Cardamonin Modulates Neuropathic Pain through the Possible Involvement of Serotonergic 5-HT1A Receptor Pathway in CCI-Induced Neuropathic Pain Mice Model
Abstract
1. Introduction
2. Results
2.1. Involvement of the 5-HT System in the Antihyperalgesic and Antiallodynic Effects of Cardamonin
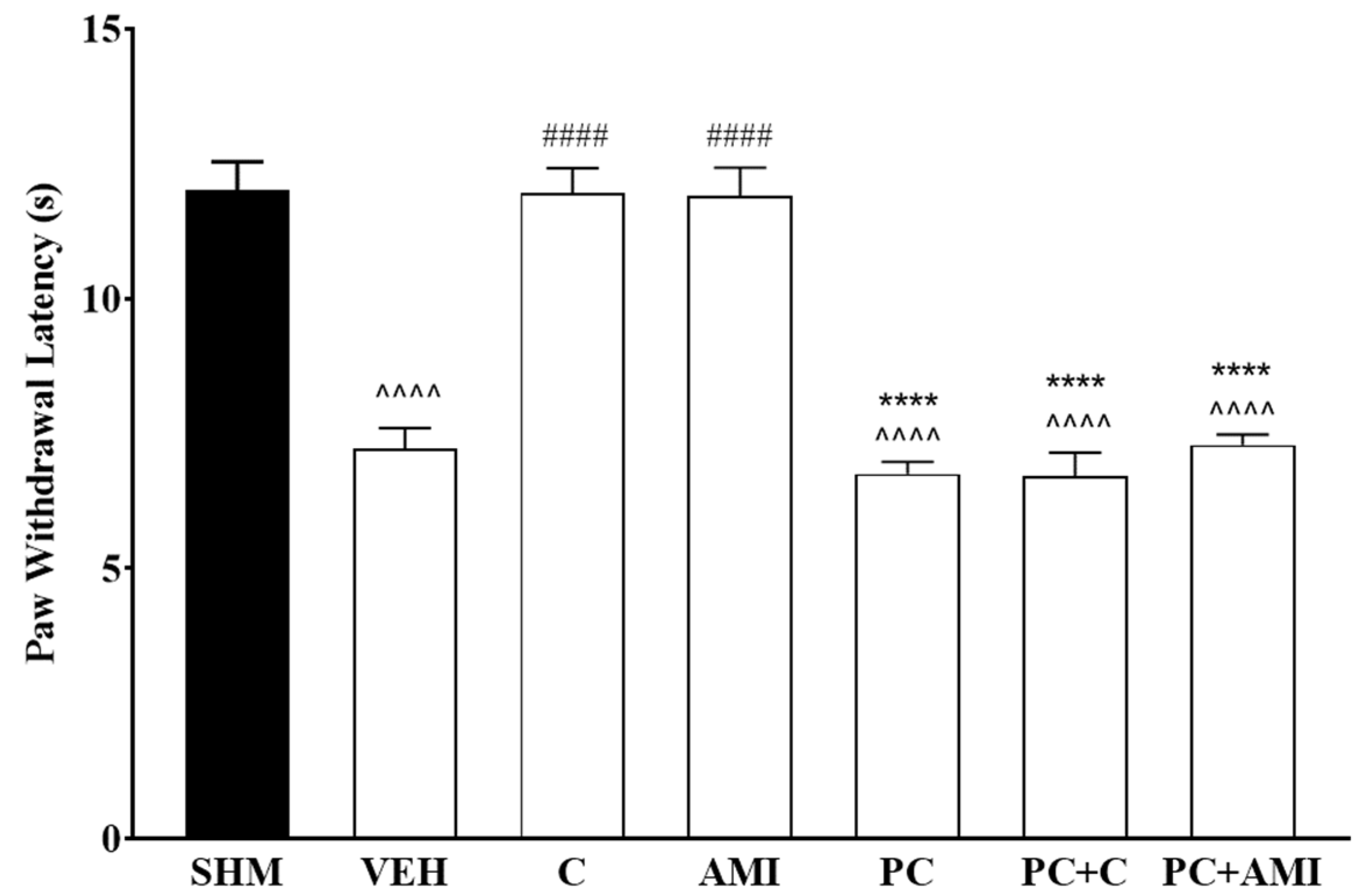
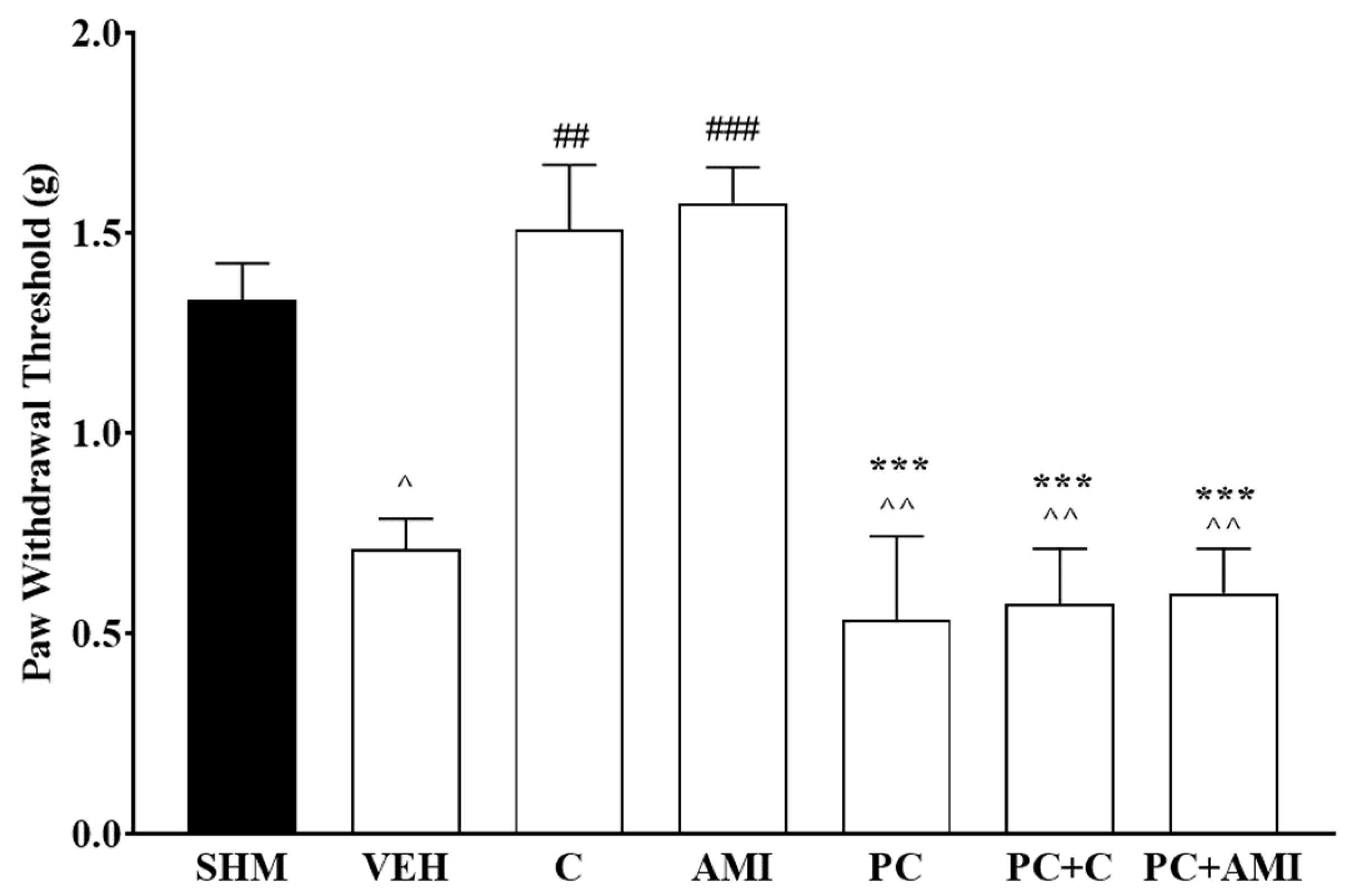
2.2. Involvement of 5-HT Receptor Subtypes to Induce Antihyperalgesic and Antiallodynic Effects of Cardamonin
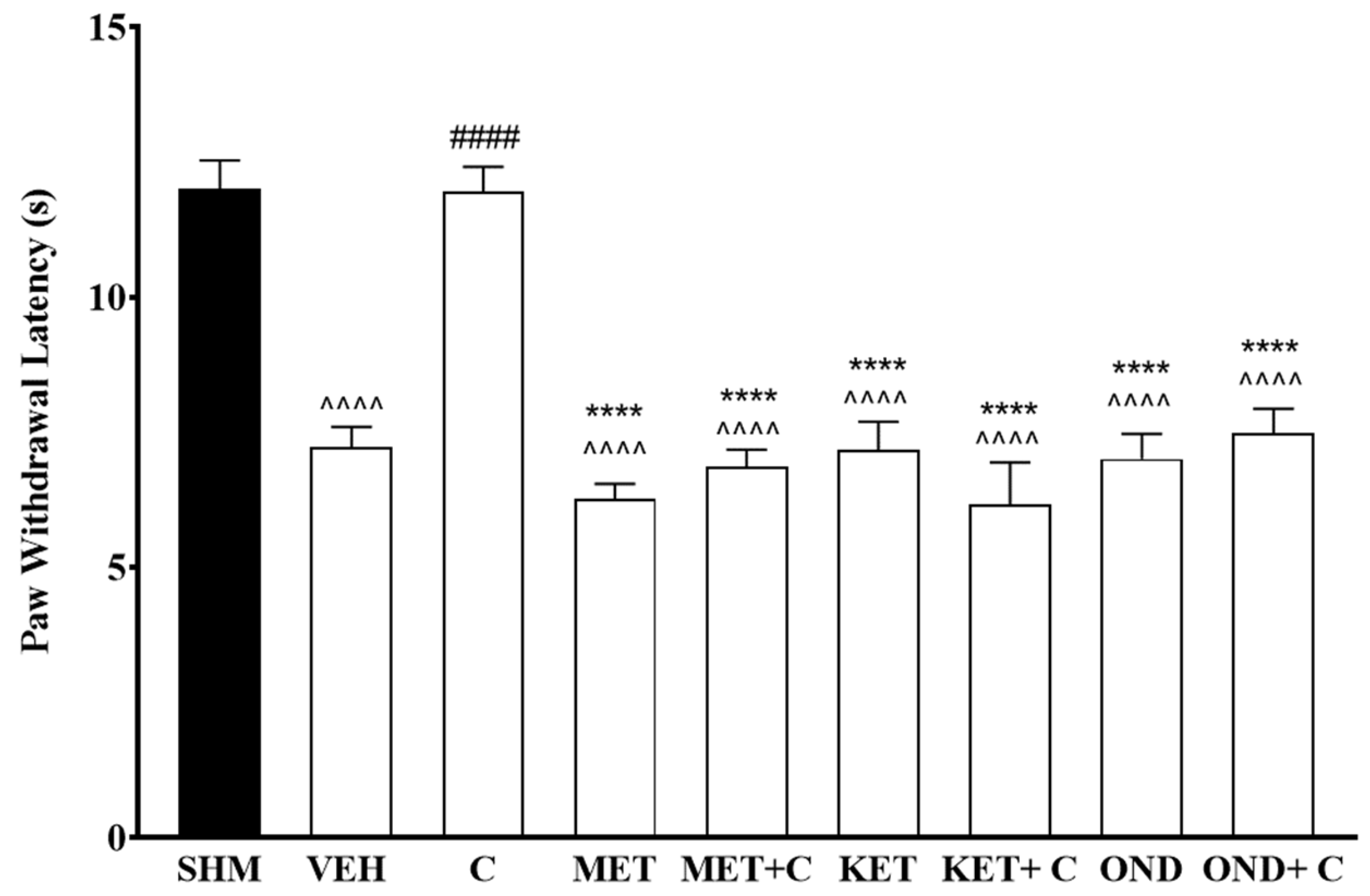
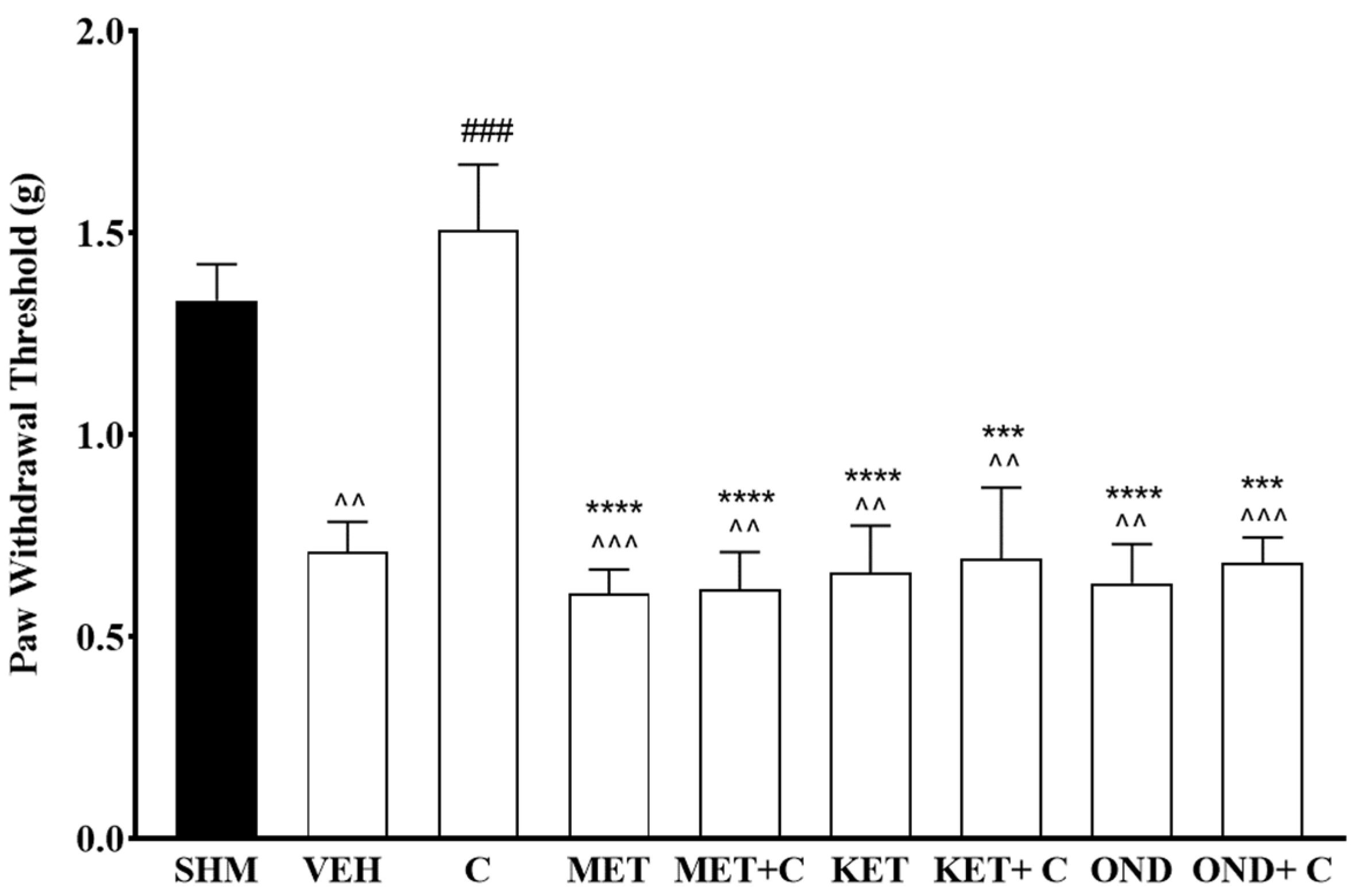
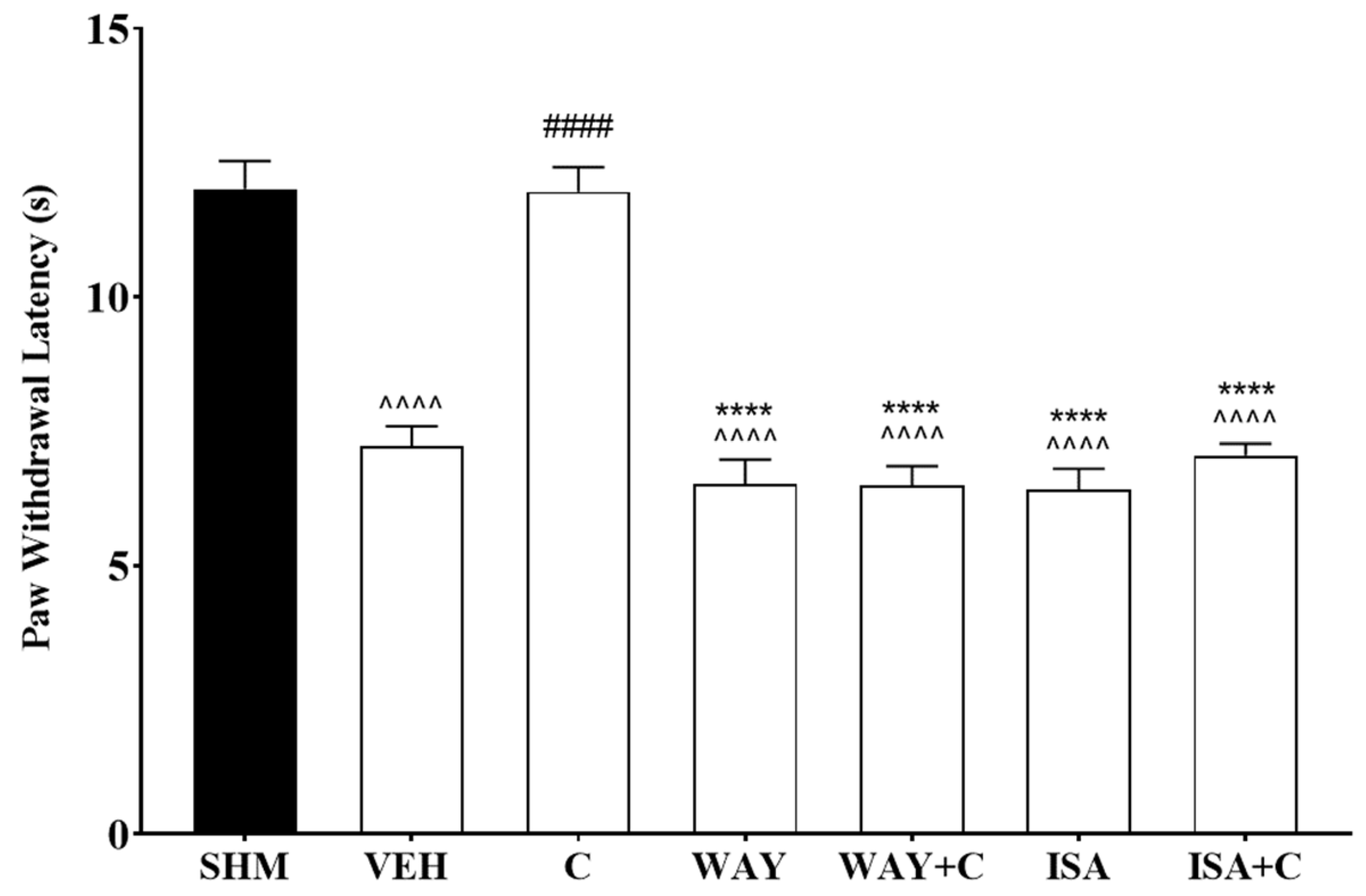
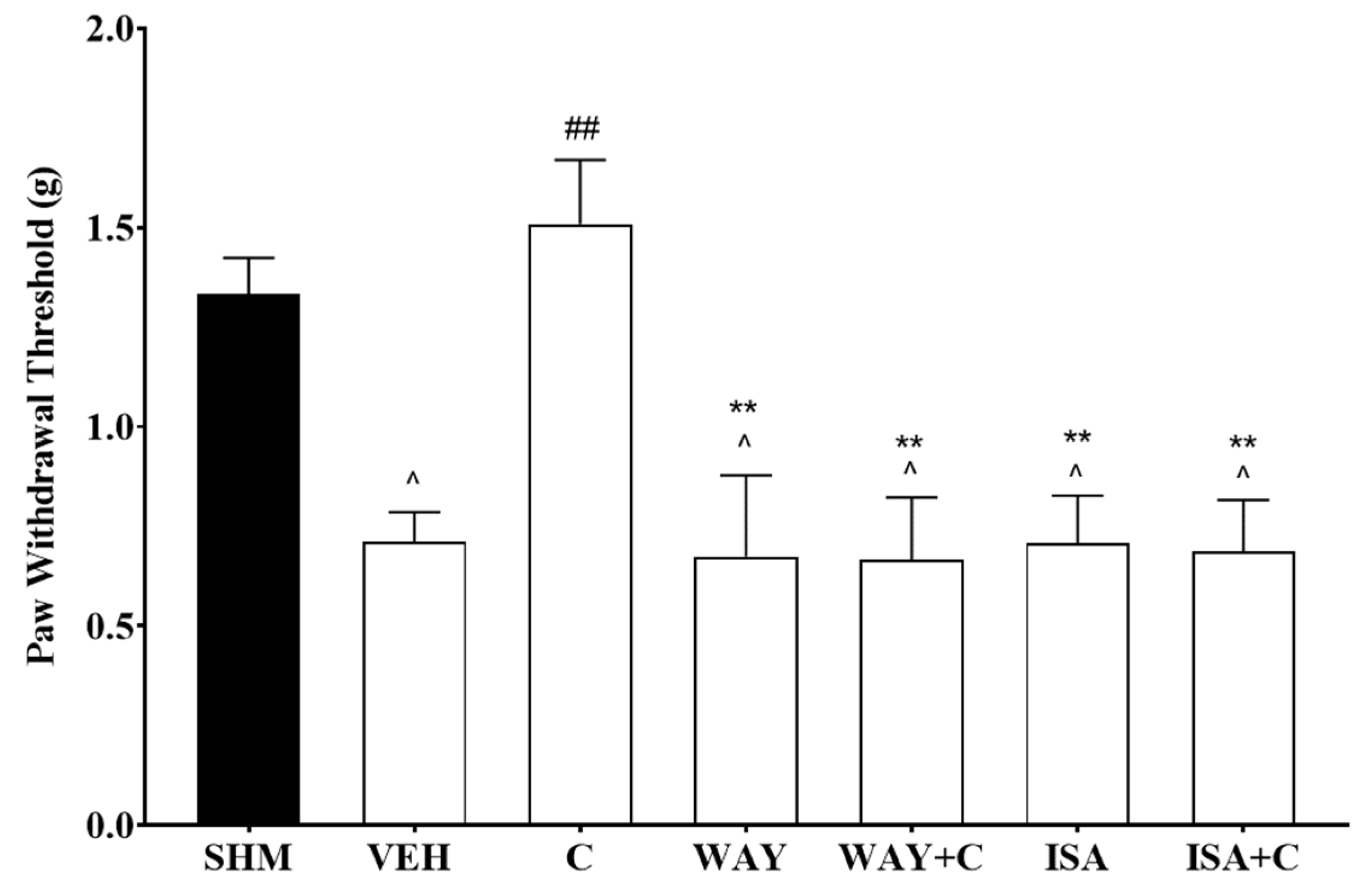
2.3. 5-HT1 Receptor Subtype Mediates the Antihyperalgesic and Antiallodynic Effects of Cardamonin
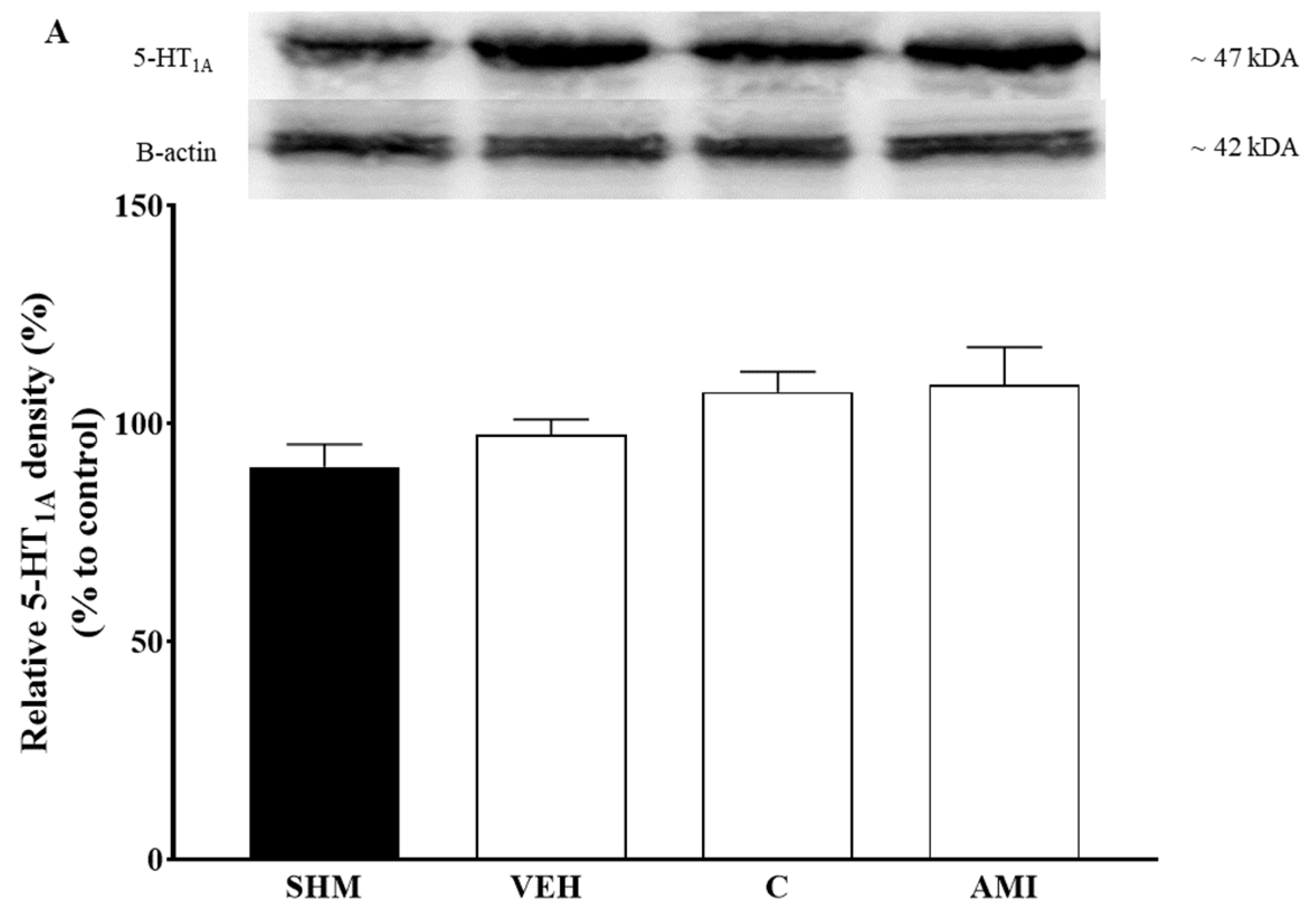
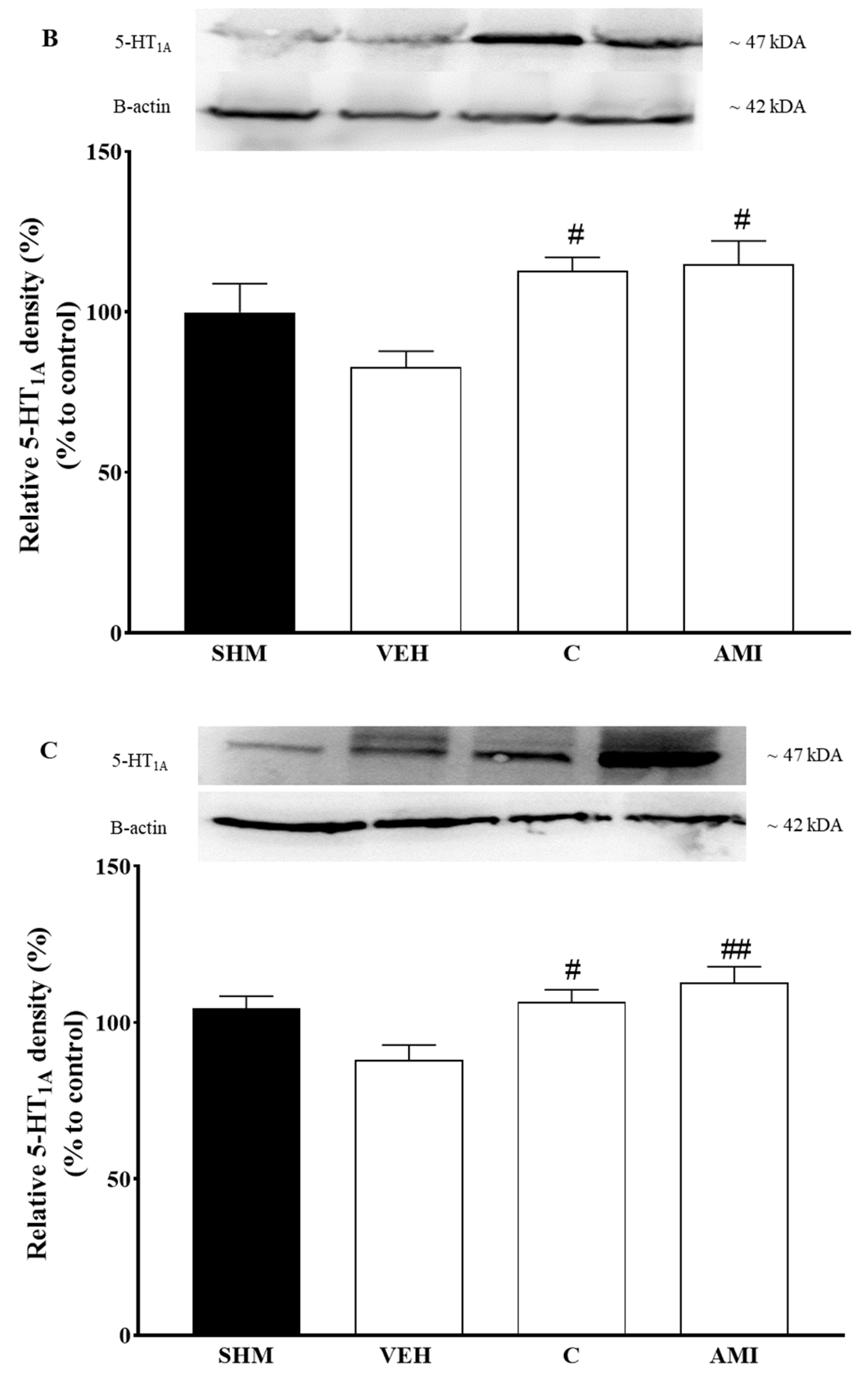
2.4. 5-HT1A Receptor Expression in Cardamonin-Treated Neuropathic Pain Mice Model
3. Discussion
4. Material and Method
4.1. Chemical
4.2. Materials
4.3. Experimental Animals
4.4. Chronic Constriction Injury (CCI)
4.5. Drug, Compound Preparation, and Experimental Groupings
4.6. Nociceptive Testing
4.6.1. Assessment of Thermal Hyperalgesia
4.6.2. Assessment of Mechanical Allodynia
4.7. Involvement of Serotonergic System
4.7.1. Serotonin Depletion
4.7.2. Serotonin Antagonists
4.8. Western Blot Analysis
4.9. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ni, L.; Meng, C.Q.; Sikorski, J.A. Recent advances in therapeutic chalcones. Expert Opin. Ther. Pat. 2004, 14, 1669–1691. [Google Scholar] [CrossRef]
- Rao, C.B.; Rao, T.N.; Suryaprakasam, S. Cardamonin and alpinetin from the seeds of Amomum subulatum. Planta Med. 1976, 29, 391–392. [Google Scholar] [CrossRef] [PubMed]
- Daimary, U.D.; Parama, D.; Rana, V.; Banik, K.; Kumar, A.; Harsha, C.; Kunnumakkara, A.B. Emerging roles of cardamonin, a multitargeted nutraceutical in the prevention and treatment of chronic diseases. Curr. Res. Pharmacol. Drug Discov. 2020, 2, 100008. [Google Scholar] [CrossRef]
- Nawaz, J.; Rasul, A.; Shah, M.A.; Hussain, G.; Riaz, A.; Sarfraz, I.; Zafar, S.; Adnan, M.; Khan, A.H.; Selamoglu, Z. Cardamonin: A new player to fight cancer via multiple cancer signaling pathways. Life Sci. 2020, 250, 117591. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Israf, D.A.; Lajis, N.H.; Shaari, K.; Mohamed, H.; Wahab, A.A.; Ariffin, K.T.; Hoo, W.Y.; Aziz, N.A.; Kadir, A.A. Cardamonin, inhibits pro-inflammatory mediators in activated RAW 264.7 cells and whole blood. Eur. J. Pharmacol. 2006, 538, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Sun, A.; Deng, C.; Zhang, J.; Wu, X.; Wei, X.; Mani, S.; Dou, W.; Wang, Z. The anti-inflammatory effect and potential mechanism of cardamonin in DSS-induced colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G517–G527. [Google Scholar] [CrossRef]
- Ping, C.P.; Tengku Mohamad, T.A.S.; Akhtar, M.N.; Perimal, E.K.; Akira, A.; Israf Ali, D.A.; Sulaiman, M.R. Antinociceptive effects of cardamonin in mice: Possible involvement of TRPV1, glutamate, and opioid receptors. Molecules 2018, 23, 2237. [Google Scholar] [CrossRef] [PubMed]
- De Spirt, S.; Eckers, A.; Wehrend, C.; Micoogullari, M.; Sies, H.; Stahl, W.; Steinbrenner, H. Interplay between the chalcone cardamonin and selenium in the biosynthesis of Nrf2-regulated antioxidant enzymes in intestinal Caco-2 cells. Free Radic. Biol. Med. 2016, 91, 164–171. [Google Scholar] [CrossRef]
- Jin, J.; Qiu, S.; Wang, P.; Liang, X.; Huang, F.; Wu, H.; Zhang, B.; Zhang, W.; Tian, X.; Xu, R. Cardamonin inhibits breast cancer growth by repressing HIF-1α-dependent metabolic reprogramming. J. Exp. Clin. Cancer Res. 2019, 38, 377. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, R.; Li, Q.; Jie, X.; Hong, J.; Zong, Y.; Dong, X.; Zhang, S.; Li, Z.; Wu, G. Cardamonin inhibits the proliferation and metastasis of non-small-cell lung cancer cells by suppressing the PI3K/Akt/mTOR pathway. Anti-Cancer Drugs 2019, 30, 241–250. [Google Scholar] [CrossRef]
- Sambasevam, Y.; Farouk, A.A.O.; Mohamad, T.A.S.T.; Sulaiman, M.R.; Bharatham, B.H.; Perimal, E.K. Cardamonin attenuates hyperalgesia and allodynia in a mouse model of chronic constriction injury-induced neuropathic pain: Possible involvement of the opioid system. Eur. J. Pharmacol. 2017, 796, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Van Hecke, O.; Austin, S.K.; Khan, R.A.; Smith, B.; Torrance, N. Neuropathic pain in the general population: A systematic review of epidemiological studies. PAIN® 2014, 155, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Baron, R.; Binder, A.; Wasner, G. Neuropathic pain: Diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol. 2010, 9, 807–819. [Google Scholar] [CrossRef]
- Colloca, L.; Ludman, T.; Bouhassira, D.; Baron, R.; Dickenson, A.H.; Yarnitsky, D.; Freeman, R.; Truini, A.; Attal, N.; Finnerup, N.B. Neuropathic pain. Nat. Rev. Dis. Primers 2017, 3, 17002. [Google Scholar] [CrossRef]
- Dworkin, R.H. An overview of neuropathic pain: Syndromes, symptoms, signs, and several mechanisms. Clin. J. Pain 2002, 18, 343–349. [Google Scholar] [CrossRef]
- Dworkin, R.H.; Backonja, M.; Rowbotham, M.C.; Allen, R.R.; Argoff, C.R.; Bennett, G.J.; Bushnell, M.C.; Farrar, J.T.; Galer, B.S.; Haythornthwaite, J.A. Advances in neuropathic pain: Diagnosis, mechanisms, and treatment recommendations. Arch. Neurol. 2003, 60, 1524–1534. [Google Scholar] [CrossRef]
- Pasero, C. Pathophysiology of neuropathic pain. Pain Manag. Nurs. 2004, 5, 3–8. [Google Scholar] [CrossRef]
- Finnerup, N.B.; Kuner, R.; Jensen, T.S. Neuropathic pain: From mechanisms to treatment. Physiol. Rev. 2021, 101, 259–301. [Google Scholar] [CrossRef]
- Truini, A.; Cruccu, G. Pathophysiological mechanisms of neuropathic pain. Neurol. Sci. 2006, 27, s179–s182. [Google Scholar] [CrossRef]
- Kwon, M.; Altin, M.; Duenas, H.; Alev, L. The role of descending inhibitory pathways on chronic pain modulation and clinical implications. Pain Pract. 2014, 14, 656–667. [Google Scholar] [CrossRef]
- Cohen, S.P.; Mao, J. Neuropathic pain: Mechanisms and their clinical implications. BMJ 2014, 348, f7656. [Google Scholar] [CrossRef]
- Bravo, L.; Llorca-Torralba, M.; Berrocoso, E.; Micó, J.A. Monoamines as drug targets in chronic pain: Focusing on neuropathic pain. Front. Neurosci. 2019, 13, 1268. [Google Scholar] [CrossRef]
- Benarroch, E.E. Descending monoaminergic pain modulation: Bidirectional control and clinical relevance. Neurology 2008, 71, 217–221. [Google Scholar] [CrossRef]
- Sagalajev, B.; Bourbia, N.; Beloushko, E.; Wei, H.; Pertovaara, A. Bidirectional amygdaloid control of neuropathic hypersensitivity mediated by descending serotonergic pathways acting on spinal 5-HT3 and 5-HT1A receptors. Behav. Brain Res. 2015, 282, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Ossipov, M.H.; Morimura, K.; Porreca, F. Descending pain modulation and chronification of pain. Curr. Opin. Supportive Palliat. Care 2014, 8, 143. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Hu, R.; Xu, T.; Zhang, Z.N.; Li, W.; Lu, J. Characterization of induced pluripotent stem cell-derived human serotonergic neurons. Front. Cell. Neurosci. 2017, 11, 131. [Google Scholar] [CrossRef] [PubMed]
- Dogrul, A.; Ossipov, M.H.; Porreca, F. Differential mediation of descending pain facilitation and inhibition by spinal 5HT-3 and 5HT-7 receptors. Brain Res. 2009, 1280, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Taniguchi, W.; Chen, Q.-Y.; Tozaki-Saitoh, H.; Song, Q.; Liu, R.-H.; Koga, K.; Matsuda, T.; Kaito-Sugimura, Y.; Wang, J.; et al. Top-down descending facilitation of spinal sensory excitatory transmission from the anterior cingulate cortex. Nat. Commun. 2018, 9, 1886. [Google Scholar] [CrossRef]
- Martins, I.; Tavares, I. Reticular formation and pain: The past and the future. Front. Neuroanat. 2017, 11. [Google Scholar] [CrossRef]
- Zhuo, M. Descending facilitation: From basic science to the treatment of chronic pain. Mol. Pain 2017, 13, 1744806917699212. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.S.; Schambra, U.B.; Wilson, K.H.; Page, S.O.; Schwinn, D.A. α1-Adrenergic receptors in human spinal cord: Specific localized expression of mRNA encoding α1-adrenergic receptor subtypes at four distinct levels. Mol. Brain Res. 1999, 63, 254–261. [Google Scholar] [CrossRef]
- Tavares, I.; Lima, D. The caudal ventrolateral medulla as an important inhibitory modulator of pain transmission in the spinal cord. J. Pain 2002, 3, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Gu, M.; Chu, Y.X. New tricks for an old slug: Descending serotonergic system in pain. Acta Physiol. Sin. 2012, 64, 520–530. [Google Scholar]
- Pertovaara, A.; Almeida, A. Descending inhibitory systems. Handb. Clin. Neurol. 2006, 81, 179–192. [Google Scholar] [PubMed]
- Hannon, J.; Hoyer, D. Molecular biology of 5-HT receptors. Behav. Brain Res. 2008, 195, 198–213. [Google Scholar] [CrossRef] [PubMed]
- Barnes, N.M.; Ahern, G.P.; Becamel, C.; Bockaert, J.; Camilleri, M.; Chaumont-Dubel, S.; Claeysen, S.; Cunningham, K.A.; Fone, K.C.; Gershon, M. International Union of Basic and Clinical Pharmacology. CX. Classification of receptors for 5-hydroxytryptamine; pharmacology and function. Pharmacol. Rev. 2021, 73, 310–520. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-Y.; Wu, S.-X.; Wang, Y.-Y.; Wang, W.; Zhou, L.; Li, Y.-Q. Changes of 5-HT receptor subtype mRNAs in rat dorsal root ganglion by bee venom-induced inflammatory pain. Neurosci. Lett. 2005, 375, 42–46. [Google Scholar] [CrossRef]
- Wu, S.-X.; Zhu, M.; Wang, W.; Wang, Y.-Y.; Li, Y.-Q.; Yew, D.T. Changes of the expression of 5-HT receptor subtype mRNAs in rat dorsal root ganglion by complete Freund’s adjuvant-induced inflammation. Neurosci. Lett. 2001, 307, 183–186. [Google Scholar] [CrossRef]
- Sasaki, M.; Ishizaki, K.; Obata, H.; Goto, F. Effects of 5-HT2 and 5-HT3 receptors on the modulation of nociceptive transmission in rat spinal cord according to the formalin test. Eur. J. Pharmacol. 2001, 424, 45–52. [Google Scholar] [CrossRef]
- Yanarates, O.; Dogrul, A.; Yildirim, V.; Sahin, A.; Sizlan, A.; Seyrek, M.; Akgül, Ö.; Kozak, O.; Kurt, E.; Aypar, U. Spinal 5-HT7 receptors play an important role in the antinociceptive and antihyperalgesic effects of tramadol and its metabolite, O-desmethyltramadol, via activation of descending serotonergic pathways. Anesthesiol. J. Am. Soc. Anesthesiol. 2010, 112, 696–710. [Google Scholar] [CrossRef]
- Cortes-Altamirano, J.L.; Olmos-Hernandez, A.; Jaime, H.B.; Carrillo-Mora, P.; Bandala, C.; Reyes-Long, S.; Alfaro-Rodríguez, A. 5-HT1, 5-HT2, 5-HT3 and 5-HT7 receptors and their role in the modulation of pain response in the central nervous system. Curr. Neuropharmacol. 2018, 16, 210–221. [Google Scholar] [CrossRef]
- Nasirinezhad, F.; Hosseini, M.; Karami, Z.; Yousefifard, M.; Janzadeh, A. Spinal 5-HT3 receptor mediates nociceptive effect on central neuropathic pain; possible therapeutic role for tropisetron. J. Spinal Cord Med. 2016, 39, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Rahman, W.; Bannister, K.; Bee, L.A.; Dickenson, A.H. A pronociceptive role for the 5-HT2 receptor on spinal nociceptive transmission: An in vivo electrophysiological study in the rat. Brain Res. 2011, 1382, 29–36. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Viguier, F.; Michot, B.; Hamon, M.; Bourgoin, S. Multiple roles of serotonin in pain control mechanisms—Implications of 5-HT7 and other 5-HT receptor types. Eur. J. Pharmacol. 2013, 716, 8–16. [Google Scholar] [CrossRef]
- Pui Ping, C.; Akhtar, M.N.; Israf, D.A.; Perimal, E.K.; Sulaiman, M.R. Possible participation of ionotropic glutamate receptors and l-arginine-nitric oxide-cyclic guanosine monophosphate-ATP-sensitive K+ channel pathway in the antinociceptive activity of cardamonin in acute pain animal models. Molecules 2020, 25, 5385. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, S.; Shukla, M.; Sharma, A.; Rangaraj, N.; Vaghasiya, K.; Malik, M.Y.; Lal, J. Preclinical pharmacokinetics and ADME characterization of a novel anticancer chalcone, cardamonin. Drug Test. Anal. 2017, 9, 1124–1136. [Google Scholar] [CrossRef]
- Sambasevam, Y.; Siong Jiun, W.; Ghazali, F.H.; Amir Ramadan, A.I.; Omar Farouk, A.A.; Sulaiman, M.R.; Hussain, M.K.; Perimal, E.K. Inhibitory effects of cardamonin on compound action potentials in frog sciatic nerves and the possible involvement of opioidergic pathway. Life Sci. Med. Biomed. 2017, 1, 1. [Google Scholar] [CrossRef]
- Kaswan, N.K.; Mohd Suhaimi, N.S.; Mohammed Izham, N.A.; Tengku Mohamad TA, S.; Sulaiman, M.R.; Perimal, E.K. Cardamonin inhibits nitric oxide production modulated through NMDA receptor in LPS-induced SH-SY5Y cell in vitro model. Life Sci. Med. Biomed. 2020, 4, 9. [Google Scholar] [CrossRef]
- De Felice, M.; Sanoja, R.; Wang, R.; Vera-Portocarrero, L.; Oyarzo, J.; King, T.; Ossipov, M.H.; Vanderah, T.W.; Lai, J.; Dussor, G.O. Engagement of descending inhibition from the rostral ventromedial medulla protects against chronic neuropathic pain. Pain 2011, 152, 2701–2709. [Google Scholar] [CrossRef]
- Ossipov, M.H.; Dussor, G.O.; Porreca, F. Central modulation of pain. J. Clin. Investig. 2010, 120, 3779–3787. [Google Scholar] [CrossRef]
- Wei, F.; Dubner, R.; Zou, S.; Ren, K.; Bai, G.; Wei, D.; Guo, W. Molecular depletion of descending serotonin unmasks its novel facilitatory role in the development of persistent pain. J. Neurosci. 2010, 30, 8624–8636. [Google Scholar] [CrossRef] [PubMed]
- Mohammad-Zadeh, L.; Moses, L.; Gwaltney-Brant, S. Serotonin: A review. J. Vet. Pharmacol. Ther. 2008, 31, 187–199. [Google Scholar] [CrossRef]
- Bardin, L. The complex role of serotonin and 5-HT receptors in chronic pain. Behav. Pharmacol. 2011, 22, 390–404. [Google Scholar] [CrossRef]
- Paredes, S.; Cantillo, S.; Candido, K.D.; Knezevic, N.N. An association of serotonin with pain disorders and its modulation by estrogens. Int. J. Mol. Sci. 2019, 20, 5729. [Google Scholar] [CrossRef]
- Fox, M.A.; Stein, A.R.; French, H.T.; Murphy, D.L. Functional interactions between 5-HT2A and presynaptic 5-HT1A receptor-based responses in mice genetically deficient in the serotonin 5-HT transporter (SERT). Br. J. Pharmacol. 2010, 159, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, K.; Iijima, M.; Chaki, S. Serotonin-1A receptor stimulation mediates effects of a metabotropic glutamate 2/3 receptor antagonist, 2S-2-amino-2-(1S, 2S-2-carboxycycloprop-1-yl)-3-(xanth-9-yl) propanoic acid (LY341495), and an N-methyl-D-aspartate receptor antagonist, ketamine, in the novelty-suppressed feeding test. Psychopharmacology 2014, 231, 2291–2298. [Google Scholar] [PubMed]
- Cathryn, R.H.; Lee, T.; Keele, N.B. 5-HT 2A receptor activation normalizes exaggerated fear behavior in p-chlorophenylalanine (PCPA)-treated rats. J. Behav. Brain Sci. 2012, 2012, 25212. [Google Scholar]
- Xu, Y.; Lin, D.; Yu, X.; Xie, X.; Wang, L.; Lian, L.; Fei, N.; Chen, J.; Zhu, N.; Wang, G. The antinociceptive effects of ferulic acid on neuropathic pain: Involvement of descending monoaminergic system and opioid receptors. Oncotarget 2016, 7, 20455. [Google Scholar] [CrossRef]
- Chia, J.S.M.; Farouk, A.A.O.; Mohamad, A.S.; Sulaiman, M.R.; Perimal, E.K. Zerumbone alleviates chronic constriction injury-induced allodynia and hyperalgesia through serotonin 5-HT receptors. Biomed. Pharmacother. 2016, 83, 1303–1310. [Google Scholar] [CrossRef]
- Sommer, C. Serotonin in pain and analgesia. Mol. Neurobiol. 2004, 30, 117–125. [Google Scholar] [CrossRef]
- Jeong, H.J.; Mitchell, V.A.; Vaughan, C.W. Role of 5-HT1 receptor subtypes in the modulation of pain and synaptic transmission in rat spinal superficial dorsal horn. Br. J. Pharmacol. 2012, 165, 1956–1965. [Google Scholar] [CrossRef]
- Iwasaki, T.; Otsuguro, K.-i.; Kobayashi, T.; Ohta, T.; Ito, S. Endogenously released 5-HT inhibits A and C fiber-evoked synaptic transmission in the rat spinal cord by the facilitation of GABA/glycine and 5-HT release via 5-HT2A and 5-HT3 receptors. Eur. J. Pharmacol. 2013, 702, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Brenchat, A.; Nadal, X.; Romero, L.; Ovalle, S.; Muro, A.; Sánchez-Arroyos, R.; Portillo-Salido, E.; Pujol, M.; Montero, A.; Codony, X. Pharmacological activation of 5-HT7 receptors reduces nerve injury-induced mechanical and thermal hypersensitivity. Pain 2010, 149, 483–494. [Google Scholar] [CrossRef]
- Reis, G.M.; Rossaneis, A.; Silveira, J.; Prado, W. μ1-and 5-HT1-dependent mechanisms in the anterior pretectal nucleus mediate the antinociceptive effects of retrosplenial cortex stimulation in rats. Life Sci. 2012, 90, 950–955. [Google Scholar] [CrossRef] [PubMed]
- Waeber, C.; Died, M.; Hoyer, D.; Palacios, J. 5. HT 1 receptors in the vertebrate brain. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1989, 340, 486–494. [Google Scholar]
- McAllister, G.; Charlesworth, A.; Snodin, C.; Beer, M.; Noble, A.; Middlemiss, D.; Iversen, L.; Whiting, P. Molecular cloning of a serotonin receptor from human brain (5HT1E): A fifth 5HT1-like subtype. Proc. Natl. Acad. Sci. USA 1992, 89, 5517–5521. [Google Scholar] [CrossRef] [PubMed]
- Agosti, R.M. 5HT1F-and 5HT7-receptor agonists for the treatment of migraines. CNS Neurol. Disord. Drug Targets 2007, 6, 235–237. [Google Scholar] [CrossRef]
- Ohno, Y. Serotonin receptors as the therapeutic target for central nervous system disorders. In Serotonin; Elsevier: Amsterdam, The Netherlands, 2019; pp. 369–390. [Google Scholar]
- Ji, R.-R.; Kawasaki, Y.; Zhuang, Z.-Y.; Wen, Y.-R.; Zhang, Y.-Q. Protein kinases as potential targets for the treatment of pathological pain. In Analgesia; Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2006; pp. 359–389. [Google Scholar]
- Lanfumey, L.; Hamon, M. Central 5-HT1A receptors: Regional distribution and functional characteristics. Nucl. Med. Biol. 2000, 27, 429–435. [Google Scholar] [CrossRef]
- Otoshi, C.K.; Walwyn, W.M.; Tillakaratne, N.J.; Zhong, H.; Roy, R.R.; Edgerton, V.R. Distribution and localization of 5-HT1A receptors in the rat lumbar spinal cord after transection and deafferentation. J. Neurotrauma 2009, 26, 575–584. [Google Scholar] [CrossRef]
- Millan, M.J. Descending control of pain. Prog. Neurobiol. 2002, 66, 355–474. [Google Scholar] [CrossRef]
- Nichols, D.E.; Nichols, C.D. Serotonin receptors. Chem. Rev. 2008, 108, 1614–1641. [Google Scholar] [CrossRef]
- Guo, Z.G.; Jia, X.P.; Su, X.J.; Li, P.; Hao, J.H. Gastrodin attenuates vincristine-induced mechanical hyperalgesia through serotonin 5-HT1A receptors. Bangladesh J. Pharmacol. 2013, 8, 414–419. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; Yin, D.; Chen, L.; Qu, W.-M.; Chen, C.-R.; Laudon, M.; Cheng, N.-N.; Urade, Y.; Huang, Z.-L. Piromelatine exerts antinociceptive effect via melatonin, opioid, and 5HT 1A receptors and hypnotic effect via melatonin receptors in a mouse model of neuropathic pain. Psychopharmacology 2014, 231, 3973–3985. [Google Scholar] [CrossRef]
- Bartsch, T.; Knight, Y.E.; Goadsby, P.J. Activation of 5-HT1B/1D receptor in the periaqueductal gray inhibits nociception. Ann. Neurol. 2004, 56, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Kayser, V.; Aubel, B.; Hamon, M.; Bourgoin, S. The antimigraine 5-HT1B/1D receptor agonists, sumatriptan, zolmitriptan and dihydroergotamine, attenuate pain-related behaviour in a rat model of trigeminal neuropathic pain. Br. J. Pharmacol. 2002, 137, 1287–1297. [Google Scholar] [CrossRef] [PubMed]
- Masson, J.; Emerit, M.B.; Hamon, M.; Darmon, M. Serotonergic signaling: Multiple effectors and pleiotropic effects. Wiley Interdiscip. Rev. Membr. Transp. Signal. 2012, 1, 685–713. [Google Scholar] [CrossRef]
- Muñoz-Islas, E.; Lozano-Cuenca, J.; González-Hernández, A.; Ramírez-Rosas, M.B.; Sánchez-López, A.; Centurión, D.; MaassenVanDenBrink, A.; Villalón, C.M. Spinal sumatriptan inhibits capsaicin-induced canine external carotid vasodilatation via 5-HT1B rather than 5-HT1D receptors. Eur. J. Pharmacol. 2009, 615, 133–138. [Google Scholar] [CrossRef]
- Virk, M.S.; Sagi, Y.; Medrihan, L.; Leung, J.; Kaplitt, M.G.; Greengard, P. Opposing roles for serotonin in cholinergic neurons of the ventral and dorsal striatum. Proc. Natl. Acad. Sci. USA 2016, 113, 734–739. [Google Scholar] [CrossRef]
- Yevtushenko, O.O.; Reynolds, G.P. Functional pharmacogenetics of serotonin receptors in psychiatric drug action. In Handbook of Behavioral Neuroscience; Elsevier: Amsterdam, The Netherlands, 2010; Volume 21, pp. 791–806. [Google Scholar]
- Obata, H.; Saito, S.; Sakurazawa, S.; Sasaki, M.; Usui, T.; Goto, F. Antiallodynic effects of intrathecally administered 5-HT2C receptor agonists in rats with nerve injury. Pain 2004, 108, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Cervantes-Durán, C.; Vidal-Cantú, G.C.; Godínez-Chaparro, B.; Granados-Soto, V. Role of spinal 5-HT2 receptors subtypes in formalin-induced long-lasting hypersensitivity. Pharmacol. Rep. 2016, 68, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Aira, Z.; Buesa, I.; Salgueiro, M.; Bilbao, J.; Aguilera, L.; Zimmermann, M.; Azkue, J. Subtype-specific changes in 5-HT receptor-mediated modulation of C fibre-evoked spinal field potentials are triggered by peripheral nerve injury. Neuroscience 2010, 168, 831–841. [Google Scholar] [CrossRef]
- Cervantes-Durán, C.; Pineda-Farias, J.; Bravo-Hernández, M.; Quiñonez-Bastidas, G.; Vidal-Cantú, G.; Barragán-Iglesias, P.; Granados-Soto, V. Evidence for the participation of peripheral 5-HT2A, 5-HT2B, and 5-HT2C receptors in formalin-induced secondary mechanical allodynia and hyperalgesia. Neuroscience 2013, 232, 169–181. [Google Scholar] [CrossRef]
- Faerber, L.; Drechsler, S.; Ladenburger, S.; Gschaidmeier, H.; Fischer, W. The neuronal 5-HT3 receptor network after 20 years of research—Evolving concepts in management of pain and inflammation. Eur. J. Pharmacol. 2007, 560, 1–8. [Google Scholar] [CrossRef]
- Bannister, K.; Bee, L.A.; Dickenson, A.H. Preclinical and early clinical investigations related to monoaminergic pain modulation. Neurotherapeutics 2009, 6, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.-J.; Uta, D.; Feng, P.-Y.; Wakita, M.; Shin, M.-C.; Furue, H.; Yoshimura, M. Identification of 5-HT receptor subtypes enhancing inhibitory transmission in the rat spinal dorsal horn in vitro. Mol. Pain 2012, 8. [Google Scholar] [CrossRef] [PubMed]
- Färber, L.; Haus, U.; Späth, M.; Drechsler, S. Physiology and pathophysiology of the 5-HT3 receptor. Scand. J. Rheumatol. 2004, 33, 2–8. [Google Scholar] [CrossRef]
- Kawamata, T.; Omote, K.; Toriyabe, M.; Yamamoto, H.; Namiki, A. The activation of 5-HT3 receptors evokes GABA release in the spinal cord. Brain Res. 2003, 978, 250–255. [Google Scholar] [CrossRef]
- Fukushima, T.; Ohtsubo, T.; Tsuda, M.; Yanagawa, Y.; Hori, Y. Facilitatory actions of serotonin type 3 receptors on GABAergic inhibitory synaptic transmission in the spinal superficial dorsal horn. J. Neurophysiol. 2009, 102, 1459–1471. [Google Scholar] [CrossRef] [PubMed]
- Finn, D.P.; Fone, K.C.; Beckett, S.R.; Baxter, J.A.; Ansell, L.; Marsden, C.A.; Chapman, V. The effects of pharmacological blockade of the 5-HT6 receptor on formalin-evoked nociceptive behaviour, locomotor activity and hypothalamo–pituitary–adrenal axis activity in rats. Eur. J. Pharmacol. 2007, 569, 59–63. [Google Scholar] [CrossRef]
- Brenchat, A.; Romero, L.; García, M.; Pujol, M.; Burgueño, J.; Torrens, A.; Hamon, M.; Baeyens, J.M.; Buschmann, H.; Zamanillo, D. 5-HT7 receptor activation inhibits mechanical hypersensitivity secondary to capsaicin sensitization in mice. Pain 2009, 141, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Taiwo, Y.; Levine, J. Serotonin is a directly-acting hyperalgesic agent in the rat. Neuroscience 1992, 48, 485–490. [Google Scholar] [CrossRef]
- Liu, Q.; Yao, X.; Gao, S.; Li, R.; Li, B.; Yang, W.; Cui, R. Role of 5-HT receptors in neuropathic pain: Potential therapeutic implications. Pharmacol. Res. 2020, 159, 104949. [Google Scholar] [CrossRef]
- Godínez-Chaparro, B.; Barragán-Iglesias, P.; Castañeda-Corral, G.; Rocha-González, H.I.; Granados-Soto, V. Role of peripheral 5-HT4, 5-HT6, and 5-HT7 receptors in development and maintenance of secondary mechanical allodynia and hyperalgesia. PAIN® 2011, 152, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Meuser, T.; Pietruck, C.; Gabriel, A.; Xie, G.-X.; Lim, K.-J.; Palmer, P.P. 5-HT7 receptors are involved in mediating 5-HT-induced activation of rat primary afferent neurons. Life Sci. 2002, 71, 2279–2289. [Google Scholar] [CrossRef]
- Berrocoso, E.; Mico, J.A. Role of serotonin 5-HT1A receptors in the antidepressant-like effect and the antinociceptive effect of venlafaxine in mice. Int. J. Neuropsychopharmacol. 2009, 12, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Sprouse, J.S.; Aghajanian, G.K. Electrophysiological responses of serotoninergic dorsal raphe neurons to 5-HT1A and 5-HT1B agonists. Synapse 1987, 1, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Pertovaara, A. 5-HT1A receptors in endogenous regulation of neuropathic hypersensitivity in the rat. Eur. J. Pharmacol. 2006, 535, 157–165. [Google Scholar] [CrossRef]
- Sommer, C.; Kress, M. Recent findings on how proinflammatory cytokines cause pain: Peripheral mechanisms in inflammatory and neuropathic hyperalgesia. Neurosci. Lett. 2004, 361, 184–187. [Google Scholar] [CrossRef]
- Huo, F.-Q.; Qu, C.-L.; Li, Y.-Q.; Tang, J.-S.; Jia, H. GABAergic modulation is involved in the ventrolateral orbital cortex 5-HT1A receptor activation-induced antinociception in the rat. Pain 2008, 139, 398–405. [Google Scholar] [CrossRef]
- Lin, B.; Wang, Y.; Zhang, P.; Yuan, Y.; Zhang, Y.; Chen, G. Gut microbiota regulates neuropathic pain: Potential mechanisms and therapeutic strategy. J. Headache Pain 2020, 21, 103. [Google Scholar] [CrossRef]
- Mawe, G.M.; Hoffman, J.M. Serotonin signalling in the gut—Functions, dysfunctions and therapeutic targets. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 473. [Google Scholar] [CrossRef]
- Spiller, R. Serotonin and GI clinical disorders. Neuropharmacology 2008, 55, 1072–1080. [Google Scholar] [CrossRef]
- Guo, R.; Chen, L.-H.; Xing, C.; Liu, T. Pain regulation by gut microbiota: Molecular mechanisms and therapeutic potential. Br. J. Anaesth. 2019, 123, 637–654. [Google Scholar] [CrossRef]
- Bennett, G.J.; Xie, Y.-K. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 1988, 33, 87–107. [Google Scholar] [CrossRef]
- Coudore, F.; Fialip, J.; Eschalier, A.; Lavarenne, J. Plasma and brain pharmacokinetics of amitriptyline and its demethylated and hydroxylated metabolites after acute intraperitoneal injection in mice. Eur. J. Drug Metab. Pharmacokinet. 1994, 19, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Bonin, R.P.; Bories, C.; De Koninck, Y. A simplified up-down method (SUDO) for measuring mechanical nociception in rodents using von Frey filaments. Mol. Pain 2014, 10. [Google Scholar] [CrossRef] [PubMed]
- Erdinc, M.; Uyar, E.; Kelle, I.; Akkoc, H. Anti-nociceptive effects of low dose ketamine in mice may be mediated by the serotonergic systems. Psychiatry Clin. Psychopharmacol. 2019, 29, 252–256. [Google Scholar] [CrossRef]
- Wang, R.; Xu, Y.; Wu, H.-L.; Li, Y.-B.; Li, Y.-H.; Guo, J.-B.; Li, X.-J. The antidepressant effects of curcumin in the forced swimming test involve 5-HT1 and 5-HT2 receptors. Eur. J. Pharmacol. 2008, 578, 43–50. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaswan, N.K.; Mohammed Izham, N.A.B.; Tengku Mohamad, T.A.S.; Sulaiman, M.R.; Perimal, E.K. Cardamonin Modulates Neuropathic Pain through the Possible Involvement of Serotonergic 5-HT1A Receptor Pathway in CCI-Induced Neuropathic Pain Mice Model. Molecules 2021, 26, 3677. https://doi.org/10.3390/molecules26123677
Kaswan NK, Mohammed Izham NAB, Tengku Mohamad TAS, Sulaiman MR, Perimal EK. Cardamonin Modulates Neuropathic Pain through the Possible Involvement of Serotonergic 5-HT1A Receptor Pathway in CCI-Induced Neuropathic Pain Mice Model. Molecules. 2021; 26(12):3677. https://doi.org/10.3390/molecules26123677
Chicago/Turabian StyleKaswan, Nur Khalisah, Noor Aishah Binti Mohammed Izham, Tengku Azam Shah Tengku Mohamad, Mohd Roslan Sulaiman, and Enoch Kumar Perimal. 2021. "Cardamonin Modulates Neuropathic Pain through the Possible Involvement of Serotonergic 5-HT1A Receptor Pathway in CCI-Induced Neuropathic Pain Mice Model" Molecules 26, no. 12: 3677. https://doi.org/10.3390/molecules26123677
APA StyleKaswan, N. K., Mohammed Izham, N. A. B., Tengku Mohamad, T. A. S., Sulaiman, M. R., & Perimal, E. K. (2021). Cardamonin Modulates Neuropathic Pain through the Possible Involvement of Serotonergic 5-HT1A Receptor Pathway in CCI-Induced Neuropathic Pain Mice Model. Molecules, 26(12), 3677. https://doi.org/10.3390/molecules26123677





