Screening Metal–Organic Frameworks for Separation of Binary Solvent Mixtures by Compact NMR Relaxometry
Abstract
1. Introduction
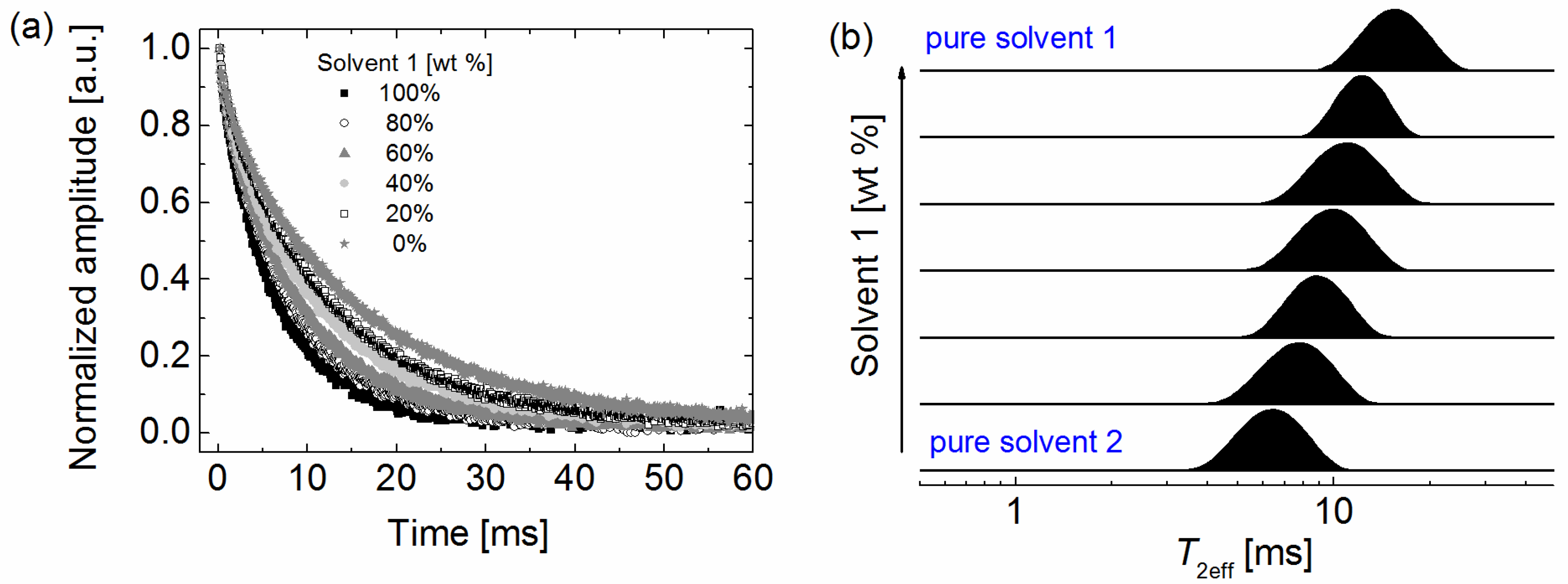
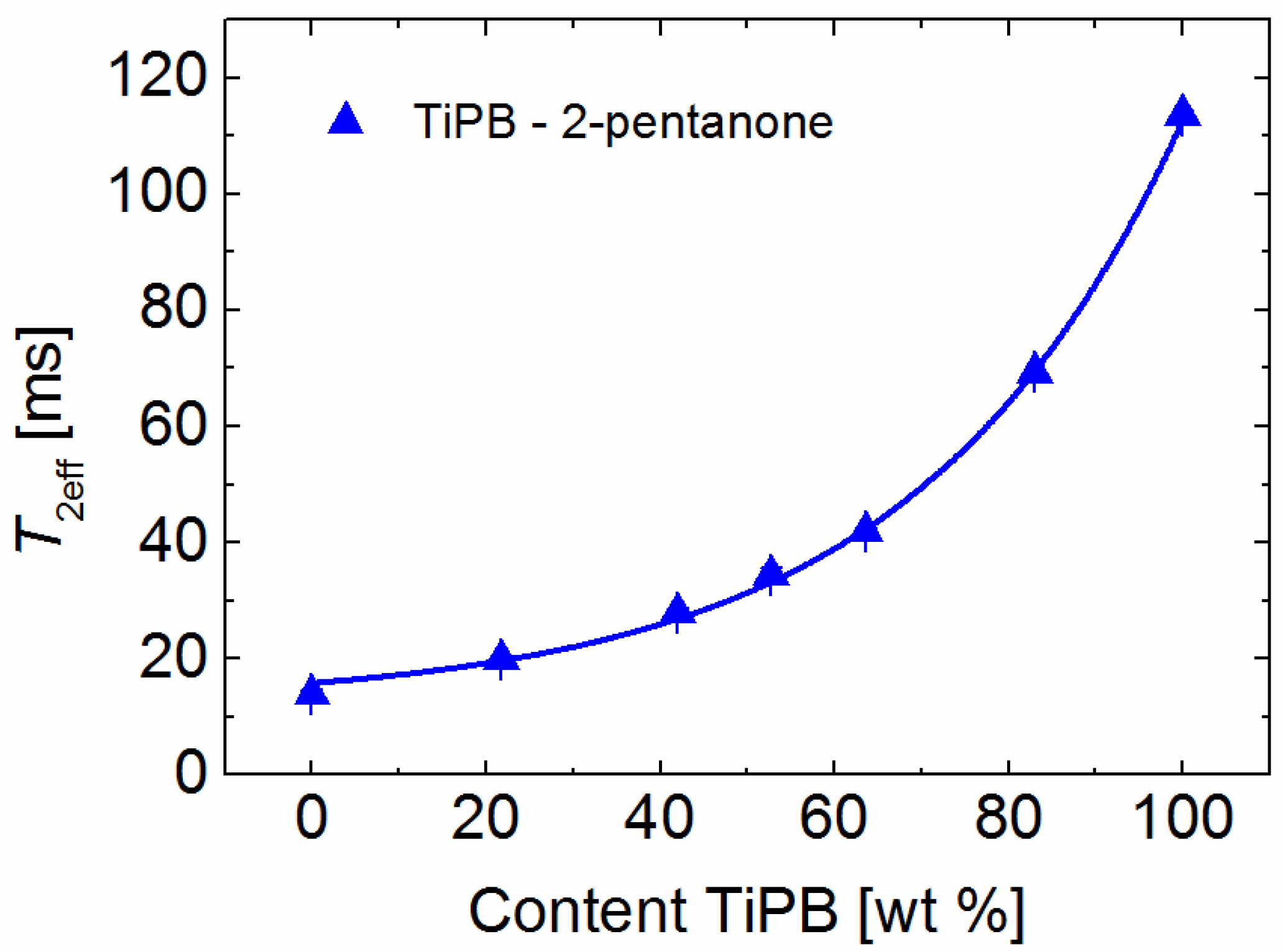
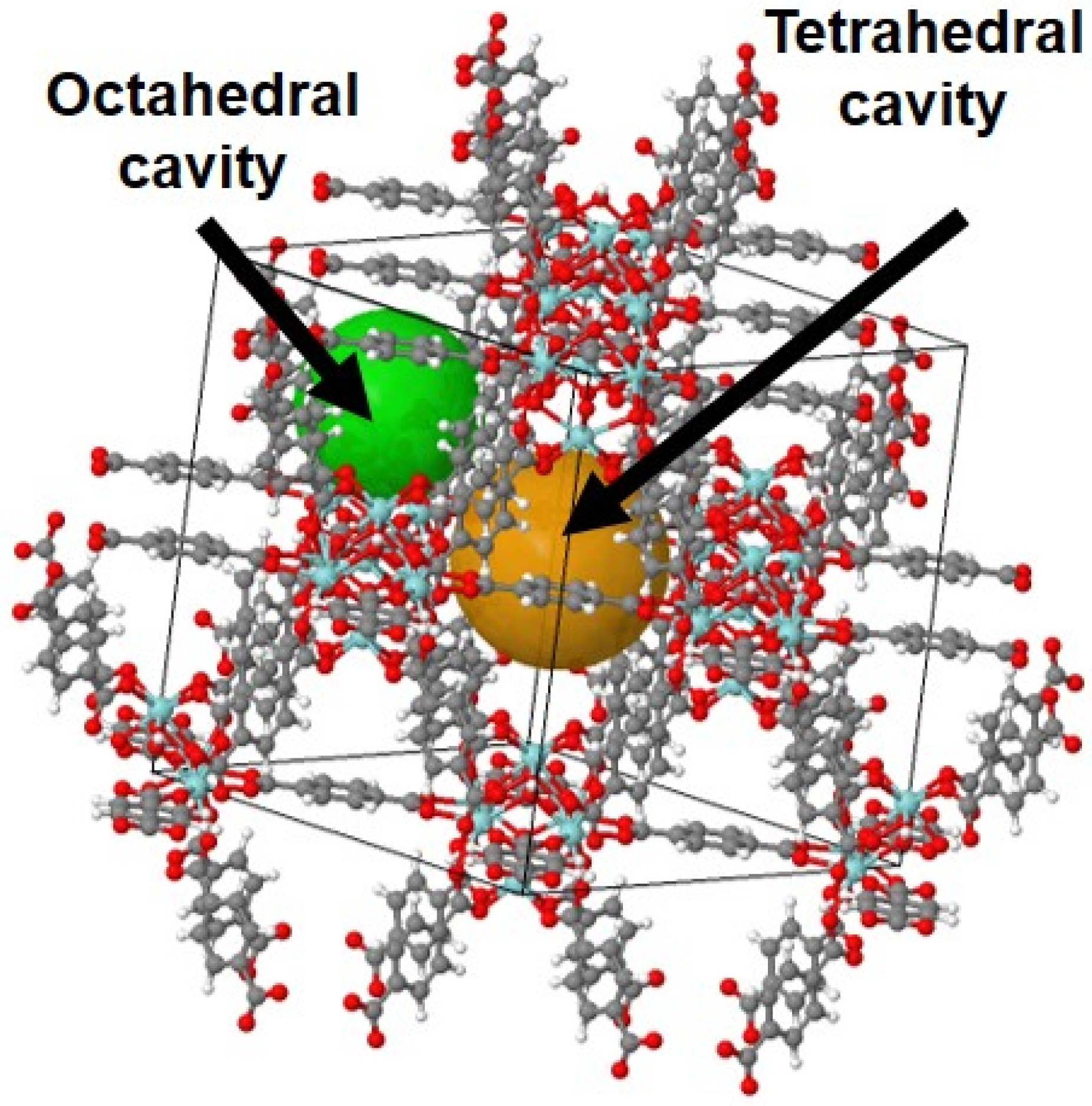
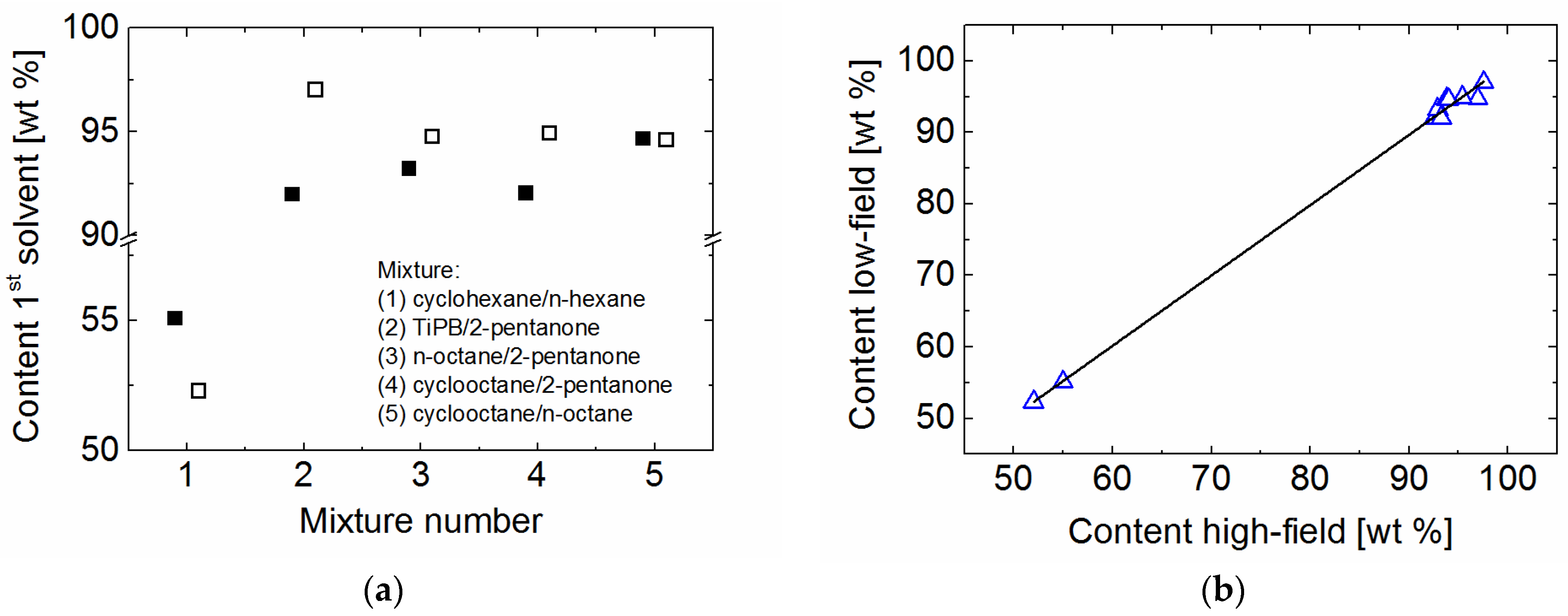
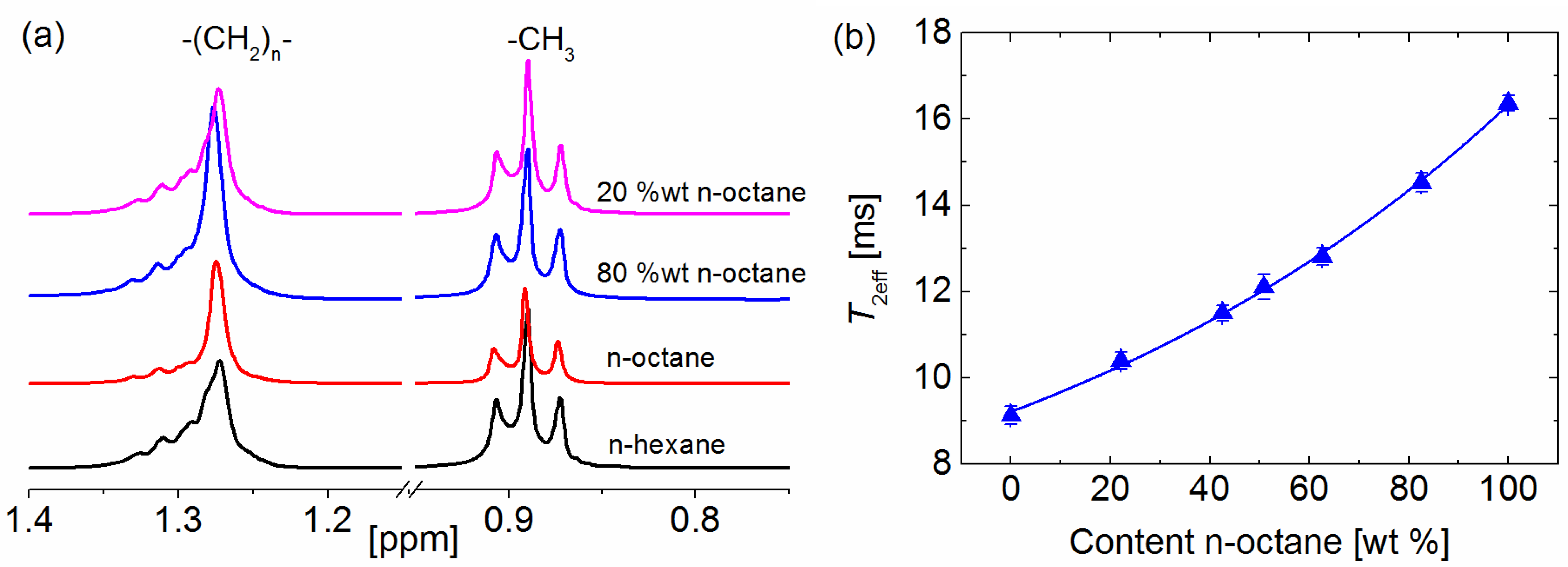
2. Results and Discussion
3. Materials and Methods
3.1. Samples
3.2. NMR Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Li, H.; Eddaoudi, M.; O’Keeffe, M.; Yaghi, O.M. Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature 1999, 402, 276–279. [Google Scholar] [CrossRef]
- Furukawa, H.; Ko, N.; Go, Y.B.; Aratani, N.; Choi, S.B.; Choi, E.; Yazaydin, A.Ö.; Snurr, R.Q.; O’Keeffe, M.; Kim, J.; et al. Ultrahigh porosity in metal-organic frameworks. Science 2010, 329, 424–428. [Google Scholar] [CrossRef]
- Davis, M.E. Ordered porous materials for emerging applications. Nature 2002, 417, 813–821. [Google Scholar] [CrossRef]
- Ferey, G. Hybrid porous solids: Past, present, future. Chem. Soc. Rev. 2008, 37, 191–214. [Google Scholar] [CrossRef]
- Zhao, D.; Timmons, D.J.; Yuan, D.; Zhou, H.C. Tuning the Topology and Functionality of Metal-Organic Frameworks by Ligand Design. Acc. Chem. Res. 2011, 44, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Eddaoudi, M.; Kim, J.; Rosi, N.; Vodak, D.; Wachter, J.; O’Keeffe, M.; Yaghi, O.M. Systematic design of pore size and functionality in isoreticular MOFs and their application in methane storage. Science 2002, 295, 469–472. [Google Scholar] [CrossRef]
- Czaja, A.U.; Trukhan, N.; Müller, U. Industrial applications of metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1284–1293. [Google Scholar] [CrossRef] [PubMed]
- Millward, A.R.; Yaghi, O.M. Metal−Organic Frameworks with Exceptionally High Capacity for Storage of Carbon Dioxide at Room Temperature. J. Am. Chem. Soc. 2005, 127, 17998–17999. [Google Scholar] [CrossRef] [PubMed]
- Murray, L.J.; Dincă, M.; Long, J.R. Hydrogen storage in metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1294–1314. [Google Scholar] [CrossRef]
- Lee, J.; Farha, O.K.; Roberts, J.; Scheidt, K.A.; Nguyen, S.T.; Hupp, J.T. Metal-organic framework materials as catalysts. Chem. Soc. Rev. 2009, 38, 1450–1459. [Google Scholar] [CrossRef]
- Kirchon, A.; Feng, L.; Drake, H.F.; Joseph, E.A.; Zhou, H. From fundamentals to applications: A toolbox for robust and multifunctional MOF materials. Chem. Soc. Rev. 2018, 47, 8611–8638. [Google Scholar] [CrossRef]
- Rasheed, T.; Rizwan, K.; Bilal, M.; Iqbal, H.M.N. Metal-Organic Framework-Based Engineered Materials—Fundamentals and Applications. Molecules 2020, 25, 1598. [Google Scholar] [CrossRef]
- Kuppler, R.J.; Timmons, D.J.; Fang, Q.; Li, J.; Makal, T.A.; Young, M.D.; Yuan, D.; Zhao, D.; Zhuang, W.; Zhou, H. Potential applications of metal-organic frameworks. Coord. Chem. Rev. 2009, 253, 3042–3066. [Google Scholar] [CrossRef]
- Bai, Y.; Dou, Y.; Xie, L.; Rutledge, W.; Li, J.; Zhou, H. Zr-based metal–organic frameworks: Design, synthesis, structure, and applications. Chem. Soc. Rev. 2016, 45, 2327–2367. [Google Scholar] [CrossRef]
- Herm, Z.R.; Wiers, B.M.; Mason, J.A.; van Baten, J.M.; Hudson, M.R.; Zajdel, P.; Brown, C.M.; Masciocchi, N.; Krishna, R.; Long, J.R. Separation of Hexane Isomers in a Metal-Organic Framework with Triangular Channels. Science 2015, 340, 960–964. [Google Scholar] [CrossRef] [PubMed]
- Bloch, E.D.; Queen, W.L.; Krishna, R.; Zadrozny, J.M.; Brown, C.M.; Long, J.R. Hydrocarbon Separations in a Metal-Organic Framework with Open Iron(II) Coordination Sites. Science 2012, 335, 1606–1610. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Jiang, J.; Wu, D.; Xu, J.; Xue, B.; Kirillov, A.M. A Highly Stable Nanotubular MOF Rotator for Selective Adsorption of Benzene and Separation of Xylene Isomers. Inorg. Chem. 2015, 54, 10524–10526. [Google Scholar] [CrossRef]
- Herm, Z.R.; Bloch, E.D.; Long, J.R. Hydrocarbon Separations in Metal−Organic Frameworks. Chem. Mater. 2014, 26, 323–338. [Google Scholar] [CrossRef]
- Mukherjee, S.; Desai, A.V.; Ghosh, S.K. Potential of metal–organic frameworks for adsorptive separation of industrially and environmentally relevant liquid mixtures. Coord. Chem. Rev. 2018, 367, 82–126. [Google Scholar] [CrossRef]
- Sholl, D.S.; Lively, R.P. Seven chemical separations to change the world. Nature 2016, 532, 435–437. [Google Scholar] [CrossRef]
- Oak Ridge National Laboratories. Materials for Separation Technologies: Energy and Emission Reduction Opportunities; Oak Ridge National Laboratories: Oak Ridge, TN, USA, 2005.
- Gadalla, M.A.; Olujic, Z.; Jansens, P.J.; Jobson, M.; Smith, R. Reducing CO2 Emissions and Energy Consumption of Heat-Integrated Distillation Systems. Environ. Sci. Technol. 2005, 39, 6860–6870. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, L.; Wang, Y.; Li, J.-R.; Li, J. Exploiting the Pore Size and Functionalization Effect in UiO Topology Structures Used for the Separation of Light Hydrocarbons. Cryst. Eng. Commun. 2017, 19, 1729–1737. [Google Scholar] [CrossRef]
- Gutierrez-Sevillano, J.; Calero, S.; Krishna, R. Selective Adsorption of Water from Mixtures with 1-Alcohols by Exploitation of Molecular Packing Effects in CuBTC. J. Phys. Chem. C 2015, 119, 3658–3666. [Google Scholar] [CrossRef]
- Lin, J.M.; He, C.T.; Liu, Y.; Liao, P.Q.; Zhou, D.D.; Zhang, J.P.; Chen, X.M. Metal-organic framework with a pore size/shape suitable for strong binding and close packing of methane. Angew. Chem. Int. Ed. 2016, 55, 4674–4678. [Google Scholar] [CrossRef] [PubMed]
- Henke, S.; Schneemann, A.; Wütscher, A.; Fischer, R.A. Directing the Breathing Behavior of Pillared-Layered Metal–Organic Frameworks via a Systematic Library of Functionalized Linkers Bearing Flexible Substituents. J. Am. Chem. Soc. 2012, 134, 9464–9474. [Google Scholar] [CrossRef]
- Grape, E.S.; Xu, H.; Cheung, O.; Calmels, M.; Zhao, J.; Dejoie, C.; Proserpio, D.M.; Zou, X.; Inge, A.K. Breathing Metal–Organic Framework Based on Flexible Inorganic Building Units. Cryst. Growth Des. 2020, 20, 320–329. [Google Scholar] [CrossRef]
- Shi, Y.X.; Li, W.X.; Zhang, W.H.; Lang, J.P. Guest-Induced Switchable Breathing Behavior in a Flexible Metal–Organic Framework with Pronounced Negative Gas Pressure. Inorg. Chem. 2018, 57, 8627–8633. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef]
- Wilmer, C.E.; Leaf, M.; Lee, C.Y.; Farka, O.K.; Hauser, B.G.; Hupp, J.T.; Snurr, R.Q. Large-scale screening of hypothetical metal-organic frameworks. Nat. Chem. 2012, 4, 83–89. [Google Scholar] [CrossRef]
- Colón, Y.J.; Snurr, R.Q. High-throughput computational screening of metal–organic frameworks. Chem. Soc. Rev. 2014, 43, 5735–5749. [Google Scholar] [CrossRef]
- Chung, Y.G.; Bai, P.; Haranczyk, M.; Leperi, K.T.; Li, P.; Zhang, H.; Wang, T.C.; Duerinck, T.; You, F.; Hupp, J.T.; et al. Computational Screening of Nanoporous Materials for Hexane and Heptane Isomer Separation. Chem. Mater. 2017, 29, 6315–6328. [Google Scholar] [CrossRef]
- Tian, F.; Zhang, X.; Chen, Y. Highly selective adsorption and separation of dichloromethane/trichloromethane on a copper-based metal–organic framework. RSC Adv. 2016, 6, 31214–31224. [Google Scholar] [CrossRef]
- Krishna, R. Screening metal-organic frameworks for mixture separations in fixed-bed adsorbers using a combined selectivity/capacity metric. RSC Adv. 2017, 7, 35724–35737. [Google Scholar] [CrossRef]
- Gutierrez-Sevillano, J.J.; Calero, S.; Krishna, R. Separation of benzene from mixtures with water, methanol, ethanol, and acetone: Highlighting hydrogen bonding and molecular clustering influences in CuBTC. Phys. Chem. Chem. Phys. 2015, 17, 20114–20124. [Google Scholar] [CrossRef]
- Zalesskiy, S.S.; Danieli, E.; Blümich, B.; Ananikov, V.P. Miniaturization of NMR systems: Desktop spectrometers, microcoil spectroscopy, and “NMR on a chip” for chemistry, biochemistry, and industry. Chem. Rev. 2014, 114, 5641–5694. [Google Scholar] [CrossRef]
- Mitchell, J.; Gladden, L.; Chandrasekera, T.; Fordham, E. Low-field permanent magnets for industrial process and quality control. Prog. Nucl. Mag. Res. Spectrosc. 2014, 76, 1–60. [Google Scholar] [CrossRef] [PubMed]
- Adams, A. Analysis of solid technical polymers by compact NMR. Trends Anal. Chem. 2016, 83, 107–119. [Google Scholar] [CrossRef]
- Adams, A.; Kwamen, R.; Woldt, B.; Graß, M. Nondestructive quantification of local plasticizer concentration in PVC by 1H NMR relaxometry. Macromol. Rapid Commun. 2015, 36, 2171–2175. [Google Scholar] [CrossRef]
- Adams, A. Non-destructive analysis of polymers and polymer-based materials by compact NMR. Magn. Reson. Imaging 2019, 56, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Kong, X.; Sumida, K.; Manumpil, M.A.; Long, J.R.; Reimer, J.A. Ex situ NMR relaxometry of metal-organic frameworks for rapid surface-area screening. Angew. Chem. Int. Ed. 2013, 52, 12043–12046. [Google Scholar] [CrossRef] [PubMed]
- Witherspoon, V.J.; Yu, L.M.; Jawahery, S.; Braun, E.; Moosavi, S.M.; Schnell, S.K.; Smit, B.; Reimer, J.A. Translational and Rotational Motion of C8 Aromatics Adsorbed in Isotropic Porous Media (MOF-5): NMR Studies and MD Simulations. J. Phys. Chem. C 2017, 121, 15456–15462. [Google Scholar] [CrossRef]
- Horch, C.; Schlayer, S.; Stallmach, F. High-pressure low-field 1H NMR relaxometry in nanoporous materials. J. Magn. Reson. 2014, 240, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Carr, H.; Purcell, E. Effects of Diffusion on Free Precession in Nuclear Magnetic Resonance Experiments. Phys. Rev. 1954, 94, 630–638. [Google Scholar] [CrossRef]
- Meiboom, S.; Gill, D. Modified Spin-Echo Method for Measuring Nuclear Relaxation Times. Rev. Sci. Instrum. 1958, 29, 688–691. [Google Scholar] [CrossRef]
- Tofts, P.S.; Lloyd, D.; Clark, C.A.; Barker, G.J.; Parker, G.J.M.; McConville, P.; Baldock, C.; Pope, J.M. Test Liquids for Quantitative MRI Measurements of Self-Diffusion Coefficient In Vivo. Magn. Reson. Med. 2000, 43, 368–374. [Google Scholar] [CrossRef]
- Korb, J.-P. Multiscale nuclear magnetic relaxation dispersion of complex liquids in bulk and confinement. Prog. Nucl. Magn. Reson. Spectrosc. 2018, 104, 12–55. [Google Scholar] [CrossRef]
- UiO-66 Metal Organic Framework. Available online: https://www.chemtube3d.com/mof-uio66/ (accessed on 23 April 2021).
- Cavka, J.H.; Jakobsen, S.; Olsbye, U.; Guillou, N.; Lamberti, C.; Bordiga, S.; Lillerud, K.P. A New Zirconium Inorganic Building Brick Forming Metal Organic Frameworks with Exceptional Stability. J. Am. Chem. Soc. 2008, 130, 13850–13851. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; van der Voort, P.A. General Strategy for the Synthesis of Functionalised UiO-66 Frameworks: Characterisation, Stability and CO2 Adsorption Properties. Eur. J. Inorg. Chem. 2013, 2154–2160. [Google Scholar] [CrossRef]
- Barcia, P.S.; Guimaraes, D.; Mendes, P.A.P.; Silva, J.A.C.; Guillerm, V.; Chevreau, H.; Serre, C.; Rodrigues, A.E. Reverse Shape Selectivity in the Adsorption of Hexane and Xylene Isomers in MOF UiO-66. Microporous Mesoporous Mater. 2011, 139, 67–73. [Google Scholar] [CrossRef]
- Duerinck, T.; Bueno-Perez, R.; Vermoortele, F.; De Vos, D.E.; Calero, S.; Baron, G.V.; Denayer, J.F.M. Understanding Hydrocarbon Adsorption in the UiO-66 Metal-Organic Framework: Separation of (Un)saturated Linear, Branched, Cyclic Adsorbates, Including Stereoisomers. J. Phys. Chem. C 2013, 117, 12567–12578. [Google Scholar] [CrossRef]
- Bozbiyik, B.; Duerinck, T.; Lannoeye, J.; De Vos, D.E.; Baron, G.V.; Denayer, J.F.M. Adsorption and separation of n-hexane and cyclohexane on the UiO-66 metal–organic framework. Microporous Mesoporous Mater. 2014, 183, 143–149. [Google Scholar] [CrossRef]
- Usman, M.; Helal, A.; Abdelnaby, M.M.; Alloush, A.M.; Zeama, M.; Yamini, Z.H. Trends and Prospects in UiO-66 Metal-Organic Framework for CO2 Capture, Separation, and Conversion. Chem. Rec. 2021, 21, 1–22. [Google Scholar] [CrossRef]
- Thomas, L.L.; Christakis, T.J.; Jorgensen, W.L. Conformation of Alkanes in the Gas Phase and Pure Liquids. J. Phys. Chem. B 2006, 110, 21198–21204. [Google Scholar] [CrossRef]
- Nikki, K.; Inakura, H.; Wu-Le; Suzuki, N.; Endo, T. Remarkable changes in conformations of n-alkanes with their carbon numbers and aromatic solvents. J. Chem. Soc. Perkin Trans. 2000, 2370–2373. [Google Scholar] [CrossRef]
- Yang, Q.; Jobic, H.; Salles, F.; Kolokolov, D.; Guillerm, V.; Serre, C.; Maurin, G. Probing the Dynamics of CO2 and CH4 within the Porous Zirconium Terephthalate UiO-66(Zr): A Synergic Combination of Neutron Scattering Measurements and Molecular Simulations. Chem. Eur. J. 2011, 17, 8882–8889. [Google Scholar] [CrossRef]
- Zhao, W.W.; Zhang, C.Y.; Yan, Z.G.; Bai, L.P.; Wang, X.; Huang, H.; Zhou, Y.Y.; Xie, Y.; Li, F.S.; Li, J.R. Separations of substituted benzenes and polycyclic aromatic hydrocarbons using normal- and reverse-phase high performance liquid chromatography with UiO-66 as the stationary phase. J. Chromatogr. A 2014, 1370, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Strem Chemicals. Available online: https://www.strem.com/catalog/v/40-1105/85/zirconium_1072413-89-8 (accessed on 23 April 2021).
- Fischer, J.; Weiss, A. Transport properties of liquids. V. self-diffusion, viscosity, and mass density of ellipsoidal shaped molecules in the pure liquid phase. Ber. Bunsenges. Phys. Chem. 1986, 90, 896–905. [Google Scholar] [CrossRef]
- Funke, H.H.; Argo, A.M.; Falconer, J.L.; Noble, R.D. Separations of Cyclic, Branched, and Linear Hydrocarbon Mixtures through Silicalite Membranes. Ind. Eng. Chem. Res. 1997, 36, 137–143. [Google Scholar] [CrossRef]
- Chua, L.M.; Hitchcock, I.; Fletcher, R.S.; Holt, E.M.; Lowe, J.; Rigby, S.P. Understanding the Spatial Distribution of Coke Deposition within Bimodal Micro-/Mesoporous Catalysts using a Novel Sorption Method in Combination with Pulsed-gradient Spin echo NMR. J. Catal. 2012, 286, 260–265. [Google Scholar] [CrossRef][Green Version]
- Van der Perre, S.; Van Assche, T.; Bozbiyik, B.; Lannoeye, J.; De Vos, D.E.; Baron, G.V.; Denayer, J.F.M. Adsorptive Characterization of the ZIF-68 Metal-Organic Framework: A Complex Structure with Amphiphilic Properties. Langmuir 2014, 30, 8416–8424. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wagemann, M.; Radzik, N.; Krzyżak, A.; Adams, A. Screening Metal–Organic Frameworks for Separation of Binary Solvent Mixtures by Compact NMR Relaxometry. Molecules 2021, 26, 3481. https://doi.org/10.3390/molecules26123481
Wagemann M, Radzik N, Krzyżak A, Adams A. Screening Metal–Organic Frameworks for Separation of Binary Solvent Mixtures by Compact NMR Relaxometry. Molecules. 2021; 26(12):3481. https://doi.org/10.3390/molecules26123481
Chicago/Turabian StyleWagemann, Marc, Natalia Radzik, Artur Krzyżak, and Alina Adams. 2021. "Screening Metal–Organic Frameworks for Separation of Binary Solvent Mixtures by Compact NMR Relaxometry" Molecules 26, no. 12: 3481. https://doi.org/10.3390/molecules26123481
APA StyleWagemann, M., Radzik, N., Krzyżak, A., & Adams, A. (2021). Screening Metal–Organic Frameworks for Separation of Binary Solvent Mixtures by Compact NMR Relaxometry. Molecules, 26(12), 3481. https://doi.org/10.3390/molecules26123481