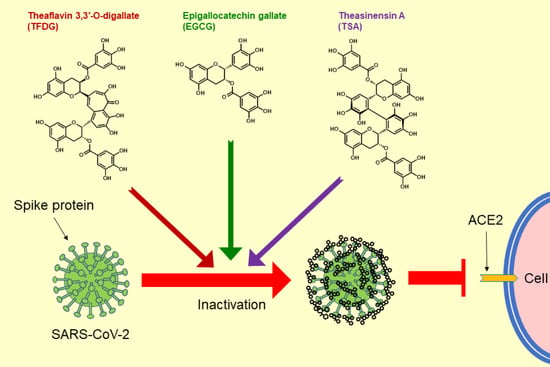
Significant Inactivation of SARS-CoV-2 In Vitro by a Green Tea Catechin, a Catechin-Derivative, and Black Tea Galloylated Theaflavins
Abstract
1. Introduction
2. Results
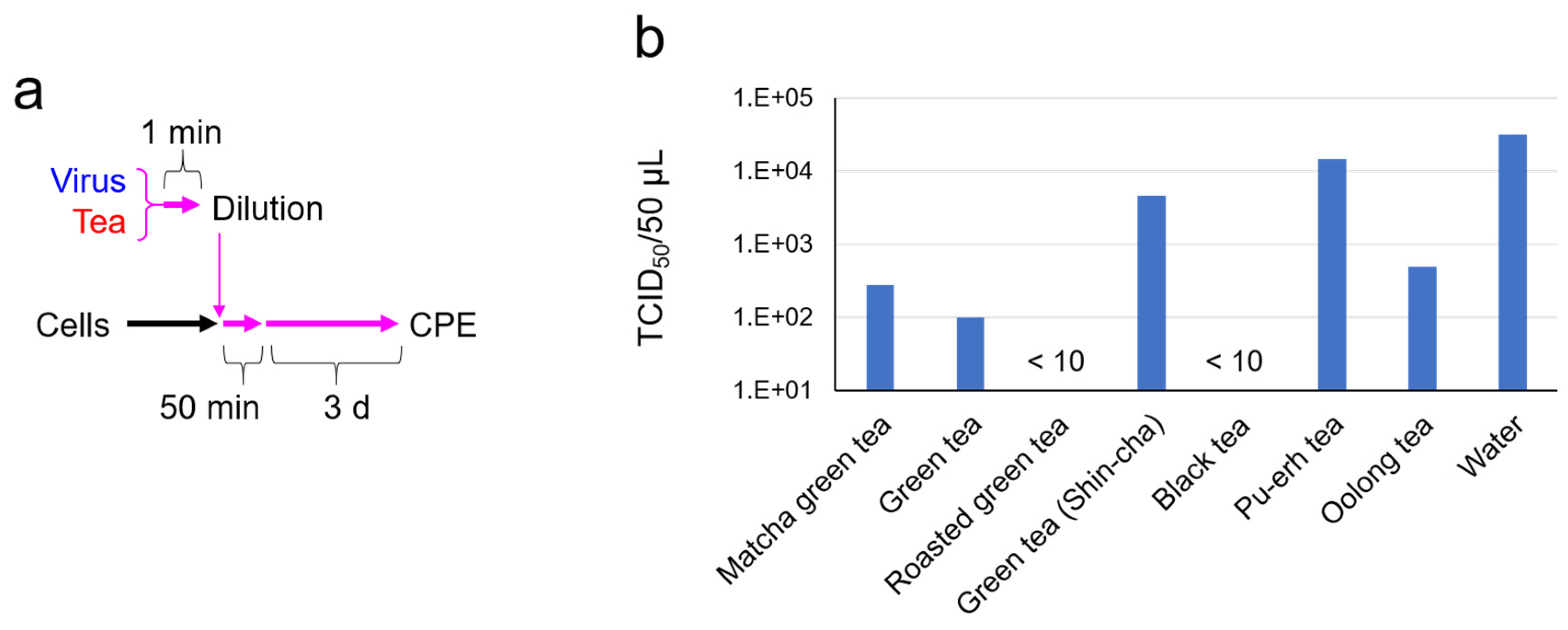
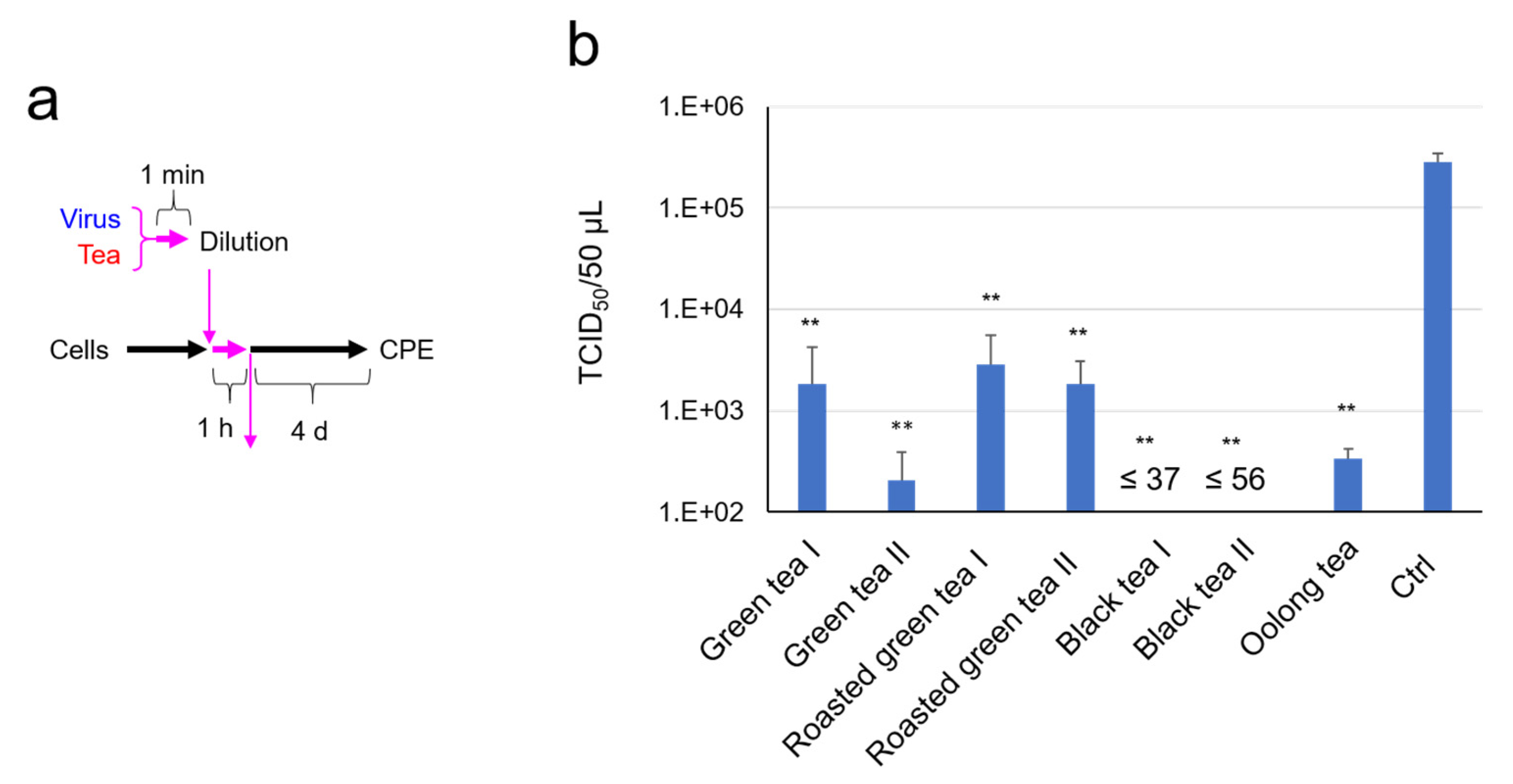
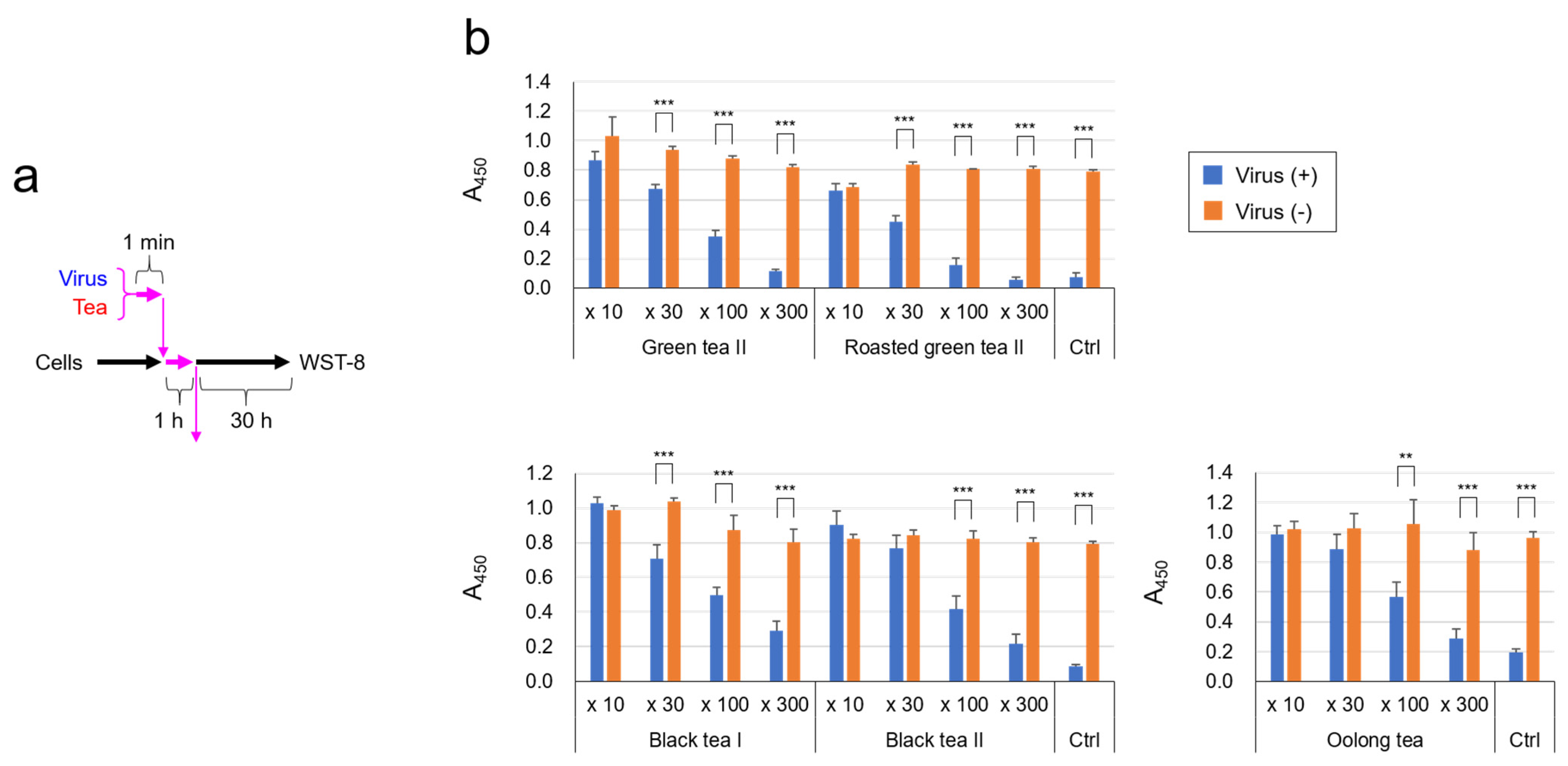
2.1. SARS-CoV-2 Was Significantly Inactivated by an Exposure to Tea
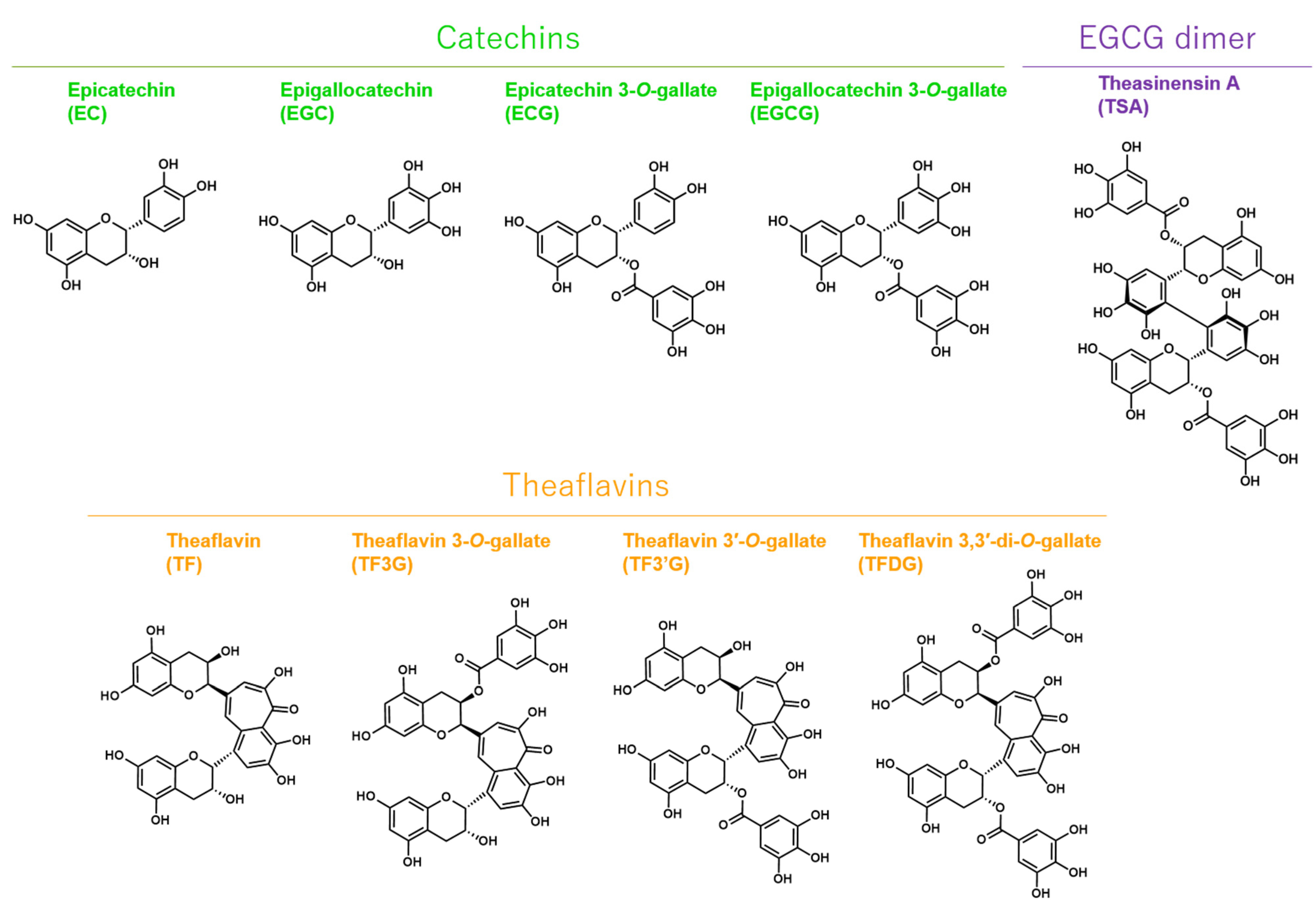
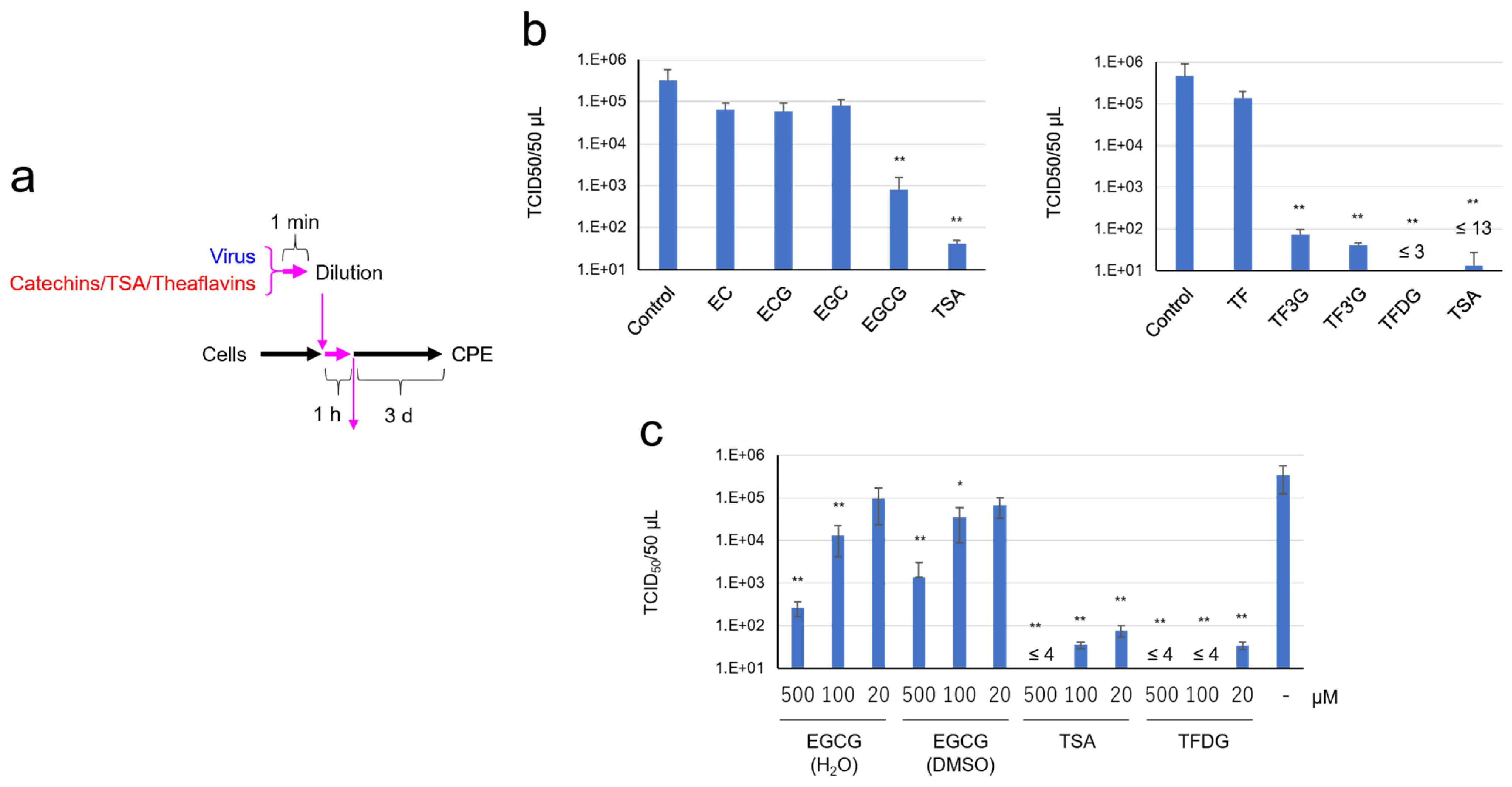
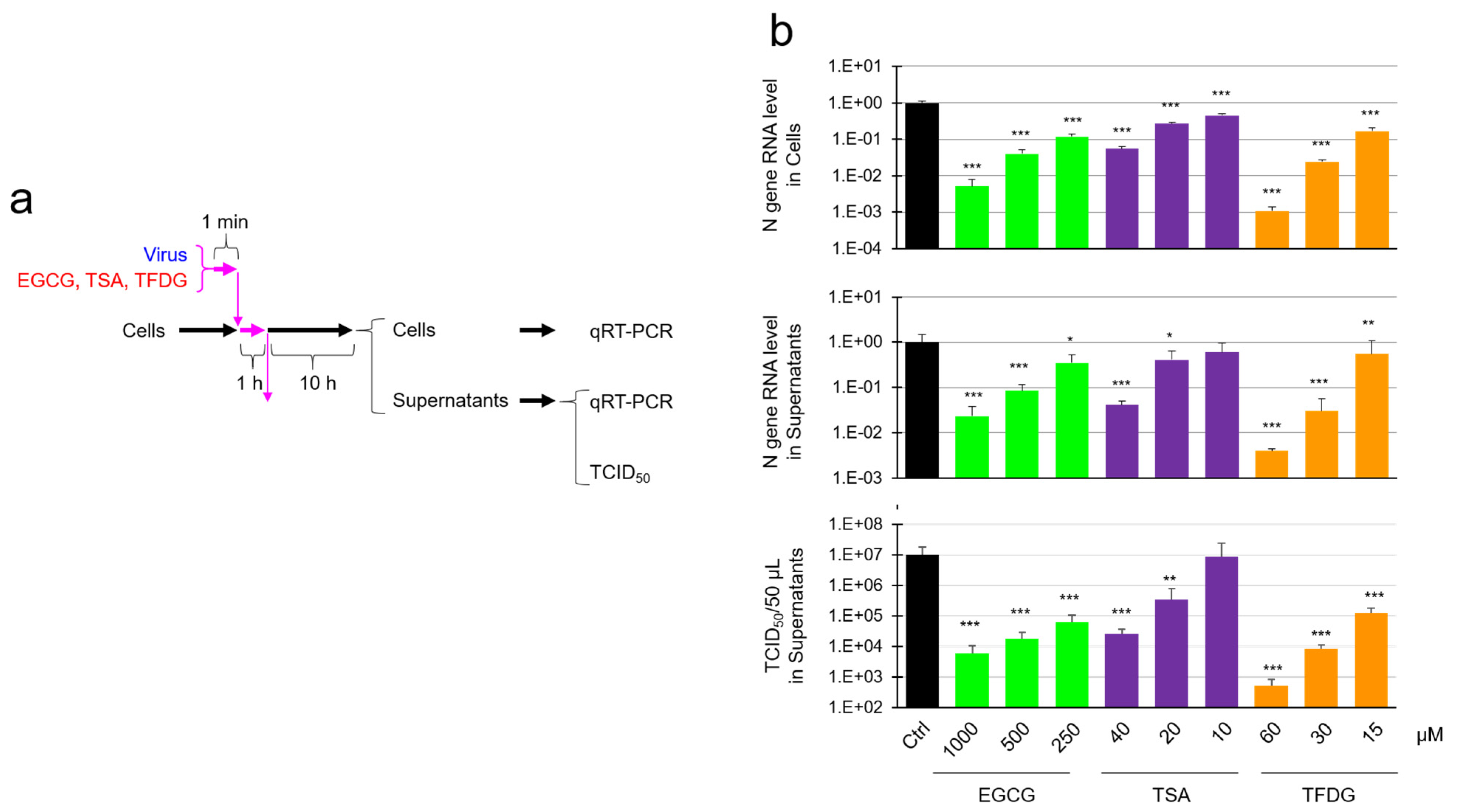
2.2. EGCG, TSA, and Galloylated Theaflavins Significantly Inactivated SARS-CoV-2
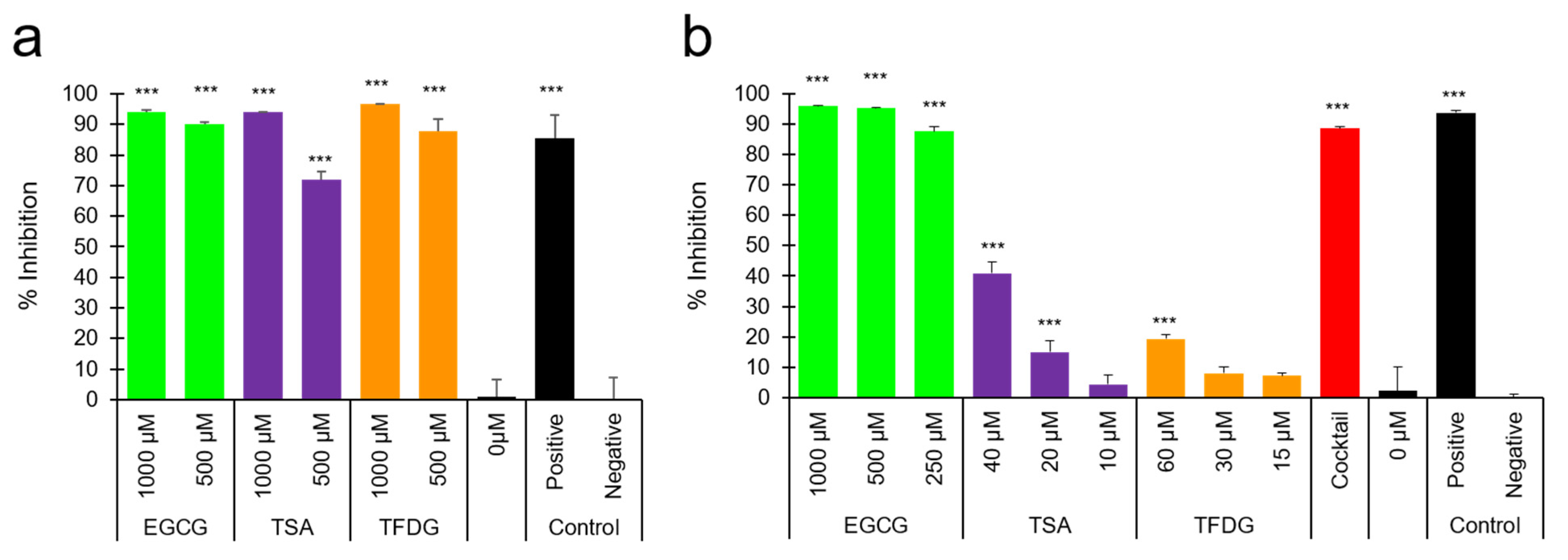
2.3. EGCG, TSA, and TFDG Blocked Interaction between ACE2 and RBD of Spike Protein
3. Discussion
4. Materials and Methods
4.1. Virus, Cells, and Culture Medium
4.2. Freeze-Dried Powders of Tea Extract
4.3. Catechins, Theaflavins, and a Catechin-Derivative
4.4. Measurement of Catechins, Theaflavins, and EGCG Dimers in Tea
4.5. TCID50 Assay for Virus Pretreated with Tea
4.6. Calculation of TCID50 Values
4.7. TCID50 Assay for Virus Pretreated with Catechins, a Catechin-Derivative, and Theaflavins
4.8. Cell Viability Assay for Virus Pretreated with Tea
4.9. Cell Viability Assay for Virus Pretreated with EGCG, TSA, and TFDG
4.10. Cell Viability Assay Using Cells Pretreated with EGCG, TSA, and TFDG
4.11. Real Time-RT-PCR
4.12. Neutralizing Assay
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Appendix A
| Catechins | Theaflavins | EGCG Dimers | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GC | EGC | C | EC | EGCG | GCG | ECg | Cg | TF | TF3G | TF3’G | TFDG | TSA | TSD | |
| Green tea I | 233 | 1213 | 120 | 440 | 1135 | 113 | 225 | 108 | - | - | - | - | N.D. | N.D. |
| Green tea II | 323 | 2053 | N.D. | 613 | 1173 | 98 | 235 | 95 | - | - | - | - | N.D. | N.D. |
| Roasted green tea I | 445 | 395 | 153 | 185 | 413 | 183 | 100 | 53 | - | - | - | - | N.D. | N.D. |
| Roasted green tea II | 370 | 240 | 103 | 95 | 255 | 198 | 73 | 70 | - | - | - | - | N.D. | N.D. |
| Oolong tea | 175 | 465 | 58 | 203 | 633 | 65 | 168 | 45 | N.D. | 1.8 | 2..3 | 3.6 | 2.0 | 7.8 |
| Black tea I | 48 | 73 | 80 | 93 | 240 | N.D. | 170 | 50 | 60.4 | 76.6 | 35.8 | 58.7 | 40.3 | 5.1 |
| Black tea II | 43 | 65 | 53 | 90 | 105 | N.D. | 130 | 73 | 51.9 | 57.1 | 21.7 | 29.2 | 29.8 | 2.0 |
References
- Hillen, H.S.; Kokic, G.; Farnung, L.; Dienemann, C.; Tegunov, D.; Cramer, P. Structure of replicating SARS-CoV-2 polymerase. Nature 2020, 584, 154–156. [Google Scholar] [CrossRef]
- Khan, N.; Mukhtar, H. Tea Polyphenols in Promotion of Human Health. Nutrients 2018, 11, 39. [Google Scholar] [CrossRef]
- Ohishi, T.; Goto, S.; Monira, P.; Isemura, M.; Nakamura, Y. Anti-inflammatory Action of Green Tea. Antiinflamm Antiallergy Agents Med. Chem. 2016, 15, 74–90. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.N.; Shankar, S.; Srivastava, R.K. Green tea catechin, epigallocatechin-3-gallate (EGCG): Mechanisms, perspectives and clinical applications. Biochem. Pharmacol. 2011, 82, 1807–1821. [Google Scholar] [CrossRef]
- Xu, J.; Xu, Z.; Zheng, W. A Review of the Antiviral Role of Green Tea Catechins. Molecules 2017, 22, 1337. [Google Scholar] [CrossRef]
- Ide, K.; Kawasaki, Y.; Kawakami, K.; Yamada, H. Anti-influenza Virus Effects of Catechins: A Molecular and Clinical Review. Curr. Med. Chem. 2016, 23, 4773–4783. [Google Scholar] [CrossRef]
- Hou, D.X.; Masuzaki, S.; Tanigawa, S.; Hashimoto, F.; Chen, J.; Sogo, T.; Fujii, M. Oolong tea theasinensins attenuate cyclooxygenase-2 expression in lipopolysaccharide (LPS)-activated mouse macrophages: Structure-activity relationship and molecular mechanisms. J. Agric. Food Chem. 2010, 58, 12735–12743. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Lu, H.; Meng, X.; Ryu, J.H.; Hara, Y.; Yang, C.S. Stability, cellular uptake, biotransformation, and efflux of tea polyphenol (-)-epigallocatechin-3-gallate in HT-29 human colon adenocarcinoma cells. Cancer Res. 2002, 62, 7241–7246. [Google Scholar]
- Sang, S.; Lee, M.J.; Hou, Z.; Ho, C.T.; Yang, C.S. Stability of tea polyphenol (-)-epigallocatechin-3-gallate and formation of dimers and epimers under common experimental conditions. J. Agric. Food Chem. 2005, 53, 9478–9484. [Google Scholar] [CrossRef] [PubMed]
- Butt, M.S.; Imran, A.; Sharif, M.K.; Ahmad, R.S.; Xiao, H.; Imran, M.; Rsool, H.A. Black tea polyphenols: A mechanistic treatise. Crit. Rev. Food Sci. Nutr. 2014, 54, 1002–1011. [Google Scholar] [CrossRef]
- Peluso, I.; Serafini, M. Antioxidants from black and green tea: From dietary modulation of oxidative stress to pharmacological mechanisms. Br. J. Pharmacol. 2017, 174, 1195–1208. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives; Nutrient Sources Added to Food; Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipic, M.; Frutos, M.J.; Galtier, P.; et al. Scientific opinion on the safety of green tea catechins. EFSA J. 2018, 16, e05239. [Google Scholar] [CrossRef] [PubMed]
- Hisanaga, A.; Ishida, H.; Sakao, K.; Sogo, T.; Kumamoto, T.; Hashimoto, F.; Hou, D.X. Anti-inflammatory activity and molecular mechanism of Oolong tea theasinensin. Food Funct. 2014, 5, 1891–1897. [Google Scholar] [CrossRef]
- Abe, I.; Umehara, K.; Morita, R.; Nemoto, K.; Degawa, M.; Noguchi, H. Green tea polyphenols as potent enhancers of glucocorticoid-induced mouse mammary tumor virus gene expression. Biochem. Biophys. Res. Commun. 2001, 281, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Naveed, M.; BiBi, J.; Kamboh, A.A.; Suheryani, I.; Kakar, I.; Fazlani, S.A.; FangFang, X.; Kalhoro, S.A.; Yunjuan, L.; Kakar, M.U.; et al. Pharmacological values and therapeutic properties of black tea (Camellia sinensis): A comprehensive overview. Biomed. Pharmacother. 2018, 100, 521–531. [Google Scholar] [CrossRef]
- Takemoto, M.; Takemoto, H. Synthesis of Theaflavins and Their Functions. Molecules 2018, 23, 918. [Google Scholar] [CrossRef] [PubMed]
- Ohgitani, E.; Shin-Ya, M.; Ichitani, M.; Kobayashi, M.; Takihara, T.; Kawamoto, M.; Kinugasa, H.; Mazda, O. Rapid inactivation in vitro of SARS-CoV-2 in saliva by black tea and green tea. bioRxiv 2020. [Google Scholar] [CrossRef]
- Lee, M.J.; Maliakal, P.; Chen, L.; Meng, X.; Bondoc, F.Y.; Prabhu, S.; Lambert, G.; Mohr, S.; Yang, C.S. Pharmacokinetics of tea catechins after ingestion of green tea and (-)-epigallocatechin-3-gallate by humans: Formation of different metabolites and individual variability. Cancer Epidemiol. Biomark. Prev. 2002, 11, 1025–1032. [Google Scholar]
- Cai, Z.Y.; Li, X.M.; Liang, J.P.; Xiang, L.P.; Wang, K.R.; Shi, Y.L.; Yang, R.; Shi, M.; Ye, J.H.; Lu, J.L.; et al. Bioavailability of Tea Catechins and Its Improvement. Molecules 2018, 23, 2346. [Google Scholar] [CrossRef]
- Pereira-Caro, G.; Moreno-Rojas, J.M.; Brindani, N.; Del Rio, D.; Lean, M.E.J.; Hara, Y.; Crozier, A. Bioavailability of Black Tea Theaflavins: Absorption, Metabolism, and Colonic Catabolism. J. Agric. Food Chem. 2017, 65, 5365–5374. [Google Scholar] [CrossRef]
- Scholl, C.; Lepper, A.; Lehr, T.; Hanke, N.; Schneider, K.L.; Brockmoller, J.; Seufferlein, T.; Stingl, J.C. Population nutrikinetics of green tea extract. PLoS ONE 2018, 13, e0193074. [Google Scholar] [CrossRef] [PubMed]
- Mulder, T.P.; van Platerink, C.J.; Wijnand Schuyl, P.J.; van Amelsvoort, J.M. Analysis of theaflavins in biological fluids using liquid chromatography-electrospray mass spectrometry. J. Chromatogr. B Biomed. Sci. Appl. 2001, 760, 271–279. [Google Scholar] [CrossRef]
- Hu, J.; Webster, D.; Cao, J.; Shao, A. The safety of green tea and green tea extract consumption in adults-Results of a systematic review. Regul. Toxicol. Pharmacol. 2018, 95, 412–433. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, S.; Nao, N.; Shirato, K.; Kawase, M.; Saito, S.; Takayama, I.; Nagata, N.; Sekizuka, T.; Katoh, H.; Kato, F.; et al. Enhanced isolation of SARS-CoV-2 by TMPRSS2-expressing cells. Proc. Natl. Acad. Sci. USA 2020, 117, 7001–7003. [Google Scholar] [CrossRef] [PubMed]
- Shii, T.; Miyamoto, M.; Matsuo, Y.; Tanaka, T.; Kouno, I. Biomimetic one-pot preparation of a black tea polyphenol theasinensin A from epigallocatechin gallate by treatment with copper(II) chloride and ascorbic acid. Chem. Pharm. Bull. 2011, 59, 1183–1185. [Google Scholar] [CrossRef]
- Pezzotti, G.; Ohgitani, E.; Shin-Ya, M.; Adachi, T.; Marin, E.; Boschetto, F.; Zhu, W.; Mazda, O. Instantaneous “catch-and-kill” inactivation of SARS-CoV-2 by nitride ceramics. Clin. Transl. Med. 2020, 10, e212. [Google Scholar] [CrossRef] [PubMed]
- Ohgitani, E.; Shin-Ya, M.; Ichitani, M.; Kobayashi, M.; Takihara, T.; Kawamoto, M.; Kinugasa, H.; Mazda, O. Significant inactivation of SARS-CoV-2 by a green tea catechin, a catechin-derivative and galloylated theaflavins in vitro. BioRxiv 2020. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohgitani, E.; Shin-Ya, M.; Ichitani, M.; Kobayashi, M.; Takihara, T.; Kawamoto, M.; Kinugasa, H.; Mazda, O. Significant Inactivation of SARS-CoV-2 In Vitro by a Green Tea Catechin, a Catechin-Derivative, and Black Tea Galloylated Theaflavins. Molecules 2021, 26, 3572. https://doi.org/10.3390/molecules26123572
Ohgitani E, Shin-Ya M, Ichitani M, Kobayashi M, Takihara T, Kawamoto M, Kinugasa H, Mazda O. Significant Inactivation of SARS-CoV-2 In Vitro by a Green Tea Catechin, a Catechin-Derivative, and Black Tea Galloylated Theaflavins. Molecules. 2021; 26(12):3572. https://doi.org/10.3390/molecules26123572
Chicago/Turabian StyleOhgitani, Eriko, Masaharu Shin-Ya, Masaki Ichitani, Makoto Kobayashi, Takanobu Takihara, Masaya Kawamoto, Hitoshi Kinugasa, and Osam Mazda. 2021. "Significant Inactivation of SARS-CoV-2 In Vitro by a Green Tea Catechin, a Catechin-Derivative, and Black Tea Galloylated Theaflavins" Molecules 26, no. 12: 3572. https://doi.org/10.3390/molecules26123572
APA StyleOhgitani, E., Shin-Ya, M., Ichitani, M., Kobayashi, M., Takihara, T., Kawamoto, M., Kinugasa, H., & Mazda, O. (2021). Significant Inactivation of SARS-CoV-2 In Vitro by a Green Tea Catechin, a Catechin-Derivative, and Black Tea Galloylated Theaflavins. Molecules, 26(12), 3572. https://doi.org/10.3390/molecules26123572