Rapid Gas Hydrate Formation—Evaluation of Three Reactor Concepts and Feasibility Study
Abstract
1. Introduction
- a gas-entry stirrer,
- spraying via a nozzle and a recycle pump,
- a combination from spraying with additional stirring (1 + 2).
2. Results and Discussion
2.1. Reactor Designs
2.2. Natural Gas Separation
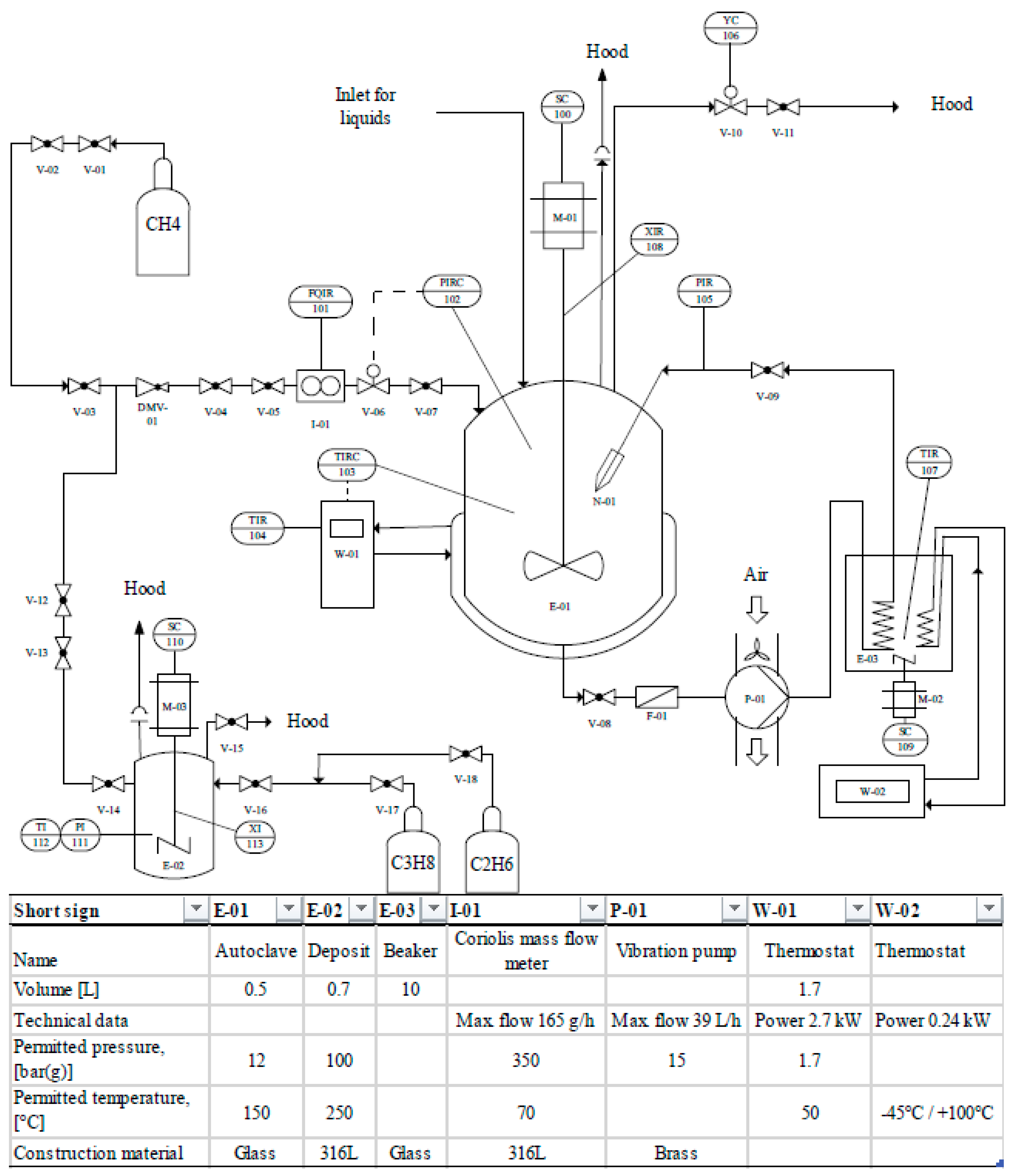
3. Materials and Methods
- Evaluate the optimal process for rapid gas hydrate formation under consideration of three different reactor concepts;
- Examine the feasibility of the rapid hydrate formation from gas mixtures for the application in (natural) gas storage and conditioning.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| CH4 | methane |
| C2H6 | ethane |
| C3H8 | propane |
| eq | equilibrium |
| GC | gas chromatography |
| i | gas component in simulated natural gas |
| j | summation index |
| rpm | rounds per minute |
| rps | rounds per second |
| sI | structure I hydrate |
| sII | structure II hydrate |
| S | Stirrer |
| SDS | sodium dodecyl sulfate |
| THF | tetrahydrofuran |
| Formula symbols: | |
| C | conversion (%) |
| d | diameter of stirrer (m) |
| D | inner diameter of reactor (m) |
| f | stirrer frequency (s−1) |
| m | mass (g) |
| M | molar mass (g/mol) |
| n | molar amount (mol) |
| ReS | stirrer Reynolds number (1) |
| S | selectivity (1) |
| x | mole fraction (1) |
| α | average separation factor (1) |
| ρ | density of hydrate forming liquid phase (kg/m3) |
| η | dynamic viscosity of hydrate forming liquid phase (Pa s) |
| Δp | pressure difference (bar) |
| ΔT | temperature difference (°C) |
References
- Sloan, E.D.; Koh, C.A. Clathrate Hydrates of Natural Gases, 3rd ed.; CRC Press Taylor & Francis: Boca Raton, FL, USA, 2008. [Google Scholar]
- Boswell, R.; Collett, T.S. Current perspectives on gas hydrate resources. Energy Environ. Sci. 2011, 4, 1206–1215. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, J.-C.; Li, X.-S.; Zhang, Y.; Chen, Z.-Y. Fluid flow mechanisms and heat transfer characteristics of gas recovery from gas-saturated and water-saturated hydrate reservoirs. Int. J. Heat Mass Transf. 2018, 118, 1115–1127. [Google Scholar] [CrossRef]
- Nair, V.C.; Prasad, S.K.; Kumar, R.; Sangwai, J.S. Energy recovery from simulated clayey gas hydrate reservoir using depressurization by constant rate gas release, thermal stimulation and their combinations. Appl. Energy 2018, 225, 755–768. [Google Scholar] [CrossRef]
- Janicki, G.; Schlüter, S.; Hennig, T.; Deerberg, G. Numerical simulation of gas hydrate exploitation from subsea reservoirs in the Black Sea. Energy Procedia 2017, 125, 467–476. [Google Scholar] [CrossRef]
- Schultz, H.J.; Fahlenkamp, H.; Deerberg, G. Simulation des Abbaus ozeanischer Gashydrate. Chem. Ing. Tech. 2004, 76, 751–754. [Google Scholar] [CrossRef]
- Zhan, L.; Wang, Y.; Li, X.-S. Experimental study on characteristics of methane hydrate formation and dissocia-tion in porous medium with different particle sizes using depressurization. Fuel 2018, 230, 37–44. [Google Scholar] [CrossRef]
- Schultz, H.J. Zum Gashydratabbau mittels Mammut-Pumpen-Prinzip; Fraunhofer IRB Verl.: Stuttgart, Germany, 2004. [Google Scholar]
- Merkel, F.S.; Schultz, H.J. Methane Extraction from Natural Gas Hydrate Reservoirs with Simultaneous Storage of Carbon Dioxide. Chem. Ing. Tech. 2015, 87, 475–483. [Google Scholar] [CrossRef]
- Chong, Z.R.; Yang, S.H.B.; Babu, P.; Linga, P.; Li, X.-S. Review of natural gas hydrates as an energy resource: Prospects and challenges. Appl. Energy 2016, 162, 1633–1652. [Google Scholar] [CrossRef]
- Lee, Y.; Seo, Y.; Ahn, T.; Lee, J.; Lee, J.Y.; Kim, S.-J.; Seo, Y. CH 4—Flue gas replacement occurring in sH hydrates and its significance for CH 4 recovery and CO 2 sequestration. Chem. Eng. J. 2017, 308, 50–58. [Google Scholar] [CrossRef]
- Lee, H.; Seo, Y.; Seo, Y.-T.; Moudrakovski, I.L.; Ripmeester, J.A. Recovering methane from solid methane hydrate with carbon dioxide. Angew. Chem. 2003, 42, 5048–5051. [Google Scholar] [CrossRef]
- Ruffine, L.; Broseta, D.; Desmedt, A. (Eds.) Gas Hydrates 2: Geoscience Issues and Potential Industrial Applications; John Wiley & Sons Inc.: Hoboken, NJ, USA, June 2018. [Google Scholar]
- Merkel, F.S.; Schmuck, C.; Schultz, H.J. Investigation of the Influence of Hydroxyl Groups on Gas Hydrate Formation at Pipeline-Like Conditions. Energy Fuels 2016, 30, 9141–9149. [Google Scholar] [CrossRef]
- Kelland, M.A. Production Chemicals for the Oil and Gas Industry, 2nd ed.; CRC Press Taylor & Francis: Boca Raton, FL, USA, 2009. [Google Scholar]
- Adamova, T.P.; Stoporev, A.S.; Semenov, A.P.; Kidyarov, B.I.; Manakov, A.Y. Methane hydrate nucleation on water—methane and water—Decane boundaries. Thermochim. Acta 2018, 668, 178–184. [Google Scholar] [CrossRef]
- Stoporev, A.S.; Svarovskaya, L.I.; Strelets, L.A.; Altunina, L.K.; Manakov, A.Y. Effect of reactor wall material on the nucleation of methane hydrate in water-in-oil emulsions. Mendeleev Commun. 2018, 28, 343–344. [Google Scholar] [CrossRef]
- Merkel, F.S.; Schmuck, C.; Schultz, H.J.; Scholz, T.A.; Wolinski, S. Research on Gas Hydrate Plug Formation under Pipeline-Like Conditions. Int. J. Chem. Eng. 2015, 2015, 1–5. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, X.-D.; Maeda, N. Synergism of Ethers on the Kinetic Inhibition Performance of Poly(N -vinyl pyrrolidone) on Methane Hydrate in a Pilot-Scale Flow Loop. Energy Fuels 2020, 34, 2790–2799. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, Y.; Zhang, J.; Pan, S.; Yu, J.; Sun, B. Flow assurance during deepwater gas well testing: Hydrate blockage prediction and prevention. J. Pet. Sci. Eng. 2018, 163, 211–216. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, J.; Zhang, J.; Liu, S.; Gao, Y.; Xiang, H.; Sun, B. Improved thermal model considering hydrate formation and deposition in gas-dominated systems with free water. Fuel 2019, 236, 870–879. [Google Scholar] [CrossRef]
- Semenov, A.P.; Mendgaziev, R.I.; Stoporev, A.S.; Istomin, V.A.; Sergeeva, D.V.; Ogienko, A.G.; Vinokurov, V.A. The pursuit of a more powerful thermodynamic hydrate inhibitor than methanol. Dimethyl sulfoxide as a case study. Chem. Eng. J. 2021, 423, 130227. [Google Scholar] [CrossRef]
- Maeda, N. Nucleation of Gas Hydrates, 1st ed.; Springer International Publishing: Cham, Germany, 2020. [Google Scholar]
- Maeda, N.; Shen, X.-D. Scaling laws for nucleation rates of gas hydrate. Fuel 2019, 253, 1597–1604. [Google Scholar] [CrossRef]
- Veluswamy, H.P.; Kumar, S.; Kumar, R.; Rangsunvigit, P.; Linga, P. Enhanced clathrate hydrate formation kinetics at near ambient temperatures and moderate pressures: Application to natural gas storage. Fuel 2016, 182, 907–919. [Google Scholar] [CrossRef]
- Veluswamy, H.P.; Kumar, A.; Kumar, R.; Linga, P. An innovative approach to enhance methane hydrate formation kinetics with leucine for energy storage application. Appl. Energy 2017, 188, 190–199. [Google Scholar] [CrossRef]
- Filarsky, F.; Schmuck, C.; Schultz, H.J. Development of a biogas production and purification process using promoted gas hydrate formation—A feasibility study. Chem. Eng. Res. Des. 2018, 134, 257–267. [Google Scholar] [CrossRef]
- Cai, J.; Zhang, Y.; Xu, C.-G.; Xia, Z.-M.; Chen, Z.-Y.; Li, X.-S. Raman spectroscopic studies on carbon dioxide separation from fuel gas via clathrate hydrate in the presence of tetrahydrofuran. Appl. Energy 2018, 214, 92–102. [Google Scholar] [CrossRef]
- Babu, P.; Nambiar, A.; He, T.; Karimi, I.A.; Lee, J.D.; Englezos, P.; Linga, P. A Review of Clathrate Hydrate Based Desalination to Strengthen Energy—Water Nexus. ACS Sustain. Chem. Eng. 2018, 6, 8093–8107. [Google Scholar] [CrossRef]
- Kang, K.C.; Linga, P.; Park, K.; Choi, S.-J.; Lee, J.D. Seawater desalination by gas hydrate process and removal characteristics of dissolved ions (Na+, K+, Mg2+, Ca2+, B3+, Cl−, SO42−). Desalination 2014, 353, 84–90. [Google Scholar] [CrossRef]
- Sun, Q.; Kang, Y.T. Review on CO2 hydrate formation/dissociation and its cold energy application. Renew. Sustain. Energy Rev. 2016, 62, 478–494. [Google Scholar] [CrossRef]
- Douzet, J.; Kwaterski, M.; Lallemand, A.; Chauvy, F.; Flick, D.; Herri, J.-M. Prototyping of a real size air-conditioning system using a tetra-n-butylammonium bromide semiclathrate hydrate slurry as secondary two-phase refrigerant—Experimental investigations and modelling. Int. J. Refrig. 2013, 36, 1616–1631. [Google Scholar] [CrossRef]
- Obara, S.; Mikawa, D. Electric power control of a power generator using dissociation expansion of a gas hydrate. Appl. Energy 2018, 222, 704–716. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Kawamura, T.; Yamamoto, Y.; Yoon, J.-H. Phase Equilibrium Studies of Tetrahydrofuran (THF) + CH4, THF + CO2, CH4 + CO2, and THF + CO2 + CH4 Hydrates. J. Chem. Eng. Data 2012, 57, 3543–3548. [Google Scholar] [CrossRef]
- Xu, C.-G.; Yu, Y.-S.; Ding, Y.-L.; Cai, J.; Li, X.-S. The effect of hydrate promoters on gas uptake. PCCP Phys. Chem. Chem. Phys. 2017, 19, 21769–21776. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Bhattacharjee, G.; Kulkarni, B.D.; Kumar, R. Role of Surfactants in Promoting Gas Hydrate Formation. Ind. Eng. Chem. Res. 2015, 54, 12217–12232. [Google Scholar] [CrossRef]
- Linga, P.; Daraboina, N.; Ripmeester, J.A.; Englezos, P. Enhanced rate of gas hydrate formation in a fixed bed column filled with sand compared to a stirred vessel. Chem. Eng. Sci. 2012, 68, 617–623. [Google Scholar] [CrossRef]
- Qureshi, M.F.; Atilhan, M.; Altamash, T.; Aparicio, S.; Aminnaji, M.; Tohidi, B. High-pressure gas hydrate auto-clave hydraulic experiments and scale-up modeling on the effect of stirring RPM effect. J. Nat. Gas Sci. Eng. 2017, 38, 50–58. [Google Scholar] [CrossRef]
- Filarsky, F.; Schmuck, C.; Schultz, H.J. Development of a Surface-Active Coating for Promoted Gas Hydrate Formation. Chem. Ing. Tech. 2019, 91, 85–91. [Google Scholar] [CrossRef]
- Linga, P.; Haligva, C.; Nam, S.C.; Ripmeester, J.A.; Englezos, P. Gas Hydrate Formation in a Variable Volume Bed of Silica Sand Particles. Energy Fuels 2009, 23, 5496–5507. [Google Scholar] [CrossRef]
- Filarsky, F.; Schmuck, C.; Schultz, H.J. Impact of Modified Silica Beads on Methane Hydrate Formation in a Fixed-Bed Reactor. Ind. Eng. Chem. Res. 2019, 58, 16687–16695. [Google Scholar] [CrossRef]
- Lang, X.; Fan, S.; Wang, Y. Intensification of methane and hydrogen storage in clathrate hydrate and future prospect. J. Nat. Gas Chem. 2010, 19, 203–209. [Google Scholar] [CrossRef]
- Xu, H.; Khan, M.N.; Peters, C.J.; Sloan, E.D.; Koh, C.A. Hydrate-Based Desalination Using Cyclopentane Hy-drates at Atmospheric Pressure. J. Chem. Eng. Data 2018, 63, 1081–1087. [Google Scholar] [CrossRef]
- Brown, T.D.; Taylor, C.E.; Bernardo, M.P. Rapid Gas Hydrate Formation Processes: Will They Work? Energies 2010, 3, 1154–1175. [Google Scholar] [CrossRef]
- Mori, Y. On the Scale-up of Gas-Hydrate-Forming Reactors: The Case of Gas-Dispersion-Type Reactors. Energies 2015, 8, 1317–1335. [Google Scholar] [CrossRef]
- Murakami, T.; Kuritsuka, H.; Fujii, H.; Mori, Y.H. Forming a Structure-H Hydrate Using Water and Methylcy-clohexane Jets Impinging on Each Other in a Methane Atmosphere. Energy Fuels 2009, 23, 1619–1625. [Google Scholar] [CrossRef]
- Kobayashi, T.; Imura, N.; Ohmura, R.; Mori, Y.H. Clathrate Hydrate Formation by Water Spraying in a Me-thane + Ethane + Propane Gas Mixture: Search for the Rate-Controlling Mechanism of Hydrate Formation in the Presence of Methylcyclohexane. Energy Fuels 2007, 21, 545–553. [Google Scholar] [CrossRef]
- Ohmura, R.; Kashiwazaki, S.; Shiota, S.; Tsuji, H.; Mori, Y.H. Structure-I and Structure-H Hydrate Formation Using Water Spraying. Energy Fuels 2002, 16, 1141–1147. [Google Scholar] [CrossRef]
- Lucia, B.; Castellani, B.; Rossi, F.; Cotana, F.; Morini, E.; Nicolini, A.; Filipponi, M. Experimental investigations on scaled-up methane hydrate production with surfactant promotion: Energy considerations. J. Pet. Sci. Eng. 2014, 120, 187–193. [Google Scholar] [CrossRef]
- Castellani, B.; Filipponi, M.; Nicolini, A.; Cotana, F.; Rossi, F. Carbon Dioxide Capture Using Gas Hydrate Technology. In Proceedings of the 25th International Conference on Efficinecy, Cost, Optimization, Simulation and Environmental Impact of Energy Systems, Perugia, Italy, 15–17 June 2012; p. 25. [Google Scholar]
- Kondo, W.; Ogawa, H.; Ohmura, R.; Mori, Y.H. Clathrate Hydrate Formation from a Hydrocarbon Gas Mixture: Evolution of Gas-Phase Composition in a Hydrate-Forming Reactor. Energy Fuels 2010, 24, 6375–6383. [Google Scholar] [CrossRef]
- Naeiji, P.; Mottahedin, M.; Varaminian, F. Separation of methane–ethane gas mixtures via gas hydrate formation. Sep. Purif. Technol. 2014, 123, 139–144. [Google Scholar] [CrossRef]
- Soltanimehr, S.; Javanmardi, J.; Nasrifar, K. Liquid Water-Hydrate-Vapor Equilibrium for Methane + Ethane Gas Mixtures: Application of Gas Hydrates for Separation. J. Chem. Eng. Data 2017, 62, 2143–2148. [Google Scholar] [CrossRef]
- Sun, C.-Y.; Chen, G.-J.; Zhang, L.-W. Hydrate phase equilibrium and structure for (methane + ethane + tetrahydrofuran + water) system. J. Chem. Thermodyn. 2010, 42, 1173–1179. [Google Scholar] [CrossRef]
- Ma, Q.; CHEN, G.; Zhang, L. Vapor-hydrate phases equilibrium of (CH4+C2H6) and (CH4+C2H4) systems. Pet. Sci. 2008, 5, 359–366. [Google Scholar] [CrossRef][Green Version]
- Zhang, L.-W.; Chen, G.-J.; Guo, X.-Q.; Sun, C.-Y.; Yang, L.-Y. The partition coefficients of ethane between vapor and hydrate phase for methane + ethane + water and methane + ethane + THF + water systems. Fluid Phase Equilibria 2004, 225, 141–144. [Google Scholar] [CrossRef]
- Stichlmair, J.G.; Fair, J.R. Distillation: Principles and Practices; Wiley-VCH: New York, NY, USA, 1998. [Google Scholar]
- VDI, e.V. VDI-Wärmeatlas: Mit 320 Tabellen, 11th ed.; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Bosch, H.; Haan, A.B. Industrial Separation Processes; DE GRUYTER: Berlin, Germany, 2013. [Google Scholar]
- Morita, K.; Nakano, S.; Ohgaki, K. Structure and stability of ethane hydrate crystal. Fluid Phase Equilibria 2000, 169, 167–175. [Google Scholar] [CrossRef]
- Darbouret, M.; Cournil, M.; Herri, J.-M. Rheological study of TBAB hydrate slurries as secondary two-phase refrigerants. Int. J. Refrig. 2005, 28, 663–671. [Google Scholar] [CrossRef]
- Luyben, W.L. NGL Demethanizer Control. Ind. Eng. Chem. Res. 2013, 52, 11626–11638. [Google Scholar] [CrossRef]
- Uchida, T.; Moriwaki, M.; Takeya, S.; Ikeda, I.Y.; Ohmura, R.; Nagao, J.; Minagawa, H.; Ebinuma, T.; Narita, H.; Gohara, K.; et al. Two-step formation of methane-propane mixed gas hydrates in a batch-type reactor. AIChE J. 2004, 50, 518–523. [Google Scholar] [CrossRef]
- Dong Lee, J.; Susilo, R.; Englezos, P. Methane-ethane and methane–propane hydrate formation and decomposition on water droplets. Chem. Eng. Sci. 2005, 60, 4203–4212. [Google Scholar] [CrossRef]
- Al-Otaibi, F.; Clarke, M.; Maini, B.; Bishnoi, P.R. Kinetics of structure II gas hydrate formation for propane and ethane using an in-situ particle size analyzer and a Raman spectrometer. Chem. Eng. Sci. 2011, 66, 2468–2474. [Google Scholar] [CrossRef]
- Kumar, R.; Linga, P.; Moudrakovski, I.; Ripmeester, J.A.; Englezos, P. Structure and kinetics of gas hydrates from methane/ethane/propane mixtures relevant to the design of natural gas hydrate storage and transport facilities. AIChE J. 2008, 54, 2132–2144. [Google Scholar] [CrossRef]
- Ando, N.; Kodama, T.; Kondo, W.; Mori, Y.H. Clathrate Hydrate Formation from a Methane + Ethane + Pro-pane Mixture in an Unstirred Surfactant-Containing System. Energy Fuels 2012, 26, 1798–1804. [Google Scholar] [CrossRef]

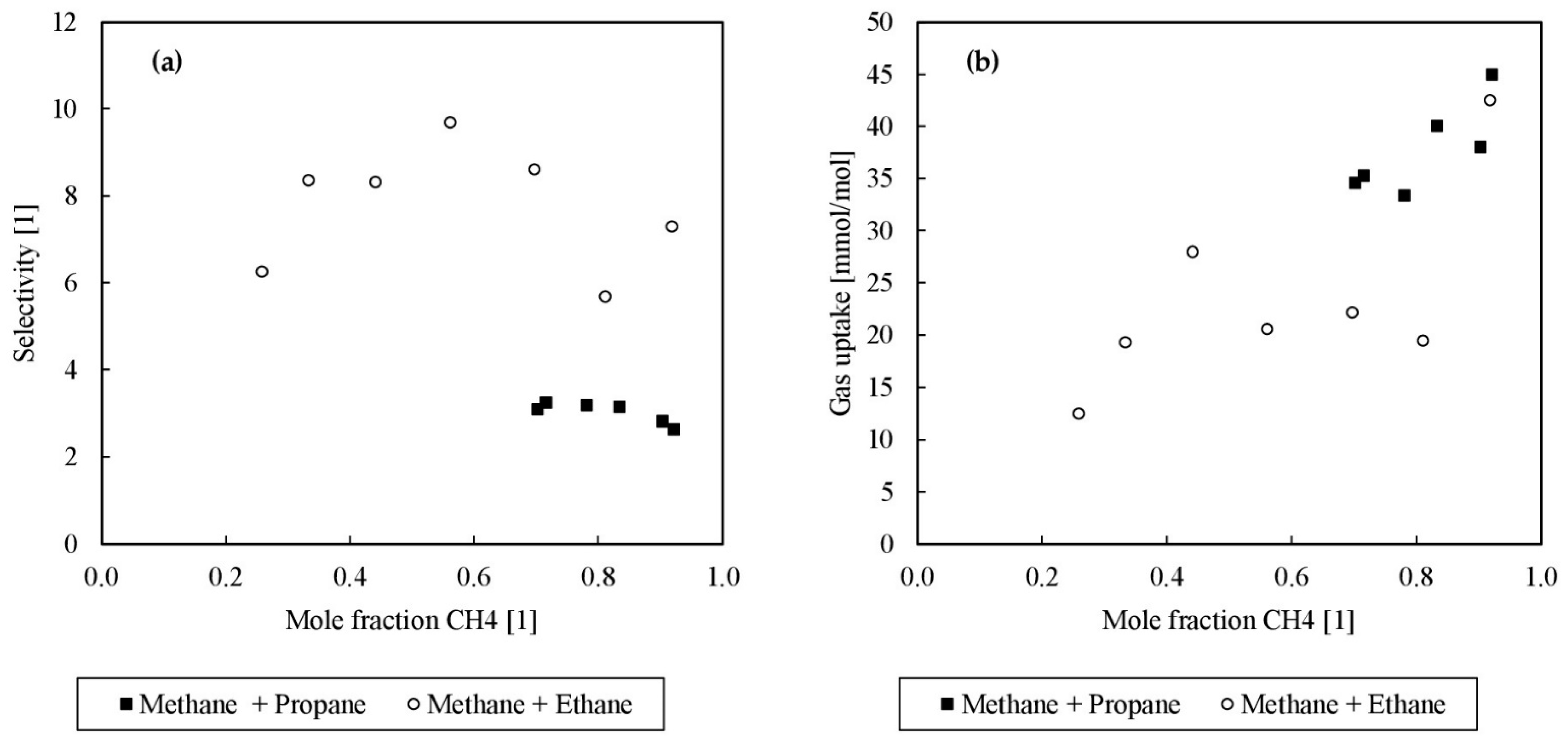
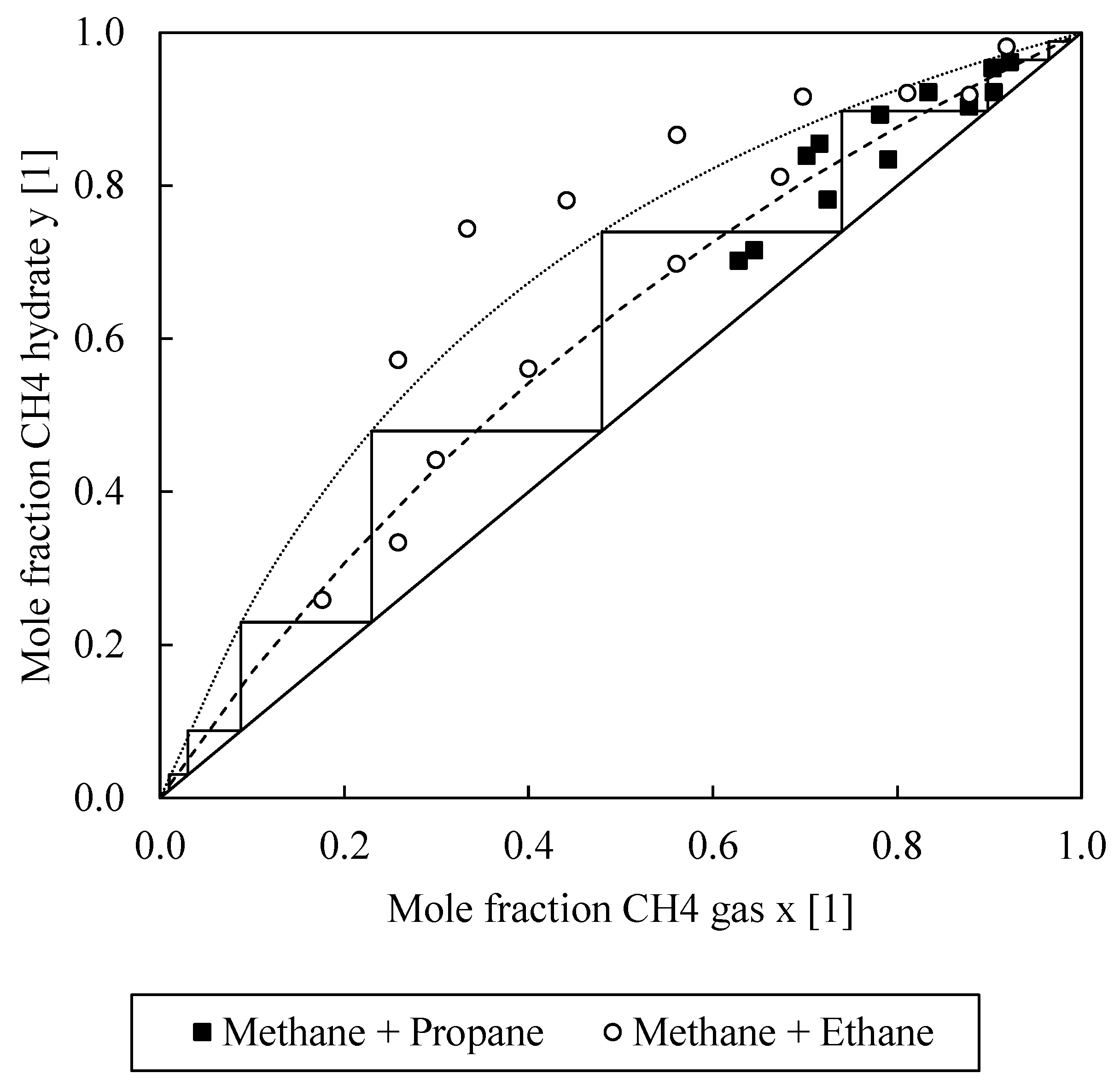

| Exp. | No. | Induction Time (s) | Ø Induction Time (s) | Final Gas Uptake (mmol/mol) | Ø Final Gas Uptake (mmol/mol) | Ø C (%) |
|---|---|---|---|---|---|---|
| Setup #1 Stirring | 1 | 287 | 218 ± 148 | 33.3 | 36.3 ± 4.0 | 30.8 ± 3.4 |
| 2 | 48 | 34.7 | ||||
| 3 | 319 | 40.9 | ||||
| Setup #2 Spraying | 1 | 0 | 60 ± 104 | 69.7 | 67.3 ± 2.2 | 57.0 ± 1.8 |
| 2 | 0 | 66.6 | ||||
| 3 | 180 | 65.5 | ||||
| Setup #3 Spraying + Stirring | 1 | 0 | 1 ± 1 | 93.9 | 95.5 ± 2.9 | 80.9 ± 2.5 |
| 2 | 2 | 98.9 | ||||
| 3 | 0 | 93.7 |
| Nr. | Initial Composition | Gas Phase | Hydrate Phase | Gas Uptake | C | Teq | S | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (mol%) | (mol%) | (mol%) | (mmol/mol) | (%) | (°C) | (1) | |||||||
| CH4 | C2H6 | C3H8 | CH4 | C2H6 | C3H8 | CH4 | C2H6 | C3H8 | |||||
| 1 | 0.92 | 0.08 | 0.90 | 0.10 | 0.96 | 0.04 | 44.97 | 38.22 | 14.24 | 2.63 | |||
| 2 | 0.90 | 0.10 | 0.88 | 0.12 | 0.95 | 0.05 | 38.01 | 32.31 | 14.15 | 2.81 | |||
| 3 | 0.83 | 0.17 | 0.79 | 0.21 | 0.92 | 0.08 | 40.04 | 34.04 | 13.63 | 3.14 | |||
| 4 | 0.78 | 0.22 | 0.72 | 0.28 | 0.89 | 0.11 | 33.34 | 28.34 | 14.11 | 3.18 | |||
| 5 | 0.72 | 0.28 | 0.64 | 0.36 | 0.85 | 0.15 | 35.26 | 29.97 | 13.74 | 3.24 | |||
| 6 | 0.70 | 0.30 | 0.63 | 0.37 | 0.84 | 0.16 | 34.58 | 29.39 | 13.45 | 3.08 | |||
| 7 | 0.92 | 0.08 | 0.88 | 0.12 | 0.98 | 0.02 | 42.50 | 36.13 | 13.45 | 7.29 | |||
| 8 | 0.81 | 0.19 | 0.67 | 0.33 | 0.92 | 0.08 | 19.44 | 16.53 | 13.20 | 5.67 | |||
| 9 | 0.70 | 0.30 | 0.56 | 0.44 | 0.92 | 0.08 | 22.17 | 18.84 | 12.80 | 8.60 | |||
| 10 | 0.56 | 0.44 | 0.40 | 0.60 | 0.87 | 0.13 | 20.58 | 17.49 | 10.91 | 9.68 | |||
| 11 | 0.44 | 0.56 | 0.30 | 0.70 | 0.78 | 0.22 | 27.96 | 23.77 | 10.29 | 8.31 | |||
| 12 | 0.33 | 0.67 | 0.26 | 0.74 | 0.74 | 0.26 | 19.28 | 16.39 | 9.78 | 8.35 | |||
| 13 | 0.26 | 0.74 | 0.18 | 0.82 | 0.57 | 0.43 | 12.44 | 10.57 | 8.74 | 6.25 | |||
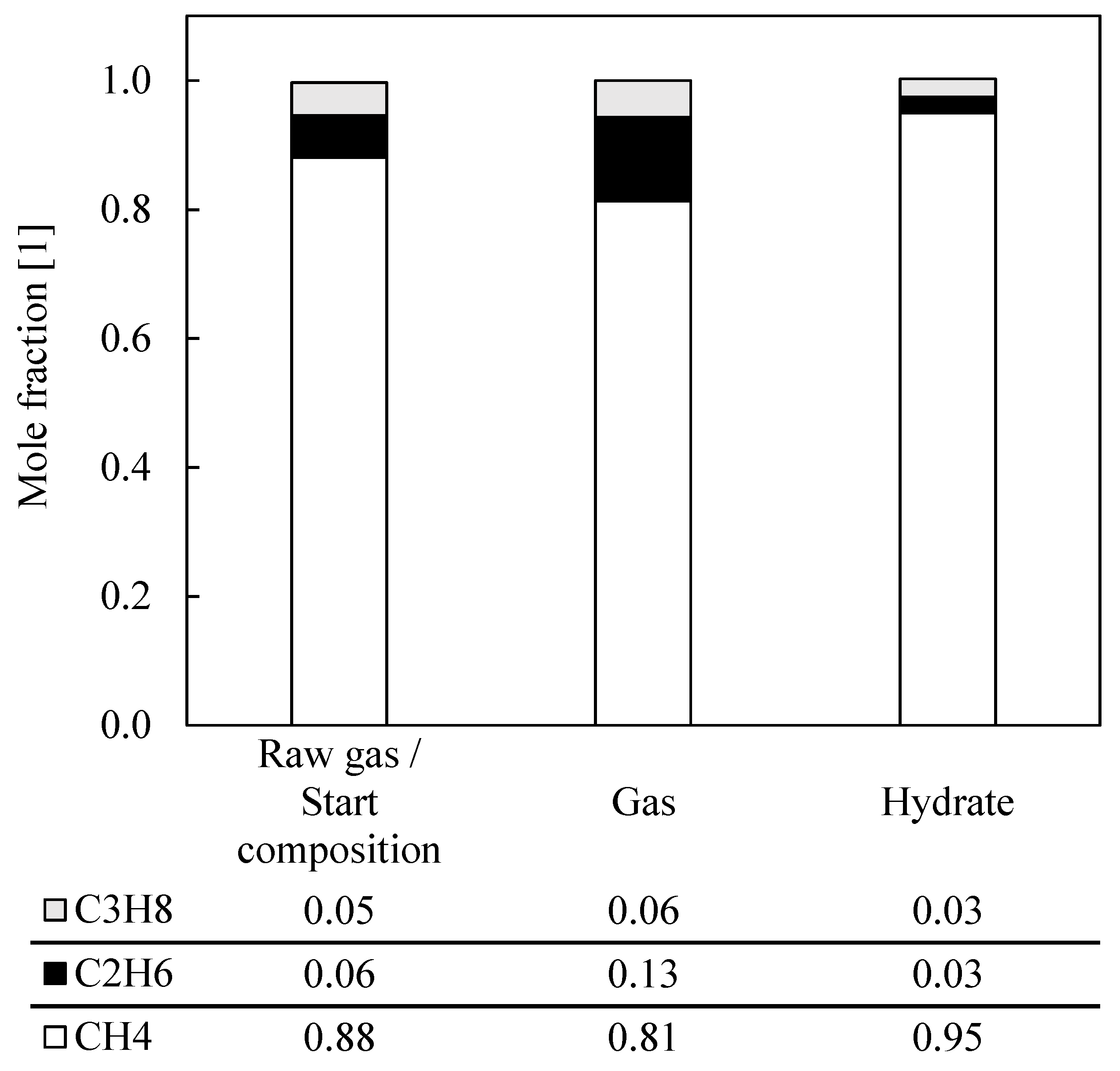
| 14 | 0.88 | 0.07 | 0.05 | 0.84 | 0.11 | 0.05 | 0.96 | 0.02 | 0.02 | 42.92 | 36.49 | 13.32 | 5.13 |
| 15 | 0.88 | 0.07 | 0.05 | 0.81 | 0.13 | 0.06 | 0.94 | 0.03 | 0.03 | 40.43 | 34.37 | 13.45 | 3.76 |
| 16 | 0.89 | 0.06 | 0.05 | 0.81 | 0.13 | 0.06 | 0.94 | 0.03 | 0.03 | 55.81 | 47.44 | 13.27 | 3.73 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filarsky, F.; Wieser, J.; Schultz, H.J. Rapid Gas Hydrate Formation—Evaluation of Three Reactor Concepts and Feasibility Study. Molecules 2021, 26, 3615. https://doi.org/10.3390/molecules26123615
Filarsky F, Wieser J, Schultz HJ. Rapid Gas Hydrate Formation—Evaluation of Three Reactor Concepts and Feasibility Study. Molecules. 2021; 26(12):3615. https://doi.org/10.3390/molecules26123615
Chicago/Turabian StyleFilarsky, Florian, Julian Wieser, and Heyko Juergen Schultz. 2021. "Rapid Gas Hydrate Formation—Evaluation of Three Reactor Concepts and Feasibility Study" Molecules 26, no. 12: 3615. https://doi.org/10.3390/molecules26123615
APA StyleFilarsky, F., Wieser, J., & Schultz, H. J. (2021). Rapid Gas Hydrate Formation—Evaluation of Three Reactor Concepts and Feasibility Study. Molecules, 26(12), 3615. https://doi.org/10.3390/molecules26123615







