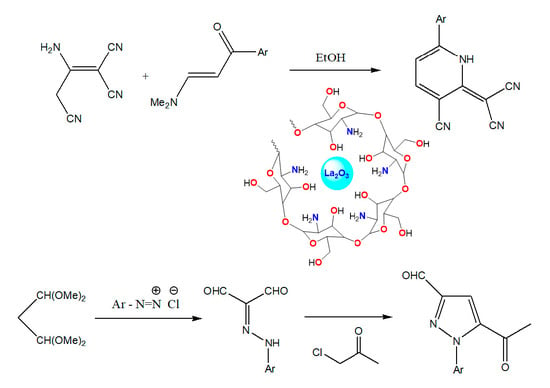
Synthesis of Chitosan-La2O3 Nanocomposite and Its Utility as a Powerful Catalyst in the Synthesis of Pyridines and Pyrazoles
Abstract
1. Introduction
2. Results and Discussion
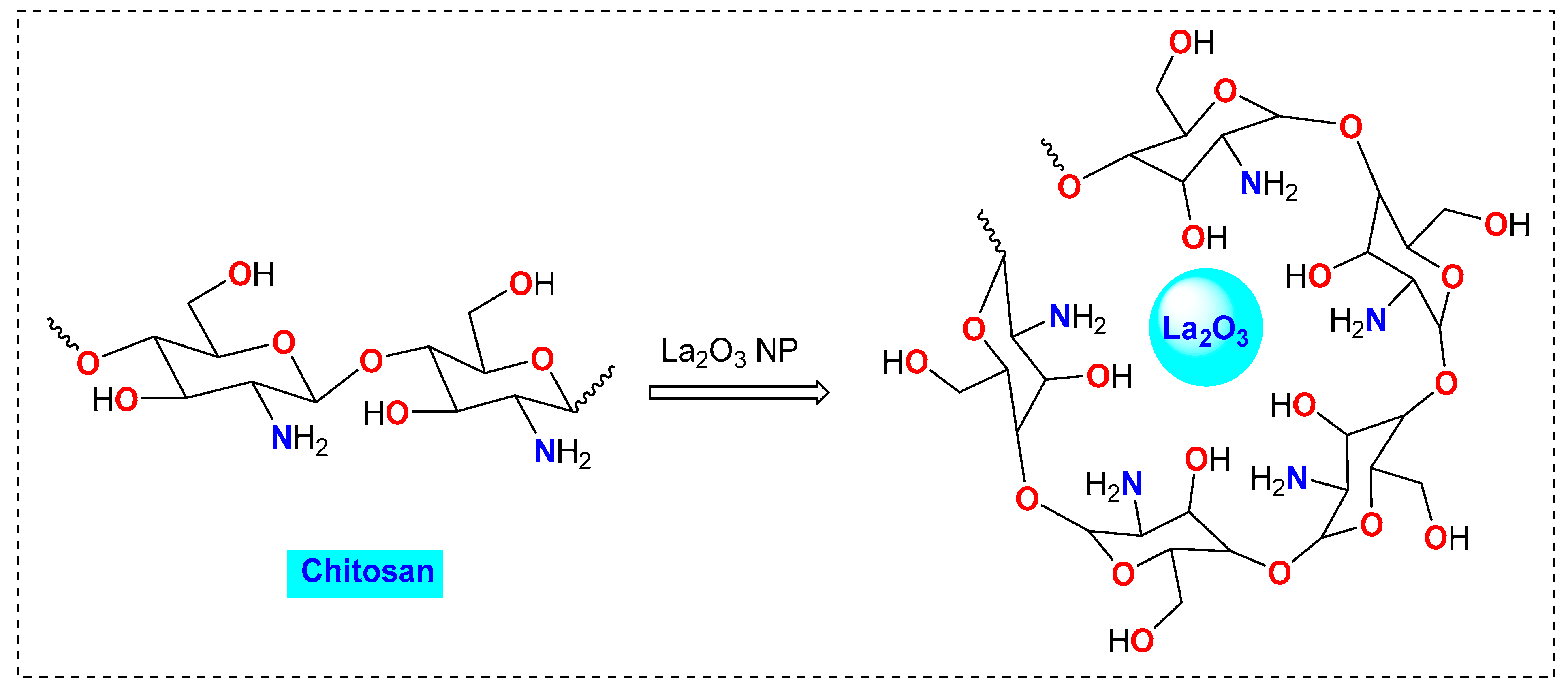
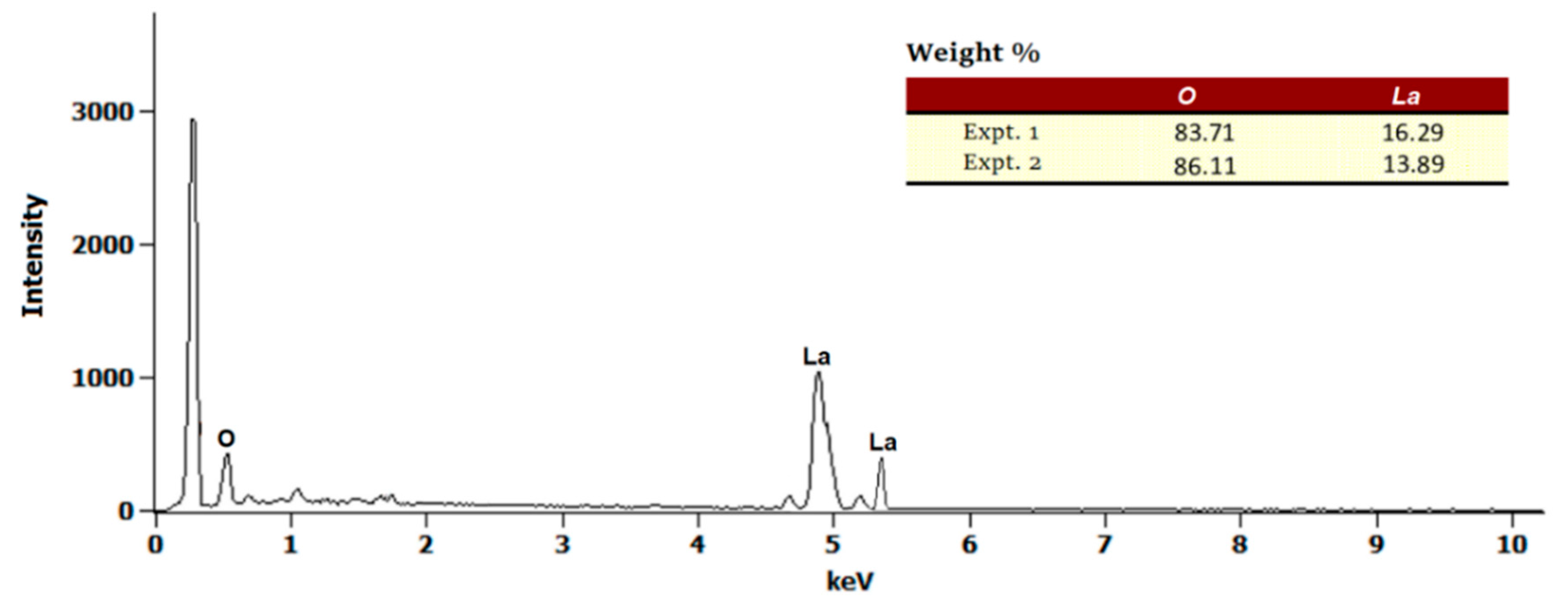
2.1. Preparation and Characterization of CS/La2O3 Nanocomposite Film
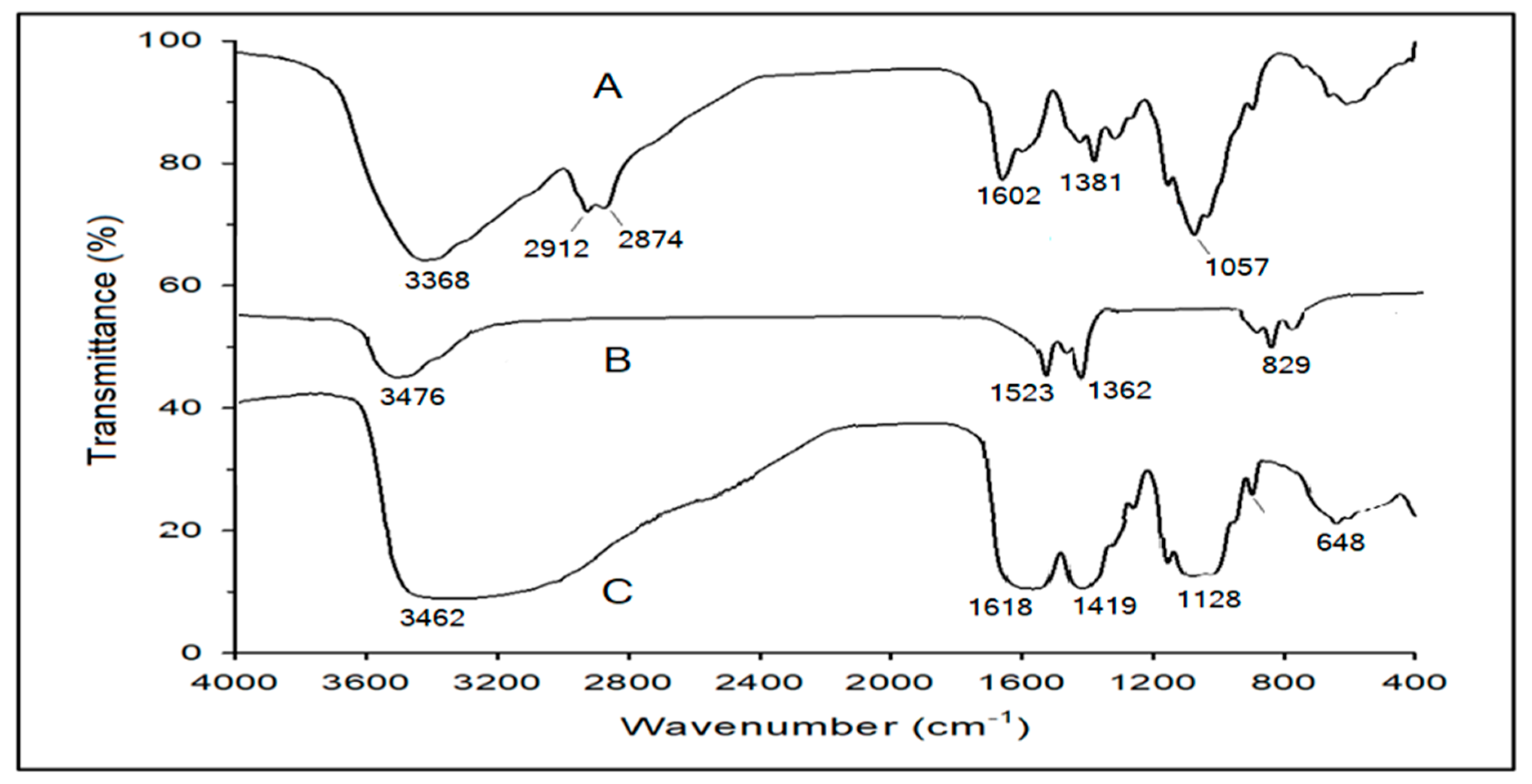
2.1.1. FTIR Characterization
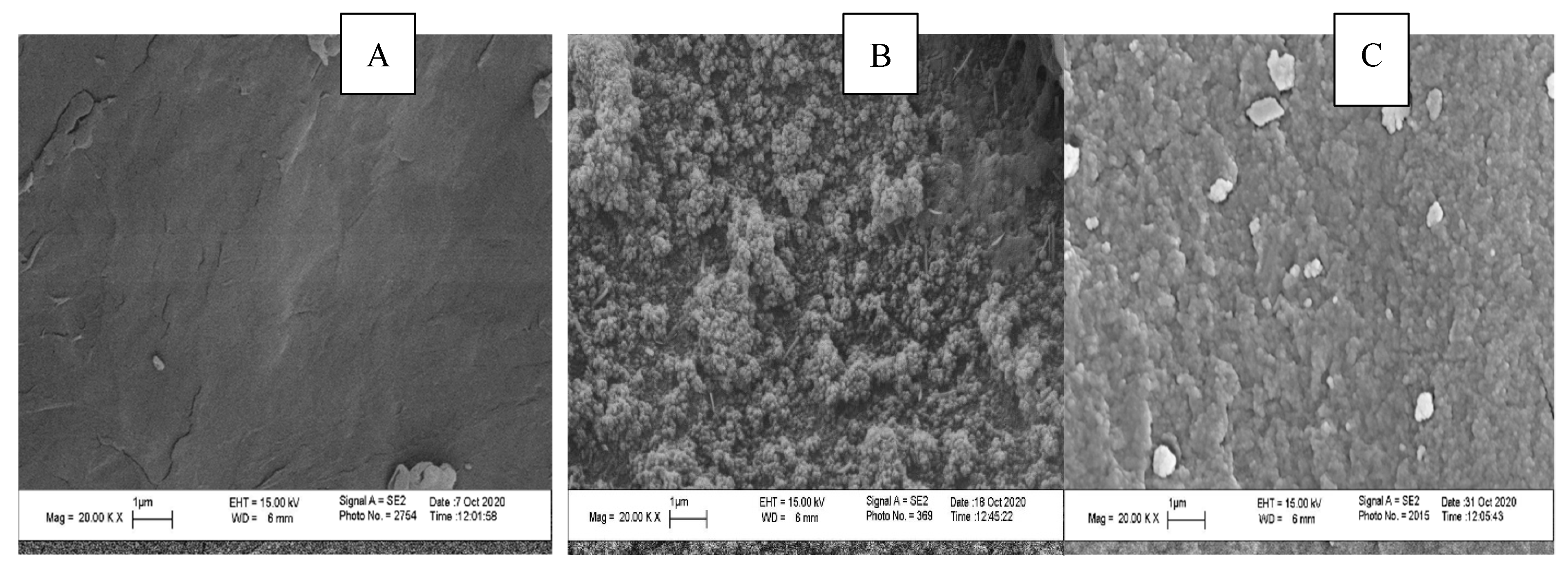
2.1.2. SEM and Morphological Changes
2.1.3. X-ray Diffraction
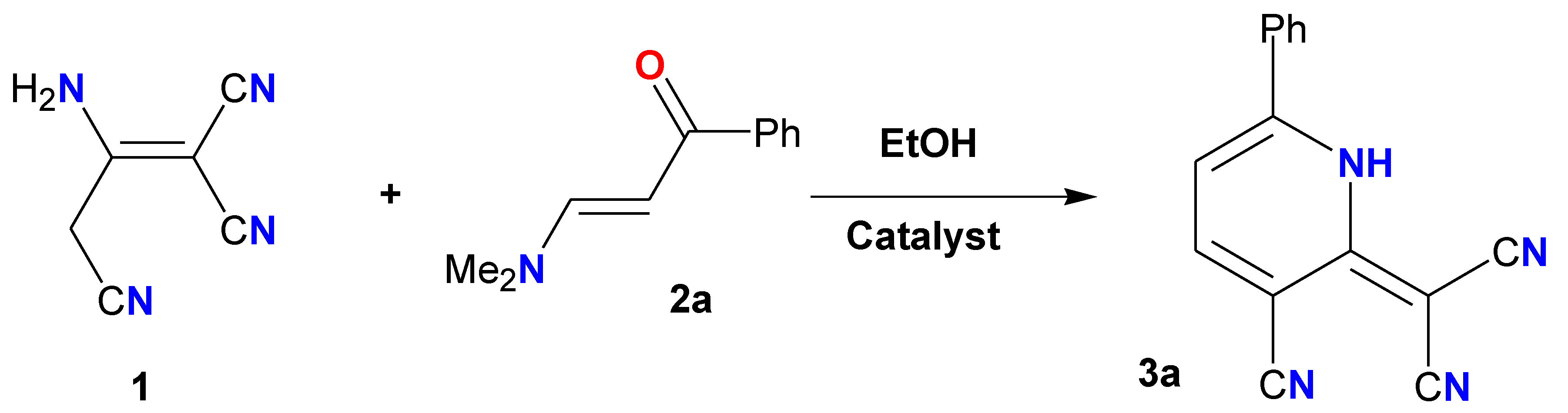
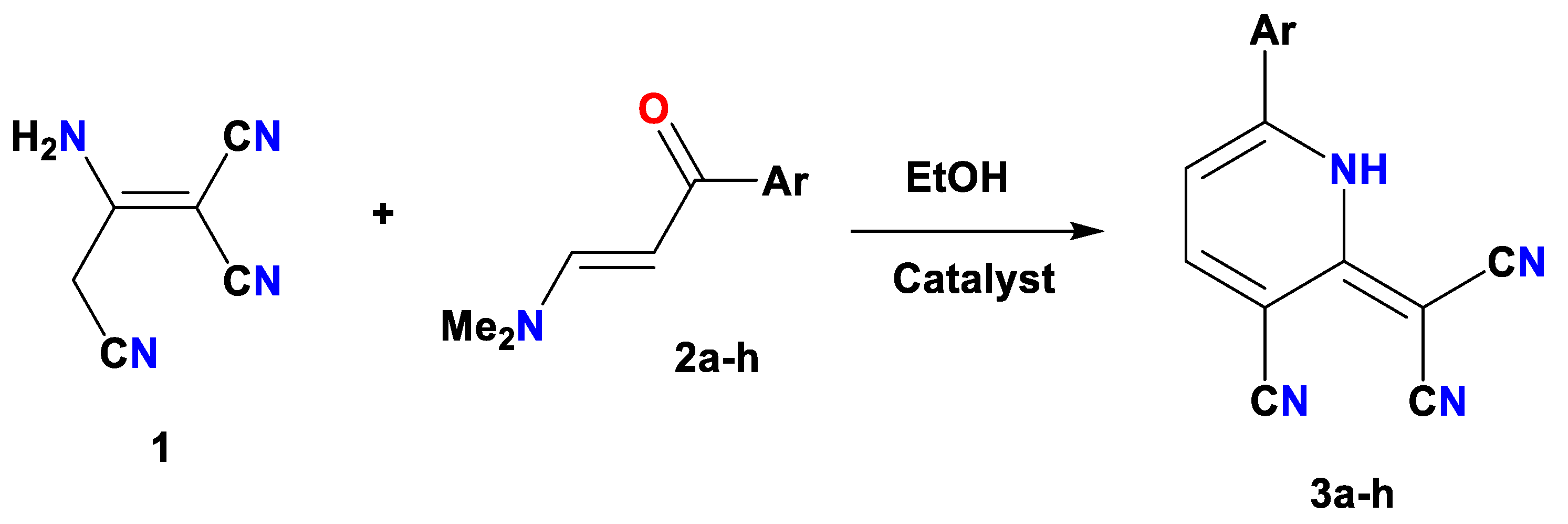
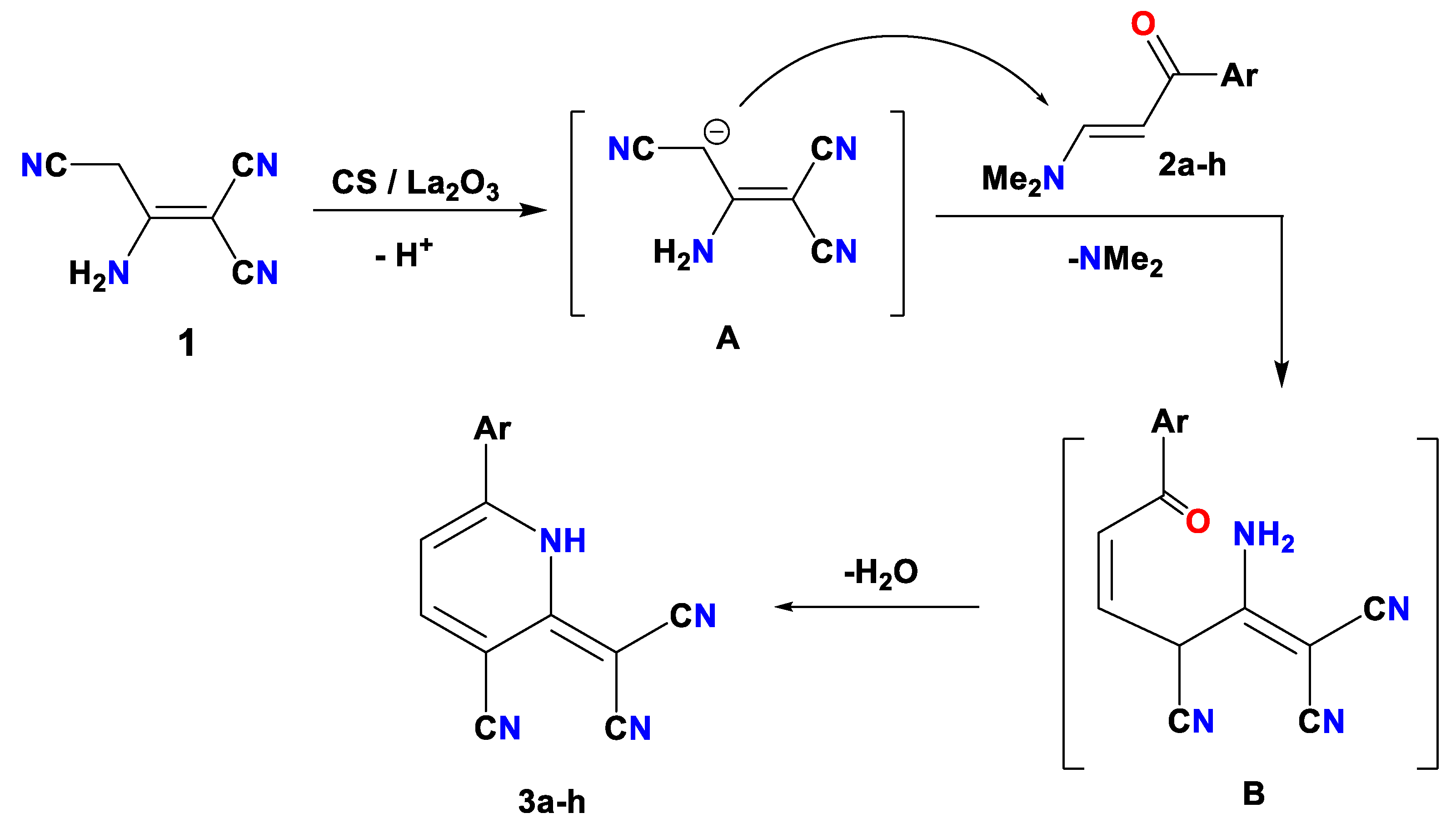
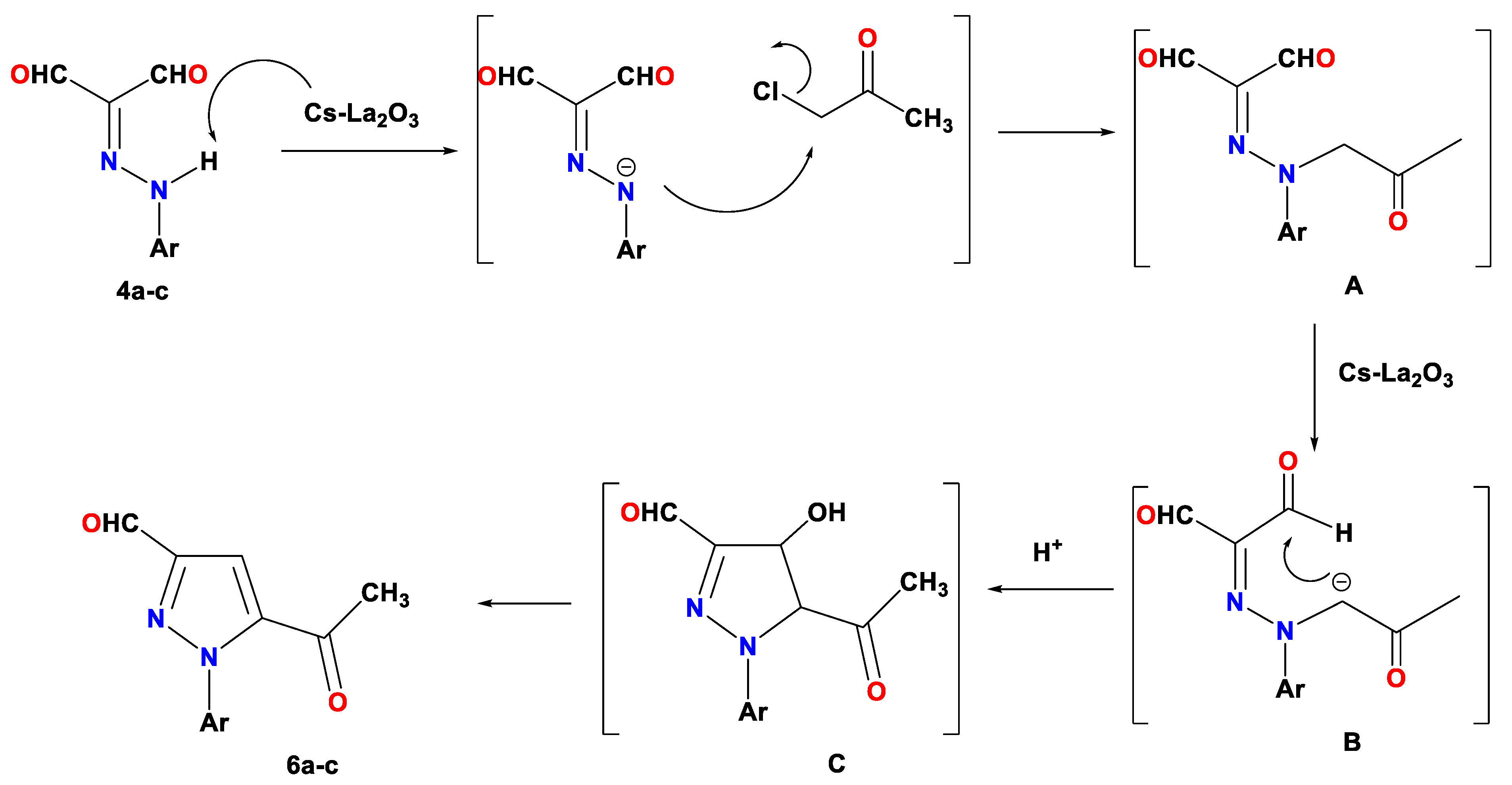
2.2. CS/La2O3 Nanocomposite Film as Basic Catalyst in Synthesis of Pyridine Derivatives
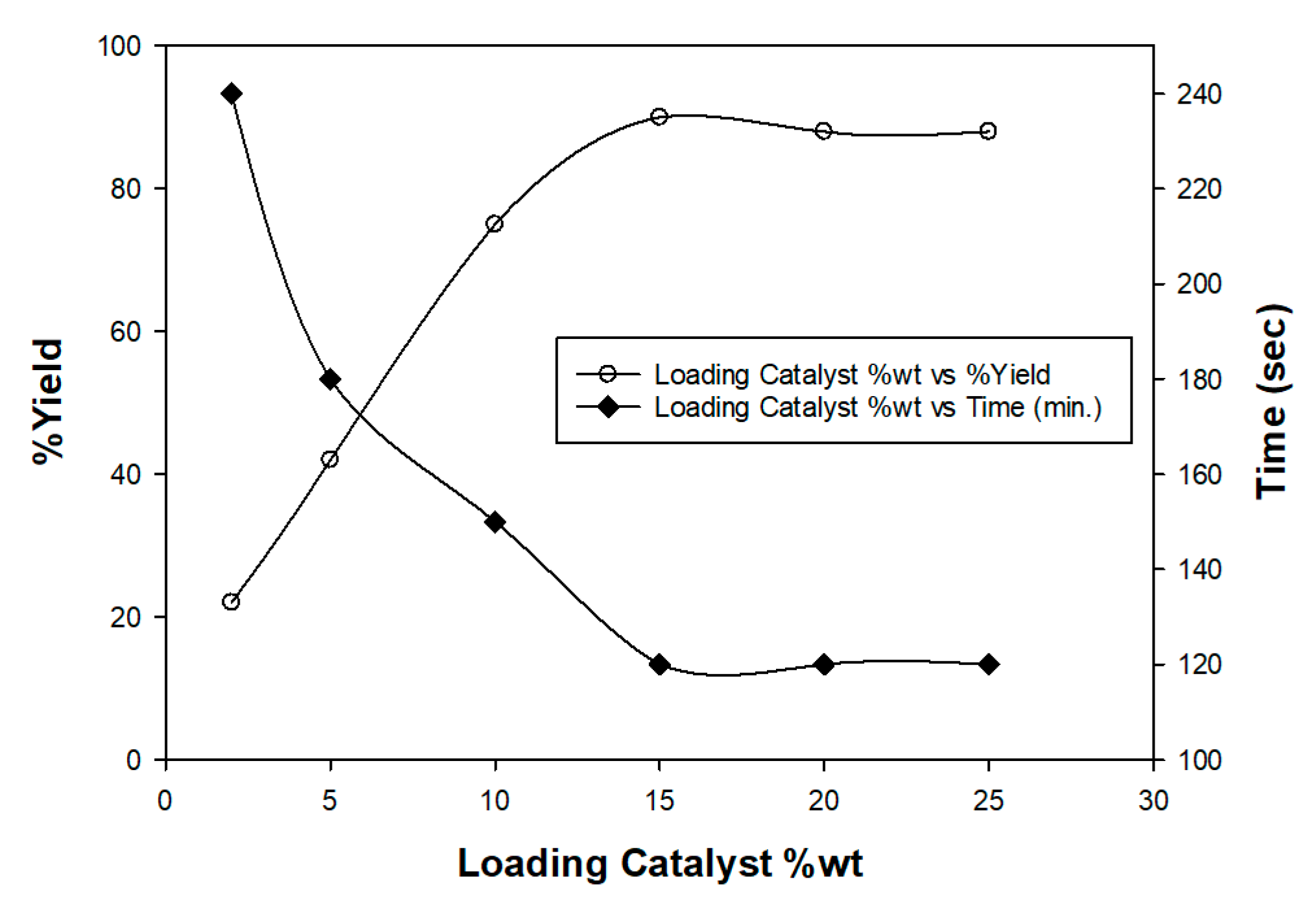
Optimizing the Catalyst Loading and the Reaction Conditions
3. Experimental
3.1. Materials and Methods
3.2. Preparation of CS/La2O3 Nanocomposite Film
3.3. Reaction of Malononitrile Dimer with Enaminone
3.4. Reaction of 2-arylazomalonaldehyde 6 with Chloroacetone
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Xu, G.; Blum, F.D. Surfactant-enhanced free radical polymerization of styrene in emulsion gels. Polymer 2008, 49, 3233–3238. [Google Scholar] [CrossRef]
- Ventra, M.; Evoy, S.; Heflin, J.R. Introduction to Nanoscale Science and Technology. Nanostructure Science and Technology; Springer: Boston, MA, USA, 2004. [Google Scholar]
- Ray, C.; Pal, T. Retracted Article: Recent advances of metal–metal oxide nanocomposites and their tailored nanostructures in numerous catalytic applications. J. Mater. Chem. A 2017, 5, 9465–9487. [Google Scholar] [CrossRef]
- Schauermann, S.; Nilius, N.; Shaikhutdinov, S.; Freund, H.-J. Nanoparticles for heterogeneous catalysis: New mechanistic insights. Acc. Chem. Res. 2013, 46, 1673–1681. [Google Scholar] [CrossRef] [PubMed]
- Riyadh, S.M.; Khalil, K.D.; Aljuhani, A. Chitosan-MgO nanocomposite: One pot preparation and its utility as an ecofriendly biocatalyst in the synthesis of thiazoles and [1,3,4] thiadiazoles. Nanomaterials 2018, 8, 928. [Google Scholar] [CrossRef] [PubMed]
- Khalil, K.D.; Riyadh, S.M.; Gomha, S.M.; Ali, I. Synthesis, characterization and application of copper oxide chitosan nanocomposite for green regioselective synthesis of [1,2,3] triazoles. Int. J. Biol. Macromol. 2019, 130, 928–937. [Google Scholar] [CrossRef] [PubMed]
- Khalil, K.D.; Ibrahim, E.I.; Al-Sagheer, F.A. A novel, efficient, and recyclable biocatalyst for Michael addition reactions and its iron (iii) complex as promoter for alkyl oxidation reactions. Catal. Sci. Technol. 2016, 6, 1410–1416. [Google Scholar] [CrossRef]
- Yilmaz, E. Chitosan: A Versatile Biomaterial. In Advances in Experimental Medicine and Biology; Hasirci, N., Hasirci, V., Eds.; Springer: Boston, MA, USA, 2004; Volume 553. [Google Scholar]
- Naskar, S.; Kuotsu, K.; Sharma, S. Chitosan-based nanoparticles as drug delivery systems: A review on two decades of research. J. Drug Target 2019, 27, 379–393. [Google Scholar] [CrossRef] [PubMed]
- Gill, R.; Singh, D.; Malik, H.K. Multifocal terahertz radiation by intense lasers in rippled plasma. J. Theor. Appl. Phys. 2017, 11, 103–108. [Google Scholar] [CrossRef]
- Singh, A.; Palakollu, V.; Pandey, A.; Kanvah, S.; Sharma, S. Green synthesis of 1, 4-benzodiazepines over La2O3 and La (OH)3 catalysts: Possibility of Langmuir–Hinshelwood adsorption. RSC Adv. 2016, 6, 103455–103462. [Google Scholar] [CrossRef]
- Li, X.-y.; Li, S.; Lu, G.-q.; Wang, D.-p.; Liu, K.-l.; Qian, X.-h.; Xue, W.-h.; Meng, F.-h. Design, synthesis and biological evaluation of novel (E)-N-phenyl-4-(pyridine-acylhydrazone) benzamide derivatives as potential antitumor agents for the treatment of multiple myeloma (MM). Bioorg. Chem. 2020, 103, 104189. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Ying, S.; Zhou, B.; Liang, X.; He, Q.; Song, P.; Hu, X.; Shi, K.; Xiong, M.; Jin, H. Discovery of novel 2-aryl-3-sulfonamido-pyridines (HoAns) as microtubule polymerization inhibitors with potent antitumor activities. Eur. J. Med. Chem. 2021, 211, 113117. [Google Scholar] [CrossRef] [PubMed]
- Abdel Hafez, N.A.; Hassaneen, H.M.; Farghaly, T.A.; Riyadh, S.M.; Elzahabi, H.S. Synthesis of new bis-spiropyrazoles as antitumor agents under ultrasound irradiation. Mini-Rev. Med. Chem. 2018, 18, 631–637. [Google Scholar] [CrossRef]
- Gouda, A.M.; El-Ghamry, H.A.; Bawazeer, T.M.; Farghaly, T.A.; Abdalla, A.N.; Aslam, A. Antitumor activity of pyrrolizines and their Cu (II) complexes: Design, synthesis and cytotoxic screening with potential apoptosis-inducing activity. Eur. J. Med. Chem. 2018, 145, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ban, H.; Yu, R.; Wang, Z.; Zhang, D. Recent progress of sodium-glucose transporter 2 inhibitors as potential antidiabetic agents. Future Med. Chem. 2018, 10, 1261–1276. [Google Scholar] [CrossRef] [PubMed]
- Eryılmaz, S.; Çelikoğlu, E.T.; İdil, Ö.; İnkaya, E.; Kozak, Z.; Mısır, E.; Gül, M. Derivatives of pyridine and thiazole hybrid: Synthesis, DFT, biological evaluation via antimicrobial and DNA cleavage activity. Bioorg. Chem. 2020, 95, 103476. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, Z.A.; Alshehrei, F.; Zayed, M.E.; Farghaly, T.A.; Abdallah, M.A. Synthesis of novel bis-pyrazole derivatives as antimicrobial agents. Mini-Rev. Med. Chem. 2019, 19, 1276–1290. [Google Scholar] [CrossRef]
- Cheng, C.C.; Huang, X.; Shipps, G.W., Jr.; Wang, Y.-S.; Wyss, D.F.; Soucy, K.A.; Jiang, C.-k.; Agrawal, S.; Ferrari, E.; He, Z. Pyridine carboxamides: Potent palm site inhibitors of HCV NS5B polymerase. ACS Med. Chem. Lett. 2010, 1, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Aydın, S.; Kaushik-Basu, N.; Özbaş-Turan, S.; Akbuğa, J.; Mega Tiber, P.; Orun, O.; Gurukumar, K.R.; Basu, A.; Güniz Küçükgüzel, Ş. Synthesis of 1-aroyl-3, 5-dimethyl-1H-pyrazoles as Anti-HCV and anticancer agents. Lett. Drug Des. 2014, 11, 121–131. [Google Scholar] [CrossRef]
- Vacher, B.; Bonnaud, B.; Funes, P.; Jubault, N.; Koek, W.; Assié, M.-B.; Cosi, C.; Kleven, M. Novel derivatives of 2-pyridinemethylamine as selective, potent, and orally active agonists at 5-HT1A receptors. J. Med. Chem. 1999, 42, 1648–1660. [Google Scholar] [CrossRef] [PubMed]
- Secci, D.; Bolasco, A.; Chimenti, P.; Carradori, S. The state of the art of pyrazole derivatives as monoamine oxidase inhibitors and antidepressant/anticonvulsant agents. Curr. Med. Chem. 2011, 18, 5114–5144. [Google Scholar] [CrossRef]
- Kabir, H.; Nandyala, S.H.; Rahman, M.M.; Kabir, M.A.; Stamboulis, A. Influence of calcination on the sol–gel synthesis of lanthanum oxide nanoparticles. Appl. Phys. A 2018, 124, 1–11. [Google Scholar] [CrossRef]
- CHERAGHALI, R.; Aghazadeh, M. A simple and facile electrochemical route to synthesis of metal hydroxides and oxides ultrafine nanoparticles (M = La, Gd, Ni and Co). Anal. Bioanal. Electrochem. 2016, 8, 64−77. [Google Scholar]
- Klug, H.P.; Alexander, L.E. X-ray Diffraction Procedures; John Wiley and Sons Inc.: New York, NY, USA, 1954; p. 633. [Google Scholar]
- Helmy, N.M.; El-Baih, F.E.; Al-Alshaikh, M.A.; Moustafa, M.S. A route to dicyanomethylene pyridines and substituted benzonitriles utilizing malononitrile dimer as a precursor. Molecules 2011, 16, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Hassanien, A.Z.A.; Ghozlan, S.A.; Elnagdi, M.H. Enaminones as building blocks in organic synthesis: A novel route to polyfunctionally substituted benzonitriles, pyridines, eneylbenzotriazoles and diazepines. J. Heterocycl. Chem. 2003, 40, 225–228. [Google Scholar] [CrossRef]
- Al-Mousawi, S.M.; Moustafa, M.S.; Elnagdi, M.H. Green synthetic approaches: Solventless synthesis of polyfunctionally substituted aromatics as potential versatile building blocks in organic synthesis utilizing enaminones and enaminonitriles as precursors. Green Chem. Lett. Rev. 2011, 4, 185–193. [Google Scholar] [CrossRef]
- Makhseed, S.; Hassaneen, H.M.; Elnagdi, M.H. Studies with 2-(arylhydrazono) aldehydes: Synthesis and chemical reactivity of mesoxalaldehyde 2-arylhydrazones and of ethyl 2-arylhydrazono-3-oxopropionates. Z. Nat. B 2007, 62, 529–536. [Google Scholar] [CrossRef]


| State of Catalyst | Fresh Catalyst | Recycled (1) | Recycled (2) | Recycled (3) |
|---|---|---|---|---|
| Product 3a (%Yield) | 90 | 89 | 88 | 88 |
| Compd. No. | Ar | Yield (%) | Ref. | |||
|---|---|---|---|---|---|---|
| Piperidine | CS | La2O3 | CS/La2O3 | |||
| 3a | C6H5- | 80 | 64 | 78 | 90 | [12] |
| 3b | 4-Me-C6H4- | 55 | 38 | 63 | 82 | [13] |
| 3c | 4-MeO-C6H4- | 78 | 70 | 81 | 88 | [14] |
| 3d | 4-Cl-C6H4- | 75 | 60 | 72 | 85 | [14] |
| 3e | 4-NO2-C6H4- | 73 | 62 | 68 | 85 | - |
| 3f | 2-furyl | 60 | 55 | 63 | 82 | - |
| 3g | 2-thienyl | 75 | 56 | 69 | 85 | [12] |
| 3h | 2-pyrrolyl | 55 | 50 | 62 | 75 | - |
| Compd. No. | Ar | Yield (%) | Ref. | |||
|---|---|---|---|---|---|---|
| Et3N | CS | La2O3 | CS/La2O3 | |||
| 6a | C6H5- | 68 | 57 | 68 | 75 | - |
| 6b | 4-Cl-C6H4- | 66 | 54 | 70 | 78 | [15] |
| 6c | 4-MeO-C6H4- | 72 | 60 | 75 | 84 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalil, K.D.; Riyadh, S.M.; Jaremko, M.; Farghaly, T.A.; Hagar, M. Synthesis of Chitosan-La2O3 Nanocomposite and Its Utility as a Powerful Catalyst in the Synthesis of Pyridines and Pyrazoles. Molecules 2021, 26, 3689. https://doi.org/10.3390/molecules26123689
Khalil KD, Riyadh SM, Jaremko M, Farghaly TA, Hagar M. Synthesis of Chitosan-La2O3 Nanocomposite and Its Utility as a Powerful Catalyst in the Synthesis of Pyridines and Pyrazoles. Molecules. 2021; 26(12):3689. https://doi.org/10.3390/molecules26123689
Chicago/Turabian StyleKhalil, Khaled D., Sayed M. Riyadh, Mariusz Jaremko, Thoraya A. Farghaly, and Mohamed Hagar. 2021. "Synthesis of Chitosan-La2O3 Nanocomposite and Its Utility as a Powerful Catalyst in the Synthesis of Pyridines and Pyrazoles" Molecules 26, no. 12: 3689. https://doi.org/10.3390/molecules26123689
APA StyleKhalil, K. D., Riyadh, S. M., Jaremko, M., Farghaly, T. A., & Hagar, M. (2021). Synthesis of Chitosan-La2O3 Nanocomposite and Its Utility as a Powerful Catalyst in the Synthesis of Pyridines and Pyrazoles. Molecules, 26(12), 3689. https://doi.org/10.3390/molecules26123689