Enhancement of the Antioxidant and Skin Permeation Properties of Betulin and Its Derivatives
Abstract
1. Introduction
2. Results
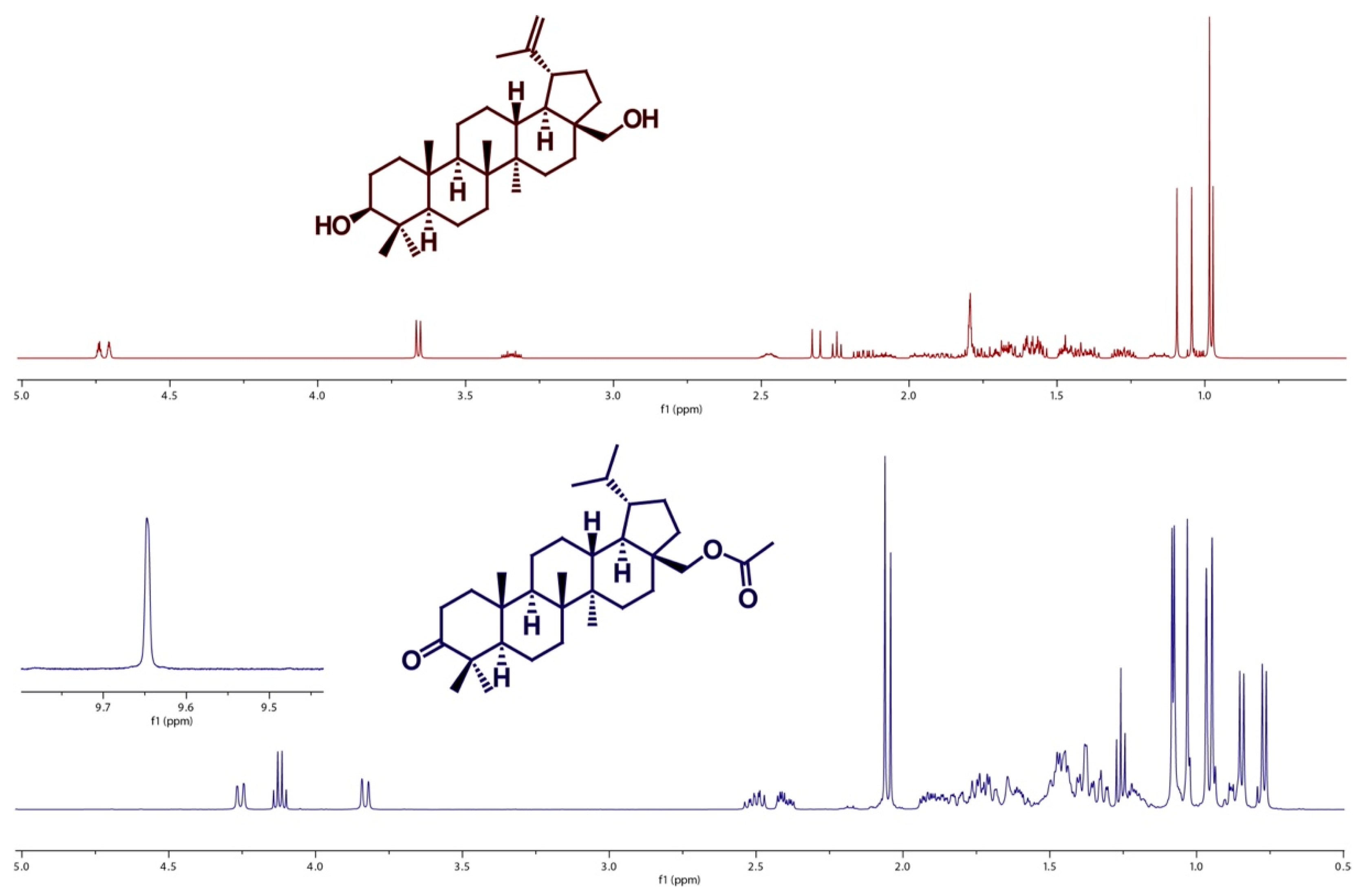
2.1. Betulin and Its Derivatives
2.2. Evaluation of Free Radical Scavenging Activity
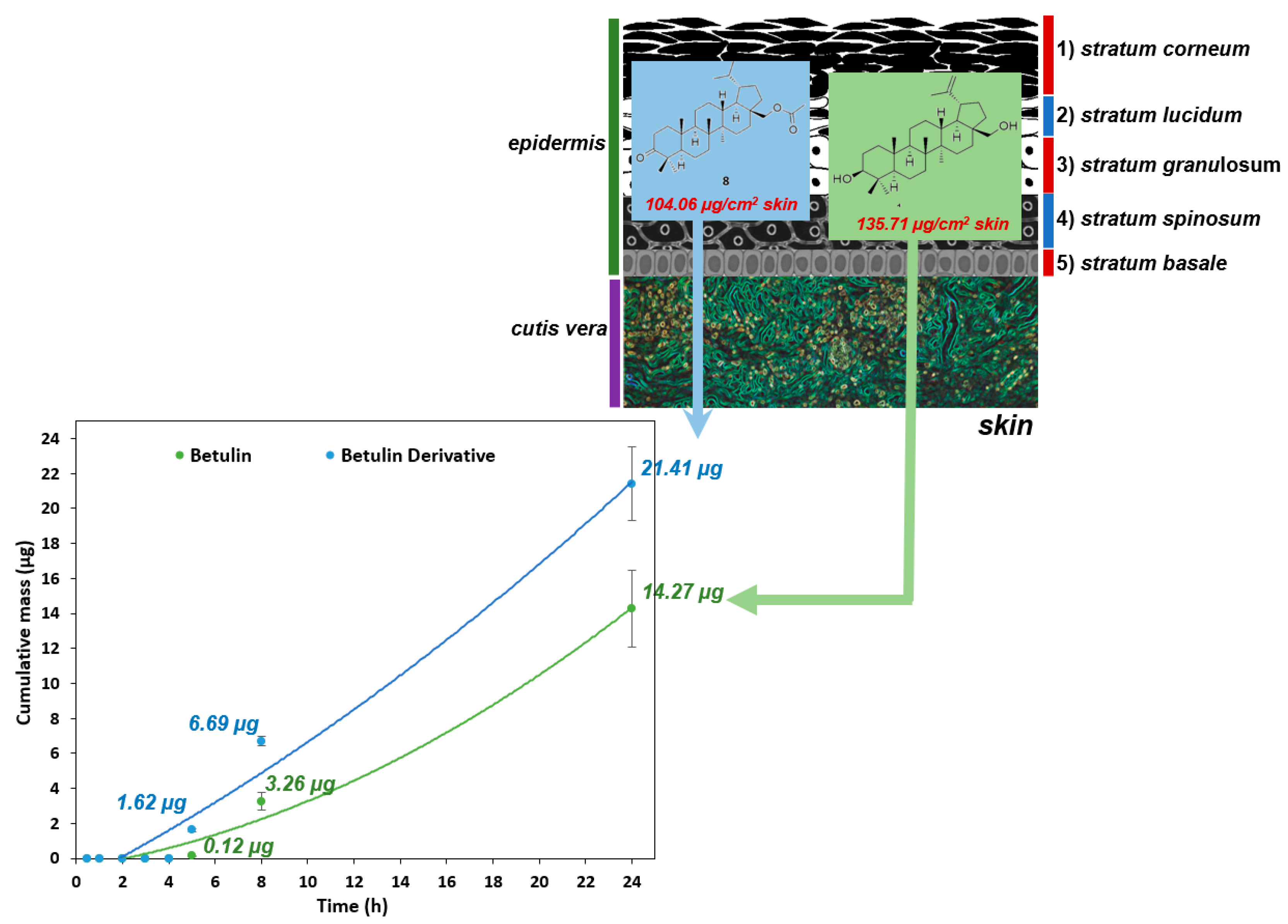
2.3. Skin Penetration and Skin Extraction
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Plant Material
4.3. Preparation of Betulin and Characterization of Betulin and Its Derivatives
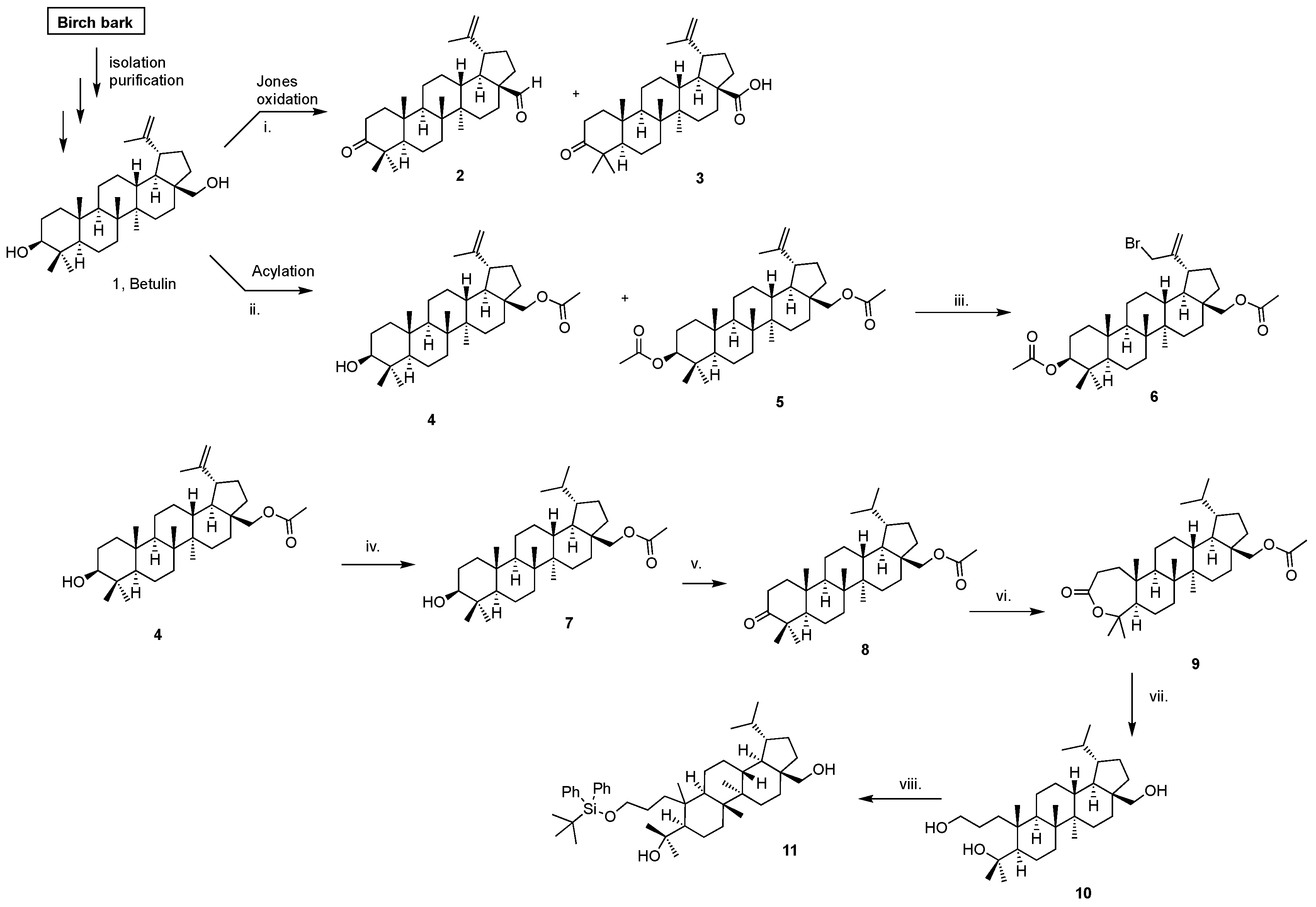
4.3.1. Preparation of the Birch Bark and Isolation of Betulin from the Birch Bark
4.3.2. Synthesis of Betulonic Aldehyde (2) and Betulonic Acid (3)
4.3.3. Synthesis of Compounds 4 and 5 by Acetylation of Betulin
4.3.4. Synthesis of Compound 6 (30-bromolup-20(29)-ene-3β,28-diyl diacetate)
4.3.5. Synthesis of Compound 7 (3β,28-lupanediol 28-acetate)
4.3.6. Synthesis of Compounds 8 (28-acetoxy-3-lupanone)
4.3.7. Synthesis of Compound 9
4.3.8. Synthesis of Compound 10
4.3.9. Synthesis of Compound 11
4.4. Evaluation of Free Radical Scavenging Activity
4.4.1. Evaluation of Free Radical Scavenging Activity Using DPPH Method
4.4.2. Evaluation of Free Radical Scavenging Activity Using ABTS Method
4.4.3. Evaluation of Total Polyphenol Content Using Folin–Ciocalteu Method
4.5. Skin Electrical Impedance
4.6. Skin Penetration
4.7. Skin Extraction
4.8. HPLC Analysis
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Fang, J.Y.; Leu, Y.L.; Hwang, T.L.; Cheng, H.C.; Hung, C.F. Development of sesquiterpenes from Alpinia oxyphylla as novel skin permeation enhancers. Eur. J. Pharm. Sci. 2003, 19, 253–262. [Google Scholar] [CrossRef]
- Torin Huzil, J.; Sivaloganathan, S.; Kohandel, M.; Foldvari, M. Drug delivery through the skin: Molecular simulations of barrier lipids to design more effective noninvasive dermal and transdermal delivery systems for small molecules, biologics, and cosmetics. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnology 2011, 3, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Puglia, C.; Bonina, F. Lipid nanoparticles as novel delivery systems for cosmetics and dermal pharmaceuticals. Expert Opin. Drug Deliv. 2012, 9, 429–441. [Google Scholar] [CrossRef]
- Ossowicz, P.; Klebeko, J.; Janus, E.; Nowak, A.; Duchnik, W.; Kucharski, Ł.; Klimowicz, A. The effect of alcohols as cosmetic formulations on the percutaneous absorption and skin retention of ibuprofen modified with l-valine alkyl esters. RSC Adv. 2020, 10, 41727–41740. [Google Scholar] [CrossRef]
- Janus, E.; Ossowicz, P.; Klebeko, J.; Nowak, A.; Duchnik, W.; Kucharski, Ł.; Klimowicz, A. Enhancement of ibuprofen solubility and skin permeation by conjugation with L-valine alkyl esters. RSC Adv. 2020, 10, 7570–7584. [Google Scholar] [CrossRef]
- Khezri, K.; Saeedi, M.; Maleki Dizaj, S. Application of nanoparticles in percutaneous delivery of active ingredients in cosmetic preparations. Biomed. Pharmacother. 2018, 106, 1499–1505. [Google Scholar] [CrossRef]
- Pinzaru, I.; Tanase, A.; Enatescu, V.; Coricovac, D.; Bociort, F.; Marcovici, I.; Watz, C.; Vlaia, L.; Soica, C.; Dehelean, C. Proniosomal gel for topical delivery of rutin: Preparation, physicochemical characterization and in vitro toxicological profile using 3d reconstructed human epidermis tissue and 2d cells. Antioxid. 2021, 10, 85. [Google Scholar] [CrossRef]
- Ramadon, D.; McCrudden, M.T.C.; Courtenay, A.J.; Donnelly, R.F. Enhancement strategies for transdermal drug delivery systems: Current trends and applications. Drug Deliv. Transl. Res. 2021, 1–34. [Google Scholar] [CrossRef]
- Tambunlertchai, S.; Geary, S.M.; Salem, A.K. Skin Penetration Enhancement Strategies Used in the Development of Melanoma Topical Treatments. AAPS J. 2021, 23, 19. [Google Scholar] [CrossRef]
- Fox, L.T.; Gerber, M.; Du Plessis, J.; Hamman, J.H. Transdermal drug delivery enhancement by compounds of natural origin. Mol. 2011, 16, 10507–10540. [Google Scholar] [CrossRef]
- Hadgraft, J. Skin the final frontier. Int. J. Pharm. 2001, 224, 1–18. [Google Scholar] [CrossRef]
- Hsieh, D.S. Drug Permeation Enhancement—Theory and Applications; Marcel Dekker: New York, NY, USA, 1993. [Google Scholar]
- Makuch, E.; Nowak, A.; Günther, A.; Pełech, R.; Kucharski, Ł.; Duchnik, W.; Klimowicz, A. Enhancement of the antioxidant and skin permeation properties of eugenol by the esterification of eugenol to new derivatives. AMB Express 2020, 10, 187. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, S.; Pandey, M.M.; Kumar, A.; Rawat, S. Medicinal plants of the genus Betula—Traditional uses and a phytochemical–pharmacological review. J. Ethnopharmacol. 2020, 159, 62–83. [Google Scholar] [CrossRef]
- Venza, M.; Visalli, M.; Beninati, C.; De Gaetano, G.V.; Teti, D.; Venza, I. Cellular Mechanisms of Oxidative Stress and Action in Melanoma. Oxid. Med. Cell. Longev. 2015, 2015, 481782. [Google Scholar] [CrossRef]
- Chrobak, E.; Kadela-Tomanek, M.; Bębenek, E.; Marciniec, K.; Wietrzyk, J.; Trynda, J.; Pawełczak, B.; Kusz, J.; Kasperczyk, J.; Chodurek, E. New phosphate derivatives of betulin as anticancer agents: Synthesis, crystal structure, and molecular docking study. Bioorg. Chem. 2019, 87, 613–628. [Google Scholar] [CrossRef] [PubMed]
- Drag-Zalesinska, M.; Kulbacka, J.; Saczko, J.; Wysocka, T.; Zabel, M.; Surowiak, P.; Drag, M. Esters of betulin and betulinic acid with amino acids have improved water solubility and are selectively cytotoxic toward cancer cells. Bioorganic Med. Chem. Lett. 2009, 19, 4814–4817. [Google Scholar] [CrossRef]
- Koch, B.P.; Rullkötter, J.; Lara, R.J. Evaluation of triterpenols and sterols as organic matter biomarkers in a mangrove ecosystem in northern Brazil. Wetl. Ecol. Manag. 2003, 11, 257–263. [Google Scholar] [CrossRef]
- Niewolik, D.; Krukiewicz, K.; Bednarczyk-Cwynar, B.; Ruszkowski, P.; Jaszcz, K. Novel polymeric derivatives of betulin with anticancer activity. RSC Adv. 2019, 9, 20892–20900. [Google Scholar] [CrossRef]
- Sousa, J.L.C.; Freire, C.S.R.; Silvestre, A.J.D.; Silva, A.M.S. Recent developments in the functionalization of betulinic acid and its natural analogues: A route to new bioactive compounds. Molecules 2019, 24, 355. [Google Scholar] [CrossRef]
- Saneja, A.; Kumar, R.; Singh, A.; Dhar Dubey, R.; Mintoo, M.J.; Singh, G.; Mondhe, D.M.; Panda, A.K.; Gupta, P.N. Development and evaluation of long-circulating nanoparticles loaded with betulinic acid for improved anti-tumor efficacy. Int. J. Pharm. 2017, 531, 153–166. [Google Scholar] [CrossRef]
- Karlina, M.V; Pozharitskaya, O.N.; Shikov, A.N.; Makarov, V.G.; Mirza, S.; Miroshnyk, I.; Hiltunen, R. Drug synthesis methods and manufacturing technology biopharmaceutical study of nanosystems containing betulin for inhalation administration. Pharm. Chem. J. 2010, 44, 501–503. [Google Scholar] [CrossRef]
- Pozharitskaya, O.N.; Karlina, M.V; Shikov, A.N.; Kosman, V.M.; Makarov, V.G.; Casals, E.; Rosenholm, J.M. Pharmacokinetics and Tissue Disposition of Nanosystem- Entrapped Betulin After Endotracheal Administration to Rats. Eur J Drug Metab Pharm. 2017, 42, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Mullauer, F.B.; van Bloois, L.; Daalhuisen, J.B.; Ten, M.S.; Storm, G.; Paul, J.; Schiffelers, R.M.; Kessler, J.H. Betulinic acid delivered in liposomes reduces growth of human lung and colon cancers in mice without causing systemic toxicity. Anti-Cancer Drugs 2011, 22, 223–233. [Google Scholar] [CrossRef]
- Niewolik, D.; Bednarczyk-cwynar, B.; Ruszkowski, P.; Sosnowski, T.R.; Jaszcz, K. Bioactive Betulin and PEG Based Polyanhydrides for Use in Drug Delivery Systems. Int. J. Mol. Sci. 2021, 22, 1090. [Google Scholar] [CrossRef] [PubMed]
- Csuk, R.; Barthel, A.; Sczepek, R.; Siewert, B.; Schwarz, S. Synthesis, encapsulation and antitumor activity of new betulin derivatives. Arch. Pharm. Chem. Life Sci. 2011, 1, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Sharaf, A.; De Michele, R.; Sharma, A.; Fakhari, S. Transcriptomic analysis reveals the roles of detoxification systems in response to mercury in Chromera velia. Biomolecules 2019, 9, 647. [Google Scholar] [CrossRef]
- Bi, Y.; Xu, J.; Wu, X.; Ye, W.; Yuan, S.; Zhang, L. Synthesis and cytotoxic activity of 17-carboxylic acid modified 23-hydroxy betulinic acid ester derivatives. Bioorganic Med. Chem. Lett. 2007, 17, 1475–1478. [Google Scholar] [CrossRef]
- Yamansarov, E.Y.; Saltykova, I.V.; Kovalev, S.V.; Petrov, R.A.; Seleznev, E.I.; Beloglazkina, E.K.; Majouga, A.G. Synthesis and cytotoxicity of new alkyne derivatives of pentacyclic triterpenoids. Russ. Chem. Bull. 2019, 68, 855–861. [Google Scholar] [CrossRef]
- Marciniec, K.; Pawełczak, B.; Latocha, M.; Skrzypek, L.; Maciązek-Jurczyk, M.; Boryczka, S.; Supuran, C.T. Synthesis, anti-breast cancer activity, and molecular docking study of a new group of acetylenic quinolinesulfonamide derivatives. Molecules 2017, 22, 300. [Google Scholar] [CrossRef]
- Nowak, A.; Cybulska, K.; Makuch, E.; Kucharski, Ł.; Różewicka-Czabańska, M.; Prowans, P.; Czapla, N.; Bargiel, P.; Petriczko, J.; Klimowicz, A. In Vitro Human Skin Penetration, Antioxidant and Antimicrobial Activity of Ethanol-Water Extract of Fireweed (Epilobium angustifolium L.). Molecules 2021, 26, 329. [Google Scholar] [CrossRef]
- Redasani, V.K.; Bari, S.B. Prodrug Design: Perspectives, Approaches and Applications in Medicinal Chemistry, 1st ed.; Academic Press: London, UK, 2015. [Google Scholar]
- Wang, X.; Wang, W.; Jin, S.; Muhammad, N.; Guo, Z. Stimuli-responsive therapeutic metallodrugs. Chem. Rev. 2019, 119, 1138–1192. [Google Scholar] [CrossRef]
- Achrem-Achremowicz, J.; Kȩpczyńska, E.; Zylewski, M.; Janeczko, Z. Synthesis of betulin derivatives and the determination of their relative lipophilicities using reversed-phase thin-layer chromatography. Biomed. Chromatogr. 2010, 24, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Sangster, J. Octanol-Water Partition Coefficients: Fundamentals and Physical Chemistry; John Wiley & Sons: Chichester, UK, 1997; p. 170. [Google Scholar]
- Kadela-Tomanek, M.; Jastrzębska, M.; Chrobak, E.; Bębenek, E.; Boryczka, S. Chromatographic and computational screening of lipophilicity and pharmacokinetics of newly synthesized betulin-1,4-quinone hybrids. Process. 2021, 9, 376. [Google Scholar] [CrossRef]
- Bȩbenek, E.; Bober-Majnusz, K.; Siudak, S.; Chrobak, E.; Kadela-Tomanek, M.; Wietrzyk, J.; Boryczka, S. Application of TLC to Evaluate the Lipophilicity of Newly Synthesized Betulin Derivatives. J. Chromatogr. Sci. 2020, 58, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Bildziukevich, U.; Vida, N.; Rárová, L.; Kolář, M.; Šaman, D.; Havlíček, L.; Drašar, P.; Wimmer, Z. Polyamine derivatives of betulinic acid and β-sitosterol: A comparative investigation. Steroids 2015, 100, 27–35. [Google Scholar] [CrossRef]
- Kuo, S.C.; Chuang, S.K.; Lin, H.Y.; Wang, L.H. Study of the aerosol fragrances of eugenol derivatives in Cananga odorata using diffuse reflectance infrared Fourier transform spectroscopy and gas chromatography. Anal. Chim. Acta. 2009, 653, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Chandra, A.; Sharma, P.K.; Irchhiaya, R. Effect of alcohols and enhancers on permeation enhancement of ketorolac. Asian J. Pharm. 2009, 3, 37. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: Concept and some practical aspects. Antioxidants 2020, 9, 852. [Google Scholar] [CrossRef]
- García-Sánchez, A.; Miranda-Díaz, A.G.; Cardona-Muñoz, E.G. The Role of Oxidative Stress in Physiopathology and Pharmacological Treatment with Pro- And Antioxidant Properties in Chronic Diseases. Oxid. Med. Cell. Longev. 2020, 2020, 2082145. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.Y.; Leem, E.; Lee, J.M.; Kim, S.R. Control of reactive oxygen species for the prevention of parkinson’s disease: The possible application of flavonoids. Antioxidants 2020, 9, 583. [Google Scholar] [CrossRef]
- Chakraborti, S.; Chakraborti, T.; Chattopadhyay, D.; Shaha, C. Oxidative stress in Microbial Diseases; Springer: Singapore, 2019. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Makuch, E.; Nowak, A.; Günther, A.; Pełech, R.; Kucharski, Ł. The effect of cream and gel vehicles on the percutaneous absorption and skin retention of a new eugenol derivative with antioxidant activity. Front. Pharmacol. 2021, 12, 1249. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C.L.W.T. Use of a free radical method to evaluate antioxidant activity. Lebensm. Wiss. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Nowak, A.; Klimowicz, A.; Duchnik, W.; Kucharski, Ł.L.; Florkowska, K.; Muzykiewicz, A.; Wira, D.; Zielonkabrzezicka, J.; Siedłowska, A.; Nadarzewska, K. Application of green-extraction technique to evaluate of antioxidative capacity of wild population of fireweed (Epilobium angustifolium). Herba Pol. 2020, 65, 18–30. [Google Scholar] [CrossRef]
- Davies, D.J.; Ward, R.J.; Heylings, J.R. Multi-species assessment of electrical resistance as a skin integrity marker for in vitro percutaneous absorption studies. Toxicol. Vitr. 2004, 18, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Sousa, J.L.C.; Gonçalves, C.; Ferreira, R.M.; Cardoso, S.M.; Freire, C.S.R.; Silvestre, A.J.D.; Silva, A.M.S. Functionalization of betulinic acid with polyphenolic fragments for the development of new amphiphilic antioxidants. Antioxidants 2021, 10, 148. [Google Scholar] [CrossRef] [PubMed]
- Amiri, S.; Dastghaib, S.; Ahmadi, M.; Mehrbod, P.; Khadem, F.; Behrouj, H.; Aghanoori, M.R.; Machaj, F.; Ghamsari, M.; Rosik, J. Betulin and its derivatives as novel compounds with different pharmacological effects. Biotechnol. Adv. 2020, 38, 107409. [Google Scholar] [CrossRef]
- Gomes Castro, A.J.; Cazarolli, L.H.; Bretanha, L.C.; Sulis, P.M.; Rey Padilla, D.P.; Aragón Novoa, D.M.; Dambrós, B.F.; Pizzolatti, M.G.; Mena Barreto Silva, F.R. The potent insulin secretagogue effect of betulinic acid is mediated by potassium and chloride channels. Arch. Biochem. Biophys. 2018, 648, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.S.G.; Oliveira, P.J.; Duarte, M.F. Oleanolic, Ursolic, and Betulinic Acids as Food Supplements or Pharmaceutical Agents for Type 2 Diabetes: Promise or Illusion? J. Agric. Food Chem. 2016, 64, 2991–3008. [Google Scholar] [CrossRef] [PubMed]
- Melnikova, N.; Knyazev, A.; Nikolskiy, V.; Peretyagin, P.; Belyaeva, K.; Nazarova, N.; Liyaskina, E.; Malygina, D.; Revin, V. Wound healing composite materials of bacterial cellulose and zinc oxide nanoparticles with immobilized betulin diphosphate. Nanomaterials 2021, 11, 713. [Google Scholar] [CrossRef]
- Sułkowska-Ziaja, K.; Grabowska, K.; Apola, A.; Kryczyk-Poprawa, A.; Muszyńska, B. Mycelial culture extracts of selected wood-decay mushrooms as a source of skin-protecting factors. Biotechnol. Lett. 2021, 43, 1051–1061. [Google Scholar] [CrossRef]
- Zagórska-Dziok, M.; Ziemlewska, A.; Bujak, T.; Nizioł-Łukaszewska, Z.; Hordyjewicz-Baran, Z. Cosmetic and Dermatological Properties of Selected Ayurvedic Plant Extracts. Molecules 2021, 26, 614. [Google Scholar] [CrossRef]
- Bober, K.; Bȩbenek, E.; Boryczka, S. Application of TLC for evaluation of the lipophilicity of newly synthetized esters: Betulin derivatives. J. Anal. Methods Chem. 2019, 3, 1297659–1297667. [Google Scholar] [CrossRef] [PubMed]
- Bębenek, E.; Kadela-Tomanek, M.; Chrobak, E.; Wietrzyk, J.; Sadowska, J.; Boryczka, S. New acetylenic derivatives of betulin and betulone, synthesis and cytotoxic activity. Med. Chem. Res. 2017, 26, 1–8. [Google Scholar] [CrossRef]
- Dubinin, M.V.; Semenova, A.A.; Ilzorkina, A.I.; Penkov, N.V.; Nedopekina, D.A.; Sharapov, V.A.; Khoroshavina, E.I.; Davletshin, E.V.; Belosludtseva, N.V.; Spivak, A.Y. Mitochondria-targeted prooxidant effects of betulinic acid conjugated with delocalized lipophilic cation F16. Free Radic. Biol. Med. 2021, 168, 55–69. [Google Scholar] [CrossRef]
- Cichewicz, R.H.; Kouzi, S.A. Chemistry, Biological Activity, and Chemotherapeutic Potential of Betulinic Acid for the Prevention and Treatment of Cancer and HIV Infection. Med. Res. Rev. 2004, 24, 90–114. [Google Scholar] [CrossRef]
- Haq, A.; Michniak-Kohn, B. Effects of solvents and penetration enhancers on transdermal delivery of thymoquinone: Permeability and skin deposition study. Drug Deliv. 2018, 25, 1943. [Google Scholar] [CrossRef] [PubMed]
- Kopečná, M.; Macháček, M.; Prchalová, E.; Štěpánek, P.; Drašar, P.; Kotora, M.; Vávrová, K. Galactosyl Pentadecene Reversibly Enhances Transdermal and Topical Drug Delivery. Pharm. Res. 2017, 34, 2097–2108. [Google Scholar] [CrossRef] [PubMed]
- Szuster-Ciesielska, A.; Plewka, K.; Daniluk, J.; Kandefer-Szerszen, M. Betulin and betulinic acid attenuate ethanol-induced liver stellate cell activation by inhibiting reactive oxygen species (ROS), cytokine (TNF-alpha, TGF-beta) production and by influencing intracellular signaling. Toxicology 2011, 280, 152–163. [Google Scholar] [CrossRef]

| Compounds | Formula | Molecular Weight[Da] | log P |
|---|---|---|---|
| 1 | C30H50O2 | 442.73 | 5.642 |
| 2 | C30H46O2 | 438.7 | 5.372 |
| 3 | C30H46O3 | 454.7 | 4.777 |
| 4 | C32H52O3 | 484.77 | 6.430 |
| 5 | C34H54O4 | 526.8 | 7.000 |
| 6 | C34H53BrO4 | 605.7 | 6.997 |
| 7 | C32H54O3 | 486.78 | 6.779 |
| 8 | C32H52O3 | 484.77 | 6.304 |
| 9 | C32H52O4 | 500.76 | 6.613 |
| 10 | C30H54O3 | 462.76 | 5.006 |
| 11 | C46H72O3Si | 701.16 | 9.684 |
| Compounds | * Antioxidant Activity (DPPH Method): | |
|---|---|---|
| % RSA | mmol TE/dm3 | |
| 1 | 2.81 ± 0.007 | 0.087 ± 0.003 |
| 2 | 1.50 ± 0.007 | 0.079 ± 0.011 |
| 3 | 7.04 ± 0.007 | 0.091 ± 0.001 |
| 4 | n.a. | n.a. |
| 5 | n.a. | n.a. |
| 6 | 14.78 ± 0.014 | 0.100 ± 0.002 |
| 7 | 2.80 ± 0.012 | 0.087 ± 0.001 |
| 8 | 61.83 ± 0.004 | 0.436 ± 0.005 |
| 9 | 14.11 ± 0.013 | 0.099 ± 0.002 |
| 10 | 4.84 ± 0.011 | 0.089 ± 0.001 |
| 11 | 12.68 ± 0.013 | 0.098 ± 0.001 |
| *Antioxidant Activity (ABTS method): | ||
| % RSA | mmol TE/dm3 | |
| 1 | n.a. | n.a. |
| 2 | n.a. | n.a. |
| 3 | n.a. | n.a. |
| 4 | n.a. | n.a. |
| 5 | n.a. | n.a. |
| 6 | n.a. | n.a. |
| 7 | n.a. | n.a. |
| 8 | 12.79 ± 0.005 | 0.237 ± 0.002 |
| 9 | n.a. | n.a. |
| 10 | n.a. | n.a. |
| 11 | 5.65 ± 0.015 | 0.105 ± 0.002 |
| *Antioxidant Activity (Folin–Ciocalteu Method): | ||
| mmol TE/dm3 | ||
| 1 | - | n.a. |
| 2 | - | n.a. |
| 3 | - | 0.847 ± 0.037 |
| 4 | - | n.a. |
| 5 | - | n.a. |
| 6 | - | 0.367 ± 0.081 |
| 7 | - | n.a. |
| 8 | - | 0.050 ± 0.000 |
| 9 | - | 0.413 ± 0.015 |
| 10 | - | n.a. |
| 11 | - | n.a. |
| Compounds | The Cumulative Mass of Substance after 24 h of Permeation Test: (µg) | Skin Accumulation of Substance: (µg/cm2 Skin) |
|---|---|---|
| * Betulin | 14.27 ± 2.20 a | 135.71 ± 9.11 a |
| * Betulin derivative 8 | 21.41 ± 2.10 b | 104.06 ± 15.79 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Günther, A.; Makuch, E.; Nowak, A.; Duchnik, W.; Kucharski, Ł.; Pełech, R.; Klimowicz, A. Enhancement of the Antioxidant and Skin Permeation Properties of Betulin and Its Derivatives. Molecules 2021, 26, 3435. https://doi.org/10.3390/molecules26113435
Günther A, Makuch E, Nowak A, Duchnik W, Kucharski Ł, Pełech R, Klimowicz A. Enhancement of the Antioxidant and Skin Permeation Properties of Betulin and Its Derivatives. Molecules. 2021; 26(11):3435. https://doi.org/10.3390/molecules26113435
Chicago/Turabian StyleGünther, Andrzej, Edyta Makuch, Anna Nowak, Wiktoria Duchnik, Łukasz Kucharski, Robert Pełech, and Adam Klimowicz. 2021. "Enhancement of the Antioxidant and Skin Permeation Properties of Betulin and Its Derivatives" Molecules 26, no. 11: 3435. https://doi.org/10.3390/molecules26113435
APA StyleGünther, A., Makuch, E., Nowak, A., Duchnik, W., Kucharski, Ł., Pełech, R., & Klimowicz, A. (2021). Enhancement of the Antioxidant and Skin Permeation Properties of Betulin and Its Derivatives. Molecules, 26(11), 3435. https://doi.org/10.3390/molecules26113435








