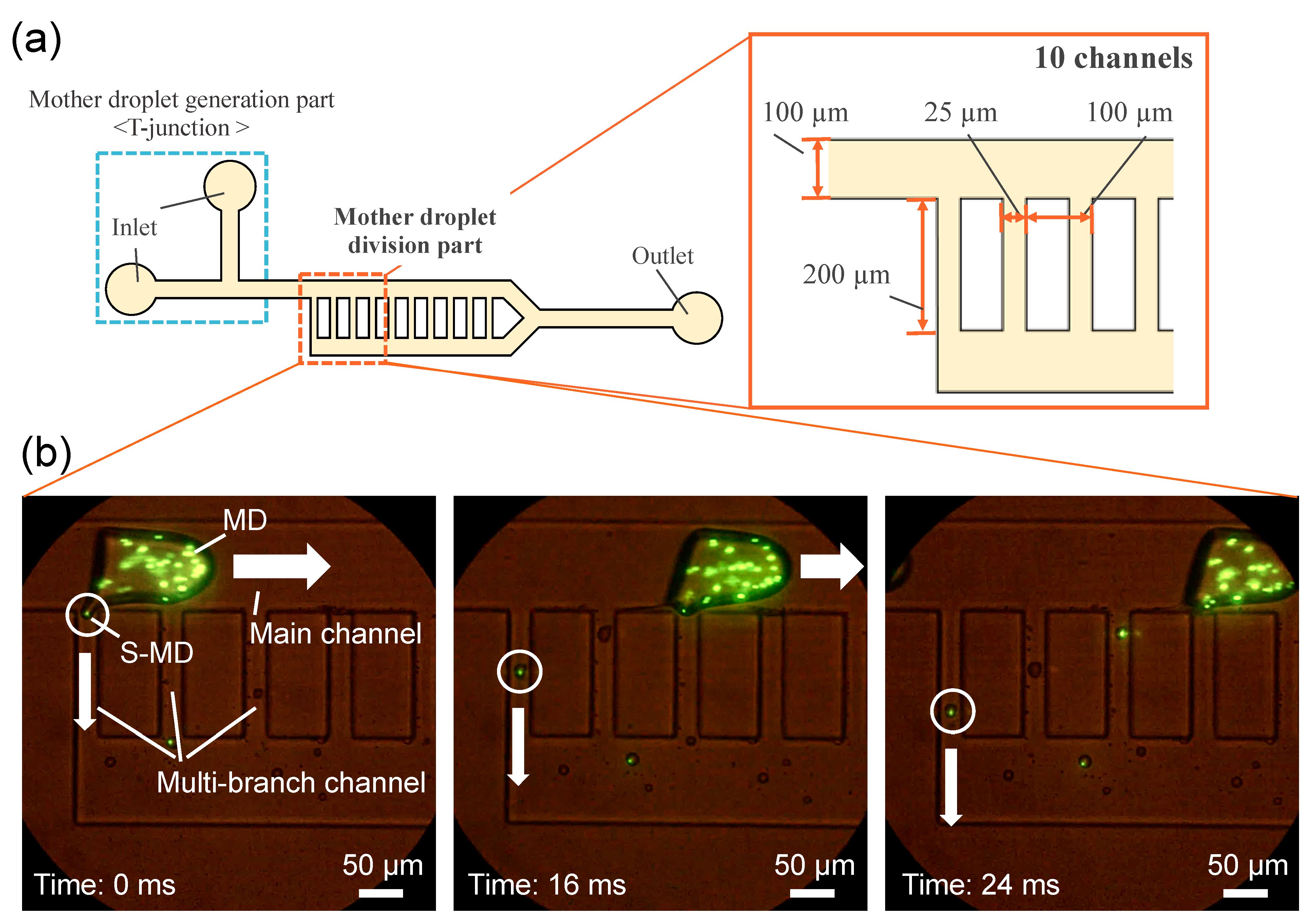
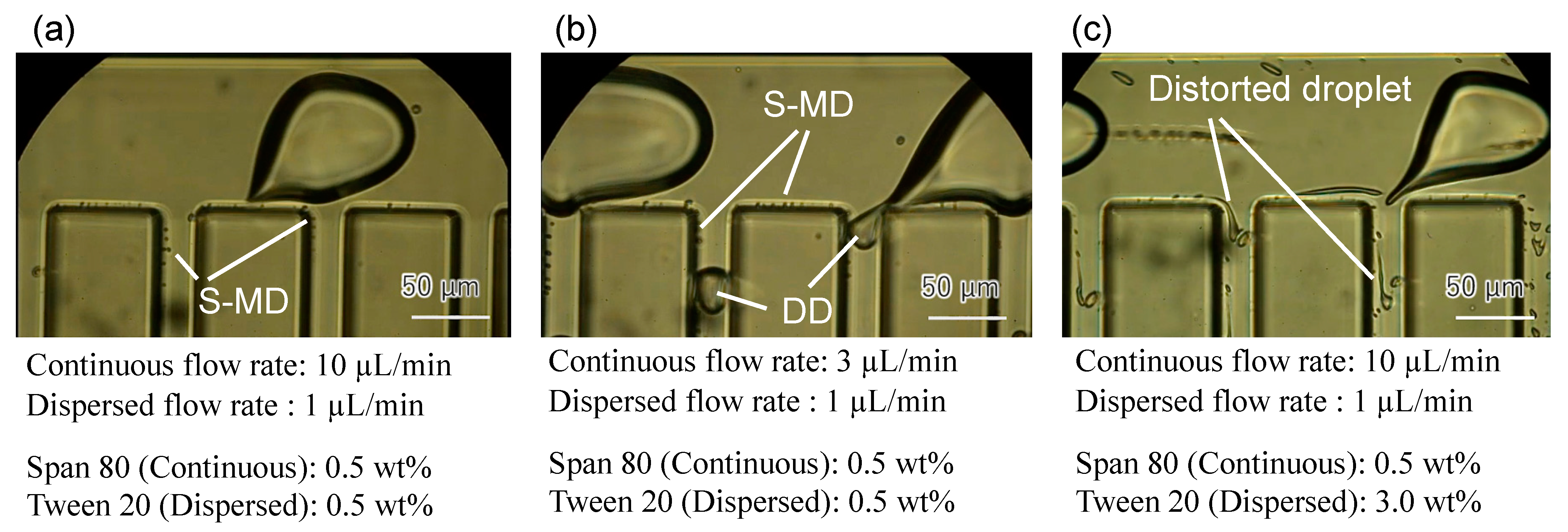
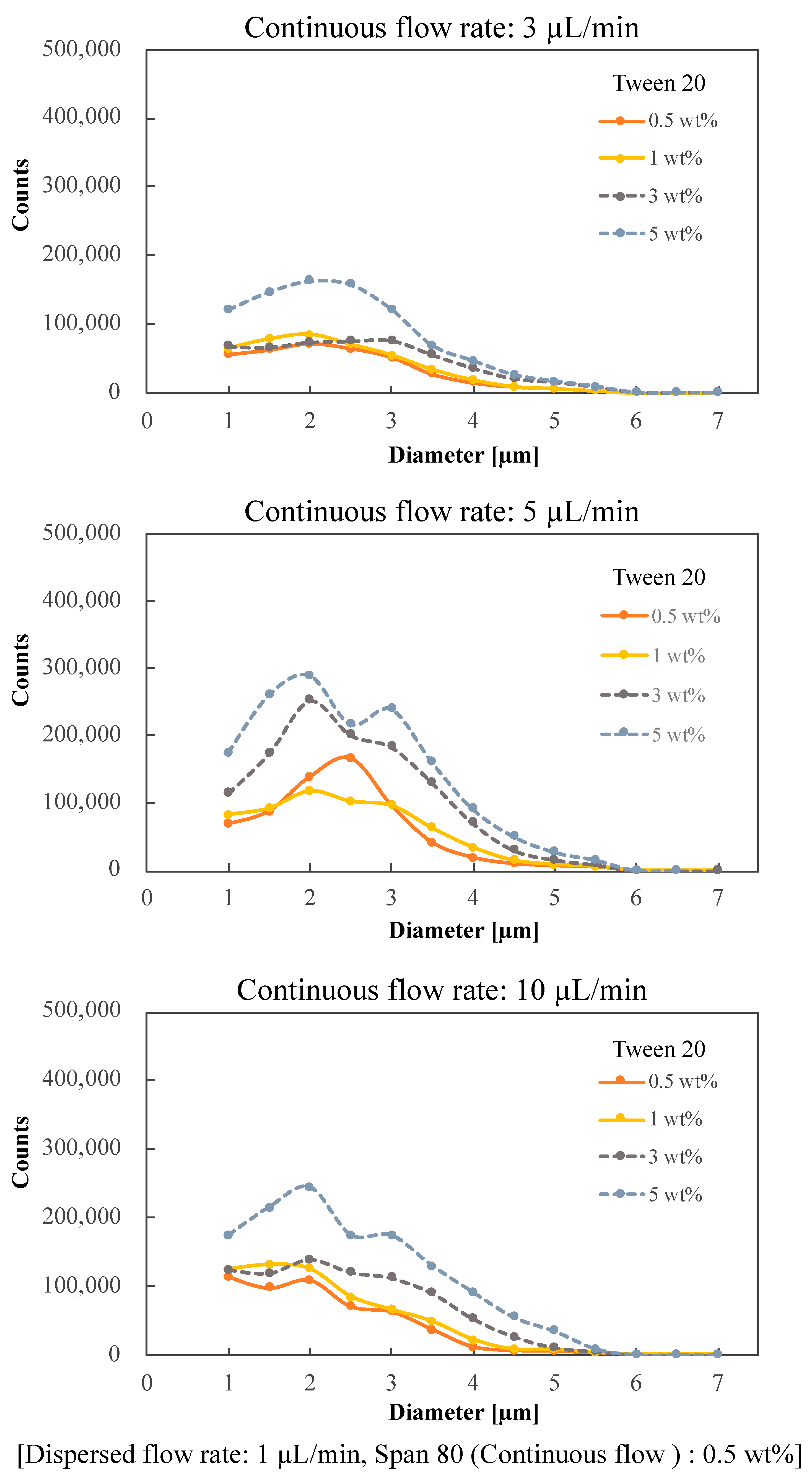
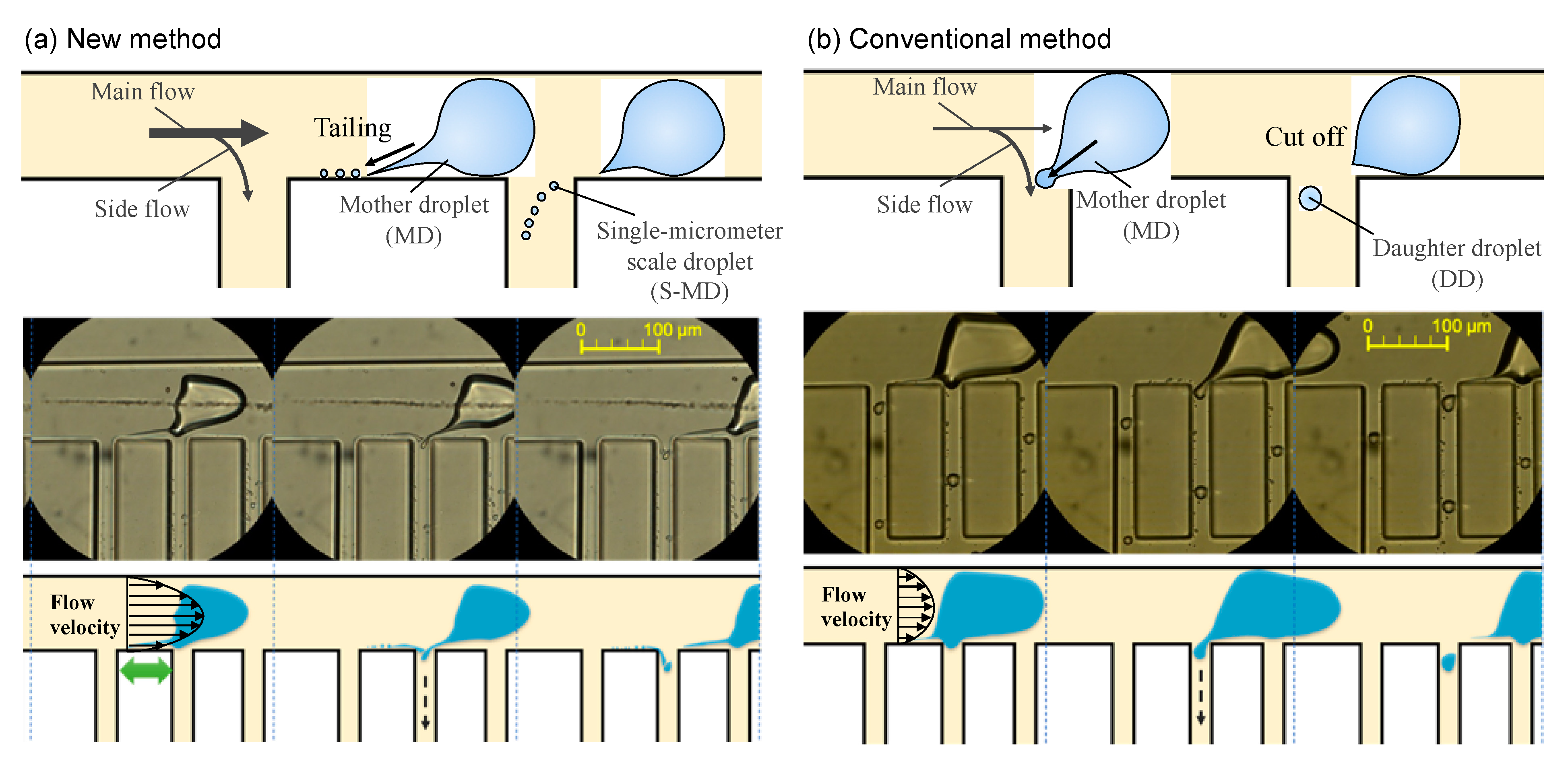
2. Results and Discussion
Device Design and Fabrication
3. Materials and Methods
Experimental Setup and Materials
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Zeng, Y.; Novak, R.; Shuga, J.; Smith, M.T.; Mathies, R.A. High-performance single cell genetic analysis using microfluidic emulsion generator arrays. Anal. Chem. 2010, 82, 3183–3190. [Google Scholar] [CrossRef] [PubMed]
- Eastburn, D.J.; Sciambi, A.; Abate, A.R. Ultrahigh-throughput mammalian single-cell reverse-transcriptase polymerase chain reaction in microfluidic drops. Anal. Chem. 2013, 85, 8016–8021. [Google Scholar] [CrossRef]
- Kinry, T.; Golberg, A.; Yarmush, M. Live single cell functional phenotyping in droplet nano-liter reactors. Sci. Rep. 2013, 3, 3179. [Google Scholar] [CrossRef]
- Li, L.; Wang, Q.; Feng, J.; Tong, L.; Tang, B. Highly sensitive and homogeneous detection of membrane protein on a single living cell by aptamer and micking enzyme assisted signal amplification based on microfluidic droplets. Anal. Chem. 2014, 86, 5101–5107. [Google Scholar] [CrossRef]
- Banerjee, S.; Gnanamani, E.; Yan, X.; Zare, R.N. Can all bulk-phase reactions be accelerated in microdroplets? Analyst 2017, 142, 1399–1402. [Google Scholar] [CrossRef] [PubMed]
- Rondelez, Y.; Tresset, G.; Tabata, K.V.; Arata, H.; Fujita, H.; Takeuchi, S.; Noji, H. Microfabricated arrays of femtoliter chambers allow single molecule enzymology. Nat. Biotech. 2005, 3, 361–365. [Google Scholar] [CrossRef]
- Rondelez, Y.; Tresset, G.; Nakashima, T.; Kato-Yamada, Y.; Fujita, H.; Takeuchi, S.; Noji, H. Highly coupled ATP synthesis by F1-ATPase single molecules. Nature 2005, 433, 773–777. [Google Scholar] [CrossRef]
- Tanaka, D.; Sawai, S.; Hattori, S.; Nozaki, Y.; Yoon, D.H.; Fujita, H.; Sekiguchi, T.; Akitsu, T.; Shoji, S. Microdroplet synthesis of azo compounds with simple microfluidics-based pH control. RSC Adv. 2020, 10, 38900–38905. [Google Scholar] [CrossRef]
- Hattori, S.; Tang, C.; Tanaka, D.; Yoon, D.H.; Nozaki, Y.; Fujita, H.; Akitsu, T.; Sekiguchi, T.; Shoji, S. Development of microdroplet generation method for organic solvents used in chemical synthesis. Molecules 2020, 25, 5360. [Google Scholar] [CrossRef] [PubMed]
- Garstecki, P.; Fuerstman, M.J.; Stone, H.A.; Whitesides, G.M. Formation of droplets and bubbles in a microfluidic T-junction—scaling and mechanism of break-up. Lab Chip 2006, 6, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Baroud, C.N.; Gallaire, F.; Dangla, R. Dynamics of microfluidic droplets. Lab Chip 2010, 10, 2032–2045. [Google Scholar] [CrossRef]
- Nisisako, T.; Torii, T.; Higuchi, T. Droplet formation in a microchannel network. Lab Chip 2002, 2, 24–26. [Google Scholar] [CrossRef] [PubMed]
- Anna, S.L.; Bontoux, N.; Stone, H.A. Formation of dispersions using “flow focusing” in microchannels. Appl. Phys. Lett. 2003, 82, 364–366. [Google Scholar] [CrossRef]
- Christopher, G.F.; Anna, S.L. Microfluidic methods for generating continuous droplet streams. J. Phys. D Appl. Phys. 2007, 40, 319–336. [Google Scholar] [CrossRef]
- Nooranidoost, M.; Izbassarov, D.; Muradoglu, M. Droplet formation in a flow focusing configuration: Effects of viscoelasticity. Phys. Fluids 2016, 28, 123102. [Google Scholar] [CrossRef]
- Loo, S.V.; Stoukatch, S.; Kraft, M.; Gilet, T. Droplet formation by squeezing in a microfluidic cross-junction. Microfluid. Nanofluid. 2016, 20, 146. [Google Scholar]
- Zhu, P.; Kong, T.; Lei, L.; Tian, X.; Kang, Z.; Wang, L. Droplet breakup in expansion-contraction microchannels. Sci. Rep. 2016, 6, 21527. [Google Scholar] [CrossRef]
- Taylor, G.I. The formation of emulsions in definable fields of flow. Proc. R. Soc. Lond. A 1934, 146, 501–523. [Google Scholar]
- Martz, T.D.; Bardin, D.; Sheeran, P.S.; Lee, A.P.; Dayton, P.A. Microfluidic generation of acoustically active nanodroplets. Small 2012, 8, 1876–1879. [Google Scholar] [CrossRef] [PubMed]
- Jeong, W.C.; Lim, J.M.; Choi, J.H.; Kim, J.H.; Lee, Y.J.; Kim, S.H.; Lee, G.; Kim, J.D.; Yi, G.R.; Yang, S.M. Controlled generation of submicron emulsion droplets via highly stable. Lab Chip 2012, 12, 1446–1453. [Google Scholar] [CrossRef]
- Xu, X.; Song, R.; He, M.; Peng, C.; Yu, M.; Hou, Y.; Oiu, H.; Zou, R.; Yao, S. Microfluidic production of nanoscale perfluorocarbon droplets as liquid contrast agents for ultrasound imaging. Lab Chip 2017, 17, 3504–3513. [Google Scholar] [CrossRef] [PubMed]
- Moyle, T.M.; Walker, L.M.; Anna, S.L. Controlling thread formation during tipstreaming through an active feedback control loop. Lab Chip 2013, 13, 4534–4541. [Google Scholar] [CrossRef]
- Steinhaus, B.; Shena, A.Q.; Sureshkumar, R. Dynamics of viscoelastic fluid filaments in microfluidic devices. Phys. Fluids 2007, 19, 073103. [Google Scholar] [CrossRef]
- Mulligan, M.K.; Rothstein, J.P. The effect of confinement-induced shear on drop deformation and breakup in microfluidic extensional flows. Phys. Fluids 2011, 23, 022004. [Google Scholar] [CrossRef]
- Wu, Y.; Fu, T.; Zhu, C.; Wang, X.; Ma, Y.; Li, H.Z. Shear-induced tail breakup of droplets (bubbles) flowing in a straight microfluidic channel. Chem. Eng. Sci. 2015, 135, 61–66. [Google Scholar] [CrossRef]
- De Brujin, R.A. Tipstreaming of drops in simple shear flows. Chem. Eng. Sci. 1993, 48, 277–284. [Google Scholar] [CrossRef]
- Anna, S.L.; Mayer, H.C. Microscale tipstreaming in a microfluidic flow focusing device. Phys. Fluids 2006, 18, 121512. [Google Scholar] [CrossRef]
- Moritani, T.; Yamada, M.; Seki, M. Generation of uniform-size droplets by multistep hydrodynamic droplet division in microfluidic circuits. Microfluid. Nanofluid. 2011, 11, 601–610. [Google Scholar] [CrossRef]
- Daunay, B.; Lambert, P.; Jalabert, L.; Kumemura, M.; Renaudot, R.; Agache, V.; Fujita, H. Effect of substrate wettability in liquid dielectrophoresis (LDEP) based droplet generation: Theoretical analysis and experimental confirmation. Lab Chip 2012, 12, 361. [Google Scholar] [CrossRef]
- Shabahang, S.; Kaufman, J.J.; Deng, D.S.; Abouraddy, A.F. Observation of the Plateau-Rayleigh capillary instability in multi-material optical fibers. Appl. Phys. Lett. 2011, 99, 161909. [Google Scholar] [CrossRef]
- Tang, Y.; Yue, M.; Fang, X.; Feng, X. Evolution of surface droplets and flow patterns on C/SiC during thermal T ablation. J. Eur. Ceram. Soc. 2019, 39, 3566–3574. [Google Scholar] [CrossRef]
| Surfactant Concentration (wt%) | Flow Rate (µL/min) | |||||
|---|---|---|---|---|---|---|
| Tween 20 | 0.5 | 1.0 | 3.0 | 5.0 | 0.5 | Dispersed: 1 |
| Span 80 | 0.5 | 0.5 | 0.5 | 0.5 | 3.0 | Continuous: 3 |
| Tween 20 | 0.5 | 1.0 | 3.0 | 5.0 | 0.5 | Dispersed: 1 |
| Span 80 | 0.5 | 0.5 | 0.5 | 0.5 | 3.0 | Continuous: 5 |
| Tween 20 | 0.5 | 1.0 | 3.0 | 5.0 | 0.5 | Dispersed: 1 |
| Span 80 | 0.5 | 0.5 | 0.5 | 0.5 | 3.0 | Continuous: 10 |
| Surfactant Concentration (wt%) | ||||
|---|---|---|---|---|
| 0.5 | 1 | 3 | 5 | |
| Water + Tween 20 (mPa·s) | 0.8788 | 0.8788 | 1.014 | 1.128 |
| Oil + Span 80 (mPa·s) | 24.51 | 24.69 | 25.31 | 26.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanaka, D.; Kajiya, S.; Shijo, S.; Yoon, D.H.; Furuya, M.; Nozaki, Y.; Fujita, H.; Sekiguchi, T.; Shoji, S. Efficient Generation of Microdroplets Using Tail Breakup Induced with Multi-Branch Channels. Molecules 2021, 26, 3707. https://doi.org/10.3390/molecules26123707
Tanaka D, Kajiya S, Shijo S, Yoon DH, Furuya M, Nozaki Y, Fujita H, Sekiguchi T, Shoji S. Efficient Generation of Microdroplets Using Tail Breakup Induced with Multi-Branch Channels. Molecules. 2021; 26(12):3707. https://doi.org/10.3390/molecules26123707
Chicago/Turabian StyleTanaka, Daiki, Satsuki Kajiya, Seito Shijo, Dong Hyun Yoon, Masahiro Furuya, Yoshito Nozaki, Hiroyuki Fujita, Tetsushi Sekiguchi, and Shuichi Shoji. 2021. "Efficient Generation of Microdroplets Using Tail Breakup Induced with Multi-Branch Channels" Molecules 26, no. 12: 3707. https://doi.org/10.3390/molecules26123707
APA StyleTanaka, D., Kajiya, S., Shijo, S., Yoon, D. H., Furuya, M., Nozaki, Y., Fujita, H., Sekiguchi, T., & Shoji, S. (2021). Efficient Generation of Microdroplets Using Tail Breakup Induced with Multi-Branch Channels. Molecules, 26(12), 3707. https://doi.org/10.3390/molecules26123707







