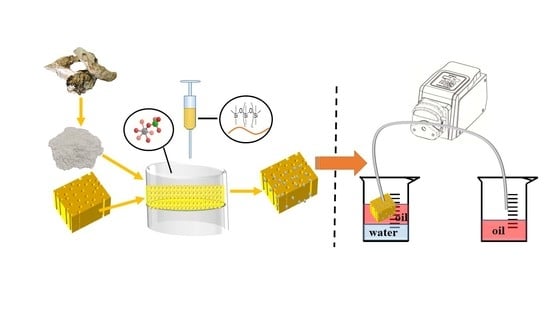
Application of Nano-Hydroxyapatite Derived from Oyster Shell in Fabricating Superhydrophobic Sponge for Efficient Oil/Water Separation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of HAp Coated Polyurethane Sponge (HAp/PU Sponge)
2.3. Physiochemical Characterization
2.4. Adsorption Performance Test and Stability Investigation
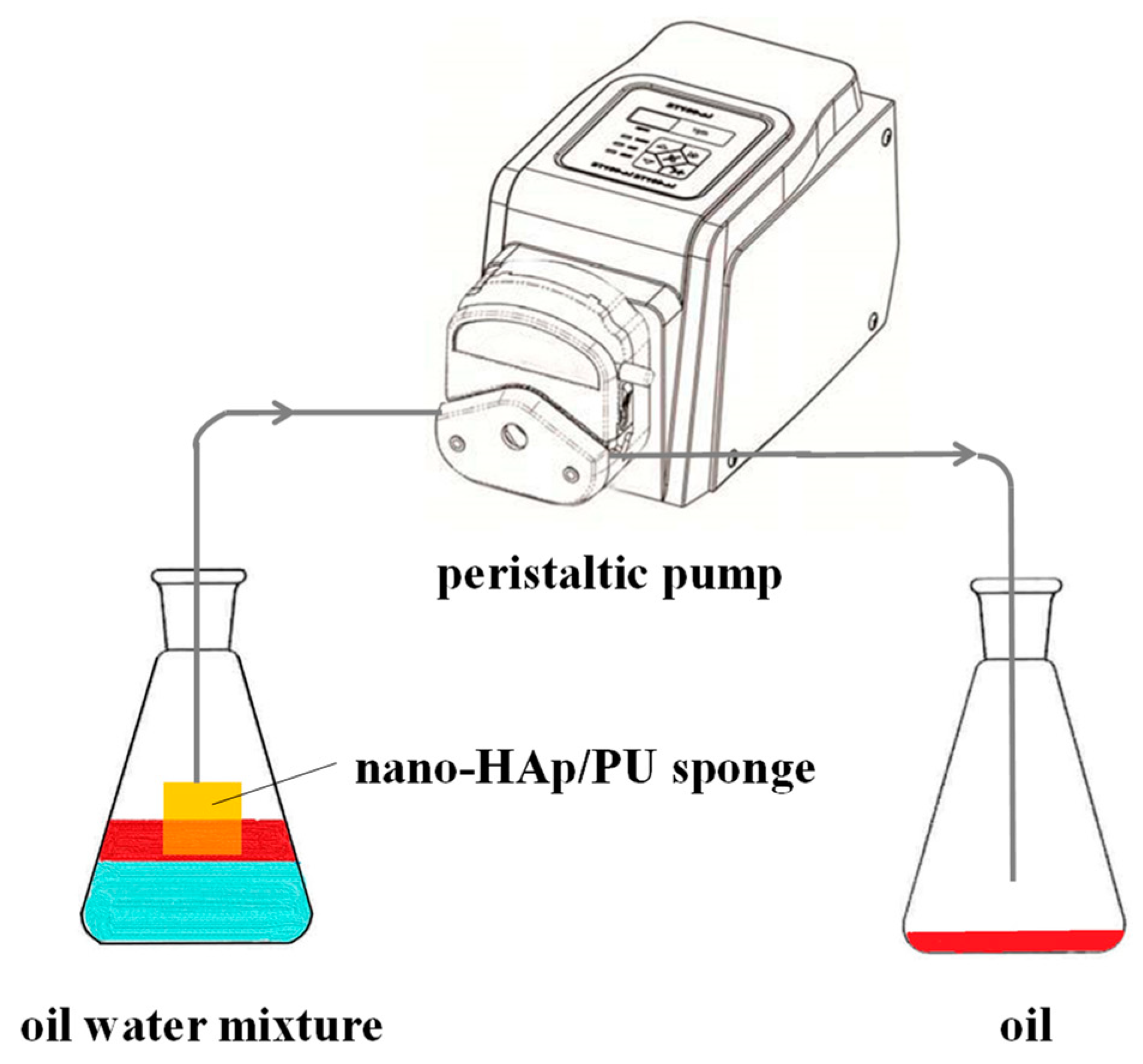
2.5. Oil−Water Separation
3. Results and Discussion
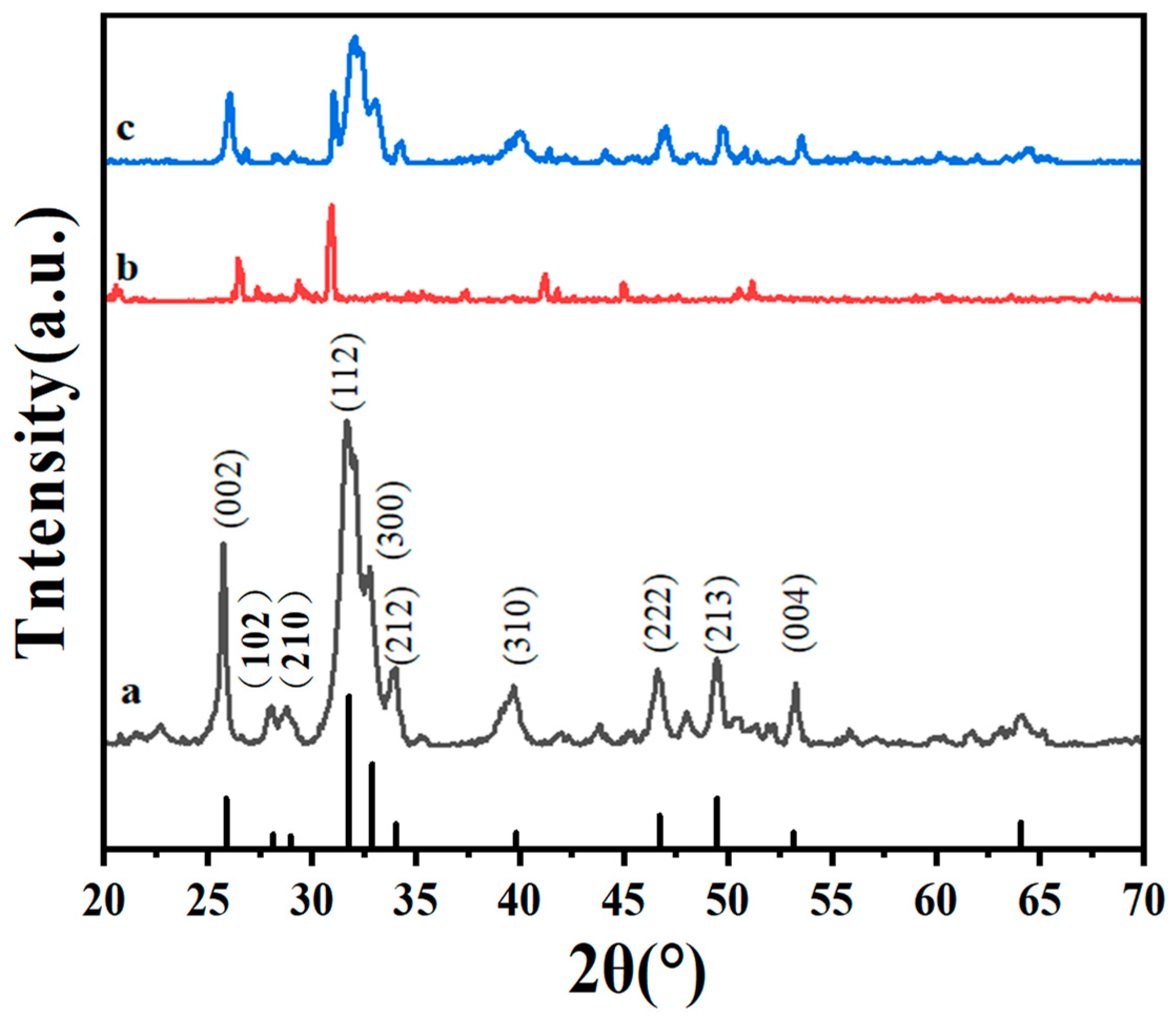
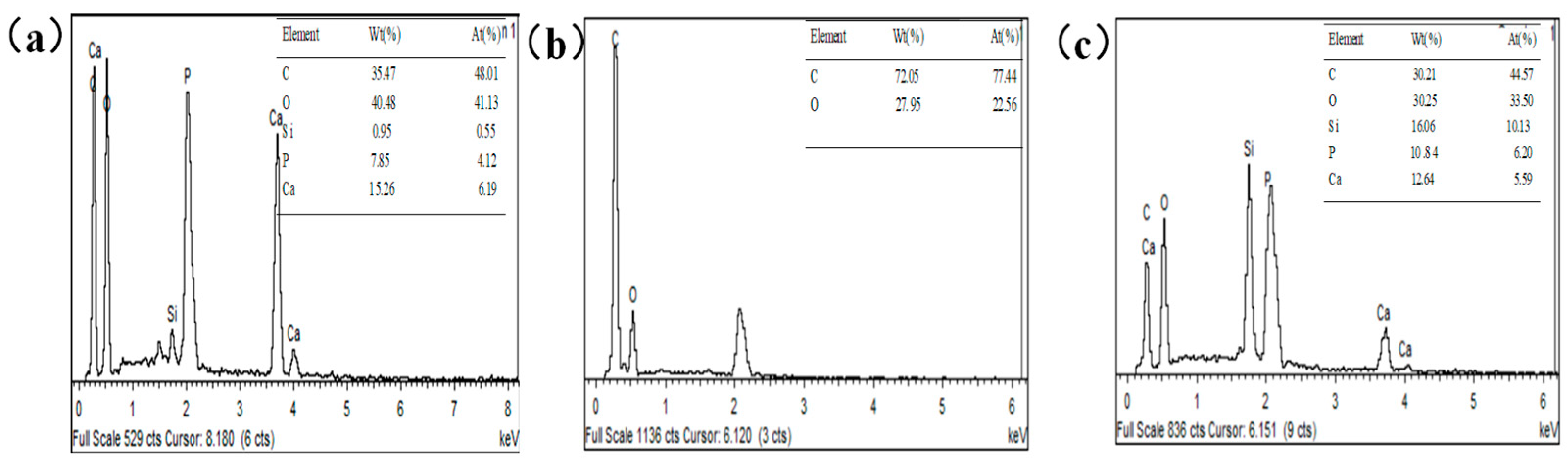
3.1. Chemical Composition Analysis
3.2. Surface Morphology Analysis
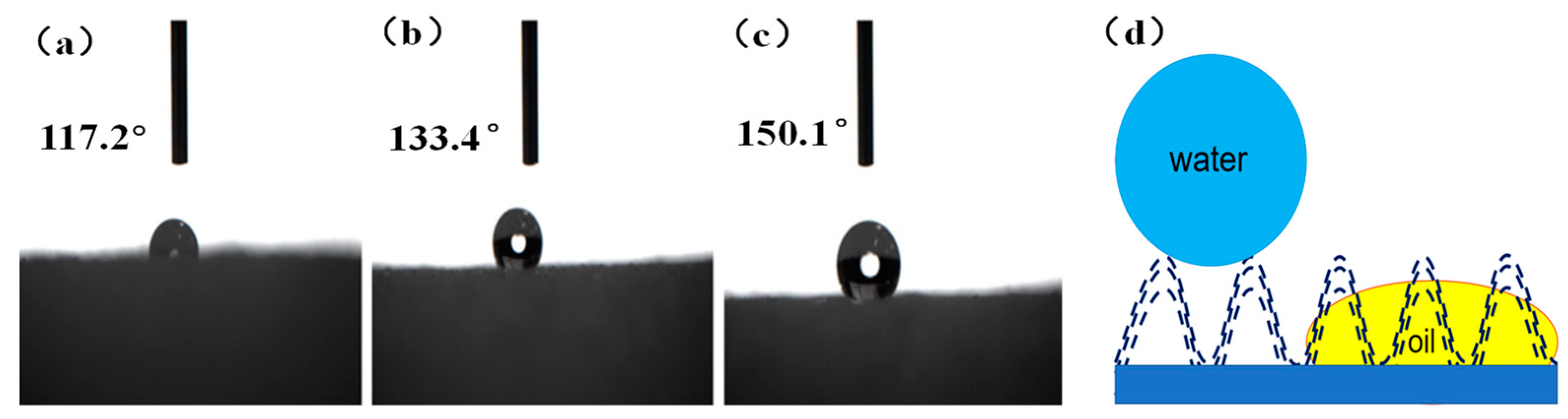
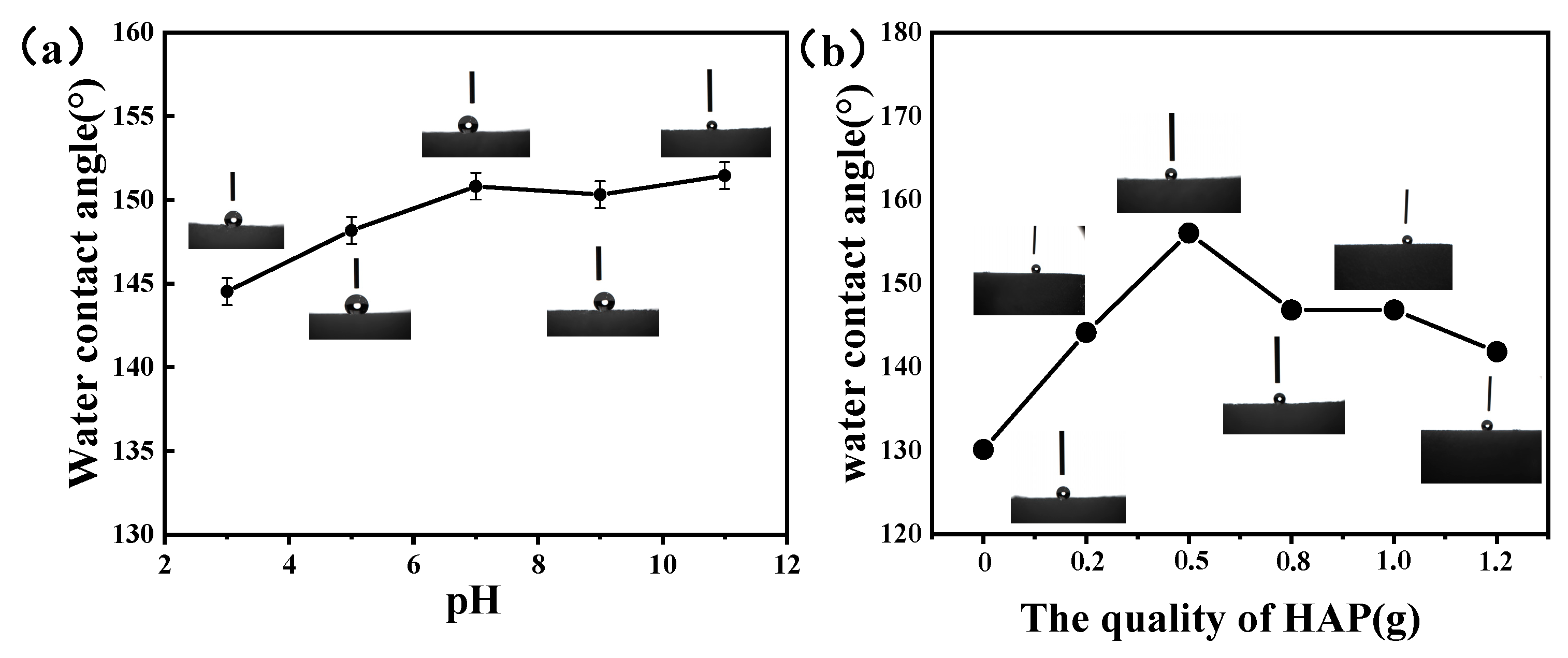
3.3. Wetting Performance of the Superhydrophobic Sponge
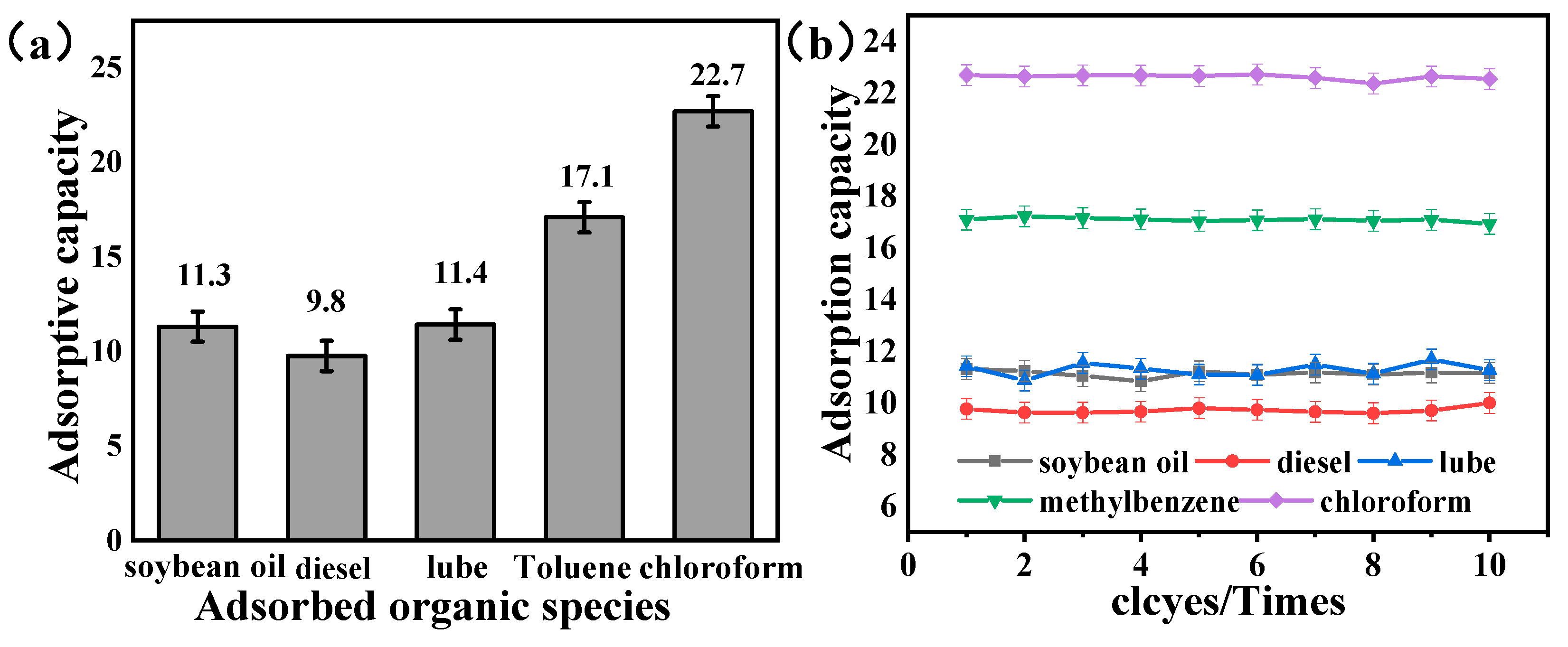
3.4. Performance of Evaluation of Nano-HAp/PU Sponge as Oil Absorbent
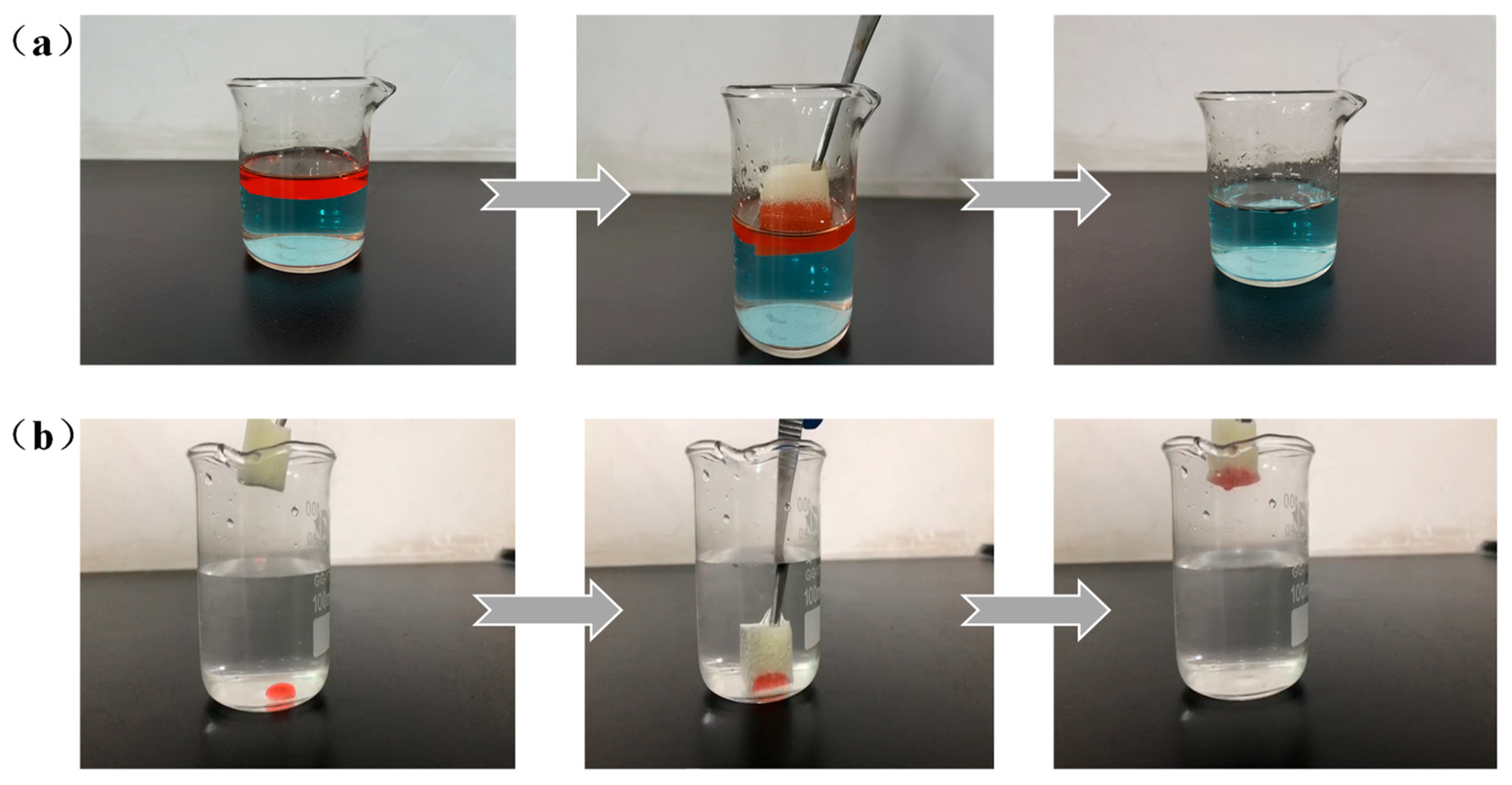
3.5. In Situ Separation of Oil/Water Mixtures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Lye, C.M. Impact of oestrogenic substances from oil production at sea. Toxicol. Lett. 2000, 112–113, 265–272. [Google Scholar] [CrossRef]
- Beyer, J.; Sundt, R.C.; Sanni, S.; Sydnes, M.O.; Jonsson, G. Alkylphenol Metabolites in Fish Bile As Biomarkers of Exposure to Offshore Oil Industry Produced Water in Feral Fish. J. Toxicol. Environ. Health Part A 2011, 74, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Obida, C.B.; Blackburn, G.A.; Whyatt, J.D.; Semple, K.T. Quantifying the exposure of humans and the environment to oil pollution in the Niger Delta using advanced geostatistical techniques. Environ. Int. 2018, 111, 32–42. [Google Scholar] [CrossRef]
- Anifowose, B.A.; Odubela, M.T. Oil facility operations: A multivariate analyses of water pollution parameters. J. Clean. Prod. 2018, 187, 180–189. [Google Scholar] [CrossRef]
- China, Discharge Standards for Petroleum Refining Industry; China Environmental Science Press: Beijing, China, 2015.
- Pintor, A.M.A.; Botelho, C.M.S.; Boaventura, R.A.R. Oil and grease removal from wastewaters: Sorption treatment as an alternative to state-of-the-art technologies. A critical review. Chem. Eng. J. 2016, 297, 229–255. [Google Scholar] [CrossRef]
- Santos, A.S.; Oliveira, L.F.S.; Marques, A.M.; Silva, D.C.; Mansur, C.R. Evaluation of the efficiency of polyethylenimine as flocculants in the removal of oil present in produced water. Colloids Surf. A Physicochem. Eng. Asp. 2018, 558, 200–210. [Google Scholar] [CrossRef]
- Gobbi, I.L.; Nascimento, E.P.; Muniz, S.M.S.; Rocha, P.S.S. Porto, Electrocoagulation with polarity switch for fast oil re-moval from oil in water emulsions. J. Environ. Manag. 2018, 213, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Regar, R.; Manickam, N. Improved polycyclic aromatic hydrocarbon degradation in a crude oil by individual and a consortium of bacteria. Bioresour. Technol. 2018, 254, 174–179. [Google Scholar] [CrossRef]
- Annunciado, T.; Sydenstricker, T.; Amico, S. Experimental investigation of various vegetable fibers as sorbent materials for oil spills. Mar. Pollut. Bull. 2005, 50, 1340–1346. [Google Scholar] [CrossRef]
- Radetić, M.M.; Jovančić, P.M.; Petrović, Z.L.; Thomas, H.F. Recycled wool-based nonwoven material as an oil sorbent. Environ. Sci. Technol. 2003, 37, 1008–1012. [Google Scholar] [CrossRef] [PubMed]
- Mota, M.F.; Silva, J.A.; Queiroz, M.B.; Laborde, H.M.; Rodrigues, M.G.F. ORGANOPHILIC CLAY FOR OIL/WATER SEPARATION PROCESS BY FINITE BATH TESTS. Braz. J. Pet. Gas 2011, 5, 97–107. [Google Scholar] [CrossRef]
- Wang, G.; Sun, Q.; Zhang, Y.; Fan, J.; Ma, L. Sorption and regeneration of magnetic exfoliated graphite as a new sorbent for oil pollution. Desalination 2010, 263, 183–188. [Google Scholar] [CrossRef]
- Kugler, S.; Spychaj, T.; Wilpiszewska, K. Starch-graft copolymers of N-vinylformamide and acrylamide modified with montmorillonite manufactured by reactive extrusion. J. Appl. Polym. Sci. 2013, 127, 2847–2854. [Google Scholar] [CrossRef]
- Chhajed, M.; Yadav, C.; Agrawal, A.K.; Maji, P.K. Esterified superhydrophobic nanofibrillated cellulose based aerogel for oil spill treatment. Carbohydr. Polym. 2019, 226, 115286. [Google Scholar] [CrossRef]
- Sabir, S. Approach of Cost-Effective Adsorbents for Oil Removal from Oily Water. Crit. Rev. Environ. Sci. Technol. 2015, 45, 1916–1945. [Google Scholar] [CrossRef]
- Cheng, Y.; Lu, S.; Xu, W.; Wen, H.; Wang, J. Fabrication of superhydrophobic Au–Zn alloy surface on a zinc substrate for roll-down, self-cleaning and anti-corrosion properties. J. Mater. Chem. A 2015, 3, 16774–16784. [Google Scholar] [CrossRef]
- Gao, D.; Jia, M. Hierarchical ZnO particles grafting by fluorocarbon polymer derivative: Preparation and superhydrophobic behavior. Appl. Surf. Sci. 2015, 343, 172–180. [Google Scholar] [CrossRef]
- Zieba, J. Modeling of textile magnetic core. Smartex Res. J. 2012, 1, 102–110. [Google Scholar]
- Peng, M.; Zhu, Y.; Li, H.; He, K.; Zeng, G.; Chen, A.; Huang, Z.; Huang, T.; Yuan, L.; Chen, G. Synthesis and application of modified commercial sponges for oil-water separation. Chem. Eng. J. 2019, 373, 213–226. [Google Scholar] [CrossRef]
- Zhu, Q.; Chu, Y.; Wang, Z.; Chen, N.; Lin, L.; Liu, F.; Pan, Q. Robust superhydrophobic polyurethane sponge as a highly reusable oil-absorption material. J. Mater. Chem. A 2013, 1, 5386–5393. [Google Scholar] [CrossRef]
- Chen, J.A.; Weibel, S.V. Garimella, Continuous Oil–Water Separation Using Polydimethylsiloxane-Functionalized Mela-mine Sponge. Ind. Eng. Chem. Res. 2016, 55, 3596–3602. [Google Scholar] [CrossRef]
- Peng, H.; Wang, H.; Wu, J.; Meng, G.; Wang, Y.; Shi, Y.; Liu, Z.; Guo, X. Preparation of Superhydrophobic Magnetic Cellulose Sponge for Removing Oil from Water. Ind. Eng. Chem. Res. 2016, 55, 832–838. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Z.; Xu, X.; Zhu, X.; Men, X.; Zhou, X. Superhydrophilic–superoleophobic coatings. J. Mater. Chem. A 2012, 22, 2834–2837. [Google Scholar] [CrossRef]
- Gao, X.; Guo, Z. Biomimetic superhydrophobic surfaces with transition metals and their oxides: A review. J. Bionic Eng. 2017, 14, 401–439. [Google Scholar] [CrossRef]
- Yang, Y.; Yao, Q.; Pu, X.; Hou, Z.; Zhang, Q. Biphasic calcium phosphate macroporous scaffolds derived from oyster shells for bone tissue engineering. Chem. Eng. J. 2011, 173, 837–845. [Google Scholar] [CrossRef]
- Vecchio, K.S.; Zhang, X.; Massie, J.B.; Wang, M.; Kim, C.W. Conversion of bulk seashells to biocompatible hydroxyapatite for bone implants. Acta Biomater. 2007, 3, 910–918. [Google Scholar] [CrossRef] [PubMed]
- ROY, D.M.; Linnehan, S.K. Hydroxyapatite formed from coral skeletal carbonate by hydrothermal exchange. Nature 1974, 246, 220–222. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.D.; Ahn, J.W.; Nam, G. Environmental benign synthesis, characterization and mechanism studies of green calcium hydroxide nano-plates derived from waste oyster shells. J. Environ. Manag. 2018, 223, 947–951. [Google Scholar] [CrossRef]
- Hao, W.; Xu, J.; Li, R.; Zhao, X.; Qiu, L.; Yang, W. Developing superhydrophobic rock wool for high-viscosity oil/water separa-tion. Chem. Eng. J. 2019, 368, 837–846. [Google Scholar] [CrossRef]
- Alinda Shaly, A.; Hannah Priya, G.; Mary Linet, J. An outlook on the mechanical attributes and load curve analysis of hydro-thermally acquired hydroxyapatite bioceramic nanoparticles. Phys. B Condens. Matter 2020, 590, 412223. [Google Scholar] [CrossRef]
- Guan, L.-Z.; Gao, J.-F.; Pei, Y.-B.; Zhao, L.; Gong, L.-X.; Wan, Y.-J.; Zhou, H.; Zheng, N.; Du, X.-S.; Wu, L.-B.; et al. Silane bonded graphene aerogels with tunable functionality and reversible compressibility. Carbon 2016, 107, 573–582. [Google Scholar] [CrossRef]
- Chen, C.; Zhu, X.; Chen, B. Durable Superhydrophobic/Superoleophilic Graphene-Based Foam for High-Efficiency Oil Spill Cleanups and Recovery. Environ. Sci. Technol. 2019, 53, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Yao, X.; Jiang, L. Recent developments in bio-inspired special wettability. Chem. Soc. Rev. 2010, 39, 3240–3255. [Google Scholar] [CrossRef] [PubMed]
- Rameshbabu, N.; Rao, K.P.; Kumar, T.S.S. Acclerated microwave processing of nanocrystalline hydroxyapatite. J. Mater. Sci. 2005, 40, 6319–6323. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Chen, S.-H.; Yang-Zhou, C.-H.; Zhang, Q.-G.; Michael, R.N. Application of Nano-Hydroxyapatite Derived from Oyster Shell in Fabricating Superhydrophobic Sponge for Efficient Oil/Water Separation. Molecules 2021, 26, 3703. https://doi.org/10.3390/molecules26123703
Liu C, Chen S-H, Yang-Zhou C-H, Zhang Q-G, Michael RN. Application of Nano-Hydroxyapatite Derived from Oyster Shell in Fabricating Superhydrophobic Sponge for Efficient Oil/Water Separation. Molecules. 2021; 26(12):3703. https://doi.org/10.3390/molecules26123703
Chicago/Turabian StyleLiu, Chao, Su-Hua Chen, Chi-Hao Yang-Zhou, Qiu-Gen Zhang, and Ruby N. Michael. 2021. "Application of Nano-Hydroxyapatite Derived from Oyster Shell in Fabricating Superhydrophobic Sponge for Efficient Oil/Water Separation" Molecules 26, no. 12: 3703. https://doi.org/10.3390/molecules26123703
APA StyleLiu, C., Chen, S.-H., Yang-Zhou, C.-H., Zhang, Q.-G., & Michael, R. N. (2021). Application of Nano-Hydroxyapatite Derived from Oyster Shell in Fabricating Superhydrophobic Sponge for Efficient Oil/Water Separation. Molecules, 26(12), 3703. https://doi.org/10.3390/molecules26123703