1. Introduction
Spider plant (
Cleome gynandra L.) is an important indigenous leafy vegetable that is important particularly in the diet of many rural communities in Africa, including South Africa [
1]. It belongs to the family
Cleomaceae and it is widely distributed as a weed in tropical and subtropical regions of the world, including South Africa [
2]. This vegetable can play a vital role in food security and income generation particularly for marginalized rural communities in Africa. The vegetable is collected from the wild during the rainy season in many parts of the world [
3,
4]. However, in parts of East Africa, the vegetable is cultivated by smallholder growers [
5]. Compared to other leafy vegetables, it is highly nutritious and is classified as a functional food due to the presence of phenolic compounds that are beneficial for human health [
6].
In Africa, the consumption of indigenous leafy vegetables such as spider plant depends on a variety of factors such as poverty status and degree of urbanization [
4]. In general, the leafy indigenous vegetables are an inexpensive source of dietary minerals, trace elements, and antioxidant phytochemicals [
6]. The young tender leaves that are preferred by end-users are boiled and consumed as a side dish or dried for consumption during the off-season [
7]. Indigenous leafy vegetables are also rich in flavonoids, which have anti-inflammatory, antibacterial, antihistaminic, and antimutagenic properties. They also possess micro- and macro-elements.
Micronutrient deficiency is a huge problem that affects the health of many people worldwide [
8]. This is mainly due to changes in dietary preferences resulting from social economic development [
9]. In many African countries, there is a decline in the consumption of indigenous leafy vegetables such as the spider plant in exchange for exotic vegetables [
10]. Consumption of spider plant can be an alternative means to improve human health since the vegetable is rich in minerals such as, iron (Fe), manganese (Mn), and zinc (Zn).
The variation in the nutritional and phytochemical content of plant foods is influenced by a wide range of factors such as geographical location, season, cultivar, and physiological state [
11,
12]. A study conducted in South Africa showed variation in the nutritional content of spider plant across two provinces due to geographical and climatic factors [
6]. Conversely, a similar study carried out in Malawi showed no significant differences in protein, fiber, and vitamin C content of the vegetables [
7]. However, limited research has been conducted in South Africa on the nutritional composition of spider plant. Moreover, the studies utilized samples that were collected from the wild or purchased from the market [
6,
13,
14] or planted in the field [
15]. However, for those planted in the field, chicken and cattle manure were added [
15]. There is merit in evaluating the nutrient composition of this vegetable cultivated in fertilizer-free environments. Most of the studies conducted in South Africa concentrated largely on the mineral, vitamin, and total phenolics profiling of spider plant [
6,
13,
14,
15].
The spider plant is also rich in secondary metabolites such as glucosinolates and flavonoids [
11,
16]. Glucosinolates are the sulfur and nitrogen-containing secondary metabolites responsible for the bitter taste in cruciferous vegetables. Genetic and environmental factors contribute to the variation in the amount and pattern of glucosinolates [
17]. Flavonoids are the largest and most abundant group of secondary metabolites with marked antioxidant properties [
18]. Some of the properties of flavonoids include free radical scavenging, strong antioxidant activities in preventing oxidation, inhibition of low lipoproteins, inhibition of hydrolytic and oxidative enzymes, and anti-inflammatory actions [
19]. The determination of the radical scavenging activity of the spider plant using the 2,2 diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging activity method is dependent on concentration [
13] and time [
20]. The leaves of the spider plant also contain phenolic compounds and other phytochemicals that have health beneficial properties [
21]. Plant phenolics include phenolic acids, flavonoids, and condensed tannins.
Limited information is available on the quantification and identification of flavonoids of spider plant in South Africa. In addition, there is inadequate information regarding the variation in the mineral and phenolic content in spider plant in South Africa. Few studies utilized germplasm from different ecological regions in Kenya [
16] and in the Netherlands [
22]. Glucosinolates including glucocapparin, glucobrassicin, and 3-hydroxypropylglucosinolate as well as quercetin-3-O-rutinoside and several hydroxycinnamic acid derivatives were reported [
11,
16,
22]. The content and distribution of flavonoids among 91 edible plant species including spider plant were evaluated [
23]. Also, plant secondary metabolites in six selected species (African nightshade, spider plant, amaranth, cowpea, common kale, and Ethiopian kale) from East Africa were identified and quantified [
11]. The major flavonoids identified in the leaves and shoots of spider plant include quercetin, kaempferol, and isorhamnetin [
11,
16,
22,
23]. The objective of this study was to determine the nutritional composition in a wide range of spider plant genotypes that were collected from different agro-ecological zones in South Africa and exotic accessions from different countries as a prerequisite for their subsequent selection and genetic improvement. The study also identified and quantified the flavonoids in the leaves of the germplasm.
3. Discussion
The results that were reported showed strong evidence that the leaves of spider plant contained a wide range of minerals that are useful in the human diet. The concentration of both micro- and macro-elements in the leaf tissue of the vegetable was generally comparable with those reported previously in similar studies [
13,
16,
24]. Nonetheless, where differences exist in some individual minerals, it is likely due to the environmental factors such as edaphic attributes and management of the cropping systems. In the present study, the spider plants were raised without applying any fertilizers. In contrast, chemical [
25] and organic [
16] fertilizers were utilized in similar studies involving spider plant. Previous reports observed that the application of calcium ammonium nitrate fertilizers enhanced leaf calcium content and farmyard manure decreased Fe content but did not influence Zn or K content in spider plant [
26]. In addition, the difference in agro-ecological regions between this study and similar investigations probably contributed to some of the individual differences in individual mineral levels. There is a considerable degree of diversity and environmental variations in Sub-Saharan Africa particularly in soil moisture availability, temperatures, and cropping systems [
27].
Variation in mineral concentration between spider plant germplasm suggested that there is potential for the genetic improvement of the local germplasm in terms of leaf mineral content. Therefore, future genetic improvement activities aimed at enhancing the leaf mineral content in this vegetable will need to identify genotypes that are stable in mineral content in the target production areas. In addition, genotypes that were significantly higher in specific minerals could be utilized as source materials for introgressing such traits (also referred to as attributes or distinct plant characteristics) into the inferior genotypes. For instance, the two local genotypes (‘ML-13-SDM’ and ‘ML-12-TMP’) that were comparable to exotic genotypes ‘GPS’ and ’IP-3’ in TPC could be utilized in a breeding program aimed at enhancing this trait.
The leaf extract of the local spider plant genotypes (‘ML-6-BTK’, MP-B-1-CG’, and ‘MP-B-4-CG’) produced the highest FRAP values and were superior to both the exotic genotypes and all the standards. The DPPH and FRAP values in the leaf extract are an indication of the antioxidant potentials of the local and exotic spider plant genotypes showing that some of the local genotypes were superior in antioxidant properties. Therefore, the high antioxidant activities and ferric-reducing antioxidant power of the leaf extracts strongly supported the utilization of these vegetables. The relatively low antioxidant activity as measured by DPPH may be due to the difference in the concentration or dosage of the extract used. Previous studies reported that an increase in radical scavenging activity was dose or concentration dependent [
13].
This study observed that quercetin glycosides were present in spider plant and the three standards (cowpea, jute mallow, and pumpkin) but differed in their concentrations. This agreed with the findings from previous studies [
11,
23]. The present study also showed that quercetin was the most abundant flavonoid in the spider plant leaf tissue, but the flavonoid content was generally high in the genotypes originating from South Africa (‘MP-B-2-CG’ and ‘ML-6-BTK’). Quercetin represents the main flavonoid in our daily diets among the polyphenols [
28]. Quercetin is a versatile molecule with many pharmacological properties such as antioxidant, neurological, antiviral, anticancer, cardiovascular, and antimicrobial activities as well as the ability to protect the reproductive system [
29,
30,
31,
32]. Quercetin was found to have therapeutic potential for the treatment of breast cancer [
33]. In a study involving male rats, quercetin in conjunction with sulfasalazine-induced alterations in steroidogenic enzyme activity, which enhanced organ weights, sperm integrity, and plasma hormone management, among other beneficial activities [
34]. In addition, quercetin possessed antiviral activity during the early stage of infection by the influenza A virus [
35]. The absence of detectable levels of phenolic compounds in swiss chard but their abundance in spider plant suggested that indigenous leafy vegetables such as the spider plant maybe superior to commercial leafy vegetables in some of the nutritional attributes. Therefore, the consumption of both indigenous and non-indigenous leafy vegetable types can provide a relatively wider spectrum of valuable nutrients that are necessary for human health. Nonetheless, the absence of some of the flavonoids (such as 3-hydroxypropyl glucosinolate and 4-methoxyglucobrassicin) that were detected in spider plant in other studies [
11,
22] could be attributed to the limited number of standards that were used in the present study. Furthermore, in a previous study, alteration of plants regulatory network which led to an accumulation of flavonoid, was attributed to UV-B radiation [
36].
However, the presence of crotonoside (glycoside) in spider plant leaves was interesting in this study, since it is a potent tyrosine inhibitor with immunosuppressive effects on immune cells [
37], antitumor activity [
38], as well as selective inhibition in acute myeloid leukemia cells [
39]. The study showed a positive relationship between quercetin and the derivatives (quercetin-3-rutinoside and quercetin-3-β-
d-glucoside). The absence of a strong correlation between the antioxidant activity (using FRAP and DPPH) with the phenolic content agreed with the observations reported in a similar study involving the spider plant [
40]. This suggests that the antioxidant activity is not entirely influenced by the phenolic compounds but other factors such as the presence of non-phenolic compounds. Antioxidant activity is partially influenced by other non-phenolic compounds such as ascorbates, reducing carbohydrates, tocopherols, carotenoids, and terpenes probably acting synergistically to produce the total antioxidant activity [
41], and pigments as well as the synergistic effect among them could possibly contribute to the total antioxidant activity. The significant positive relationship between total phenolic content, quercetin, quercetin-3-β-
d-glucoside, and quercetin-3-rutinoside could be useful in selection programs that are aimed at the concomitant improvement of both total phenolic content and flavonoids in spider plant. On the other hand, a positive relationship that was observed between Cu and Zn could be attributed partly to the role of Cu as a cofactor of the antioxidant enzyme Cu, Zn-superoxide [
42].
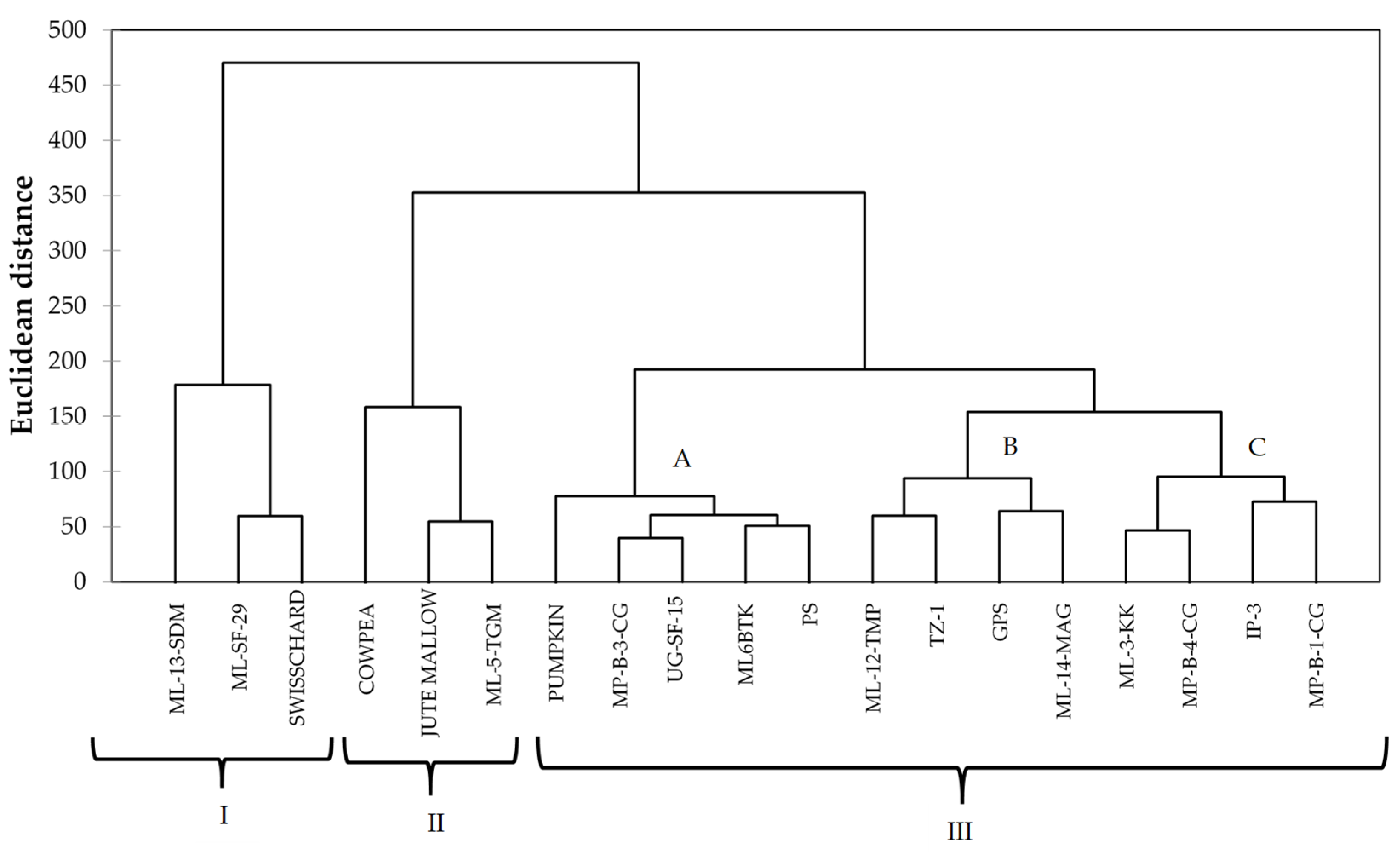
The PCA biplot showed a high level of similarity between the spider plant genotypes and the four standards. All the spider plant accessions were clustered together in the first and second quadrant, while the standards (swiss chard, jute mallow, cowpea, and pumpkin) were isolated from the spider plant genotypes. This is an indication that the indigenous leafy vegetable spider plant is superior in terms of the nutritional composition when compared to the standards in this study. Four exotic genotypes and three local genotypes were associated with high Al, Fe, Zn, N, and TPC and low Ca, TFC, and DPPH and were positively correlated with each other. These genotypes could be used for spider plant genetic improvement. The cluster analysis showed the diversity between spider plant genotypes from different agro-ecological regions and the four common leafy vegetables that were used as standards in the study. The clustering pattern showed that some of the exotic genotypes were similar to the local genotypes. The clusters showed three groups consisting of local genotypes from Limpopo ‘ML-12-TMP’ and ‘ML-14- MAG’ clustering with exotic genotypes ‘TZ-1’ and ‘GPS’ and indicating a high level of similarity. In cases where clusters consisted of both local and exotic genotypes, it indicated that some of the local genotypes are comparable to exotic genotypes in terms of the nutritional composition.